User login
Scalp Psoriasis Considerations
1. Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33-40.
2. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
3. Merola JF, Li T, Li WQ, et al. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41:486-489.
4. Frez ML, Asawanonda P, Gunasekara C, et al. Recommendations for a patient-centered approach to the assessment and treatment of scalp psoriasis: a consensus statement from the Asia Scalp Psoriasis Study Group. J Dermatol Treat. 2014;25:38-45.
5. van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. diagnosis and management. Am J Clin Dermatol. 2001;2:159-165.
6. Chan CS, Van Voorhees AS, Lebwohl MG, et al. Treatment of severe scalp psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60:962-971.
7. Aldredge LM, Higham RC. Manifestations and management of difficult-to-treat psoriasis. J Dermatol Nurses Assoc. 2018;10:189-197.
8. Dopytalska K, Sobolewski P, Blaszczak A, et al. Psoriasis in special localizations. Reumatologia. 2018;56:392-398.
9. Papp K, Berth-Jones J, Kragballe K, et al. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21:1151-1160.
10. Kircik LH, Kumar S. Scalp psoriasis. J Drugs Dermatol. 2010;9(8 suppl):S101-S105.
11. Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26:448-459.
12. Sampogna F, Linder D, Piaserico S, et al. Quality of life assessment of patients with scalp dermatitis using the Italian version of the Scalpdex. Acta Dermato-Venereologica. 2014;94:411-414.
13. Crowley J. Scalp psoriasis: an overview of the disease and available therapies. J Drugs Dermatol. 2010;9:912-918.
14. Shah VV, Lee EB, Reddy SP, et al. Scalp psoriasis with increased hair density. Cutis. 2018;102:63-64.
15. George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
16. Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
17. Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
18. Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
1. Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33-40.
2. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
3. Merola JF, Li T, Li WQ, et al. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41:486-489.
4. Frez ML, Asawanonda P, Gunasekara C, et al. Recommendations for a patient-centered approach to the assessment and treatment of scalp psoriasis: a consensus statement from the Asia Scalp Psoriasis Study Group. J Dermatol Treat. 2014;25:38-45.
5. van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. diagnosis and management. Am J Clin Dermatol. 2001;2:159-165.
6. Chan CS, Van Voorhees AS, Lebwohl MG, et al. Treatment of severe scalp psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60:962-971.
7. Aldredge LM, Higham RC. Manifestations and management of difficult-to-treat psoriasis. J Dermatol Nurses Assoc. 2018;10:189-197.
8. Dopytalska K, Sobolewski P, Blaszczak A, et al. Psoriasis in special localizations. Reumatologia. 2018;56:392-398.
9. Papp K, Berth-Jones J, Kragballe K, et al. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21:1151-1160.
10. Kircik LH, Kumar S. Scalp psoriasis. J Drugs Dermatol. 2010;9(8 suppl):S101-S105.
11. Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26:448-459.
12. Sampogna F, Linder D, Piaserico S, et al. Quality of life assessment of patients with scalp dermatitis using the Italian version of the Scalpdex. Acta Dermato-Venereologica. 2014;94:411-414.
13. Crowley J. Scalp psoriasis: an overview of the disease and available therapies. J Drugs Dermatol. 2010;9:912-918.
14. Shah VV, Lee EB, Reddy SP, et al. Scalp psoriasis with increased hair density. Cutis. 2018;102:63-64.
15. George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
16. Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
17. Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
18. Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
1. Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33-40.
2. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
3. Merola JF, Li T, Li WQ, et al. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41:486-489.
4. Frez ML, Asawanonda P, Gunasekara C, et al. Recommendations for a patient-centered approach to the assessment and treatment of scalp psoriasis: a consensus statement from the Asia Scalp Psoriasis Study Group. J Dermatol Treat. 2014;25:38-45.
5. van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. diagnosis and management. Am J Clin Dermatol. 2001;2:159-165.
6. Chan CS, Van Voorhees AS, Lebwohl MG, et al. Treatment of severe scalp psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60:962-971.
7. Aldredge LM, Higham RC. Manifestations and management of difficult-to-treat psoriasis. J Dermatol Nurses Assoc. 2018;10:189-197.
8. Dopytalska K, Sobolewski P, Blaszczak A, et al. Psoriasis in special localizations. Reumatologia. 2018;56:392-398.
9. Papp K, Berth-Jones J, Kragballe K, et al. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21:1151-1160.
10. Kircik LH, Kumar S. Scalp psoriasis. J Drugs Dermatol. 2010;9(8 suppl):S101-S105.
11. Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26:448-459.
12. Sampogna F, Linder D, Piaserico S, et al. Quality of life assessment of patients with scalp dermatitis using the Italian version of the Scalpdex. Acta Dermato-Venereologica. 2014;94:411-414.
