User login
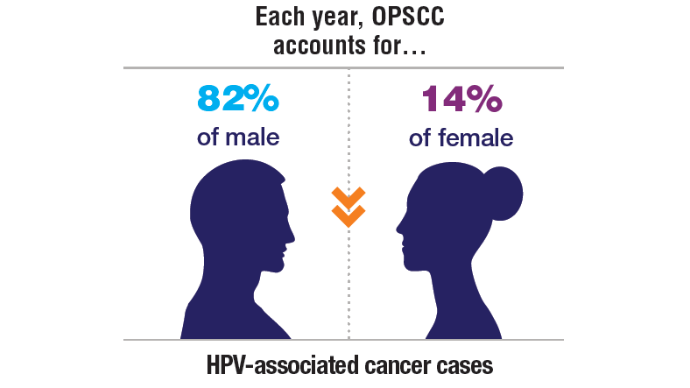
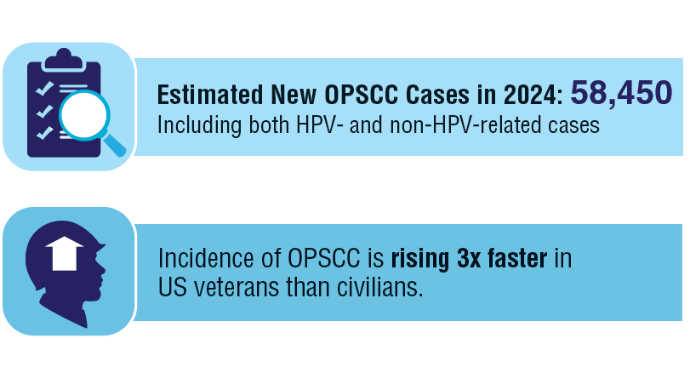
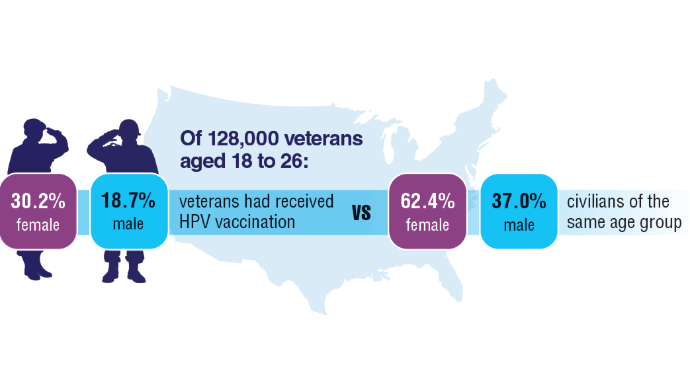
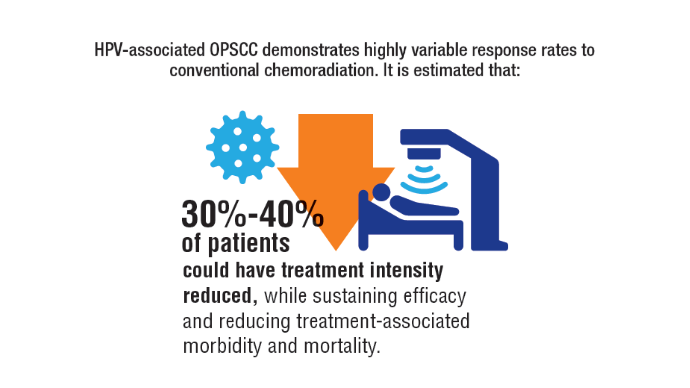
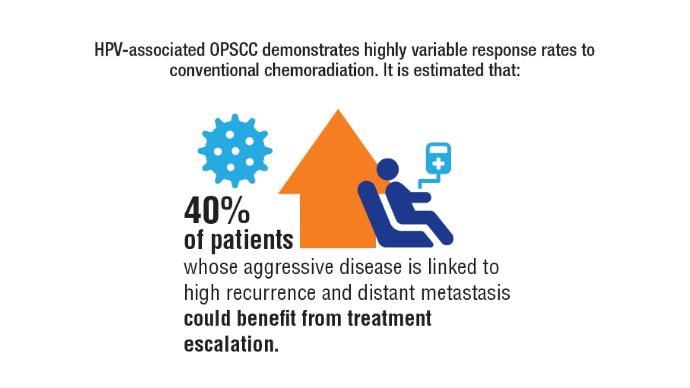
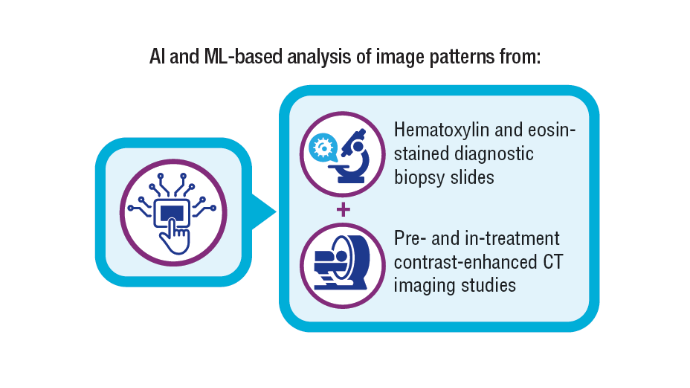
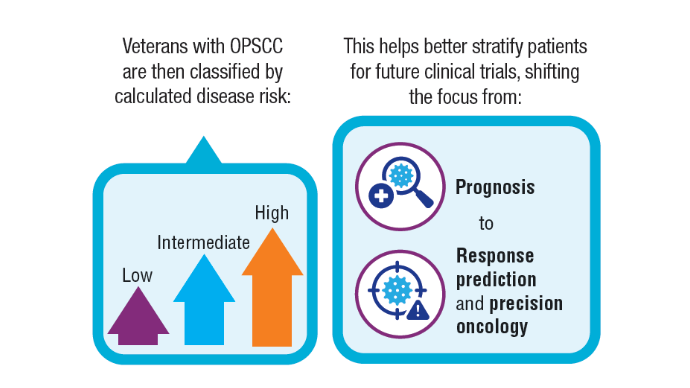
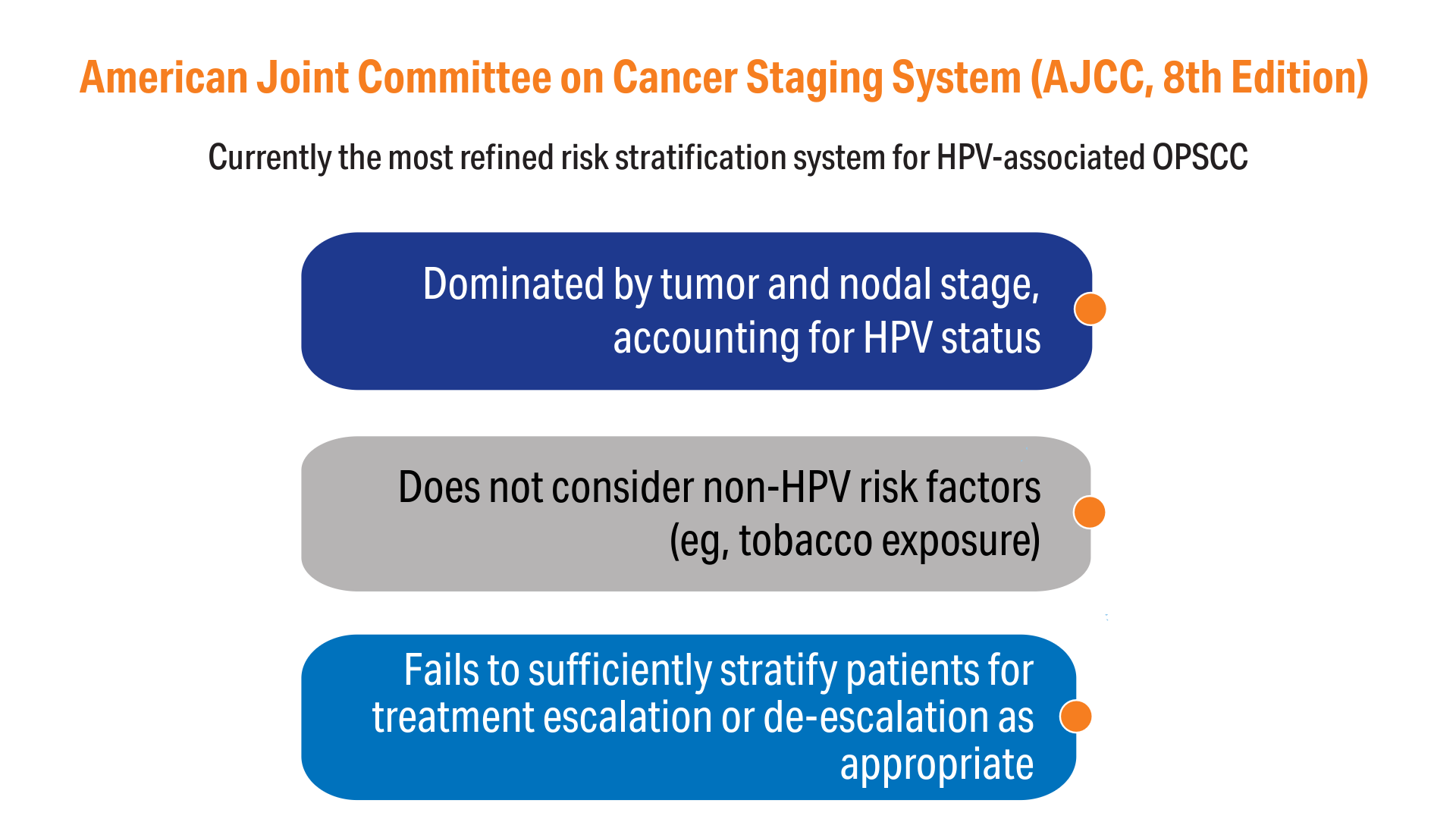
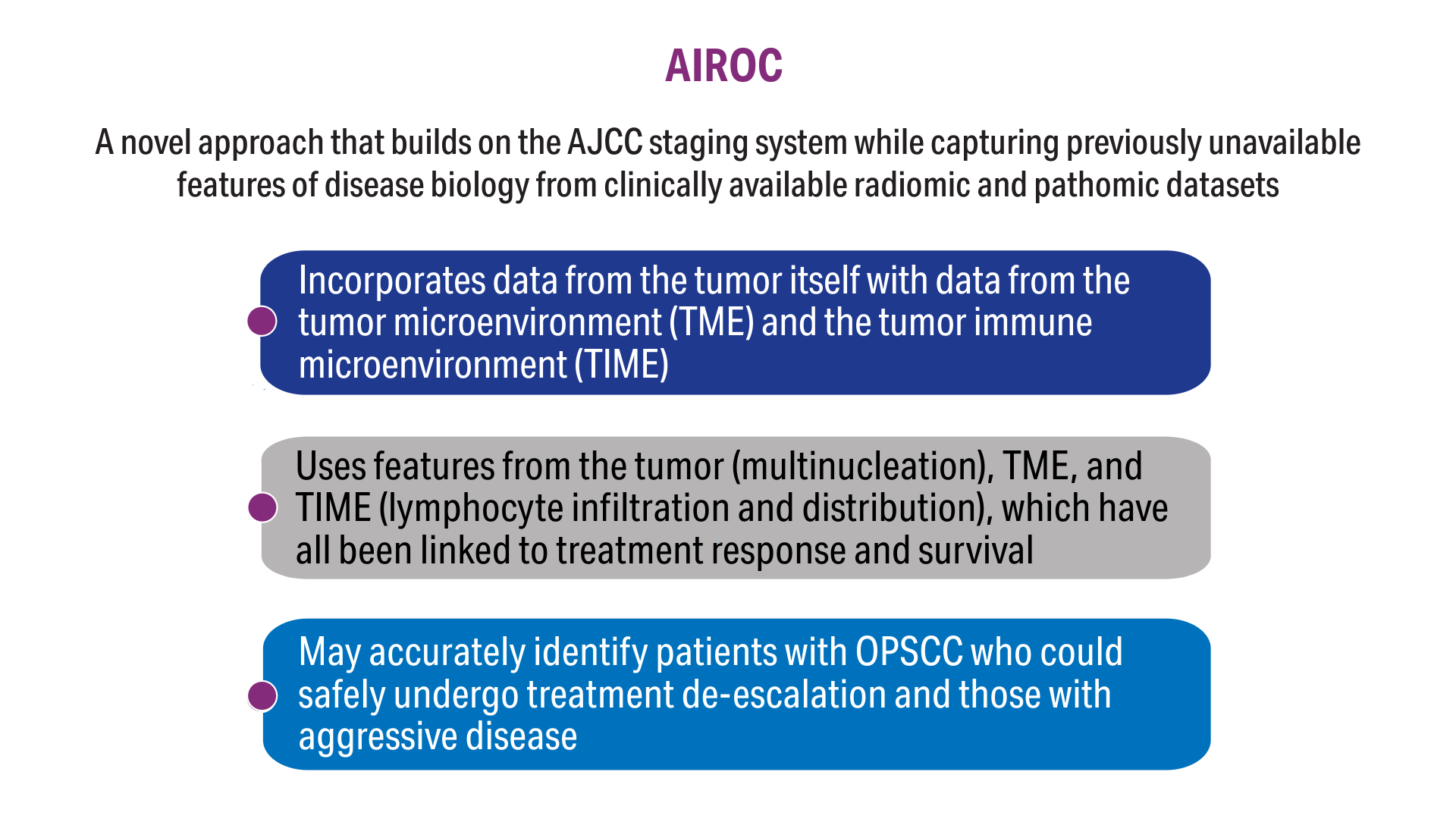
AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
Click here to view more from Cancer Data Trends 2025.
1. Zevallos JP, Kramer JR, Sandulache VC, et al. National trends in oropharyngeal cancer incidence and survival within the Veterans Affairs Health Care System. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
2. Fakhry C, Blackford AL, Neuner G, et al. Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol. 2019;5(7):985-992. doi:10.1001/jamaoncol.2019.0439
3. Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: implications for risk-based therapeutic intensity trials. Cancer. 2019;125(12):2027-2038. doi:10.1002/cncr.32025
4. O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440-451. doi:10.1016/S1470-2045(15)00560-4
5. Koyuncu CF, Lu C, Bera K, et al. Computerized tumor multinucleation index (MuNI) is prognostic in p16+ oropharyngeal carcinoma. J Clin Invest. 2021;131(8):e145488. doi:10.1172/JCI145488
6. Lu C, Lewis JS Jr, Dupont WD, Plummer WD Jr, Janowczyk A, Madabhushi A. An oral cavity squamous cell carcinoma quantitative histomorphometric-based image classifier of nuclear morphology can risk stratify patients for disease-specific survival. Mod Pathol. 2017;30(12):1655-1665. doi:10.1038/modpathol.2017.98
7. Corredor G, Toro P, Koyuncu C, et al. An imaging biomarker of tumor-infiltrating lymphocytes to risk-stratify patients with HPV-associated oropharyngeal cancer. J Natl Cancer Inst. 2022;114(4):609-617. doi:10.1093/jnci/djab215
8. Cancer stat facts: oral cavity and pharynx cancer. National Cancer Institute, SEER Program. Accessed November 5, 2024. https://seer.cancer.gov/statfacts/html/oralcav.html
9. Cancers associated with human papillomavirus. Centers for Disease Control and Prevention. September 18, 2024. Accessed November 5, 2024. https://www.cdc.gov/united-states-cancer-statistics/publications/hpv-associated-cancers.html
10. Chidambaram S, Chang SH, Sandulache VC, Mazul AL, Zevallos JP. Human papillomavirus vaccination prevalence and disproportionate cancer burden among US veterans. JAMA Oncol. 2023;9(5):712-714. doi:10.1001/jamaoncol.2022.7944
11. Corredor G, Wang X, Zhou Y, et al. Spatial architecture and arrangement of tumor-infiltrating lymphocytes for predicting likelihood of recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2019;25(5):1526-1534. doi:10.1158/1078-0432.CCR-18-2013
12. Alilou M, Orooji M, Beig N, et al. Quantitative vessel tortuosity: a potential CT imaging biomarker for distinguishing lung granulomas from adenocarcinomas. Sci Rep. 2018;8(1):15290. doi:10.1038/s41598-018-33473-0
13. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi:10.3322/caac.21388
Click here to view more from Cancer Data Trends 2025.
Click here to view more from Cancer Data Trends 2025.
1. Zevallos JP, Kramer JR, Sandulache VC, et al. National trends in oropharyngeal cancer incidence and survival within the Veterans Affairs Health Care System. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
2. Fakhry C, Blackford AL, Neuner G, et al. Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol. 2019;5(7):985-992. doi:10.1001/jamaoncol.2019.0439
3. Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: implications for risk-based therapeutic intensity trials. Cancer. 2019;125(12):2027-2038. doi:10.1002/cncr.32025
4. O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440-451. doi:10.1016/S1470-2045(15)00560-4
5. Koyuncu CF, Lu C, Bera K, et al. Computerized tumor multinucleation index (MuNI) is prognostic in p16+ oropharyngeal carcinoma. J Clin Invest. 2021;131(8):e145488. doi:10.1172/JCI145488
6. Lu C, Lewis JS Jr, Dupont WD, Plummer WD Jr, Janowczyk A, Madabhushi A. An oral cavity squamous cell carcinoma quantitative histomorphometric-based image classifier of nuclear morphology can risk stratify patients for disease-specific survival. Mod Pathol. 2017;30(12):1655-1665. doi:10.1038/modpathol.2017.98
7. Corredor G, Toro P, Koyuncu C, et al. An imaging biomarker of tumor-infiltrating lymphocytes to risk-stratify patients with HPV-associated oropharyngeal cancer. J Natl Cancer Inst. 2022;114(4):609-617. doi:10.1093/jnci/djab215
8. Cancer stat facts: oral cavity and pharynx cancer. National Cancer Institute, SEER Program. Accessed November 5, 2024. https://seer.cancer.gov/statfacts/html/oralcav.html
9. Cancers associated with human papillomavirus. Centers for Disease Control and Prevention. September 18, 2024. Accessed November 5, 2024. https://www.cdc.gov/united-states-cancer-statistics/publications/hpv-associated-cancers.html
10. Chidambaram S, Chang SH, Sandulache VC, Mazul AL, Zevallos JP. Human papillomavirus vaccination prevalence and disproportionate cancer burden among US veterans. JAMA Oncol. 2023;9(5):712-714. doi:10.1001/jamaoncol.2022.7944
11. Corredor G, Wang X, Zhou Y, et al. Spatial architecture and arrangement of tumor-infiltrating lymphocytes for predicting likelihood of recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2019;25(5):1526-1534. doi:10.1158/1078-0432.CCR-18-2013
12. Alilou M, Orooji M, Beig N, et al. Quantitative vessel tortuosity: a potential CT imaging biomarker for distinguishing lung granulomas from adenocarcinomas. Sci Rep. 2018;8(1):15290. doi:10.1038/s41598-018-33473-0
13. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi:10.3322/caac.21388
1. Zevallos JP, Kramer JR, Sandulache VC, et al. National trends in oropharyngeal cancer incidence and survival within the Veterans Affairs Health Care System. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
2. Fakhry C, Blackford AL, Neuner G, et al. Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol. 2019;5(7):985-992. doi:10.1001/jamaoncol.2019.0439
3. Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: implications for risk-based therapeutic intensity trials. Cancer. 2019;125(12):2027-2038. doi:10.1002/cncr.32025
4. O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440-451. doi:10.1016/S1470-2045(15)00560-4
5. Koyuncu CF, Lu C, Bera K, et al. Computerized tumor multinucleation index (MuNI) is prognostic in p16+ oropharyngeal carcinoma. J Clin Invest. 2021;131(8):e145488. doi:10.1172/JCI145488
6. Lu C, Lewis JS Jr, Dupont WD, Plummer WD Jr, Janowczyk A, Madabhushi A. An oral cavity squamous cell carcinoma quantitative histomorphometric-based image classifier of nuclear morphology can risk stratify patients for disease-specific survival. Mod Pathol. 2017;30(12):1655-1665. doi:10.1038/modpathol.2017.98
7. Corredor G, Toro P, Koyuncu C, et al. An imaging biomarker of tumor-infiltrating lymphocytes to risk-stratify patients with HPV-associated oropharyngeal cancer. J Natl Cancer Inst. 2022;114(4):609-617. doi:10.1093/jnci/djab215
8. Cancer stat facts: oral cavity and pharynx cancer. National Cancer Institute, SEER Program. Accessed November 5, 2024. https://seer.cancer.gov/statfacts/html/oralcav.html
9. Cancers associated with human papillomavirus. Centers for Disease Control and Prevention. September 18, 2024. Accessed November 5, 2024. https://www.cdc.gov/united-states-cancer-statistics/publications/hpv-associated-cancers.html
10. Chidambaram S, Chang SH, Sandulache VC, Mazul AL, Zevallos JP. Human papillomavirus vaccination prevalence and disproportionate cancer burden among US veterans. JAMA Oncol. 2023;9(5):712-714. doi:10.1001/jamaoncol.2022.7944
11. Corredor G, Wang X, Zhou Y, et al. Spatial architecture and arrangement of tumor-infiltrating lymphocytes for predicting likelihood of recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2019;25(5):1526-1534. doi:10.1158/1078-0432.CCR-18-2013
12. Alilou M, Orooji M, Beig N, et al. Quantitative vessel tortuosity: a potential CT imaging biomarker for distinguishing lung granulomas from adenocarcinomas. Sci Rep. 2018;8(1):15290. doi:10.1038/s41598-018-33473-0
13. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi:10.3322/caac.21388
AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
Rising Kidney Cancer Cases and Emerging Treatments for Veterans
Rising Kidney Cancer Cases and Emerging Treatments for Veterans
Click here to view more from Cancer Data Trends 2025.
1. American Cancer Society website. Key Statistics About Kidney Cancer. Revised May 2024. Accessed December 18, 2024. https://www.cancer.org/cancer/types/kidney-cancer/about/key-statistics.html
2. American Cancer Society website. Cancer Facts & Figures 2024. 2024—First Year the US Expects More than 2M New Cases of Cancer. Published January 17, 2024. Accessed December 18, 2024. https://www.cancer.org/research/acs-research-news/facts-and-figures-2024.html
3.United States Department of Veterans Affairs factsheet. Pact Act & Gulf War, Post-911 Era Veterans. Published July 2023. Accessed December 18, 2024. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.va.gov/files/2023-08/PACT%20Act%20and%20Gulf%20War%2C%20Post-911%20Veterans%20NEW%20July%202023.pdf
4. Li M, Li L, Zheng J, Li Z, Li S, Wang K, Chen X. Liquid biopsy at the frontier in renal cell carcinoma: recent analysis of techniques and clinical application. Mol Cancer. 2023 Feb 21;22(1):37. doi:10.1186/s12943-023-01745-7
5. Bellman NL. Incidental Finding of Renal Cell Carcinoma: Detected by a Thrombus in the Inferior Vena Cava. Journal of Diagnostic Medical Sonography. 2015;31(2):118-121. doi:10.1177/8756479314546691
6. Brown JT. Adjuvant Therapy for Non-Clear Cell Renal Cell Carcinoma—The Ascent Continues. JAMA Network Open. 2024 Aug 1;7(8):e2425251. doi:10.1001/jamanetworkopen.2024.25251
7. Siva S, Louie AV, Kotecha R, et al. Stereotactic body radiotherapy for primary renal cell carcinoma: a systematic review and practice guideline from the International Society of Stereotactic Radiosurgery (ISRS). Lancet Oncol. 2024 Jan;25(1):e18-e28. doi: 10.1016/S1470-2045(23)00513-2.
8. Choueiri TK, Tomczak P, Park SH, et al; for the KEYNOTE-564 Investigators. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N Engl J Med. 2024 Apr 18;390(15):1359-1371. doi:10.1056/NEJMoa2312695
9. Bytnar JA, McGlynn KA, Kern SQ, Shriver CD, Zhu K. Incidence rates of bladder and kidney cancers among US military servicemen: comparison with the rates in the general US population. Eur J Cancer Prev. 2024 Nov 1;33(6):505-511. doi:10.1097/CEJ.0000000000000886
Click here to view more from Cancer Data Trends 2025.
Click here to view more from Cancer Data Trends 2025.
1. American Cancer Society website. Key Statistics About Kidney Cancer. Revised May 2024. Accessed December 18, 2024. https://www.cancer.org/cancer/types/kidney-cancer/about/key-statistics.html
2. American Cancer Society website. Cancer Facts & Figures 2024. 2024—First Year the US Expects More than 2M New Cases of Cancer. Published January 17, 2024. Accessed December 18, 2024. https://www.cancer.org/research/acs-research-news/facts-and-figures-2024.html
3.United States Department of Veterans Affairs factsheet. Pact Act & Gulf War, Post-911 Era Veterans. Published July 2023. Accessed December 18, 2024. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.va.gov/files/2023-08/PACT%20Act%20and%20Gulf%20War%2C%20Post-911%20Veterans%20NEW%20July%202023.pdf
4. Li M, Li L, Zheng J, Li Z, Li S, Wang K, Chen X. Liquid biopsy at the frontier in renal cell carcinoma: recent analysis of techniques and clinical application. Mol Cancer. 2023 Feb 21;22(1):37. doi:10.1186/s12943-023-01745-7
5. Bellman NL. Incidental Finding of Renal Cell Carcinoma: Detected by a Thrombus in the Inferior Vena Cava. Journal of Diagnostic Medical Sonography. 2015;31(2):118-121. doi:10.1177/8756479314546691
6. Brown JT. Adjuvant Therapy for Non-Clear Cell Renal Cell Carcinoma—The Ascent Continues. JAMA Network Open. 2024 Aug 1;7(8):e2425251. doi:10.1001/jamanetworkopen.2024.25251
7. Siva S, Louie AV, Kotecha R, et al. Stereotactic body radiotherapy for primary renal cell carcinoma: a systematic review and practice guideline from the International Society of Stereotactic Radiosurgery (ISRS). Lancet Oncol. 2024 Jan;25(1):e18-e28. doi: 10.1016/S1470-2045(23)00513-2.
8. Choueiri TK, Tomczak P, Park SH, et al; for the KEYNOTE-564 Investigators. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N Engl J Med. 2024 Apr 18;390(15):1359-1371. doi:10.1056/NEJMoa2312695
9. Bytnar JA, McGlynn KA, Kern SQ, Shriver CD, Zhu K. Incidence rates of bladder and kidney cancers among US military servicemen: comparison with the rates in the general US population. Eur J Cancer Prev. 2024 Nov 1;33(6):505-511. doi:10.1097/CEJ.0000000000000886
1. American Cancer Society website. Key Statistics About Kidney Cancer. Revised May 2024. Accessed December 18, 2024. https://www.cancer.org/cancer/types/kidney-cancer/about/key-statistics.html
2. American Cancer Society website. Cancer Facts & Figures 2024. 2024—First Year the US Expects More than 2M New Cases of Cancer. Published January 17, 2024. Accessed December 18, 2024. https://www.cancer.org/research/acs-research-news/facts-and-figures-2024.html
3.United States Department of Veterans Affairs factsheet. Pact Act & Gulf War, Post-911 Era Veterans. Published July 2023. Accessed December 18, 2024. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.va.gov/files/2023-08/PACT%20Act%20and%20Gulf%20War%2C%20Post-911%20Veterans%20NEW%20July%202023.pdf
4. Li M, Li L, Zheng J, Li Z, Li S, Wang K, Chen X. Liquid biopsy at the frontier in renal cell carcinoma: recent analysis of techniques and clinical application. Mol Cancer. 2023 Feb 21;22(1):37. doi:10.1186/s12943-023-01745-7
5. Bellman NL. Incidental Finding of Renal Cell Carcinoma: Detected by a Thrombus in the Inferior Vena Cava. Journal of Diagnostic Medical Sonography. 2015;31(2):118-121. doi:10.1177/8756479314546691
6. Brown JT. Adjuvant Therapy for Non-Clear Cell Renal Cell Carcinoma—The Ascent Continues. JAMA Network Open. 2024 Aug 1;7(8):e2425251. doi:10.1001/jamanetworkopen.2024.25251
7. Siva S, Louie AV, Kotecha R, et al. Stereotactic body radiotherapy for primary renal cell carcinoma: a systematic review and practice guideline from the International Society of Stereotactic Radiosurgery (ISRS). Lancet Oncol. 2024 Jan;25(1):e18-e28. doi: 10.1016/S1470-2045(23)00513-2.
8. Choueiri TK, Tomczak P, Park SH, et al; for the KEYNOTE-564 Investigators. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N Engl J Med. 2024 Apr 18;390(15):1359-1371. doi:10.1056/NEJMoa2312695
9. Bytnar JA, McGlynn KA, Kern SQ, Shriver CD, Zhu K. Incidence rates of bladder and kidney cancers among US military servicemen: comparison with the rates in the general US population. Eur J Cancer Prev. 2024 Nov 1;33(6):505-511. doi:10.1097/CEJ.0000000000000886
Rising Kidney Cancer Cases and Emerging Treatments for Veterans
Rising Kidney Cancer Cases and Emerging Treatments for Veterans
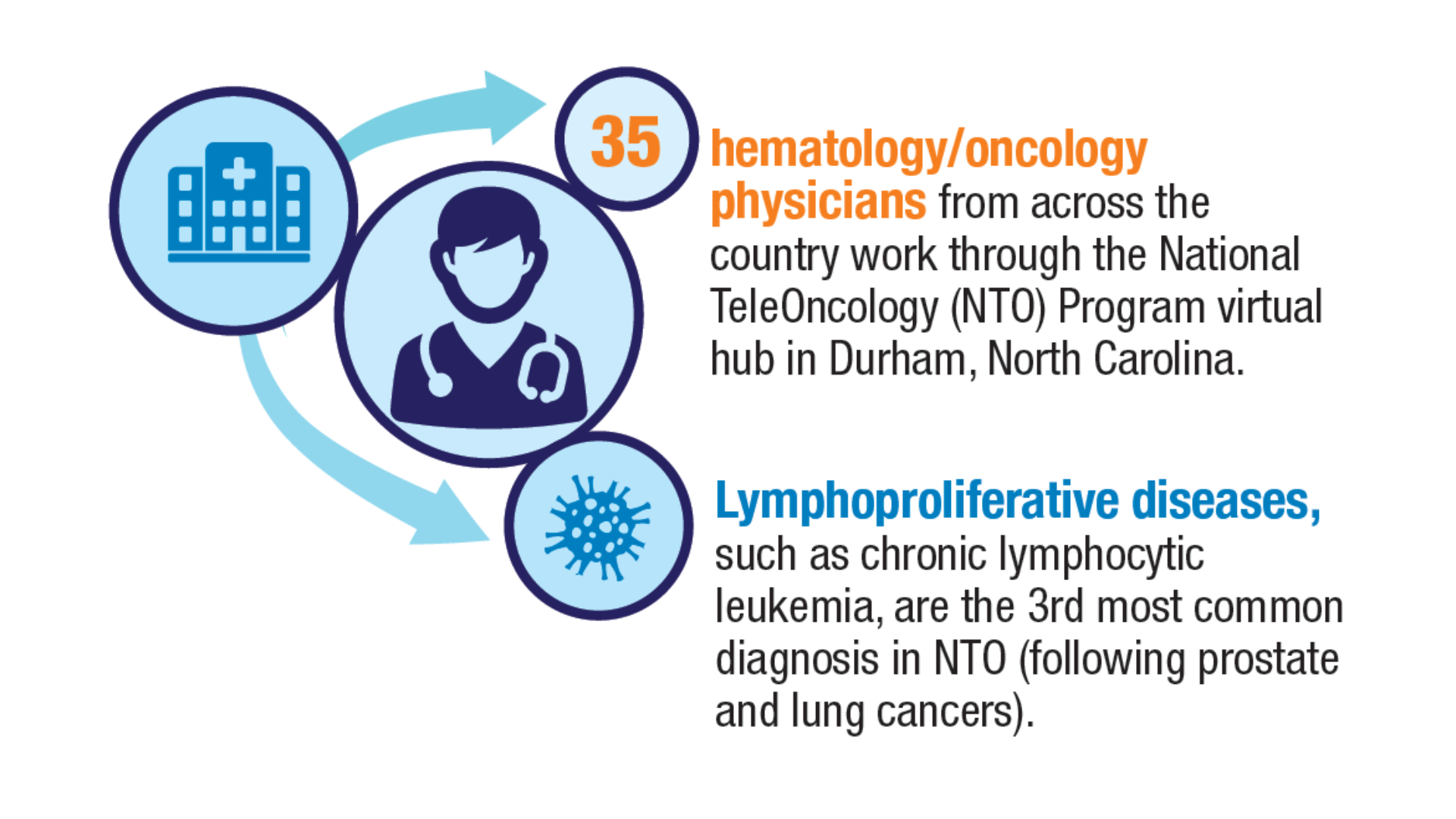
Advances in Blood Cancer Care for Veterans
Advances in Blood Cancer Care for Veterans
Click to view more from Cancer Data Trends 2025.