13. Crowley J. Scalp psoriasis: an overview of the disease and available therapies. J Drugs Dermatol. 2010;9:912-918.
14. Shah VV, Lee EB, Reddy SP, et al. Scalp psoriasis with increased hair density. Cutis. 2018;102:63-64.
15. George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
16. Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
17. Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
18. Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Scalp Psoriasis: A Challenge for Patients and Dermatologists
Prevalence of Scalp Psoriasis
Scalp psoriasis is a common and difficult-to-treat manifestation of psoriasis.1,2 The prevalence of scalp psoriasis in patients with psoriasis is estimated to be 45% to 56%.3 Other studies have shown 80% to 90% of patients with psoriasis have scalp involvement at some point during the course of their disease.2,4-6
Clinical Presentation
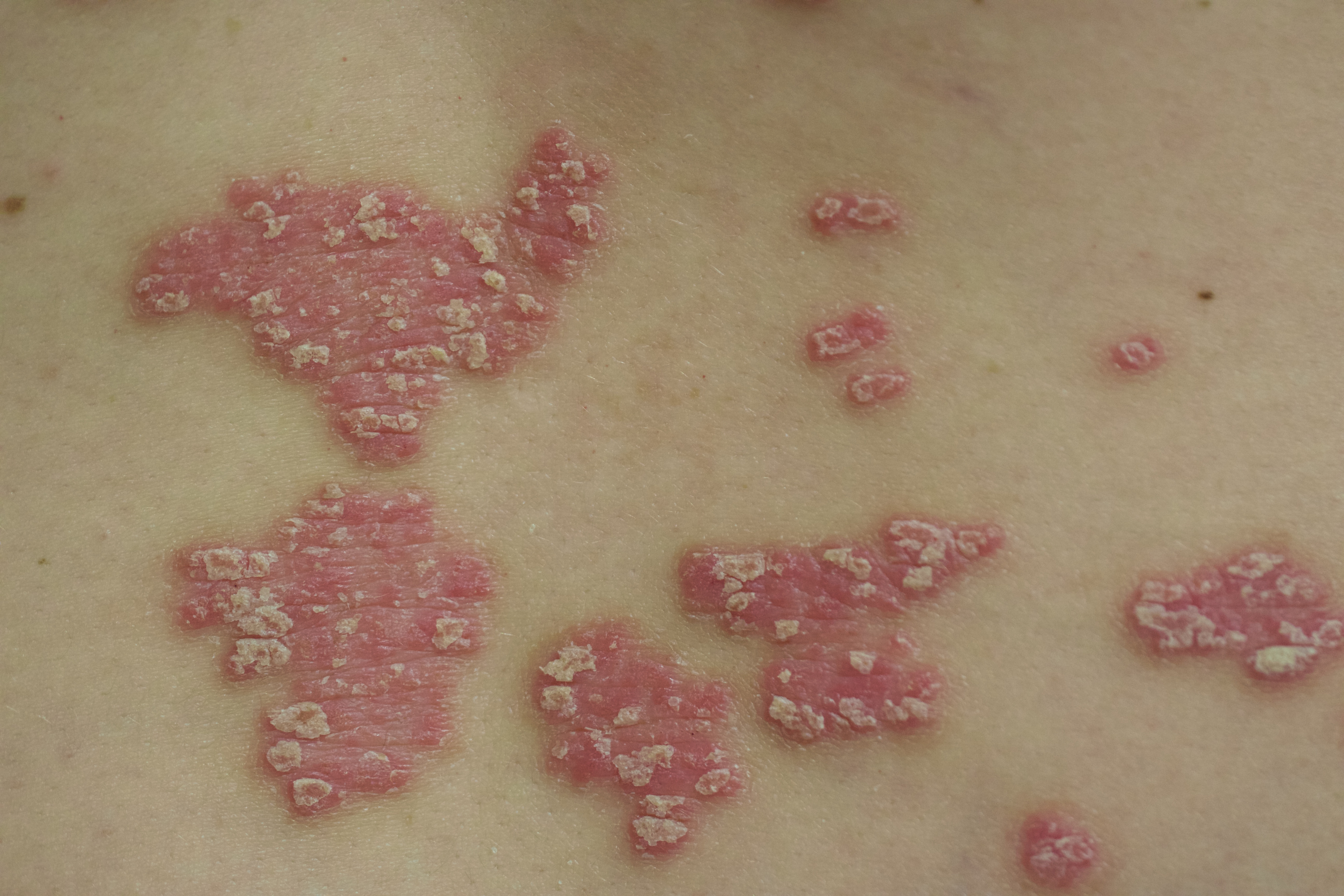
Scalp psoriasis typically presents as red thickened patches with silvery white scales that flake and may be mistaken for dandruff.7,8
The lesions may be limited to the hairline or may extend to the forehead, ears, and back of the neck.1,9 Patients often report intense itching, feelings of soreness, and burning.10,11 Patients with scalp psoriasis also are vulnerable to Koebner phenomenon because normal hair care along with scratching or picking at lesions can result in skin trauma and a cycle of exacerbating disease.11
Quality of Life Implications
Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.2,12,13 In one study, more than 70% of patients with scalp psoriasis reported difficulty with daily life.12 Patients frequently report feelings of shame, embarrassment, or self-consciousness about scalp psoriasis; many grow their hair long or wear hats to hide scalp lesions. Others report that flaking sometimes, often, or always affects their choice of clothing color.7,12
Psoriatic Alopecia
Alopecia is another common sequala in the setting of scalp psoriasis, though it is not well understood.14,15 First described by Shuster16 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.14,17 Clinically, it presents as pink scaly plaques consistent with psoriasis with overlying alopecia. In most patients, hair loss is usually reversible following effective treatment of psoriasis; however, instances of psoriatic alopecia have been reported as cicatricial (permanent) hair loss and generalized telogen effluvium. Cicatricial alopecia is increasingly being linked with chronic relapsing episodes of psoriasis.14,15 Patients with psoriatic alopecia are known to have a higher proportion of telogen and catagen hairs.14,18 Moreover, patients with psoriasis have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients.14
The patient described here had scalp psoriasis with increased and preserved hair density. The case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.14,15
Therapeutic Options
Although numerous treatment options for psoriasis are available, the scalp remains a difficult area to treat.1,14 Increased hair density can complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.14 The presence of hair also has been shown to strongly influence treatment adherence.1,8 Patients often discuss the greasy effect of medications in this area and difficulty removing products from the hair.1
Topical corticosteroids, with or without the addition of the vitamin D analogs calcipotriol or calcipotriene, remain the first-line treatment of mild scalp psoriasis. It is possible that the development of new formulations in recent years—foams, shampoos, and sprays—may improve adherence. Systemic treatment should be considered in severe or intractable cases.