- Li W, ed. The 5th Edition of the World Health Organization Classification of
Hematolymphoid Tumors. In: Leukemia [Internet]. Brisbane (AU): Exon Publications;
October 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK586208/ - Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with
hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin
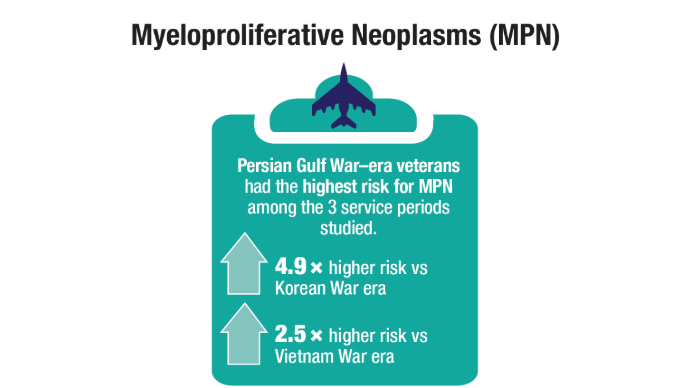
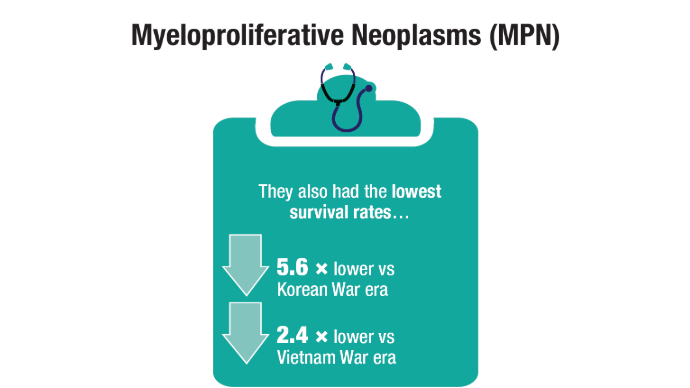
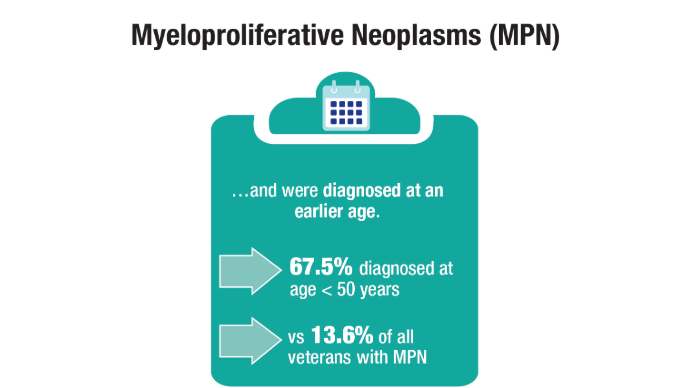
Oncol. 2020;47(1):94-101. doi:10.1053/j.seminoncol.2020.02.010. - Tiu A, McKinnell Z, Liu S, et al. Risk of myeloproliferative neoplasms among
U.S. Veterans from Korean, Vietnam, and Persian Gulf War eras. Am J Hematol.
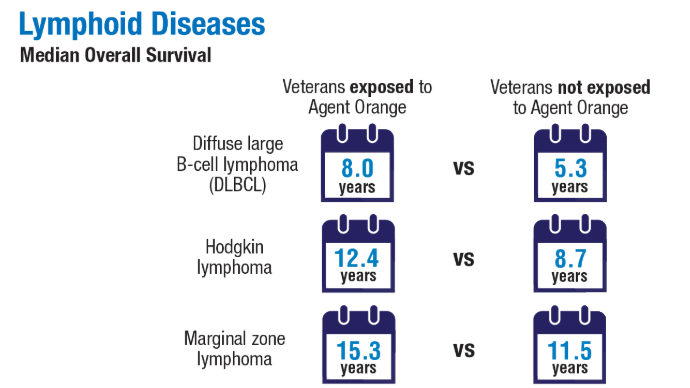
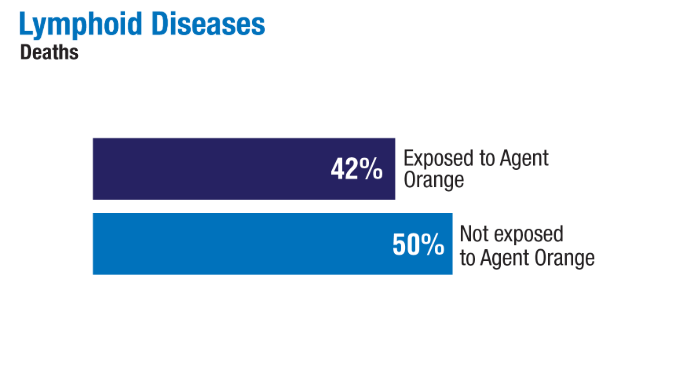
2024;99(10):1969-1978. doi:10.1002/ajh.27438 - Ma H, Wan JY, Cortessis VK, Gupta P, Cozen W. Survival in Agent Orange
exposed and unexposed Vietnam-era veterans who were diagnosed with
lymphoid malignancies. Blood Adv. 2024;8(4):1037-1041. doi:10.1182/
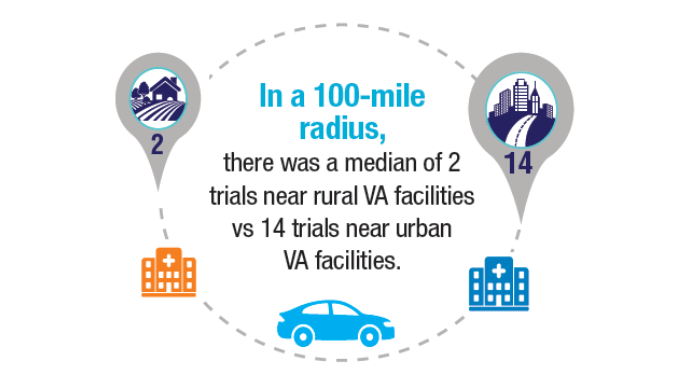
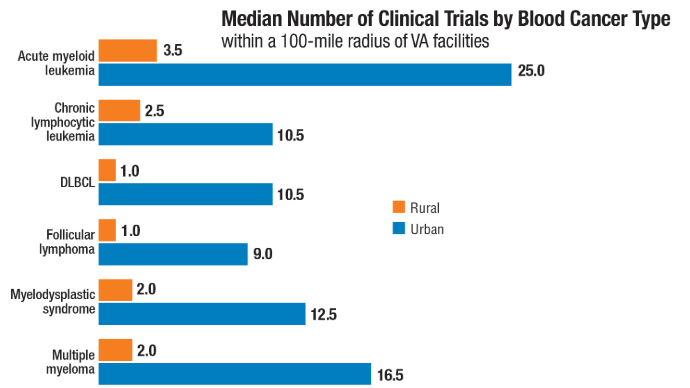
bloodadvances.2023011999 - Friedman DR, Rodgers TD, Kovalick C, Yellapragada S, Szumita L, Weiss ES. Veterans
with blood cancers: Clinical trial navigation and the challenge of rurality. J Rural
Health. 2024;40(1):114-120. doi:10.1111/jrh.12773 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(3):e100042. doi:10.1200/EDBK_100042 - Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological
advancements in cancer diagnostics: Improvements and limitations. Cancer Rep
(Hoboken). 2023;6(2):e1764. doi:10.1002/cnr2.1764
Click to view more from Cancer Data Trends 2025.
Click to view more from Cancer Data Trends 2025.
- Li W, ed. The 5th Edition of the World Health Organization Classification of
Hematolymphoid Tumors. In: Leukemia [Internet]. Brisbane (AU): Exon Publications;
October 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK586208/ - Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with
hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin
Oncol. 2020;47(1):94-101. doi:10.1053/j.seminoncol.2020.02.010. - Tiu A, McKinnell Z, Liu S, et al. Risk of myeloproliferative neoplasms among
U.S. Veterans from Korean, Vietnam, and Persian Gulf War eras. Am J Hematol.
2024;99(10):1969-1978. doi:10.1002/ajh.27438 - Ma H, Wan JY, Cortessis VK, Gupta P, Cozen W. Survival in Agent Orange
exposed and unexposed Vietnam-era veterans who were diagnosed with
lymphoid malignancies. Blood Adv. 2024;8(4):1037-1041. doi:10.1182/
bloodadvances.2023011999 - Friedman DR, Rodgers TD, Kovalick C, Yellapragada S, Szumita L, Weiss ES. Veterans
with blood cancers: Clinical trial navigation and the challenge of rurality. J Rural
Health. 2024;40(1):114-120. doi:10.1111/jrh.12773 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(3):e100042. doi:10.1200/EDBK_100042 - Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological
advancements in cancer diagnostics: Improvements and limitations. Cancer Rep
(Hoboken). 2023;6(2):e1764. doi:10.1002/cnr2.1764
- Li W, ed. The 5th Edition of the World Health Organization Classification of
Hematolymphoid Tumors. In: Leukemia [Internet]. Brisbane (AU): Exon Publications;
October 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK586208/ - Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with
hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin
Oncol. 2020;47(1):94-101. doi:10.1053/j.seminoncol.2020.02.010. - Tiu A, McKinnell Z, Liu S, et al. Risk of myeloproliferative neoplasms among
U.S. Veterans from Korean, Vietnam, and Persian Gulf War eras. Am J Hematol.
2024;99(10):1969-1978. doi:10.1002/ajh.27438 - Ma H, Wan JY, Cortessis VK, Gupta P, Cozen W. Survival in Agent Orange
exposed and unexposed Vietnam-era veterans who were diagnosed with
lymphoid malignancies. Blood Adv. 2024;8(4):1037-1041. doi:10.1182/
bloodadvances.2023011999 - Friedman DR, Rodgers TD, Kovalick C, Yellapragada S, Szumita L, Weiss ES. Veterans
with blood cancers: Clinical trial navigation and the challenge of rurality. J Rural
Health. 2024;40(1):114-120. doi:10.1111/jrh.12773 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(3):e100042. doi:10.1200/EDBK_100042 - Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological
advancements in cancer diagnostics: Improvements and limitations. Cancer Rep
(Hoboken). 2023;6(2):e1764. doi:10.1002/cnr2.1764
Advances in Blood Cancer Care for Veterans
Advances in Blood Cancer Care for Veterans
HCC Updates: Quality Care Framework and Risk Stratification Data
HCC Updates: Quality Care Framework and Risk Stratification Data
Click here to view more from Cancer Data Trends 2025.
1. Rogal SS, Taddei TH, Monto A, et al. Hepatocellular Carcinoma Diagnosis and Management in 2021: A National Veterans Affairs Quality Improvement Project. Clin Gastroenterol Hepatol. 2024 Feb;22(2):324-338. doi:10.1016/j.cgh.2023.07.002
2. John BV, Dang Y, Kaplan DE, et al. Liver Stiffness Measurement and Risk Prediction of Hepatocellular Carcinoma After HCV Eradication in Veterans With Cirrhosis. Clin Gastroenterol Hepatol. 2024 Apr;22(4):778-788.e7. doi:10.1016/j.cgh.2023.11.020
Click here to view more from Cancer Data Trends 2025.
Click here to view more from Cancer Data Trends 2025.
1. Rogal SS, Taddei TH, Monto A, et al. Hepatocellular Carcinoma Diagnosis and Management in 2021: A National Veterans Affairs Quality Improvement Project. Clin Gastroenterol Hepatol. 2024 Feb;22(2):324-338. doi:10.1016/j.cgh.2023.07.002
2. John BV, Dang Y, Kaplan DE, et al. Liver Stiffness Measurement and Risk Prediction of Hepatocellular Carcinoma After HCV Eradication in Veterans With Cirrhosis. Clin Gastroenterol Hepatol. 2024 Apr;22(4):778-788.e7. doi:10.1016/j.cgh.2023.11.020
1. Rogal SS, Taddei TH, Monto A, et al. Hepatocellular Carcinoma Diagnosis and Management in 2021: A National Veterans Affairs Quality Improvement Project. Clin Gastroenterol Hepatol. 2024 Feb;22(2):324-338. doi:10.1016/j.cgh.2023.07.002
2. John BV, Dang Y, Kaplan DE, et al. Liver Stiffness Measurement and Risk Prediction of Hepatocellular Carcinoma After HCV Eradication in Veterans With Cirrhosis. Clin Gastroenterol Hepatol. 2024 Apr;22(4):778-788.e7. doi:10.1016/j.cgh.2023.11.020
HCC Updates: Quality Care Framework and Risk Stratification Data
HCC Updates: Quality Care Framework and Risk Stratification Data
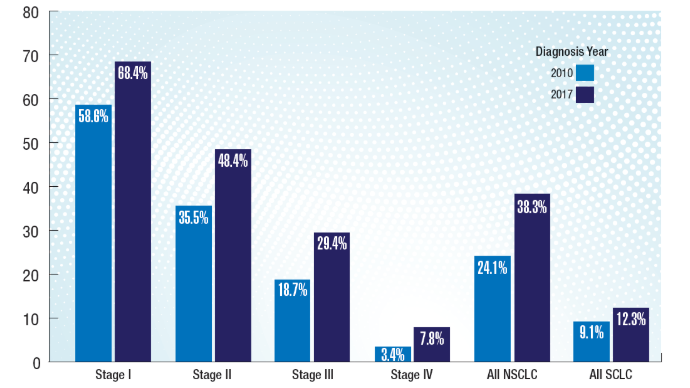
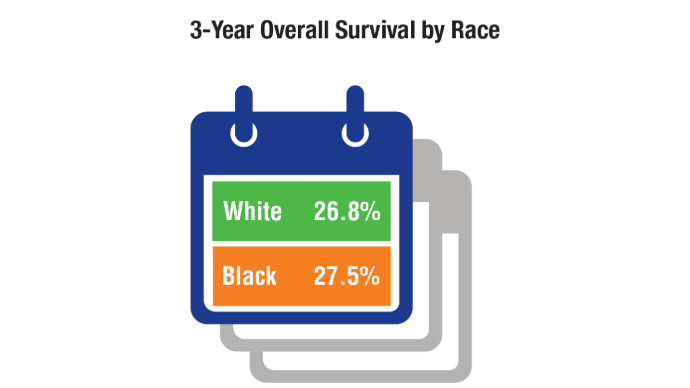
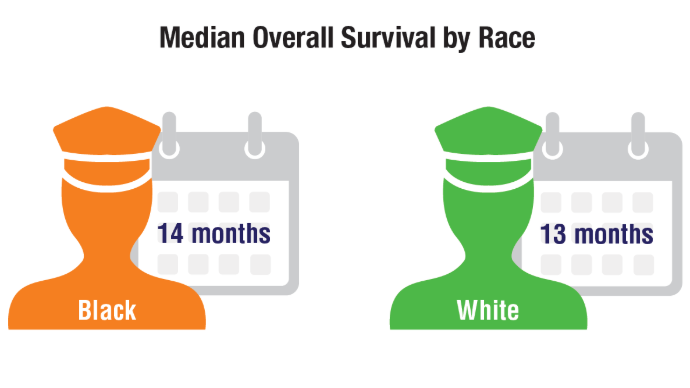
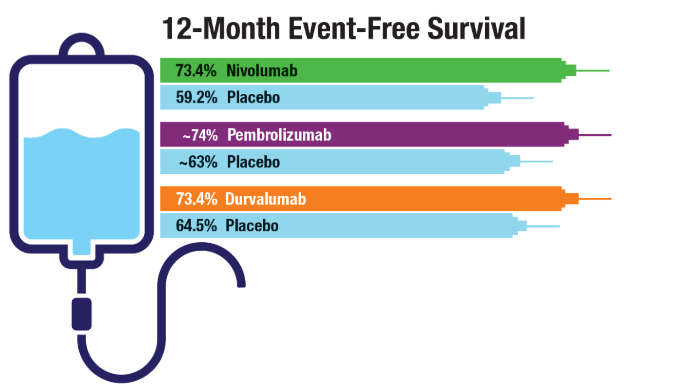
Lung Cancer: Mortality Trends in Veterans and New Treatments
Lung Cancer: Mortality Trends in Veterans and New Treatments
Click to view more from Cancer Data Trends 2025.
- Tehzeeb J, Mahmood F, Gemoets D, Azem A, Mehdi SA. Epidemiology and survival
trends of lung carcinoids in the veteran population. J Clin Oncol. 2023;41:e21049.
doi:10.1200/JCO.2023.41.16_suppl.e21049 - Moghanaki D, Taylor J, Bryant AK, et al. Lung Cancer Survival Trends in the Veterans
Health Administration. Clin Lung Cancer. 2024;25(3):225-232. doi:10.1016/j.
cllc.2024.02.009 - Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in
lung carcinoma from the VA National Precision Oncology Program. Semin Oncol.
2022;49(3-4):265-274. doi:10.1053/j.seminoncol.2022.06.014 - Cascone T, Awad MM, Spicer JD, et al; for the CheckMate 77T Investigators.
Perioperative Nivolumab in Resectable Lung Cancer. N Engl J Med.
2024;390(19):1756-1769. doi:10.1056/NEJMoa2311926 - Wakelee H, Liberman M, Kato T, et al; for the KEYNOTE-671 Investigators.
Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J
Med. 2023;389(6):491-503. doi:10.1056/NEJMoa2302983 - Heymach JV, Harpole D, Mitsudomi T, et al; for the AEGEAN Investigators.
Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J
Med. 2023;389(18):1672-1684. doi:10.1056/NEJMoa2304875 - Duncan FC, Al Nasrallah N, Nephew L, et al. Racial disparities in staging, treatment,
and mortality in non-small cell lung cancer. Transl Lung Cancer Res. 2024;13(1):76-
94. doi:10.21037/tlcr-23-407
Click to view more from Cancer Data Trends 2025.
Click to view more from Cancer Data Trends 2025.
- Tehzeeb J, Mahmood F, Gemoets D, Azem A, Mehdi SA. Epidemiology and survival
trends of lung carcinoids in the veteran population. J Clin Oncol. 2023;41:e21049.
doi:10.1200/JCO.2023.41.16_suppl.e21049 - Moghanaki D, Taylor J, Bryant AK, et al. Lung Cancer Survival Trends in the Veterans
Health Administration. Clin Lung Cancer. 2024;25(3):225-232. doi:10.1016/j.
cllc.2024.02.009 - Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in
lung carcinoma from the VA National Precision Oncology Program. Semin Oncol.
2022;49(3-4):265-274. doi:10.1053/j.seminoncol.2022.06.014 - Cascone T, Awad MM, Spicer JD, et al; for the CheckMate 77T Investigators.
Perioperative Nivolumab in Resectable Lung Cancer. N Engl J Med.
2024;390(19):1756-1769. doi:10.1056/NEJMoa2311926 - Wakelee H, Liberman M, Kato T, et al; for the KEYNOTE-671 Investigators.
Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J
Med. 2023;389(6):491-503. doi:10.1056/NEJMoa2302983 - Heymach JV, Harpole D, Mitsudomi T, et al; for the AEGEAN Investigators.
Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J
Med. 2023;389(18):1672-1684. doi:10.1056/NEJMoa2304875 - Duncan FC, Al Nasrallah N, Nephew L, et al. Racial disparities in staging, treatment,
and mortality in non-small cell lung cancer. Transl Lung Cancer Res. 2024;13(1):76-
94. doi:10.21037/tlcr-23-407
- Tehzeeb J, Mahmood F, Gemoets D, Azem A, Mehdi SA. Epidemiology and survival
trends of lung carcinoids in the veteran population. J Clin Oncol. 2023;41:e21049.
doi:10.1200/JCO.2023.41.16_suppl.e21049 - Moghanaki D, Taylor J, Bryant AK, et al. Lung Cancer Survival Trends in the Veterans
Health Administration. Clin Lung Cancer. 2024;25(3):225-232. doi:10.1016/j.
cllc.2024.02.009 - Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in
lung carcinoma from the VA National Precision Oncology Program. Semin Oncol.
2022;49(3-4):265-274. doi:10.1053/j.seminoncol.2022.06.014 - Cascone T, Awad MM, Spicer JD, et al; for the CheckMate 77T Investigators.
Perioperative Nivolumab in Resectable Lung Cancer. N Engl J Med.
2024;390(19):1756-1769. doi:10.1056/NEJMoa2311926 - Wakelee H, Liberman M, Kato T, et al; for the KEYNOTE-671 Investigators.
Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J
Med. 2023;389(6):491-503. doi:10.1056/NEJMoa2302983 - Heymach JV, Harpole D, Mitsudomi T, et al; for the AEGEAN Investigators.
Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J
Med. 2023;389(18):1672-1684. doi:10.1056/NEJMoa2304875 - Duncan FC, Al Nasrallah N, Nephew L, et al. Racial disparities in staging, treatment,
and mortality in non-small cell lung cancer. Transl Lung Cancer Res. 2024;13(1):76-
94. doi:10.21037/tlcr-23-407
Lung Cancer: Mortality Trends in Veterans and New Treatments
Lung Cancer: Mortality Trends in Veterans and New Treatments
FDA Approves Avastin Biosimilar Agent, Jobevne
The United States Food and Drug Administration (FDA) has approved bevacizumab-nwgd (Jobevne, Biocon Biologics Ltd), a biosimilar to bevacizumab (Avastin, Genentech), for intravenous use across multiple cancer types.
Approval was based on “a comprehensive package of comparative pharmacokinetic, safety, efficacy, nonclinical, structural, analytical and functional data, which confirmed the Jobevne is highly similar to Avastin,” according to a Biocon Biologics Ltd press release.
“The data demonstrated that there were no clinically meaningful differences between Jobevne and Avastin in terms of pharmacokinetics, safety, efficacy, and immunogenicity,” the company stated.
The biosimilar agent is indicated as part of various combinations for the treatment of metastatic colorectal cancer, certain types of non-squamous non–small cell lung cancer, recurrent glioblastoma, metastatic renal cell carcinoma, certain advanced cervical cancers, and epithelial ovarian, fallopian tube, or primary peritoneal cancers, the company noted.
The agent is not indicated for adjuvant treatment of colon cancer, according to the press release, which includes detailed information about the indications, as well as a list of warnings and precautions.
A version of this article first appeared on Medscape.com.
The United States Food and Drug Administration (FDA) has approved bevacizumab-nwgd (Jobevne, Biocon Biologics Ltd), a biosimilar to bevacizumab (Avastin, Genentech), for intravenous use across multiple cancer types.
Approval was based on “a comprehensive package of comparative pharmacokinetic, safety, efficacy, nonclinical, structural, analytical and functional data, which confirmed the Jobevne is highly similar to Avastin,” according to a Biocon Biologics Ltd press release.
“The data demonstrated that there were no clinically meaningful differences between Jobevne and Avastin in terms of pharmacokinetics, safety, efficacy, and immunogenicity,” the company stated.
The biosimilar agent is indicated as part of various combinations for the treatment of metastatic colorectal cancer, certain types of non-squamous non–small cell lung cancer, recurrent glioblastoma, metastatic renal cell carcinoma, certain advanced cervical cancers, and epithelial ovarian, fallopian tube, or primary peritoneal cancers, the company noted.
The agent is not indicated for adjuvant treatment of colon cancer, according to the press release, which includes detailed information about the indications, as well as a list of warnings and precautions.
A version of this article first appeared on Medscape.com.
The United States Food and Drug Administration (FDA) has approved bevacizumab-nwgd (Jobevne, Biocon Biologics Ltd), a biosimilar to bevacizumab (Avastin, Genentech), for intravenous use across multiple cancer types.
Approval was based on “a comprehensive package of comparative pharmacokinetic, safety, efficacy, nonclinical, structural, analytical and functional data, which confirmed the Jobevne is highly similar to Avastin,” according to a Biocon Biologics Ltd press release.
“The data demonstrated that there were no clinically meaningful differences between Jobevne and Avastin in terms of pharmacokinetics, safety, efficacy, and immunogenicity,” the company stated.
The biosimilar agent is indicated as part of various combinations for the treatment of metastatic colorectal cancer, certain types of non-squamous non–small cell lung cancer, recurrent glioblastoma, metastatic renal cell carcinoma, certain advanced cervical cancers, and epithelial ovarian, fallopian tube, or primary peritoneal cancers, the company noted.
The agent is not indicated for adjuvant treatment of colon cancer, according to the press release, which includes detailed information about the indications, as well as a list of warnings and precautions.
A version of this article first appeared on Medscape.com.
Medical Centers Address Unique Needs of Young Adults With Cancer
Adam DuVall, MD, MPH, is a rare medical oncologist who trained in both adult and pediatric hematology/oncology.
This distinction, which DuVall said he shares with only a handful of oncologists in the world, matches his role at University of Chicago (UChicago) Medicine, Chicago. Since joining UChicago in 2020, DuVall has helped expand its Adolescent and Young Adult (AYA) Oncology Program, which aims to provide comprehensive, one-stop care and support for patients with cancer aged from 15 to 39 years.
Started in 2012, the program is one of the oldest in a growing array of initiatives nationwide that seek to address the specific psychosocial and other support needs of patients with cancer who fall into the gap between young children and the older patients who more typically have cancer. Along with DuVall and other oncologists, UChicago’s AYA Program offers dedicated nurse practitioners, social workers, psychologists, a physical therapist, and a program administrator. A community health worker, who does home visits, helps patients coordinate travel, works with their insurance, and generally navigates the medical system, DuVall added.
The program receives about 1500-2000 visits a year, according to DuVall. What the young adult population with cancer has in common that distinguishes it from other age cohorts, he said, are its members’ particular psychosocial needs. “Going through adolescence and young adulthood without cancer, there’s plenty of things that are hard,” DuVall observed. “Put cancer on top of that, and it impacts every aspect of life.”
‘Millennials Have Higher Risk’
The proliferation of AYA programs comes as more and more studies have been published recently showing that young adults are increasingly getting cancer.
According to American Cancer Society research published in December in The Lancet Oncology, incidence rates of colorectal cancer (CRC) among young adults aged 25-49 years rose in the decade through 2017 in more than half of the 50 countries and territories examined. For the past 5 years studied, the incidence rate of early-onset CRC was highest in Australia, Puerto Rico, New Zealand, the United States, and South Korea. At the same time, the study found, rates among older adults in all of those places except South Korea were stable or declining.
Hyuna Sung, PhD, the study’s lead author, said, “Research has shown that Gen X and millennials have higher risk of multiple types of cancer compared to the older generations.”
Some of the cancers found to be increasing among younger adults are linked to “excess body obesity,” Sung said, including not only CRC but also cancers of the uterine corpus, gallbladder, kidney, pancreas, breast, and stomach cardia, as well as myeloma. Early onset of cancers not linked to obesity, such as testicular cancer and small intestinal cancer, has also been shown to be on the rise, Sung noted.
As cancer rates among young adults have risen, nonprofits have stepped in to help medical institutions open programs geared to their needs. Teen Cancer America, founded by members of rock band The Who, has partnered with 64 hospitals in 36 cities to develop AYA-focused programs, funding 85 hospital positions, according to a spokesperson. The Los Angeles–based nonprofit has also provided free consultation to 130 hospitals without formally providing a grant, the spokesperson said.
A map on Teen Cancer America’s website illustrates the nationwide spread of AYA programs, from UCLA Santa Monica Medical Center to Memorial Sloan Kettering Cancer Center in New York City, with more in between.
‘Setting Them Up for a Life of Meaning’
Michael Roth, MD, co-director of the AYA Program at the University of Texas MD Anderson Cancer Center in Houston, likes to say, “If you’ve seen one AYA Program, you’ve seen one AYA Program.”
In other words, offerings vary. “Most centers do not have comprehensive AYA programs,” Roth said, noting that at many sites the AYA Program might consist of oncofertility support. “That said, programs are doing the best they can, knowing that the AYA population is growing exponentially globally.”
Almost 90,000 AYA patients are diagnosed with cancer each year, and 85% will be at least 5-year survivors, Roth said. There are more than 2 million survivors of AYA cancer, he added, and if the median age of diagnosis is 30, they can live five decades beyond their cancer treatment. “Their life matters,” Roth said. “It matters during treatment. Their life after cancer matters.”
The AYA Program at MD Anderson began in 2017, and it sees more than 2000 AYAs diagnosed with cancer every year, according to Roth. The program is designed to complement the care that patients with cancer aged from 15 to 39 years or older may already be receiving from their primary treatment teams. New patients see a medical provider, a social worker, and a vocational counselor for discussions about their needs and concerns, and they have access to a nutritionist and genetic counselor.
The program offers psychosocial and supportive care for patients who may be facing challenges with school, work, relationships, having young children, and mental health, Roth said. Along with assessments and counseling around fertility risks and genetic predisposition, MD Anderson also provides patients in the program with a long-term survivorship plan.
“It’s not just increasing cures,” Roth observed. “We’re also setting them up for a life of meaning and happiness and productivity and health.”
Almost 40% of visits to the program are conducted virtually, according to Roth. “Our goal is to meet the patients where they are,” he said. “We want to be convenient, not be a burden.”
‘The Face of Cancer Has Changed’
Patients with AYA cancer diagnoses may be finishing up school or starting a job, developing their body image and sexual identity, or caring for young children or older parents.
“They feel incredibly isolated,” said Ann LaCasce, MD, MMSc, co-director of the Center for Adolescent and Young Adult Oncology at Dana-Farber Cancer Institute in Boston. “They go into the cancer center or their community practice, and everyone is double, triple their age.”
Last year, Dana-Farber opened a Young Adult Lounge meant for patients aged 18 years or older to be able to relax and, if they wish, interact between appointments. “When you talk to these patients, they want to meet each other,” LaCasce said. “They want to share experiences.”
The Young Adults With Cancer Program at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee, opened its doors in 2019. It recently hired an interim nurse navigator, said Cathy Eng, MD, FACP, FASCO, director of the Program, which concentrates on patients aged 25-45 years.
“The face of cancer has changed,” Eng said.
She advises other oncologists to talk to their young adult patients as much as possible. “Really talk to them as an individual and see what other needs they have,” she said. “Even if they don’t tell you the first time, ask them the second time, ask them the third time.”
Christopher Cann, MD, executive director of the Young Adult Cancer Program at Fox Chase Cancer Center in Philadelphia, did his fellowship under Eng. He joined Fox Chase in 2023, and the Young Adult Cancer Program started accepting patients around the end of last year, zeroing in on patients aged 18-39 years.
Following the implementation of a new best practice advisory that pops up in the medical records system, he said, oncofertility referrals increased by 400% within 6 months.
“My hope is that if every institution throughout the country can have a young adult program, even something small like this can provide a large impact for patients,” Cann said.
The University of North Carolina (UNC) AYA Cancer Program, part of the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, formed in 2015. It has expanded into a team of about 12 including a nurse practitioner, fertility counselor, and psychologist, said Jacob Stein, MD, MPH, the program’s AYA oncology liaison. UNC sees about 400 AYA patients with cancer annually, and the program interacts with slightly more than 100 of them, according to Stein.
“Our program has taken a very different approach, to target services, contact, and engagement with AYAs who we perceive to be at the highest need, either clinically or socially, for support,” he said.
Stein was the lead author on research presented last year at the ASCO Quality Care Symposium in San Francisco finding that patients engaged in the program were more likely to participate in clinical trials and received higher rates of fertility preservation and palliative care than AYA patients at UNC without program contact.
Andrew Smitherman, MD, MS, medical director of the UNC AYA Cancer Center Program, said the AYA field has grown impressively since a progress review group was started in 2005, which was backed by the National Cancer Institute and LIVESTRONG Young Adult Alliance. The group developed recommendations to address AYA oncology nationwide, in hopes of acting as a catalyst for future initiatives. Clearly, others caring for patients with cancer heard the message.
“If a colleague comes to me and says, ‘Where do I start, how do I make this change at my institution,’ I usually lead with changing the culture,” Smitherman said. “Educating hospital leadership about the importance of this population, educating colleagues, finding partners. And then start thinking about ways to make structural changes, like creating space. That’s worked really well for us.”
DuVall, Sung, Roth, LaCasce, Eng, Cann, Stein, and Smitherman declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Adam DuVall, MD, MPH, is a rare medical oncologist who trained in both adult and pediatric hematology/oncology.
This distinction, which DuVall said he shares with only a handful of oncologists in the world, matches his role at University of Chicago (UChicago) Medicine, Chicago. Since joining UChicago in 2020, DuVall has helped expand its Adolescent and Young Adult (AYA) Oncology Program, which aims to provide comprehensive, one-stop care and support for patients with cancer aged from 15 to 39 years.
Started in 2012, the program is one of the oldest in a growing array of initiatives nationwide that seek to address the specific psychosocial and other support needs of patients with cancer who fall into the gap between young children and the older patients who more typically have cancer. Along with DuVall and other oncologists, UChicago’s AYA Program offers dedicated nurse practitioners, social workers, psychologists, a physical therapist, and a program administrator. A community health worker, who does home visits, helps patients coordinate travel, works with their insurance, and generally navigates the medical system, DuVall added.
The program receives about 1500-2000 visits a year, according to DuVall. What the young adult population with cancer has in common that distinguishes it from other age cohorts, he said, are its members’ particular psychosocial needs. “Going through adolescence and young adulthood without cancer, there’s plenty of things that are hard,” DuVall observed. “Put cancer on top of that, and it impacts every aspect of life.”
‘Millennials Have Higher Risk’
The proliferation of AYA programs comes as more and more studies have been published recently showing that young adults are increasingly getting cancer.
According to American Cancer Society research published in December in The Lancet Oncology, incidence rates of colorectal cancer (CRC) among young adults aged 25-49 years rose in the decade through 2017 in more than half of the 50 countries and territories examined. For the past 5 years studied, the incidence rate of early-onset CRC was highest in Australia, Puerto Rico, New Zealand, the United States, and South Korea. At the same time, the study found, rates among older adults in all of those places except South Korea were stable or declining.
Hyuna Sung, PhD, the study’s lead author, said, “Research has shown that Gen X and millennials have higher risk of multiple types of cancer compared to the older generations.”