Bottom Line
Although hair loss is more common, scalp psoriasis also may present with increased hair density, which may make topical medications more difficult to apply and can affect treatment adherence. Topical corticosteroids, with or without the addition of the vitamin D analog calcipotriol, remain the first-line treatment of mild scalp psoriasis. Systemic therapy should be considered in severe or recalcitrant cases.
- Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33-40.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- Merola JF, Li T, Li WQ, et al. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41:486-489.
- Frez ML, Asawanonda P, Gunasekara C, et al. Recommendations for a patient-centered approach to the assessment and treatment of scalp psoriasis: a consensus statement from the Asia Scalp Psoriasis Study Group. J Dermatol Treat. 2014;25:38-45.
- van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. diagnosis and management. Am J Clin Dermatol. 2001;2:159-165.
- Chan CS, Van Voorhees AS, Lebwohl MG, et al. Treatment of severe scalp psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60:962-971.
- Aldredge LM, Higham RC. Manifestations and management of difficult-to-treat psoriasis. J Dermatol Nurses Assoc. 2018;10:189-197.
- Dopytalska K, Sobolewski P, Blaszczak A, et al. Psoriasis in special localizations. Reumatologia. 2018;56:392-398.
- Papp K, Berth-Jones J, Kragballe K, et al. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21:1151-1160.
- Kircik LH, Kumar S. Scalp psoriasis. J Drugs Dermatol. 2010;9(8 suppl):S101-S105.
- Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26:448-459.
- Sampogna F, Linder D, Piaserico S, et al. Quality of life assessment of patients with scalp dermatitis using the Italian version of the Scalpdex. Acta Dermato-Venereologica. 2014;94:411-414.
- Crowley J. Scalp psoriasis: an overview of the disease and available therapies. J Drugs Dermatol. 2010;9:912-918.
- Shah VV, Lee EB, Reddy SP, et al. Scalp psoriasis with increased hair density. Cutis. 2018;102:63-64.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Prevalence of Scalp Psoriasis
Scalp psoriasis is a common and difficult-to-treat manifestation of psoriasis.1,2 The prevalence of scalp psoriasis in patients with psoriasis is estimated to be 45% to 56%.3 Other studies have shown 80% to 90% of patients with psoriasis have scalp involvement at some point during the course of their disease.2,4-6
Clinical Presentation
Scalp psoriasis typically presents as red thickened patches with silvery white scales that flake and may be mistaken for dandruff.7,8
The lesions may be limited to the hairline or may extend to the forehead, ears, and back of the neck.1,9 Patients often report intense itching, feelings of soreness, and burning.10,11 Patients with scalp psoriasis also are vulnerable to Koebner phenomenon because normal hair care along with scratching or picking at lesions can result in skin trauma and a cycle of exacerbating disease.11
Quality of Life Implications
Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.2,12,13 In one study, more than 70% of patients with scalp psoriasis reported difficulty with daily life.12 Patients frequently report feelings of shame, embarrassment, or self-consciousness about scalp psoriasis; many grow their hair long or wear hats to hide scalp lesions. Others report that flaking sometimes, often, or always affects their choice of clothing color.7,12
Psoriatic Alopecia
Alopecia is another common sequala in the setting of scalp psoriasis, though it is not well understood.14,15 First described by Shuster16 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.14,17 Clinically, it presents as pink scaly plaques consistent with psoriasis with overlying alopecia. In most patients, hair loss is usually reversible following effective treatment of psoriasis; however, instances of psoriatic alopecia have been reported as cicatricial (permanent) hair loss and generalized telogen effluvium. Cicatricial alopecia is increasingly being linked with chronic relapsing episodes of psoriasis.14,15 Patients with psoriatic alopecia are known to have a higher proportion of telogen and catagen hairs.14,18 Moreover, patients with psoriasis have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients.14
The patient described here had scalp psoriasis with increased and preserved hair density. The case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.14,15
Therapeutic Options
Although numerous treatment options for psoriasis are available, the scalp remains a difficult area to treat.1,14 Increased hair density can complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.14 The presence of hair also has been shown to strongly influence treatment adherence.1,8 Patients often discuss the greasy effect of medications in this area and difficulty removing products from the hair.1
Topical corticosteroids, with or without the addition of the vitamin D analogs calcipotriol or calcipotriene, remain the first-line treatment of mild scalp psoriasis. It is possible that the development of new formulations in recent years—foams, shampoos, and sprays—may improve adherence. Systemic treatment should be considered in severe or intractable cases.