Some of the cancers found to be increasing among younger adults are linked to “excess body obesity,” Sung said, including not only CRC but also cancers of the uterine corpus, gallbladder, kidney, pancreas, breast, and stomach cardia, as well as myeloma. Early onset of cancers not linked to obesity, such as testicular cancer and small intestinal cancer, has also been shown to be on the rise, Sung noted.
As cancer rates among young adults have risen, nonprofits have stepped in to help medical institutions open programs geared to their needs. Teen Cancer America, founded by members of rock band The Who, has partnered with 64 hospitals in 36 cities to develop AYA-focused programs, funding 85 hospital positions, according to a spokesperson. The Los Angeles–based nonprofit has also provided free consultation to 130 hospitals without formally providing a grant, the spokesperson said.
A map on Teen Cancer America’s website illustrates the nationwide spread of AYA programs, from UCLA Santa Monica Medical Center to Memorial Sloan Kettering Cancer Center in New York City, with more in between.
‘Setting Them Up for a Life of Meaning’
Michael Roth, MD, co-director of the AYA Program at the University of Texas MD Anderson Cancer Center in Houston, likes to say, “If you’ve seen one AYA Program, you’ve seen one AYA Program.”
In other words, offerings vary. “Most centers do not have comprehensive AYA programs,” Roth said, noting that at many sites the AYA Program might consist of oncofertility support. “That said, programs are doing the best they can, knowing that the AYA population is growing exponentially globally.”
Almost 90,000 AYA patients are diagnosed with cancer each year, and 85% will be at least 5-year survivors, Roth said. There are more than 2 million survivors of AYA cancer, he added, and if the median age of diagnosis is 30, they can live five decades beyond their cancer treatment. “Their life matters,” Roth said. “It matters during treatment. Their life after cancer matters.”
The AYA Program at MD Anderson began in 2017, and it sees more than 2000 AYAs diagnosed with cancer every year, according to Roth. The program is designed to complement the care that patients with cancer aged from 15 to 39 years or older may already be receiving from their primary treatment teams. New patients see a medical provider, a social worker, and a vocational counselor for discussions about their needs and concerns, and they have access to a nutritionist and genetic counselor.
The program offers psychosocial and supportive care for patients who may be facing challenges with school, work, relationships, having young children, and mental health, Roth said. Along with assessments and counseling around fertility risks and genetic predisposition, MD Anderson also provides patients in the program with a long-term survivorship plan.
“It’s not just increasing cures,” Roth observed. “We’re also setting them up for a life of meaning and happiness and productivity and health.”
Almost 40% of visits to the program are conducted virtually, according to Roth. “Our goal is to meet the patients where they are,” he said. “We want to be convenient, not be a burden.”
‘The Face of Cancer Has Changed’
Patients with AYA cancer diagnoses may be finishing up school or starting a job, developing their body image and sexual identity, or caring for young children or older parents.
“They feel incredibly isolated,” said Ann LaCasce, MD, MMSc, co-director of the Center for Adolescent and Young Adult Oncology at Dana-Farber Cancer Institute in Boston. “They go into the cancer center or their community practice, and everyone is double, triple their age.”
Last year, Dana-Farber opened a Young Adult Lounge meant for patients aged 18 years or older to be able to relax and, if they wish, interact between appointments. “When you talk to these patients, they want to meet each other,” LaCasce said. “They want to share experiences.”
The Young Adults With Cancer Program at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee, opened its doors in 2019. It recently hired an interim nurse navigator, said Cathy Eng, MD, FACP, FASCO, director of the Program, which concentrates on patients aged 25-45 years.
“The face of cancer has changed,” Eng said.
She advises other oncologists to talk to their young adult patients as much as possible. “Really talk to them as an individual and see what other needs they have,” she said. “Even if they don’t tell you the first time, ask them the second time, ask them the third time.”
Christopher Cann, MD, executive director of the Young Adult Cancer Program at Fox Chase Cancer Center in Philadelphia, did his fellowship under Eng. He joined Fox Chase in 2023, and the Young Adult Cancer Program started accepting patients around the end of last year, zeroing in on patients aged 18-39 years.
Following the implementation of a new best practice advisory that pops up in the medical records system, he said, oncofertility referrals increased by 400% within 6 months.
“My hope is that if every institution throughout the country can have a young adult program, even something small like this can provide a large impact for patients,” Cann said.
The University of North Carolina (UNC) AYA Cancer Program, part of the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, formed in 2015. It has expanded into a team of about 12 including a nurse practitioner, fertility counselor, and psychologist, said Jacob Stein, MD, MPH, the program’s AYA oncology liaison. UNC sees about 400 AYA patients with cancer annually, and the program interacts with slightly more than 100 of them, according to Stein.
“Our program has taken a very different approach, to target services, contact, and engagement with AYAs who we perceive to be at the highest need, either clinically or socially, for support,” he said.
Stein was the lead author on research presented last year at the ASCO Quality Care Symposium in San Francisco finding that patients engaged in the program were more likely to participate in clinical trials and received higher rates of fertility preservation and palliative care than AYA patients at UNC without program contact.
Andrew Smitherman, MD, MS, medical director of the UNC AYA Cancer Center Program, said the AYA field has grown impressively since a progress review group was started in 2005, which was backed by the National Cancer Institute and LIVESTRONG Young Adult Alliance. The group developed recommendations to address AYA oncology nationwide, in hopes of acting as a catalyst for future initiatives. Clearly, others caring for patients with cancer heard the message.
“If a colleague comes to me and says, ‘Where do I start, how do I make this change at my institution,’ I usually lead with changing the culture,” Smitherman said. “Educating hospital leadership about the importance of this population, educating colleagues, finding partners. And then start thinking about ways to make structural changes, like creating space. That’s worked really well for us.”
DuVall, Sung, Roth, LaCasce, Eng, Cann, Stein, and Smitherman declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Adam DuVall, MD, MPH, is a rare medical oncologist who trained in both adult and pediatric hematology/oncology.
This distinction, which DuVall said he shares with only a handful of oncologists in the world, matches his role at University of Chicago (UChicago) Medicine, Chicago. Since joining UChicago in 2020, DuVall has helped expand its Adolescent and Young Adult (AYA) Oncology Program, which aims to provide comprehensive, one-stop care and support for patients with cancer aged from 15 to 39 years.
Started in 2012, the program is one of the oldest in a growing array of initiatives nationwide that seek to address the specific psychosocial and other support needs of patients with cancer who fall into the gap between young children and the older patients who more typically have cancer. Along with DuVall and other oncologists, UChicago’s AYA Program offers dedicated nurse practitioners, social workers, psychologists, a physical therapist, and a program administrator. A community health worker, who does home visits, helps patients coordinate travel, works with their insurance, and generally navigates the medical system, DuVall added.
The program receives about 1500-2000 visits a year, according to DuVall. What the young adult population with cancer has in common that distinguishes it from other age cohorts, he said, are its members’ particular psychosocial needs. “Going through adolescence and young adulthood without cancer, there’s plenty of things that are hard,” DuVall observed. “Put cancer on top of that, and it impacts every aspect of life.”
‘Millennials Have Higher Risk’
The proliferation of AYA programs comes as more and more studies have been published recently showing that young adults are increasingly getting cancer.
According to American Cancer Society research published in December in The Lancet Oncology, incidence rates of colorectal cancer (CRC) among young adults aged 25-49 years rose in the decade through 2017 in more than half of the 50 countries and territories examined. For the past 5 years studied, the incidence rate of early-onset CRC was highest in Australia, Puerto Rico, New Zealand, the United States, and South Korea. At the same time, the study found, rates among older adults in all of those places except South Korea were stable or declining.
Hyuna Sung, PhD, the study’s lead author, said, “Research has shown that Gen X and millennials have higher risk of multiple types of cancer compared to the older generations.”
Some of the cancers found to be increasing among younger adults are linked to “excess body obesity,” Sung said, including not only CRC but also cancers of the uterine corpus, gallbladder, kidney, pancreas, breast, and stomach cardia, as well as myeloma. Early onset of cancers not linked to obesity, such as testicular cancer and small intestinal cancer, has also been shown to be on the rise, Sung noted.
As cancer rates among young adults have risen, nonprofits have stepped in to help medical institutions open programs geared to their needs. Teen Cancer America, founded by members of rock band The Who, has partnered with 64 hospitals in 36 cities to develop AYA-focused programs, funding 85 hospital positions, according to a spokesperson. The Los Angeles–based nonprofit has also provided free consultation to 130 hospitals without formally providing a grant, the spokesperson said.
A map on Teen Cancer America’s website illustrates the nationwide spread of AYA programs, from UCLA Santa Monica Medical Center to Memorial Sloan Kettering Cancer Center in New York City, with more in between.
‘Setting Them Up for a Life of Meaning’
Michael Roth, MD, co-director of the AYA Program at the University of Texas MD Anderson Cancer Center in Houston, likes to say, “If you’ve seen one AYA Program, you’ve seen one AYA Program.”
In other words, offerings vary. “Most centers do not have comprehensive AYA programs,” Roth said, noting that at many sites the AYA Program might consist of oncofertility support. “That said, programs are doing the best they can, knowing that the AYA population is growing exponentially globally.”
Almost 90,000 AYA patients are diagnosed with cancer each year, and 85% will be at least 5-year survivors, Roth said. There are more than 2 million survivors of AYA cancer, he added, and if the median age of diagnosis is 30, they can live five decades beyond their cancer treatment. “Their life matters,” Roth said. “It matters during treatment. Their life after cancer matters.”
The AYA Program at MD Anderson began in 2017, and it sees more than 2000 AYAs diagnosed with cancer every year, according to Roth. The program is designed to complement the care that patients with cancer aged from 15 to 39 years or older may already be receiving from their primary treatment teams. New patients see a medical provider, a social worker, and a vocational counselor for discussions about their needs and concerns, and they have access to a nutritionist and genetic counselor.
The program offers psychosocial and supportive care for patients who may be facing challenges with school, work, relationships, having young children, and mental health, Roth said. Along with assessments and counseling around fertility risks and genetic predisposition, MD Anderson also provides patients in the program with a long-term survivorship plan.
“It’s not just increasing cures,” Roth observed. “We’re also setting them up for a life of meaning and happiness and productivity and health.”
Almost 40% of visits to the program are conducted virtually, according to Roth. “Our goal is to meet the patients where they are,” he said. “We want to be convenient, not be a burden.”
‘The Face of Cancer Has Changed’
Patients with AYA cancer diagnoses may be finishing up school or starting a job, developing their body image and sexual identity, or caring for young children or older parents.
“They feel incredibly isolated,” said Ann LaCasce, MD, MMSc, co-director of the Center for Adolescent and Young Adult Oncology at Dana-Farber Cancer Institute in Boston. “They go into the cancer center or their community practice, and everyone is double, triple their age.”
Last year, Dana-Farber opened a Young Adult Lounge meant for patients aged 18 years or older to be able to relax and, if they wish, interact between appointments. “When you talk to these patients, they want to meet each other,” LaCasce said. “They want to share experiences.”
The Young Adults With Cancer Program at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee, opened its doors in 2019. It recently hired an interim nurse navigator, said Cathy Eng, MD, FACP, FASCO, director of the Program, which concentrates on patients aged 25-45 years.
“The face of cancer has changed,” Eng said.
She advises other oncologists to talk to their young adult patients as much as possible. “Really talk to them as an individual and see what other needs they have,” she said. “Even if they don’t tell you the first time, ask them the second time, ask them the third time.”
Christopher Cann, MD, executive director of the Young Adult Cancer Program at Fox Chase Cancer Center in Philadelphia, did his fellowship under Eng. He joined Fox Chase in 2023, and the Young Adult Cancer Program started accepting patients around the end of last year, zeroing in on patients aged 18-39 years.
Following the implementation of a new best practice advisory that pops up in the medical records system, he said, oncofertility referrals increased by 400% within 6 months.
“My hope is that if every institution throughout the country can have a young adult program, even something small like this can provide a large impact for patients,” Cann said.
The University of North Carolina (UNC) AYA Cancer Program, part of the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, formed in 2015. It has expanded into a team of about 12 including a nurse practitioner, fertility counselor, and psychologist, said Jacob Stein, MD, MPH, the program’s AYA oncology liaison. UNC sees about 400 AYA patients with cancer annually, and the program interacts with slightly more than 100 of them, according to Stein.
“Our program has taken a very different approach, to target services, contact, and engagement with AYAs who we perceive to be at the highest need, either clinically or socially, for support,” he said.
Stein was the lead author on research presented last year at the ASCO Quality Care Symposium in San Francisco finding that patients engaged in the program were more likely to participate in clinical trials and received higher rates of fertility preservation and palliative care than AYA patients at UNC without program contact.
Andrew Smitherman, MD, MS, medical director of the UNC AYA Cancer Center Program, said the AYA field has grown impressively since a progress review group was started in 2005, which was backed by the National Cancer Institute and LIVESTRONG Young Adult Alliance. The group developed recommendations to address AYA oncology nationwide, in hopes of acting as a catalyst for future initiatives. Clearly, others caring for patients with cancer heard the message.
“If a colleague comes to me and says, ‘Where do I start, how do I make this change at my institution,’ I usually lead with changing the culture,” Smitherman said. “Educating hospital leadership about the importance of this population, educating colleagues, finding partners. And then start thinking about ways to make structural changes, like creating space. That’s worked really well for us.”
DuVall, Sung, Roth, LaCasce, Eng, Cann, Stein, and Smitherman declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
FDA OKs First-line Nivolumab + Ipilimumab in Advanced HCC
The US Food and Drug Administration (FDA) has approved nivolumab (Opdivo, Bristol Myers Squibb) with ipilimumab (Yervoy, Bristol Myers Squibb) as a first-line treatment for adults with unresectable or metastatic hepatocellular carcinoma (HCC).
The decision, which follows the FDA’s 2020 accelerated approval in the second-line setting for advanced HCC and adds to the list of other indications for the combination therapy, was based on efficacy demonstrated in the randomized, open-label CHECKMATE-9DW trial. The trial enrolled 668 patients with unresectable or metastatic HCC and no prior systemic therapy for advanced disease, according to the FDA approval notice.
Median overall survival, the primary outcome measure, was 23.7 months in those randomized to receive nivolumab + ipilimumab, compared with 20.6 months in those randomized to receive investigators’ choice of lenvatinib or sorafenib (hazard ratio, 0.79). The overall response rate was 36.1% vs 13.2% in the arms, respectively.
Those in the treatment arm received 1 mg/kg intravenous (IV) nivolumab with 3 mg/kg IV ipilimumab every 3 weeks for up to four doses, followed by single-agent IV nivolumab at 480 mg every 4 weeks. Those in the control arm received either 8 or 12 mg lenvatinib daily or 400 mg sorafenib twice daily until disease progression or unacceptable toxicity, according to early results from the trial, which were presented at the 2024 American Society of Clinical Oncology meeting.
Adverse reactions occurring in more than 20% of patients included rash, pruritus, fatigue, and diarrhea.
The recommended nivolumab dose for this indication is 1 mg/kg with 3 mg/kg ipilimumab given intravenously every 3 weeks for up to four doses, followed by 240 mg nivolumab every 2 weeks or 480 mg nivolumab every 4 weeks. Full prescribing information will be available at Drugs@FDA.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved nivolumab (Opdivo, Bristol Myers Squibb) with ipilimumab (Yervoy, Bristol Myers Squibb) as a first-line treatment for adults with unresectable or metastatic hepatocellular carcinoma (HCC).
The decision, which follows the FDA’s 2020 accelerated approval in the second-line setting for advanced HCC and adds to the list of other indications for the combination therapy, was based on efficacy demonstrated in the randomized, open-label CHECKMATE-9DW trial. The trial enrolled 668 patients with unresectable or metastatic HCC and no prior systemic therapy for advanced disease, according to the FDA approval notice.
Median overall survival, the primary outcome measure, was 23.7 months in those randomized to receive nivolumab + ipilimumab, compared with 20.6 months in those randomized to receive investigators’ choice of lenvatinib or sorafenib (hazard ratio, 0.79). The overall response rate was 36.1% vs 13.2% in the arms, respectively.
Those in the treatment arm received 1 mg/kg intravenous (IV) nivolumab with 3 mg/kg IV ipilimumab every 3 weeks for up to four doses, followed by single-agent IV nivolumab at 480 mg every 4 weeks. Those in the control arm received either 8 or 12 mg lenvatinib daily or 400 mg sorafenib twice daily until disease progression or unacceptable toxicity, according to early results from the trial, which were presented at the 2024 American Society of Clinical Oncology meeting.
Adverse reactions occurring in more than 20% of patients included rash, pruritus, fatigue, and diarrhea.
The recommended nivolumab dose for this indication is 1 mg/kg with 3 mg/kg ipilimumab given intravenously every 3 weeks for up to four doses, followed by 240 mg nivolumab every 2 weeks or 480 mg nivolumab every 4 weeks. Full prescribing information will be available at Drugs@FDA.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved nivolumab (Opdivo, Bristol Myers Squibb) with ipilimumab (Yervoy, Bristol Myers Squibb) as a first-line treatment for adults with unresectable or metastatic hepatocellular carcinoma (HCC).
The decision, which follows the FDA’s 2020 accelerated approval in the second-line setting for advanced HCC and adds to the list of other indications for the combination therapy, was based on efficacy demonstrated in the randomized, open-label CHECKMATE-9DW trial. The trial enrolled 668 patients with unresectable or metastatic HCC and no prior systemic therapy for advanced disease, according to the FDA approval notice.
Median overall survival, the primary outcome measure, was 23.7 months in those randomized to receive nivolumab + ipilimumab, compared with 20.6 months in those randomized to receive investigators’ choice of lenvatinib or sorafenib (hazard ratio, 0.79). The overall response rate was 36.1% vs 13.2% in the arms, respectively.
Those in the treatment arm received 1 mg/kg intravenous (IV) nivolumab with 3 mg/kg IV ipilimumab every 3 weeks for up to four doses, followed by single-agent IV nivolumab at 480 mg every 4 weeks. Those in the control arm received either 8 or 12 mg lenvatinib daily or 400 mg sorafenib twice daily until disease progression or unacceptable toxicity, according to early results from the trial, which were presented at the 2024 American Society of Clinical Oncology meeting.
Adverse reactions occurring in more than 20% of patients included rash, pruritus, fatigue, and diarrhea.
The recommended nivolumab dose for this indication is 1 mg/kg with 3 mg/kg ipilimumab given intravenously every 3 weeks for up to four doses, followed by 240 mg nivolumab every 2 weeks or 480 mg nivolumab every 4 weeks. Full prescribing information will be available at Drugs@FDA.
A version of this article first appeared on Medscape.com.
Radiation Oncology Reimbursement: New Bill Rocks the Boat
A renewed effort to modernize and stabilize Medicare reimbursement for radiation therapy services is underway.
In mid-March, members of Congress reintroduced bipartisan federal legislation that would shift Medicare reimbursement for radiation oncology services from quantity-based payments to episode-based payments and help stabilize the declining rates of reimbursement in the field.
The Radiation Oncology Case Rate (ROCR) Value Based Payment Program Act, sponsored by two senators and four representatives, would not only “transform” how Medicare reimburses radiation therapy services, it would also “protect access to high quality cancer care and improve outcomes for patients nationwide, while generating savings for Medicare,” according to a recent American Society for Radiation Oncology (ASTRO) press release praising the bill.
However, the reaction among those in the field has been mixed. Whereas some radiation oncologists are aligned with the bill, others argue that the legislation was crafted without meaningful input from many who will be affected.
“There’s consensus across multiple groups within the house of radiation oncology, hospital groups, and industry, which is incredibly important,” according to Mustafa Basree, DO, a radiation oncology resident who serves on ASTRO’s government relations committee and was part of the discussion on drafting the bill.
But, Basree acknowledged, “not everybody likes the bill.”
A core complaint is a lack of communication and input from clinicians in the field. “If we’re going to decide to design our own quality program — which is really like a dream from a clinician’s standpoint — we need a meaningful way to come together as a unified field,” said Matthew Spraker, MD, PhD, a radiation oncologist practicing in Denver. In this bill, “we’re not getting any of that.”
Impetus for the Bill
Amid dramatic drops in Medicare reimbursement — and with more probably on the horizon — ASTRO announced in January 2024 that the society had partnered with the American College of Radiation Oncology, the American College of Radiology, and the American Society of Clinical Oncology to lobby for payment reform.
Cuts to Medicare reimbursement were approaching 25% at the time. These declines were related, in part, to changes in how radiation treatment was being delivered. Reimbursement has historically been based on the fraction of radiation given, but the field has increasingly embraced hypofractionated regimens and deescalated approaches, which have led to fewer billable fractions and consequently lower reimbursement.
A recent study highlighted significant declines in reimbursement based on this shift in care. For instance, greater use of hypofractionation led to declines in reimbursement for technical services in freestanding radiation oncology offices by nearly 17% for breast cancer and 14.2% for prostate cancer between 2016 and 2022. Inflation-adjusted Medicare conversion factors fell 12.2% in hospital outpatient centers and 20.8% in freestanding offices.
These declining reimbursement rates have occurred alongside changes to radiation oncology practice patterns. A recent analysis reported a 51% increase in the number of US practices with at least 10 radiation oncologists between 2015 and 2023, and a 27% decrease in the number of solo practices during the same period. The number of practicing radiation oncologists increased by 16%, but the number of practices employing them decreased by 13%, indicating a trend toward practice consolidation.
These changes, the analysis found, may affect patients’ access to care. In rural areas, retirement rates were higher and rates of entry of new radiation oncologists was lower compared with urban areas.
The current payment structure “has become untenable,” leading to practice consolidation that threaten patient access, especially in rural and underserved areas, a spokesperson for ASTRO told this news organization last year.
What Is the ROCR Act?
The ROCR ACT was drafted by ASTRO to address these issues and reverse declines in Medicare reimbursement.
In addition to shifting radiation oncology reimbursement from fraction-based to episode-based, the bill also aims to encourage clinicians to adoptevidence-based shorter treatment regimens, improve safety and quality by supporting new technologies, and generate savings to Medicare by eliminating outdated and costly practices that have not been shown to improve patient outcomes.
When first introduced last year, the bill did not receive a vote in Congress.
A 2025 version of the bill, introduced last month, largely aligns with the 2024 legislation but contains some “enhancements,” such as improving accreditation with increased incentive payments, outlining a revised exemption for practices with limited resources and instituting a transitional payment period for adaptive radiation therapy to allow billing to continue while a new code is created.
Mixed Reactions
How has the radiation oncology community reacted to the latest ROCR Act?
A recent survey, which included more than 500 practicing radiation oncologists, found that 61% of respondents supported implementing an episode-based payment model such as that proposed in the 2025 legislation, 17.3% neither supported nor opposed it, and 21.6% opposed the model.
“I think this supports this idea that our field would have benefited from much more open discussion in the design phases of the bill,” Spraker told this news organization.
Jason Beckta, MD, PhD, a radiation oncologist at Rutland Regional Medical Center, Vermont, agreed.
While on board with the concept of reform and episode-based payment, given what Beckta called “the absolute absurdity of the cuts in radiation oncology,” he took issue with the lack of transparency in the rollout of the bill.
The announcement about the 2025 version of the ROCR Act came as “a complete surprise — out of nowhere — except to insiders,” Beckta said.
ASTRO held a town hall in February 2025 “featuring new information and discussion” regarding ROCR 2025, but the bill had already been finalized for submission at that point, Beckta said.
And although Beckta and Spraker believe ASTRO had good intentions, the physicians highlighted concerns with several aspects of the bill.
“What upsets me most is the blatant regulatory capture,” Beckta said. The legislation will require all practices to be accredited by either ASTRO, the American College of Radiation Oncology, or the American College of Radiology, essentially capturing their business through a regulation or having practices “face a 2.5% penalty, which is up 1% from the prior version,” Beckta explained.
The bill also shortens the runway to get accredited from 3 years to 2 years, Beckta noted, stressing the arduousness of the accreditation process as a hurdle for many practices.
But doing the accreditation program does not mean care will get better, Spraker said. In fact, “that is absolutely not the case.”
Another issue: Requirements for medical equipment and quality review periods seem to favor industry over patients and practices, he said, highlighting the potential role of manufacturers in determining if or when equipment updates are required.
“A field like ours has rapidly exploding technology with not-always-clear patient benefit,” said Spraker. “We’re seeing too many examples where people are leveraging that, basically to sell devices instead of to help patients,” he added.
Furthermore, Beckta noted, the bill allows for reduced reimbursement of between 4% and 7%, depending on the circumstances — a cut to reimbursement that is being justified by saying it’s the only way the bill will get through Congress. But “it’s just a less-bad option than continued cuts to fee-for-service,” Beckta said.
ASTRO leadership has expressed strong support for the bill. In the recent ASTRO press release, Howard M. Sandler, MD, chair of the ASTRO Board of Directors, called the ROCR Act “the only viable policy solution designed to provide payment stability for the field of radiation oncology in 2026 and beyond.”
The ROCR Act, which is broadly supported by more than 80 organizations, “represents a balanced, evidence-based policy solution to safeguard access to high value cancer treatment for Americans,” Sandler said.
“I believe in this bill,” Basree added.
Basree touted the replacement of a fee-for-service model with a “value-based payment system, ensuring predictable, fair reimbursement for the field” as a major win for stabilizing Medicare reimbursement. The bill also includes measures to improve patient access, such as providing discounted transportation for patients — a significant need, particularly in rural areas, he explained.
Although not everyone is happy with the bill, ASTRO did aim “to build coalition of support,” Basree said. “It’s an uphill battle, for sure, but we should press forward and hope for the best,” he added.
Even with their concerns, both Spraker and Beckta are optimistic that improvements to the bill can still be made, and urge colleagues to study the bill, speak out, and engage to help promote the best possible policy.
Basree reported receiving reimbursement for meeting travel and lodging as both a Fellow and member of the Association of Residents in Radiation Oncology. Spraker and Beckta reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
A renewed effort to modernize and stabilize Medicare reimbursement for radiation therapy services is underway.
In mid-March, members of Congress reintroduced bipartisan federal legislation that would shift Medicare reimbursement for radiation oncology services from quantity-based payments to episode-based payments and help stabilize the declining rates of reimbursement in the field.
The Radiation Oncology Case Rate (ROCR) Value Based Payment Program Act, sponsored by two senators and four representatives, would not only “transform” how Medicare reimburses radiation therapy services, it would also “protect access to high quality cancer care and improve outcomes for patients nationwide, while generating savings for Medicare,” according to a recent American Society for Radiation Oncology (ASTRO) press release praising the bill.
However, the reaction among those in the field has been mixed. Whereas some radiation oncologists are aligned with the bill, others argue that the legislation was crafted without meaningful input from many who will be affected.
“There’s consensus across multiple groups within the house of radiation oncology, hospital groups, and industry, which is incredibly important,” according to Mustafa Basree, DO, a radiation oncology resident who serves on ASTRO’s government relations committee and was part of the discussion on drafting the bill.
But, Basree acknowledged, “not everybody likes the bill.”
A core complaint is a lack of communication and input from clinicians in the field. “If we’re going to decide to design our own quality program — which is really like a dream from a clinician’s standpoint — we need a meaningful way to come together as a unified field,” said Matthew Spraker, MD, PhD, a radiation oncologist practicing in Denver. In this bill, “we’re not getting any of that.”
Impetus for the Bill
Amid dramatic drops in Medicare reimbursement — and with more probably on the horizon — ASTRO announced in January 2024 that the society had partnered with the American College of Radiation Oncology, the American College of Radiology, and the American Society of Clinical Oncology to lobby for payment reform.
Cuts to Medicare reimbursement were approaching 25% at the time. These declines were related, in part, to changes in how radiation treatment was being delivered. Reimbursement has historically been based on the fraction of radiation given, but the field has increasingly embraced hypofractionated regimens and deescalated approaches, which have led to fewer billable fractions and consequently lower reimbursement.
A recent study highlighted significant declines in reimbursement based on this shift in care. For instance, greater use of hypofractionation led to declines in reimbursement for technical services in freestanding radiation oncology offices by nearly 17% for breast cancer and 14.2% for prostate cancer between 2016 and 2022. Inflation-adjusted Medicare conversion factors fell 12.2% in hospital outpatient centers and 20.8% in freestanding offices.
These declining reimbursement rates have occurred alongside changes to radiation oncology practice patterns. A recent analysis reported a 51% increase in the number of US practices with at least 10 radiation oncologists between 2015 and 2023, and a 27% decrease in the number of solo practices during the same period. The number of practicing radiation oncologists increased by 16%, but the number of practices employing them decreased by 13%, indicating a trend toward practice consolidation.
These changes, the analysis found, may affect patients’ access to care. In rural areas, retirement rates were higher and rates of entry of new radiation oncologists was lower compared with urban areas.
The current payment structure “has become untenable,” leading to practice consolidation that threaten patient access, especially in rural and underserved areas, a spokesperson for ASTRO told this news organization last year.
What Is the ROCR Act?
The ROCR ACT was drafted by ASTRO to address these issues and reverse declines in Medicare reimbursement.
In addition to shifting radiation oncology reimbursement from fraction-based to episode-based, the bill also aims to encourage clinicians to adoptevidence-based shorter treatment regimens, improve safety and quality by supporting new technologies, and generate savings to Medicare by eliminating outdated and costly practices that have not been shown to improve patient outcomes.
When first introduced last year, the bill did not receive a vote in Congress.
A 2025 version of the bill, introduced last month, largely aligns with the 2024 legislation but contains some “enhancements,” such as improving accreditation with increased incentive payments, outlining a revised exemption for practices with limited resources and instituting a transitional payment period for adaptive radiation therapy to allow billing to continue while a new code is created.
Mixed Reactions
How has the radiation oncology community reacted to the latest ROCR Act?
A recent survey, which included more than 500 practicing radiation oncologists, found that 61% of respondents supported implementing an episode-based payment model such as that proposed in the 2025 legislation, 17.3% neither supported nor opposed it, and 21.6% opposed the model.
“I think this supports this idea that our field would have benefited from much more open discussion in the design phases of the bill,” Spraker told this news organization.
Jason Beckta, MD, PhD, a radiation oncologist at Rutland Regional Medical Center, Vermont, agreed.
While on board with the concept of reform and episode-based payment, given what Beckta called “the absolute absurdity of the cuts in radiation oncology,” he took issue with the lack of transparency in the rollout of the bill.
The announcement about the 2025 version of the ROCR Act came as “a complete surprise — out of nowhere — except to insiders,” Beckta said.
ASTRO held a town hall in February 2025 “featuring new information and discussion” regarding ROCR 2025, but the bill had already been finalized for submission at that point, Beckta said.
And although Beckta and Spraker believe ASTRO had good intentions, the physicians highlighted concerns with several aspects of the bill.
“What upsets me most is the blatant regulatory capture,” Beckta said. The legislation will require all practices to be accredited by either ASTRO, the American College of Radiation Oncology, or the American College of Radiology, essentially capturing their business through a regulation or having practices “face a 2.5% penalty, which is up 1% from the prior version,” Beckta explained.
The bill also shortens the runway to get accredited from 3 years to 2 years, Beckta noted, stressing the arduousness of the accreditation process as a hurdle for many practices.
But doing the accreditation program does not mean care will get better, Spraker said. In fact, “that is absolutely not the case.”
Another issue: Requirements for medical equipment and quality review periods seem to favor industry over patients and practices, he said, highlighting the potential role of manufacturers in determining if or when equipment updates are required.
“A field like ours has rapidly exploding technology with not-always-clear patient benefit,” said Spraker. “We’re seeing too many examples where people are leveraging that, basically to sell devices instead of to help patients,” he added.
Furthermore, Beckta noted, the bill allows for reduced reimbursement of between 4% and 7%, depending on the circumstances — a cut to reimbursement that is being justified by saying it’s the only way the bill will get through Congress. But “it’s just a less-bad option than continued cuts to fee-for-service,” Beckta said.
ASTRO leadership has expressed strong support for the bill. In the recent ASTRO press release, Howard M. Sandler, MD, chair of the ASTRO Board of Directors, called the ROCR Act “the only viable policy solution designed to provide payment stability for the field of radiation oncology in 2026 and beyond.”
The ROCR Act, which is broadly supported by more than 80 organizations, “represents a balanced, evidence-based policy solution to safeguard access to high value cancer treatment for Americans,” Sandler said.
“I believe in this bill,” Basree added.
Basree touted the replacement of a fee-for-service model with a “value-based payment system, ensuring predictable, fair reimbursement for the field” as a major win for stabilizing Medicare reimbursement. The bill also includes measures to improve patient access, such as providing discounted transportation for patients — a significant need, particularly in rural areas, he explained.
Although not everyone is happy with the bill, ASTRO did aim “to build coalition of support,” Basree said. “It’s an uphill battle, for sure, but we should press forward and hope for the best,” he added.
Even with their concerns, both Spraker and Beckta are optimistic that improvements to the bill can still be made, and urge colleagues to study the bill, speak out, and engage to help promote the best possible policy.
Basree reported receiving reimbursement for meeting travel and lodging as both a Fellow and member of the Association of Residents in Radiation Oncology. Spraker and Beckta reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
A renewed effort to modernize and stabilize Medicare reimbursement for radiation therapy services is underway.
In mid-March, members of Congress reintroduced bipartisan federal legislation that would shift Medicare reimbursement for radiation oncology services from quantity-based payments to episode-based payments and help stabilize the declining rates of reimbursement in the field.