Bottom Line
Although hair loss is more common, scalp psoriasis also may present with increased hair density, which may make topical medications more difficult to apply and can affect treatment adherence. Topical corticosteroids, with or without the addition of the vitamin D analog calcipotriol, remain the first-line treatment of mild scalp psoriasis. Systemic therapy should be considered in severe or recalcitrant cases.
Prevalence of Scalp Psoriasis
Scalp psoriasis is a common and difficult-to-treat manifestation of psoriasis.1,2 The prevalence of scalp psoriasis in patients with psoriasis is estimated to be 45% to 56%.3 Other studies have shown 80% to 90% of patients with psoriasis have scalp involvement at some point during the course of their disease.2,4-6
Clinical Presentation
Scalp psoriasis typically presents as red thickened patches with silvery white scales that flake and may be mistaken for dandruff.7,8
The lesions may be limited to the hairline or may extend to the forehead, ears, and back of the neck.1,9 Patients often report intense itching, feelings of soreness, and burning.10,11 Patients with scalp psoriasis also are vulnerable to Koebner phenomenon because normal hair care along with scratching or picking at lesions can result in skin trauma and a cycle of exacerbating disease.11
Quality of Life Implications
Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.2,12,13 In one study, more than 70% of patients with scalp psoriasis reported difficulty with daily life.12 Patients frequently report feelings of shame, embarrassment, or self-consciousness about scalp psoriasis; many grow their hair long or wear hats to hide scalp lesions. Others report that flaking sometimes, often, or always affects their choice of clothing color.7,12
Psoriatic Alopecia
Alopecia is another common sequala in the setting of scalp psoriasis, though it is not well understood.14,15 First described by Shuster16 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.14,17 Clinically, it presents as pink scaly plaques consistent with psoriasis with overlying alopecia. In most patients, hair loss is usually reversible following effective treatment of psoriasis; however, instances of psoriatic alopecia have been reported as cicatricial (permanent) hair loss and generalized telogen effluvium. Cicatricial alopecia is increasingly being linked with chronic relapsing episodes of psoriasis.14,15 Patients with psoriatic alopecia are known to have a higher proportion of telogen and catagen hairs.14,18 Moreover, patients with psoriasis have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients.14
The patient described here had scalp psoriasis with increased and preserved hair density. The case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.14,15
Therapeutic Options
Although numerous treatment options for psoriasis are available, the scalp remains a difficult area to treat.1,14 Increased hair density can complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.14 The presence of hair also has been shown to strongly influence treatment adherence.1,8 Patients often discuss the greasy effect of medications in this area and difficulty removing products from the hair.1
Topical corticosteroids, with or without the addition of the vitamin D analogs calcipotriol or calcipotriene, remain the first-line treatment of mild scalp psoriasis. It is possible that the development of new formulations in recent years—foams, shampoos, and sprays—may improve adherence. Systemic treatment should be considered in severe or intractable cases.
Bottom Line
Although hair loss is more common, scalp psoriasis also may present with increased hair density, which may make topical medications more difficult to apply and can affect treatment adherence. Topical corticosteroids, with or without the addition of the vitamin D analog calcipotriol, remain the first-line treatment of mild scalp psoriasis. Systemic therapy should be considered in severe or recalcitrant cases.
- Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33-40.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- Merola JF, Li T, Li WQ, et al. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41:486-489.
- Frez ML, Asawanonda P, Gunasekara C, et al. Recommendations for a patient-centered approach to the assessment and treatment of scalp psoriasis: a consensus statement from the Asia Scalp Psoriasis Study Group. J Dermatol Treat. 2014;25:38-45.
- van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. diagnosis and management. Am J Clin Dermatol. 2001;2:159-165.
- Chan CS, Van Voorhees AS, Lebwohl MG, et al. Treatment of severe scalp psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60:962-971.
- Aldredge LM, Higham RC. Manifestations and management of difficult-to-treat psoriasis. J Dermatol Nurses Assoc. 2018;10:189-197.
- Dopytalska K, Sobolewski P, Blaszczak A, et al. Psoriasis in special localizations. Reumatologia. 2018;56:392-398.
- Papp K, Berth-Jones J, Kragballe K, et al. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21:1151-1160.
- Kircik LH, Kumar S. Scalp psoriasis. J Drugs Dermatol. 2010;9(8 suppl):S101-S105.
- Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26:448-459.
- Sampogna F, Linder D, Piaserico S, et al. Quality of life assessment of patients with scalp dermatitis using the Italian version of the Scalpdex. Acta Dermato-Venereologica. 2014;94:411-414.
- Crowley J. Scalp psoriasis: an overview of the disease and available therapies. J Drugs Dermatol. 2010;9:912-918.
- Shah VV, Lee EB, Reddy SP, et al. Scalp psoriasis with increased hair density. Cutis. 2018;102:63-64.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
- Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33-40.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- Merola JF, Li T, Li WQ, et al. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41:486-489.
- Frez ML, Asawanonda P, Gunasekara C, et al. Recommendations for a patient-centered approach to the assessment and treatment of scalp psoriasis: a consensus statement from the Asia Scalp Psoriasis Study Group. J Dermatol Treat. 2014;25:38-45.
- van de Kerkhof PC, Franssen ME. Psoriasis of the scalp. diagnosis and management. Am J Clin Dermatol. 2001;2:159-165.
- Chan CS, Van Voorhees AS, Lebwohl MG, et al. Treatment of severe scalp psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60:962-971.