The Radiation Oncology Case Rate (ROCR) Value Based Payment Program Act, sponsored by two senators and four representatives, would not only “transform” how Medicare reimburses radiation therapy services, it would also “protect access to high quality cancer care and improve outcomes for patients nationwide, while generating savings for Medicare,” according to a recent American Society for Radiation Oncology (ASTRO) press release praising the bill.
However, the reaction among those in the field has been mixed. Whereas some radiation oncologists are aligned with the bill, others argue that the legislation was crafted without meaningful input from many who will be affected.
“There’s consensus across multiple groups within the house of radiation oncology, hospital groups, and industry, which is incredibly important,” according to Mustafa Basree, DO, a radiation oncology resident who serves on ASTRO’s government relations committee and was part of the discussion on drafting the bill.
But, Basree acknowledged, “not everybody likes the bill.”
A core complaint is a lack of communication and input from clinicians in the field. “If we’re going to decide to design our own quality program — which is really like a dream from a clinician’s standpoint — we need a meaningful way to come together as a unified field,” said Matthew Spraker, MD, PhD, a radiation oncologist practicing in Denver. In this bill, “we’re not getting any of that.”
Impetus for the Bill
Amid dramatic drops in Medicare reimbursement — and with more probably on the horizon — ASTRO announced in January 2024 that the society had partnered with the American College of Radiation Oncology, the American College of Radiology, and the American Society of Clinical Oncology to lobby for payment reform.
Cuts to Medicare reimbursement were approaching 25% at the time. These declines were related, in part, to changes in how radiation treatment was being delivered. Reimbursement has historically been based on the fraction of radiation given, but the field has increasingly embraced hypofractionated regimens and deescalated approaches, which have led to fewer billable fractions and consequently lower reimbursement.
A recent study highlighted significant declines in reimbursement based on this shift in care. For instance, greater use of hypofractionation led to declines in reimbursement for technical services in freestanding radiation oncology offices by nearly 17% for breast cancer and 14.2% for prostate cancer between 2016 and 2022. Inflation-adjusted Medicare conversion factors fell 12.2% in hospital outpatient centers and 20.8% in freestanding offices.
These declining reimbursement rates have occurred alongside changes to radiation oncology practice patterns. A recent analysis reported a 51% increase in the number of US practices with at least 10 radiation oncologists between 2015 and 2023, and a 27% decrease in the number of solo practices during the same period. The number of practicing radiation oncologists increased by 16%, but the number of practices employing them decreased by 13%, indicating a trend toward practice consolidation.
These changes, the analysis found, may affect patients’ access to care. In rural areas, retirement rates were higher and rates of entry of new radiation oncologists was lower compared with urban areas.
The current payment structure “has become untenable,” leading to practice consolidation that threaten patient access, especially in rural and underserved areas, a spokesperson for ASTRO told this news organization last year.
What Is the ROCR Act?
The ROCR ACT was drafted by ASTRO to address these issues and reverse declines in Medicare reimbursement.
In addition to shifting radiation oncology reimbursement from fraction-based to episode-based, the bill also aims to encourage clinicians to adoptevidence-based shorter treatment regimens, improve safety and quality by supporting new technologies, and generate savings to Medicare by eliminating outdated and costly practices that have not been shown to improve patient outcomes.
When first introduced last year, the bill did not receive a vote in Congress.
A 2025 version of the bill, introduced last month, largely aligns with the 2024 legislation but contains some “enhancements,” such as improving accreditation with increased incentive payments, outlining a revised exemption for practices with limited resources and instituting a transitional payment period for adaptive radiation therapy to allow billing to continue while a new code is created.
Mixed Reactions
How has the radiation oncology community reacted to the latest ROCR Act?
A recent survey, which included more than 500 practicing radiation oncologists, found that 61% of respondents supported implementing an episode-based payment model such as that proposed in the 2025 legislation, 17.3% neither supported nor opposed it, and 21.6% opposed the model.
“I think this supports this idea that our field would have benefited from much more open discussion in the design phases of the bill,” Spraker told this news organization.
Jason Beckta, MD, PhD, a radiation oncologist at Rutland Regional Medical Center, Vermont, agreed.
While on board with the concept of reform and episode-based payment, given what Beckta called “the absolute absurdity of the cuts in radiation oncology,” he took issue with the lack of transparency in the rollout of the bill.
The announcement about the 2025 version of the ROCR Act came as “a complete surprise — out of nowhere — except to insiders,” Beckta said.
ASTRO held a town hall in February 2025 “featuring new information and discussion” regarding ROCR 2025, but the bill had already been finalized for submission at that point, Beckta said.
And although Beckta and Spraker believe ASTRO had good intentions, the physicians highlighted concerns with several aspects of the bill.
“What upsets me most is the blatant regulatory capture,” Beckta said. The legislation will require all practices to be accredited by either ASTRO, the American College of Radiation Oncology, or the American College of Radiology, essentially capturing their business through a regulation or having practices “face a 2.5% penalty, which is up 1% from the prior version,” Beckta explained.
The bill also shortens the runway to get accredited from 3 years to 2 years, Beckta noted, stressing the arduousness of the accreditation process as a hurdle for many practices.
But doing the accreditation program does not mean care will get better, Spraker said. In fact, “that is absolutely not the case.”
Another issue: Requirements for medical equipment and quality review periods seem to favor industry over patients and practices, he said, highlighting the potential role of manufacturers in determining if or when equipment updates are required.
“A field like ours has rapidly exploding technology with not-always-clear patient benefit,” said Spraker. “We’re seeing too many examples where people are leveraging that, basically to sell devices instead of to help patients,” he added.
Furthermore, Beckta noted, the bill allows for reduced reimbursement of between 4% and 7%, depending on the circumstances — a cut to reimbursement that is being justified by saying it’s the only way the bill will get through Congress. But “it’s just a less-bad option than continued cuts to fee-for-service,” Beckta said.
ASTRO leadership has expressed strong support for the bill. In the recent ASTRO press release, Howard M. Sandler, MD, chair of the ASTRO Board of Directors, called the ROCR Act “the only viable policy solution designed to provide payment stability for the field of radiation oncology in 2026 and beyond.”
The ROCR Act, which is broadly supported by more than 80 organizations, “represents a balanced, evidence-based policy solution to safeguard access to high value cancer treatment for Americans,” Sandler said.
“I believe in this bill,” Basree added.
Basree touted the replacement of a fee-for-service model with a “value-based payment system, ensuring predictable, fair reimbursement for the field” as a major win for stabilizing Medicare reimbursement. The bill also includes measures to improve patient access, such as providing discounted transportation for patients — a significant need, particularly in rural areas, he explained.
Although not everyone is happy with the bill, ASTRO did aim “to build coalition of support,” Basree said. “It’s an uphill battle, for sure, but we should press forward and hope for the best,” he added.
Even with their concerns, both Spraker and Beckta are optimistic that improvements to the bill can still be made, and urge colleagues to study the bill, speak out, and engage to help promote the best possible policy.
Basree reported receiving reimbursement for meeting travel and lodging as both a Fellow and member of the Association of Residents in Radiation Oncology. Spraker and Beckta reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
A ‘Fool’s Errand’? Picking a Winner for Treating Early-Stage NSCLC
For years, the default definitive treatment for patients with early-stage I non–small cell lung cancer (NSCLC) has been surgical resection, typically minimally invasive lobectomy with systematic lymph node dissection.
Guidelines from the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology, and the European Society for Medical Oncology all list surgery (in particular, lobectomy) as the primary local therapy for fit, operable patients with stage I NSCLC.
More recently, however, stereotactic body radiotherapy (SBRT), also called stereotactic ablative radiotherapy, has emerged as a definitive treatment option for stage I NSCLC, especially for older, less fit patients who are unsuitable or deemed high-risk for surgery.
“We see patients in our practice who cannot undergo surgery or who may not have adequate lung function to be able to tolerate surgery, and for these patients who are medically inoperable or surgically unresectable, radiation therapy may be a reasonable option,” Charu Aggarwal, MD, MPH, professor and lung cancer specialist, University of Pennsylvania, Philadelphia, told this news organization.
Given some encouraging data suggesting that SBRT could provide similar survival outcomes and be an alternative to surgery for operable disease, SBRT is also increasingly being considered in these early-stage patients. However, other evidence indicates that SBRT may be associated with higher rates of regional and distant recurrences and worse long-term survival, particularly in operable patients.
What may ultimately matter most is carefully selecting operable patients who undergo SBRT.
Aggarwal has encountered certain patients who are fit for surgery but would rather have radiation therapy. “This is an individual decision, and these patients are usually discussed at tumor board and in multidisciplinary discussions to really make sure that they’re making the right decision for themselves,” she explained.
The Pros and Cons of SBRT
SBRT is a nonsurgical approach in which precision high-dose radiation is delivered in just a few fractions — typically, 3, 5, or 8, depending on institutional protocols and tumor characteristics.
SBRT is performed on an outpatient basis, usually over 1-2 weeks, with most patients able to resume usual activities with minimal to no delay. Surgery, on the other hand, requires a hospital stay and takes most people about 2-6 weeks to return to regular activities. SBRT also avoids anesthesia and surgical incisions, both of which come with risks.
The data on SBRT in early-stage NSCLC are mixed. While some studies indicate that SBRT comes with promising survival outcomes, other research has reported worse survival and recurrence rates.
One potential reason for higher recurrence rates with SBRT is the lack of pathologic nodal staging, which only happens after surgery, as well as lower rates of nodal evaluation with endobronchial ultrasound or mediastinoscopy before surgery or SBRT. Without nodal assessments, clinicians may miss a more aggressive histology or more advanced nodal stage, which would go undertreated if patients received SBRT.
Latest Data in Large Cohort
A recent study published in Lung Cancer indicated that, when carefully selected, operable patients with early NSCLC have comparable survival with lobectomy or SBRT.
In the study, Dutch researchers took an in-depth look at survival and recurrence patterns in a retrospective cohort study of 2183 patients with clinical stage I NSCLC treated with minimally invasive lobectomy or SBRT. The study includes one of the largest cohorts to date, with robust data collection on baseline characteristics, comorbidities, tumor size, performance status, and follow-up.
Patients receiving SBRT were typically older (median age, 74 vs 67 years), had higher comorbidity burdens (Charlson index ≥ 5 in 57% of SBRT patients vs 23% of surgical patients), worse performance status, and lower lung function. To adjust for these differences, the researchers used propensity score weighting so the SBRT group’s baseline characteristics were comparable with those in the surgery group.
The surgery cohort had more invasive nodal evaluation: 21% underwent endobronchial ultrasound or mediastinoscopy vs only 12% in the SBRT group. The vast majority in both groups had PET-CT staging, reflecting modern imaging-based workups.
While 5-year local recurrence rates between the two groups were similar (13.1% for SBRT vs 12.1% for surgery), the 5-year regional recurrence rate was significantly higher after SBRT than lobectomy (18.1% vs 14.2%; hazard ratio [HR], 0.74), as was the distant metastasis rate (26.2% vs 20.2%; HR, 0.72).
Mortality at 30 days was higher after surgery than SBRT (1.0% vs 0.2%). And in the unadjusted analysis, 5-year overall survival was significantly better with lobectomy than SBRT (70.2% vs 40.3%).
However, when the analysis only included patients with similar baseline characteristics, overall survival was no longer significantly different in the two groups (HR, 0.89; 95% CI, 0.65-1.20). Lung cancer–specific mortality was also not significantly different between the two treatments (HR, 1.08), but the study was underpowered to detect significant differences in this outcome on the basis of a relatively low number of deaths from NSCLC.
Still, even after comparing similar patients, recurrence-free survival was notably better with surgery (HR, 0.70), due to fewer regional recurrences and distant metastases. Overall, 13% of the surgical cohort had nodal upstaging at pathology, meaning that even in clinically “node-negative” stage I disease, a subset of patients had unsuspected nodal involvement.
Patients receiving SBRT did not have pathologic nodal staging, raising the possibility of occult micrometastases. The authors noted that the proportion of SBRT patients with occult lymph node metastases is likely at least equal to that in the surgery group, but these metastases would go undetected without pathologic assessment.
Missing potential occult micrometastases in the SBRT group likely contributed to higher regional recurrence rates over time. By improving nodal staging, more patients with occult lymph node metastases who would be undertreated with SBRT may be identified before treatment, the authors said.
What Do Experts Say?
So, is SBRT an option for patients with stage I NSCLC?
Opinions vary.
“If you got one shot for a cure, then you want to do the surgery because that’s what results in a cure,” said Raja Flores, MD, chairman of Thoracic Surgery, Mount Sinai Health System, New York City.
Flores noted that the survival rate with surgery is high in this population. “There’s really nothing out there that can compare,” he said.
In his view, surgery “remains the gold standard.” However, “radiation could be considered in nonsurgical candidates,” he said.
The most recent NCCN guidelines align with Flores’ take. The guidelines say that SBRT is indicated for stage IA-IIA (N0) NSCLC in patients who are deemed “medically inoperable, high surgical risk as determined by thoracic surgeon, and those who decline surgery after thoracic surgical consultation.”
Clifford G. Robinson, MD, agreed. “In the United States, we largely treat patients with SBRT who are medically inoperable or high-risk operable and a much smaller proportion who decline surgery,” said Robinson, professor of radiation oncology and chief of SBRT at Washington University in St. Louis, St. Louis. “Many patients who are deemed operable are not offered SBRT.”
Still, for Robinson, determining which patients are best suited for surgery or SBRT remains unclear.
“Retrospective comparisons are fraught with problems because of confounding,” Robinson told this news organization. “That is, the healthier patients get surgery, and the less healthy ones get SBRT. No manner of fancy statistical manipulation can remove that fact.”
In fact, a recent meta-analysis found that the most significant variable predicting whether surgery or SBRT was superior in retrospective studies was whether the author was a surgeon or radiation oncologist.
Robinson noted that multiple randomized trials have attempted to randomize patients with medically operable early-stage NSCLC to surgery or SBRT and failed to accrue, largely due to patients’ “understandable unwillingness to be randomized between operative vs nonoperative interventions when most already prefer one or the other approach.”
Flores highlighted another point of caution about interpreting trial results: Not all early-stage NSCLC behaves similarly. “Some are slow-growing ‘turtles,’ and others are aggressive ‘rabbits’ — and the turtles are usually the ones that have been included in these radiotherapy trials, and that’s the danger,” he said.
While medical operability is the primary factor for deciding the treatment modality for early-stage NSCLC, there are other more subtle factors that can play into the decision.
These include prior surgery or radiotherapy to the chest, prior cancers, and social issues, such as the patient being a primary caregiver for another person and job insecurity, that might make recovery from surgery more challenging. And in rare instances, a patient may be medically fit to undergo surgery, but the cancer is technically challenging to resect due to anatomic issues or prior surgery to the chest, Robinson added.
A Winner?
Results from two ongoing, highly anticipated randomized trials expected in the next several years will hopefully provide additional insights and clarify ongoing uncertainties about the optimal treatment strategies for operable patients with stage I NSCLC.
STABLE-MATES is comparing outcomes after sublobar resection and SBRT in high-risk operable stage I NSCLC, and VALOR is evaluating outcomes after anatomic pulmonary resections and SBRT in patients with stage I NSCLC who have a long life expectancy and are fit enough to tolerate surgery.
But Robinson said his group believes that trying to decide on a winner is a “fool’s errand” and is instead running a pragmatic study across multiple academic and community centers around the United States and Canada where patients choose therapy based on their personal preferences and guidance from their physicians. The researchers will carefully track baseline comorbidity and frailty and assess serial quality-of-life changes over time.
“The goal is to create a calculator that a given patient might use in the future to determine what patients like them would have received, complete with expected outcomes and side effects,” Robinson said.
Robinson cautioned, however, that it “seems unlikely, given the existing literature, that one of the treatments will be truly ‘superior’ to the other one and lead to the ‘losing’ treatment fading away since both are excellent options with pros and cons.”
Aggarwal, Robinson, and Flores had no relevant disclosures.
A version of this article first appeared on Medscape.com.
For years, the default definitive treatment for patients with early-stage I non–small cell lung cancer (NSCLC) has been surgical resection, typically minimally invasive lobectomy with systematic lymph node dissection.
Guidelines from the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology, and the European Society for Medical Oncology all list surgery (in particular, lobectomy) as the primary local therapy for fit, operable patients with stage I NSCLC.
More recently, however, stereotactic body radiotherapy (SBRT), also called stereotactic ablative radiotherapy, has emerged as a definitive treatment option for stage I NSCLC, especially for older, less fit patients who are unsuitable or deemed high-risk for surgery.
“We see patients in our practice who cannot undergo surgery or who may not have adequate lung function to be able to tolerate surgery, and for these patients who are medically inoperable or surgically unresectable, radiation therapy may be a reasonable option,” Charu Aggarwal, MD, MPH, professor and lung cancer specialist, University of Pennsylvania, Philadelphia, told this news organization.
Given some encouraging data suggesting that SBRT could provide similar survival outcomes and be an alternative to surgery for operable disease, SBRT is also increasingly being considered in these early-stage patients. However, other evidence indicates that SBRT may be associated with higher rates of regional and distant recurrences and worse long-term survival, particularly in operable patients.
What may ultimately matter most is carefully selecting operable patients who undergo SBRT.
Aggarwal has encountered certain patients who are fit for surgery but would rather have radiation therapy. “This is an individual decision, and these patients are usually discussed at tumor board and in multidisciplinary discussions to really make sure that they’re making the right decision for themselves,” she explained.
The Pros and Cons of SBRT
SBRT is a nonsurgical approach in which precision high-dose radiation is delivered in just a few fractions — typically, 3, 5, or 8, depending on institutional protocols and tumor characteristics.
SBRT is performed on an outpatient basis, usually over 1-2 weeks, with most patients able to resume usual activities with minimal to no delay. Surgery, on the other hand, requires a hospital stay and takes most people about 2-6 weeks to return to regular activities. SBRT also avoids anesthesia and surgical incisions, both of which come with risks.
The data on SBRT in early-stage NSCLC are mixed. While some studies indicate that SBRT comes with promising survival outcomes, other research has reported worse survival and recurrence rates.
One potential reason for higher recurrence rates with SBRT is the lack of pathologic nodal staging, which only happens after surgery, as well as lower rates of nodal evaluation with endobronchial ultrasound or mediastinoscopy before surgery or SBRT. Without nodal assessments, clinicians may miss a more aggressive histology or more advanced nodal stage, which would go undertreated if patients received SBRT.
Latest Data in Large Cohort
A recent study published in Lung Cancer indicated that, when carefully selected, operable patients with early NSCLC have comparable survival with lobectomy or SBRT.
In the study, Dutch researchers took an in-depth look at survival and recurrence patterns in a retrospective cohort study of 2183 patients with clinical stage I NSCLC treated with minimally invasive lobectomy or SBRT. The study includes one of the largest cohorts to date, with robust data collection on baseline characteristics, comorbidities, tumor size, performance status, and follow-up.
Patients receiving SBRT were typically older (median age, 74 vs 67 years), had higher comorbidity burdens (Charlson index ≥ 5 in 57% of SBRT patients vs 23% of surgical patients), worse performance status, and lower lung function. To adjust for these differences, the researchers used propensity score weighting so the SBRT group’s baseline characteristics were comparable with those in the surgery group.
The surgery cohort had more invasive nodal evaluation: 21% underwent endobronchial ultrasound or mediastinoscopy vs only 12% in the SBRT group. The vast majority in both groups had PET-CT staging, reflecting modern imaging-based workups.
While 5-year local recurrence rates between the two groups were similar (13.1% for SBRT vs 12.1% for surgery), the 5-year regional recurrence rate was significantly higher after SBRT than lobectomy (18.1% vs 14.2%; hazard ratio [HR], 0.74), as was the distant metastasis rate (26.2% vs 20.2%; HR, 0.72).
Mortality at 30 days was higher after surgery than SBRT (1.0% vs 0.2%). And in the unadjusted analysis, 5-year overall survival was significantly better with lobectomy than SBRT (70.2% vs 40.3%).
However, when the analysis only included patients with similar baseline characteristics, overall survival was no longer significantly different in the two groups (HR, 0.89; 95% CI, 0.65-1.20). Lung cancer–specific mortality was also not significantly different between the two treatments (HR, 1.08), but the study was underpowered to detect significant differences in this outcome on the basis of a relatively low number of deaths from NSCLC.
Still, even after comparing similar patients, recurrence-free survival was notably better with surgery (HR, 0.70), due to fewer regional recurrences and distant metastases. Overall, 13% of the surgical cohort had nodal upstaging at pathology, meaning that even in clinically “node-negative” stage I disease, a subset of patients had unsuspected nodal involvement.
Patients receiving SBRT did not have pathologic nodal staging, raising the possibility of occult micrometastases. The authors noted that the proportion of SBRT patients with occult lymph node metastases is likely at least equal to that in the surgery group, but these metastases would go undetected without pathologic assessment.
Missing potential occult micrometastases in the SBRT group likely contributed to higher regional recurrence rates over time. By improving nodal staging, more patients with occult lymph node metastases who would be undertreated with SBRT may be identified before treatment, the authors said.
What Do Experts Say?
So, is SBRT an option for patients with stage I NSCLC?
Opinions vary.
“If you got one shot for a cure, then you want to do the surgery because that’s what results in a cure,” said Raja Flores, MD, chairman of Thoracic Surgery, Mount Sinai Health System, New York City.
Flores noted that the survival rate with surgery is high in this population. “There’s really nothing out there that can compare,” he said.
In his view, surgery “remains the gold standard.” However, “radiation could be considered in nonsurgical candidates,” he said.
The most recent NCCN guidelines align with Flores’ take. The guidelines say that SBRT is indicated for stage IA-IIA (N0) NSCLC in patients who are deemed “medically inoperable, high surgical risk as determined by thoracic surgeon, and those who decline surgery after thoracic surgical consultation.”
Clifford G. Robinson, MD, agreed. “In the United States, we largely treat patients with SBRT who are medically inoperable or high-risk operable and a much smaller proportion who decline surgery,” said Robinson, professor of radiation oncology and chief of SBRT at Washington University in St. Louis, St. Louis. “Many patients who are deemed operable are not offered SBRT.”
Still, for Robinson, determining which patients are best suited for surgery or SBRT remains unclear.
“Retrospective comparisons are fraught with problems because of confounding,” Robinson told this news organization. “That is, the healthier patients get surgery, and the less healthy ones get SBRT. No manner of fancy statistical manipulation can remove that fact.”
In fact, a recent meta-analysis found that the most significant variable predicting whether surgery or SBRT was superior in retrospective studies was whether the author was a surgeon or radiation oncologist.
Robinson noted that multiple randomized trials have attempted to randomize patients with medically operable early-stage NSCLC to surgery or SBRT and failed to accrue, largely due to patients’ “understandable unwillingness to be randomized between operative vs nonoperative interventions when most already prefer one or the other approach.”
Flores highlighted another point of caution about interpreting trial results: Not all early-stage NSCLC behaves similarly. “Some are slow-growing ‘turtles,’ and others are aggressive ‘rabbits’ — and the turtles are usually the ones that have been included in these radiotherapy trials, and that’s the danger,” he said.
While medical operability is the primary factor for deciding the treatment modality for early-stage NSCLC, there are other more subtle factors that can play into the decision.
These include prior surgery or radiotherapy to the chest, prior cancers, and social issues, such as the patient being a primary caregiver for another person and job insecurity, that might make recovery from surgery more challenging. And in rare instances, a patient may be medically fit to undergo surgery, but the cancer is technically challenging to resect due to anatomic issues or prior surgery to the chest, Robinson added.
A Winner?
Results from two ongoing, highly anticipated randomized trials expected in the next several years will hopefully provide additional insights and clarify ongoing uncertainties about the optimal treatment strategies for operable patients with stage I NSCLC.
STABLE-MATES is comparing outcomes after sublobar resection and SBRT in high-risk operable stage I NSCLC, and VALOR is evaluating outcomes after anatomic pulmonary resections and SBRT in patients with stage I NSCLC who have a long life expectancy and are fit enough to tolerate surgery.
But Robinson said his group believes that trying to decide on a winner is a “fool’s errand” and is instead running a pragmatic study across multiple academic and community centers around the United States and Canada where patients choose therapy based on their personal preferences and guidance from their physicians. The researchers will carefully track baseline comorbidity and frailty and assess serial quality-of-life changes over time.
“The goal is to create a calculator that a given patient might use in the future to determine what patients like them would have received, complete with expected outcomes and side effects,” Robinson said.
Robinson cautioned, however, that it “seems unlikely, given the existing literature, that one of the treatments will be truly ‘superior’ to the other one and lead to the ‘losing’ treatment fading away since both are excellent options with pros and cons.”
Aggarwal, Robinson, and Flores had no relevant disclosures.
A version of this article first appeared on Medscape.com.
For years, the default definitive treatment for patients with early-stage I non–small cell lung cancer (NSCLC) has been surgical resection, typically minimally invasive lobectomy with systematic lymph node dissection.
Guidelines from the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology, and the European Society for Medical Oncology all list surgery (in particular, lobectomy) as the primary local therapy for fit, operable patients with stage I NSCLC.
More recently, however, stereotactic body radiotherapy (SBRT), also called stereotactic ablative radiotherapy, has emerged as a definitive treatment option for stage I NSCLC, especially for older, less fit patients who are unsuitable or deemed high-risk for surgery.
“We see patients in our practice who cannot undergo surgery or who may not have adequate lung function to be able to tolerate surgery, and for these patients who are medically inoperable or surgically unresectable, radiation therapy may be a reasonable option,” Charu Aggarwal, MD, MPH, professor and lung cancer specialist, University of Pennsylvania, Philadelphia, told this news organization.
Given some encouraging data suggesting that SBRT could provide similar survival outcomes and be an alternative to surgery for operable disease, SBRT is also increasingly being considered in these early-stage patients. However, other evidence indicates that SBRT may be associated with higher rates of regional and distant recurrences and worse long-term survival, particularly in operable patients.
What may ultimately matter most is carefully selecting operable patients who undergo SBRT.
Aggarwal has encountered certain patients who are fit for surgery but would rather have radiation therapy. “This is an individual decision, and these patients are usually discussed at tumor board and in multidisciplinary discussions to really make sure that they’re making the right decision for themselves,” she explained.
The Pros and Cons of SBRT
SBRT is a nonsurgical approach in which precision high-dose radiation is delivered in just a few fractions — typically, 3, 5, or 8, depending on institutional protocols and tumor characteristics.
SBRT is performed on an outpatient basis, usually over 1-2 weeks, with most patients able to resume usual activities with minimal to no delay. Surgery, on the other hand, requires a hospital stay and takes most people about 2-6 weeks to return to regular activities. SBRT also avoids anesthesia and surgical incisions, both of which come with risks.
The data on SBRT in early-stage NSCLC are mixed. While some studies indicate that SBRT comes with promising survival outcomes, other research has reported worse survival and recurrence rates.
One potential reason for higher recurrence rates with SBRT is the lack of pathologic nodal staging, which only happens after surgery, as well as lower rates of nodal evaluation with endobronchial ultrasound or mediastinoscopy before surgery or SBRT. Without nodal assessments, clinicians may miss a more aggressive histology or more advanced nodal stage, which would go undertreated if patients received SBRT.
Latest Data in Large Cohort
A recent study published in Lung Cancer indicated that, when carefully selected, operable patients with early NSCLC have comparable survival with lobectomy or SBRT.
In the study, Dutch researchers took an in-depth look at survival and recurrence patterns in a retrospective cohort study of 2183 patients with clinical stage I NSCLC treated with minimally invasive lobectomy or SBRT. The study includes one of the largest cohorts to date, with robust data collection on baseline characteristics, comorbidities, tumor size, performance status, and follow-up.
Patients receiving SBRT were typically older (median age, 74 vs 67 years), had higher comorbidity burdens (Charlson index ≥ 5 in 57% of SBRT patients vs 23% of surgical patients), worse performance status, and lower lung function. To adjust for these differences, the researchers used propensity score weighting so the SBRT group’s baseline characteristics were comparable with those in the surgery group.
The surgery cohort had more invasive nodal evaluation: 21% underwent endobronchial ultrasound or mediastinoscopy vs only 12% in the SBRT group. The vast majority in both groups had PET-CT staging, reflecting modern imaging-based workups.
While 5-year local recurrence rates between the two groups were similar (13.1% for SBRT vs 12.1% for surgery), the 5-year regional recurrence rate was significantly higher after SBRT than lobectomy (18.1% vs 14.2%; hazard ratio [HR], 0.74), as was the distant metastasis rate (26.2% vs 20.2%; HR, 0.72).
Mortality at 30 days was higher after surgery than SBRT (1.0% vs 0.2%). And in the unadjusted analysis, 5-year overall survival was significantly better with lobectomy than SBRT (70.2% vs 40.3%).
However, when the analysis only included patients with similar baseline characteristics, overall survival was no longer significantly different in the two groups (HR, 0.89; 95% CI, 0.65-1.20). Lung cancer–specific mortality was also not significantly different between the two treatments (HR, 1.08), but the study was underpowered to detect significant differences in this outcome on the basis of a relatively low number of deaths from NSCLC.
Still, even after comparing similar patients, recurrence-free survival was notably better with surgery (HR, 0.70), due to fewer regional recurrences and distant metastases. Overall, 13% of the surgical cohort had nodal upstaging at pathology, meaning that even in clinically “node-negative” stage I disease, a subset of patients had unsuspected nodal involvement.
Patients receiving SBRT did not have pathologic nodal staging, raising the possibility of occult micrometastases. The authors noted that the proportion of SBRT patients with occult lymph node metastases is likely at least equal to that in the surgery group, but these metastases would go undetected without pathologic assessment.
Missing potential occult micrometastases in the SBRT group likely contributed to higher regional recurrence rates over time. By improving nodal staging, more patients with occult lymph node metastases who would be undertreated with SBRT may be identified before treatment, the authors said.
What Do Experts Say?
So, is SBRT an option for patients with stage I NSCLC?
Opinions vary.
“If you got one shot for a cure, then you want to do the surgery because that’s what results in a cure,” said Raja Flores, MD, chairman of Thoracic Surgery, Mount Sinai Health System, New York City.
Flores noted that the survival rate with surgery is high in this population. “There’s really nothing out there that can compare,” he said.
In his view, surgery “remains the gold standard.” However, “radiation could be considered in nonsurgical candidates,” he said.
The most recent NCCN guidelines align with Flores’ take. The guidelines say that SBRT is indicated for stage IA-IIA (N0) NSCLC in patients who are deemed “medically inoperable, high surgical risk as determined by thoracic surgeon, and those who decline surgery after thoracic surgical consultation.”
Clifford G. Robinson, MD, agreed. “In the United States, we largely treat patients with SBRT who are medically inoperable or high-risk operable and a much smaller proportion who decline surgery,” said Robinson, professor of radiation oncology and chief of SBRT at Washington University in St. Louis, St. Louis. “Many patients who are deemed operable are not offered SBRT.”
Still, for Robinson, determining which patients are best suited for surgery or SBRT remains unclear.
“Retrospective comparisons are fraught with problems because of confounding,” Robinson told this news organization. “That is, the healthier patients get surgery, and the less healthy ones get SBRT. No manner of fancy statistical manipulation can remove that fact.”
In fact, a recent meta-analysis found that the most significant variable predicting whether surgery or SBRT was superior in retrospective studies was whether the author was a surgeon or radiation oncologist.
Robinson noted that multiple randomized trials have attempted to randomize patients with medically operable early-stage NSCLC to surgery or SBRT and failed to accrue, largely due to patients’ “understandable unwillingness to be randomized between operative vs nonoperative interventions when most already prefer one or the other approach.”
Flores highlighted another point of caution about interpreting trial results: Not all early-stage NSCLC behaves similarly. “Some are slow-growing ‘turtles,’ and others are aggressive ‘rabbits’ — and the turtles are usually the ones that have been included in these radiotherapy trials, and that’s the danger,” he said.
While medical operability is the primary factor for deciding the treatment modality for early-stage NSCLC, there are other more subtle factors that can play into the decision.
These include prior surgery or radiotherapy to the chest, prior cancers, and social issues, such as the patient being a primary caregiver for another person and job insecurity, that might make recovery from surgery more challenging. And in rare instances, a patient may be medically fit to undergo surgery, but the cancer is technically challenging to resect due to anatomic issues or prior surgery to the chest, Robinson added.
A Winner?
Results from two ongoing, highly anticipated randomized trials expected in the next several years will hopefully provide additional insights and clarify ongoing uncertainties about the optimal treatment strategies for operable patients with stage I NSCLC.
STABLE-MATES is comparing outcomes after sublobar resection and SBRT in high-risk operable stage I NSCLC, and VALOR is evaluating outcomes after anatomic pulmonary resections and SBRT in patients with stage I NSCLC who have a long life expectancy and are fit enough to tolerate surgery.
But Robinson said his group believes that trying to decide on a winner is a “fool’s errand” and is instead running a pragmatic study across multiple academic and community centers around the United States and Canada where patients choose therapy based on their personal preferences and guidance from their physicians. The researchers will carefully track baseline comorbidity and frailty and assess serial quality-of-life changes over time.
“The goal is to create a calculator that a given patient might use in the future to determine what patients like them would have received, complete with expected outcomes and side effects,” Robinson said.
Robinson cautioned, however, that it “seems unlikely, given the existing literature, that one of the treatments will be truly ‘superior’ to the other one and lead to the ‘losing’ treatment fading away since both are excellent options with pros and cons.”
Aggarwal, Robinson, and Flores had no relevant disclosures.
A version of this article first appeared on Medscape.com.