- Aldredge LM, Higham RC. Manifestations and management of difficult-to-treat psoriasis. J Dermatol Nurses Assoc. 2018;10:189-197.
- Dopytalska K, Sobolewski P, Blaszczak A, et al. Psoriasis in special localizations. Reumatologia. 2018;56:392-398.
- Papp K, Berth-Jones J, Kragballe K, et al. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21:1151-1160.
- Kircik LH, Kumar S. Scalp psoriasis. J Drugs Dermatol. 2010;9(8 suppl):S101-S105.
- Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26:448-459.
- Sampogna F, Linder D, Piaserico S, et al. Quality of life assessment of patients with scalp dermatitis using the Italian version of the Scalpdex. Acta Dermato-Venereologica. 2014;94:411-414.
- Crowley J. Scalp psoriasis: an overview of the disease and available therapies. J Drugs Dermatol. 2010;9:912-918.
- Shah VV, Lee EB, Reddy SP, et al. Scalp psoriasis with increased hair density. Cutis. 2018;102:63-64.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
The Case
A 19-year-old man initially presented for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. The patient’s medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made.
Treatment
The patient was prescribed topical corticosteroids for 2 weeks and as needed thereafter.
Patient Outcomes
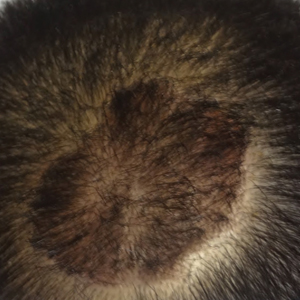
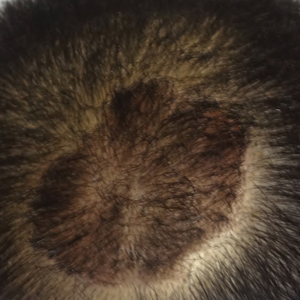
The patient remained stable for 5 years before again presenting to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye.
Clobetasol solution 0.05% twice daily was prescribed for application to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

This case was adapted from Shah VV, Lee EB, Reddy SP, et al. Scalp psoriasis with increased hair density. Cutis. 2018;102:63-64.
Nerves, Neuropeptides, and the Nervous System in the Pathogenesis of Psoriasis
Background
Psoriasis is a complex, multifactorial, systemic disease that is associated with numerous neurologic comorbidities, including stroke, multiple sclerosis, epilepsy, migraine, restless leg syndrome, Parkinson disease, and less frequently Guillain-Barré syndrome and myasthenia gravis. Anxiety and depression also are frequently seen in patients with psoriasis.1 In recent years, heightened understanding of the pathogenesis and disease mechanisms involved in psoriasis has led to the development of therapies designed to help to control the chronic inflammation associated with the disease, such as immunobiologics and small molecules.2
Although tremendous effort has gone into elucidating the immunologic underpinnings of psoriasis (certainly a worthwhile endeavor), less attention has been given to the role the nervous system plays in its pathogenesis.3,4 Nonetheless, clinical evidence suggests that the nervous system plays an important role in the pathophysiology of psoriasis and is deserving of further investigation.3
Nerves and Neuropeptides
Psychological stress is known to exacerbate psoriasis, which points to the involvement of the nervous system in psoriasis.3,5,6 In addition to provoking the sympathetic response, psychological stressors have been shown to affect the peripheral nervous system in psoriasis by modulating the skin’s network of nerves and neuropeptides.6-11 A small study divided patients with psoriasis into low-stress and high-stress groups based on their clinical examinations and answers to questionnaires. Immunohistochemical analysis showed patients in the high-stress group had elevated levels of calcitonin gene-related peptide and vasoactive intestinal polypeptide as well as reduced levels of the neuropeptide-degrading enzyme chymase compared to the low-stress group.12 Two later studies showed calcitonin gene-related peptide stimulates keratinocyte proliferation3,13 and is found at increased levels in psoriatic skin.3,14 Similarly, higher quantities of vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in psoriatic plaques compared to nonlesional and normal skin.3,15
Early research suggested that substance P (SP) released from cutaneous nerve fibers causes a local neurogenic response that elicits psoriasis in predisposed individuals.16 However, there have been conflicting reports of both higher and lower levels of SP in involved and noninvolved skin in patients with psoriasis compared with healthy individuals, making the role of SP in psoriasis ambiguous.3,15,17
Nerve growth factor (NGF), a principal mediator of neurogenic inflammation, also is suspected of playing a role in the pathogenesis of psoriasis.3,6 Studies have shown NGF prevents apoptosis of keratinocytes, activates T cells, and is found in higher levels in psoriatic skin compared to controls.3,18,19
Neuropeptides also may play a contributory role in the itching and Köbner phenomenon that are seen with psoriasis.3 The Köbner phenomenon refers to the formation of psoriatic lesions in uninvolved skin of patients with psoriasis following cutaneous trauma.20 Increased levels of NGF in nonlesional skin of patients with psoriasis are believed to contribute to the development of psoriatic plaques following trauma by triggering an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide.3 These neuropeptides generate keratinocyte proliferation, which in turn further increase NGF expression; as such, a cycle of inflammation and formation of psoriatic lesions is engendered.3,5 A noteworthy correlation also has been shown between the severity of pruritus and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.3,21
Spontaneous Clearing of Psoriasis
Spontaneous remission of psoriasis after cerebrovascular accident was first described in a case report published in 1998.22 Other cases have reported protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.23,24 Conversely, recurrence of skin lesions in areas corresponding to nervous system injury also have been reported in cases in which patients regained neurologic function; when permanent nerve damage was sustained, psoriasis did not recur,4 which confirms that peripheral nerves play a role in the pathogenesis of psoriasis.3 It is believed that peripheral nerve damage leads to reduced secretion of neuropeptides, and central nervous system injury can propagate similar downstream effects.3,25

Reports of psoriasis remission in the wake of peripheral and central nervous system injury from surgical nerve resection as well as cerebrovascular accident, as documented in the case presented here, provide clinical evidence in support of the neurocutaneous pathway’s role in psoriasis.3,4 Several reports have described clinical improvement of psoriasis following sensory cutaneous nerve damage, suggesting inflammation of the cutaneous nerves may be involved in the pathogenesis of psoriasis.3,6 Clearance of psoriatic plaques at the site of injury occurred following nerve resection; after reinnervation of the affected areas, disease recurrence occurred.6,26-28 More recently, cutaneous denervation was shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3,25 Intradermal injections of calcitonin gene-related peptide and/or a SP agonist into the denervated areas reversed this denervation-mediated improvement.3,25
Bottom Line
This case report describes spontaneous clearing of psoriasis following a cerebrovascular accident. Improvement in psoriasis in the absence of neural inputs suggest the nervous system plays a crucial role in the development of psoriatic disease.4 A better understanding of the neuropeptides involved in the neurologic-mediated clearance of psoriasis may contribute to the development of improved targeted therapies, specifically designed to target the neurologic aspects of psoriasis.3 Neuropeptides such as nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide, and possibly SP may play an important role in the pathogenesis of psoriasis and may one day be ideal targets for novel therapies.
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
- Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
- Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
- Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
- Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
Background
Psoriasis is a complex, multifactorial, systemic disease that is associated with numerous neurologic comorbidities, including stroke, multiple sclerosis, epilepsy, migraine, restless leg syndrome, Parkinson disease, and less frequently Guillain-Barré syndrome and myasthenia gravis. Anxiety and depression also are frequently seen in patients with psoriasis.1 In recent years, heightened understanding of the pathogenesis and disease mechanisms involved in psoriasis has led to the development of therapies designed to help to control the chronic inflammation associated with the disease, such as immunobiologics and small molecules.2

Although tremendous effort has gone into elucidating the immunologic underpinnings of psoriasis (certainly a worthwhile endeavor), less attention has been given to the role the nervous system plays in its pathogenesis.3,4 Nonetheless, clinical evidence suggests that the nervous system plays an important role in the pathophysiology of psoriasis and is deserving of further investigation.3
Nerves and Neuropeptides
Psychological stress is known to exacerbate psoriasis, which points to the involvement of the nervous system in psoriasis.3,5,6 In addition to provoking the sympathetic response, psychological stressors have been shown to affect the peripheral nervous system in psoriasis by modulating the skin’s network of nerves and neuropeptides.6-11 A small study divided patients with psoriasis into low-stress and high-stress groups based on their clinical examinations and answers to questionnaires. Immunohistochemical analysis showed patients in the high-stress group had elevated levels of calcitonin gene-related peptide and vasoactive intestinal polypeptide as well as reduced levels of the neuropeptide-degrading enzyme chymase compared to the low-stress group.12 Two later studies showed calcitonin gene-related peptide stimulates keratinocyte proliferation3,13 and is found at increased levels in psoriatic skin.3,14 Similarly, higher quantities of vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in psoriatic plaques compared to nonlesional and normal skin.3,15

Early research suggested that substance P (SP) released from cutaneous nerve fibers causes a local neurogenic response that elicits psoriasis in predisposed individuals.16 However, there have been conflicting reports of both higher and lower levels of SP in involved and noninvolved skin in patients with psoriasis compared with healthy individuals, making the role of SP in psoriasis ambiguous.3,15,17
Nerve growth factor (NGF), a principal mediator of neurogenic inflammation, also is suspected of playing a role in the pathogenesis of psoriasis.3,6 Studies have shown NGF prevents apoptosis of keratinocytes, activates T cells, and is found in higher levels in psoriatic skin compared to controls.3,18,19

Neuropeptides also may play a contributory role in the itching and Köbner phenomenon that are seen with psoriasis.3 The Köbner phenomenon refers to the formation of psoriatic lesions in uninvolved skin of patients with psoriasis following cutaneous trauma.20 Increased levels of NGF in nonlesional skin of patients with psoriasis are believed to contribute to the development of psoriatic plaques following trauma by triggering an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide.3 These neuropeptides generate keratinocyte proliferation, which in turn further increase NGF expression; as such, a cycle of inflammation and formation of psoriatic lesions is engendered.3,5 A noteworthy correlation also has been shown between the severity of pruritus and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.3,21
Spontaneous Clearing of Psoriasis
Spontaneous remission of psoriasis after cerebrovascular accident was first described in a case report published in 1998.22 Other cases have reported protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.23,24 Conversely, recurrence of skin lesions in areas corresponding to nervous system injury also have been reported in cases in which patients regained neurologic function; when permanent nerve damage was sustained, psoriasis did not recur,4 which confirms that peripheral nerves play a role in the pathogenesis of psoriasis.3 It is believed that peripheral nerve damage leads to reduced secretion of neuropeptides, and central nervous system injury can propagate similar downstream effects.3,25

Reports of psoriasis remission in the wake of peripheral and central nervous system injury from surgical nerve resection as well as cerebrovascular accident, as documented in the case presented here, provide clinical evidence in support of the neurocutaneous pathway’s role in psoriasis.3,4 Several reports have described clinical improvement of psoriasis following sensory cutaneous nerve damage, suggesting inflammation of the cutaneous nerves may be involved in the pathogenesis of psoriasis.3,6 Clearance of psoriatic plaques at the site of injury occurred following nerve resection; after reinnervation of the affected areas, disease recurrence occurred.6,26-28 More recently, cutaneous denervation was shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3,25 Intradermal injections of calcitonin gene-related peptide and/or a SP agonist into the denervated areas reversed this denervation-mediated improvement.3,25
Bottom Line
This case report describes spontaneous clearing of psoriasis following a cerebrovascular accident. Improvement in psoriasis in the absence of neural inputs suggest the nervous system plays a crucial role in the development of psoriatic disease.4 A better understanding of the neuropeptides involved in the neurologic-mediated clearance of psoriasis may contribute to the development of improved targeted therapies, specifically designed to target the neurologic aspects of psoriasis.3 Neuropeptides such as nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide, and possibly SP may play an important role in the pathogenesis of psoriasis and may one day be ideal targets for novel therapies.
Background
Psoriasis is a complex, multifactorial, systemic disease that is associated with numerous neurologic comorbidities, including stroke, multiple sclerosis, epilepsy, migraine, restless leg syndrome, Parkinson disease, and less frequently Guillain-Barré syndrome and myasthenia gravis. Anxiety and depression also are frequently seen in patients with psoriasis.1 In recent years, heightened understanding of the pathogenesis and disease mechanisms involved in psoriasis has led to the development of therapies designed to help to control the chronic inflammation associated with the disease, such as immunobiologics and small molecules.2

Although tremendous effort has gone into elucidating the immunologic underpinnings of psoriasis (certainly a worthwhile endeavor), less attention has been given to the role the nervous system plays in its pathogenesis.3,4 Nonetheless, clinical evidence suggests that the nervous system plays an important role in the pathophysiology of psoriasis and is deserving of further investigation.3
Nerves and Neuropeptides
Psychological stress is known to exacerbate psoriasis, which points to the involvement of the nervous system in psoriasis.3,5,6 In addition to provoking the sympathetic response, psychological stressors have been shown to affect the peripheral nervous system in psoriasis by modulating the skin’s network of nerves and neuropeptides.6-11 A small study divided patients with psoriasis into low-stress and high-stress groups based on their clinical examinations and answers to questionnaires. Immunohistochemical analysis showed patients in the high-stress group had elevated levels of calcitonin gene-related peptide and vasoactive intestinal polypeptide as well as reduced levels of the neuropeptide-degrading enzyme chymase compared to the low-stress group.12 Two later studies showed calcitonin gene-related peptide stimulates keratinocyte proliferation3,13 and is found at increased levels in psoriatic skin.3,14 Similarly, higher quantities of vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in psoriatic plaques compared to nonlesional and normal skin.3,15

Early research suggested that substance P (SP) released from cutaneous nerve fibers causes a local neurogenic response that elicits psoriasis in predisposed individuals.16 However, there have been conflicting reports of both higher and lower levels of SP in involved and noninvolved skin in patients with psoriasis compared with healthy individuals, making the role of SP in psoriasis ambiguous.3,15,17
Nerve growth factor (NGF), a principal mediator of neurogenic inflammation, also is suspected of playing a role in the pathogenesis of psoriasis.3,6 Studies have shown NGF prevents apoptosis of keratinocytes, activates T cells, and is found in higher levels in psoriatic skin compared to controls.3,18,19

Neuropeptides also may play a contributory role in the itching and Köbner phenomenon that are seen with psoriasis.3 The Köbner phenomenon refers to the formation of psoriatic lesions in uninvolved skin of patients with psoriasis following cutaneous trauma.20 Increased levels of NGF in nonlesional skin of patients with psoriasis are believed to contribute to the development of psoriatic plaques following trauma by triggering an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide.3 These neuropeptides generate keratinocyte proliferation, which in turn further increase NGF expression; as such, a cycle of inflammation and formation of psoriatic lesions is engendered.3,5 A noteworthy correlation also has been shown between the severity of pruritus and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.3,21
Spontaneous Clearing of Psoriasis
Spontaneous remission of psoriasis after cerebrovascular accident was first described in a case report published in 1998.22 Other cases have reported protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.23,24 Conversely, recurrence of skin lesions in areas corresponding to nervous system injury also have been reported in cases in which patients regained neurologic function; when permanent nerve damage was sustained, psoriasis did not recur,4 which confirms that peripheral nerves play a role in the pathogenesis of psoriasis.3 It is believed that peripheral nerve damage leads to reduced secretion of neuropeptides, and central nervous system injury can propagate similar downstream effects.3,25

Reports of psoriasis remission in the wake of peripheral and central nervous system injury from surgical nerve resection as well as cerebrovascular accident, as documented in the case presented here, provide clinical evidence in support of the neurocutaneous pathway’s role in psoriasis.3,4 Several reports have described clinical improvement of psoriasis following sensory cutaneous nerve damage, suggesting inflammation of the cutaneous nerves may be involved in the pathogenesis of psoriasis.3,6 Clearance of psoriatic plaques at the site of injury occurred following nerve resection; after reinnervation of the affected areas, disease recurrence occurred.6,26-28 More recently, cutaneous denervation was shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3,25 Intradermal injections of calcitonin gene-related peptide and/or a SP agonist into the denervated areas reversed this denervation-mediated improvement.3,25
Bottom Line
This case report describes spontaneous clearing of psoriasis following a cerebrovascular accident. Improvement in psoriasis in the absence of neural inputs suggest the nervous system plays a crucial role in the development of psoriatic disease.4 A better understanding of the neuropeptides involved in the neurologic-mediated clearance of psoriasis may contribute to the development of improved targeted therapies, specifically designed to target the neurologic aspects of psoriasis.3 Neuropeptides such as nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide, and possibly SP may play an important role in the pathogenesis of psoriasis and may one day be ideal targets for novel therapies.
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
- Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
- Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
- Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
- Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
- Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
- Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
- Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
- Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
The Case
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success; however, he had not yet tried biologic therapy. Physical examination revealed erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%.
Treatment
Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was subsequently hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcu-taneous enoxaparin with plans to initiate warfarin as an outpatient. No therapies for the treatment of psoriasis were given during his admission. Three days after being admitted, he was discharged to a skilled nursing facility.
Patient Outcome
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. Physical examination revealed his skin was clear of psoriatic lesions.
This case was adapted from Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
Role of the Nervous System in Psoriasis
1. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
2. Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
3. Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
4. Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
5. Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
6. Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
7. Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
8. Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
9. Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
10. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
11. Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
12. Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
13. He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
14. Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
15. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
16. Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
17. Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
18. Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
19. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
20. Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
21. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
22. Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
23. Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
24. Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
25. Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
26. Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
27. Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
28. Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
1. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
2. Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
3. Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
4. Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
5. Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
6. Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
7. Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
8. Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
9. Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
10. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
11. Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
12. Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
13. He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
14. Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
15. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
16. Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
17. Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
18. Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
19. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
20. Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
21. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
22. Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
23. Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
24. Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
25. Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
26. Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
27. Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
28. Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
1. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
2. Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
3. Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
4. Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
5. Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
6. Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
7. Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
8. Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
9. Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
10. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
11. Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
12. Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
13. He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
14. Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
15. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
16. Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
17. Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
18. Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
19. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
20. Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
21. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
22. Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
23. Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
24. Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
25. Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
26. Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
27. Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
28. Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to Clinical Practice
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 The disease is moderate to severe for approximately 1 in 6 individuals with psoriasis.2 These patients, particularly those with symptoms that are refractory to topical therapy and/or phototherapy, can benefit from the use of biologic agents, which are monoclonal antibodies and fusion proteins engineered to inhibit the action of cytokines that drive psoriatic inflammation.
In February 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of biologics in treating adult patients with psoriasis.3 The prior guidelines were released in 2008 when just 3 biologics—etanercept, infliximab, and adalimumab—were approved by the US Food and Drug Administration (FDA) for the management of psoriasis. These older recommendations were mostly based on studies of the efficacy and safety of biologics for patients with psoriatic arthritis.4 Over the last 11 years, 8 novel biologics have gained FDA approval, and numerous large phase 2 and phase 3 trials evaluating the risks and benefits of biologics have been conducted. The new guidelines contain considerably more detail and are based on evidence more specific to psoriasis rather than to psoriatic arthritis. Given the large repertoire of biologics available today and the increased amount of published research regarding each one, these guidelines may aid dermatologists in choosing the optimal biologic and managing therapy.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and adverse events of the 10 biologics that have been FDA approved for the treatment of psoriasis as of March 2019, plus risankizumab, which was pending FDA approval at the time of publication and was later approved in April 2019. They also address dosing regimens, potential to combine biologics with other therapies, and different forms of psoriasis for which each may be effective.3 The purpose of this discussion is to present these guidelines in a condensed form to prescribers of biologic therapies and review the most clinically significant considerations during each step of treatment. Of note, we highlight only treatment of adult patients and do not discuss information relevant to risankizumab, as it was not FDA approved when the AAD-NPF guidelines were released.
Choosing a Biologic
Biologic therapy may be considered for patients with psoriasis that affects more than 3% of the body’s surface and is recalcitrant to localized therapies. There is no particular first-line biologic recommended for all patients with psoriasis; rather, choice of therapy should be individualized to the patient, considering factors such as body parts affected, comorbidities, lifestyle, and drug cost.
All 10 FDA-approved biologics (Table) have been ranked by the AAD and NPF as having grade A evidence for efficacy as monotherapy in the treatment of moderate to severe plaque-type psoriasis. Involvement of difficult-to-treat areas may be considered when choosing a specific therapy. The tumor necrosis factor α (TNF-α) inhibitors etanercept and adalimumab, the IL-17 inhibitor secukinumab, and the IL-23 inhibitor guselkumab have the greatest evidence for efficacy in treatment of nail disease. For scalp involvement, etanercept and guselkumab have the highest-quality evidence, and for palmoplantar disease, adalimumab, secukinumab, and guselkumab are considered the most effective. The TNF-α inhibitors are considered the optimal treatment option for concurrent psoriatic arthritis, though the IL-12/IL-23 inhibitor ustekinumab and the IL-17 inhibitors secukinumab and ixekizumab also have shown grade A evidence of efficacy. Of note, because TNF-α inhibitors received the earliest FDA approval, there is most evidence available for this class. Therapies with lower evidence quality for certain forms of psoriasis may show real-world effectiveness in individual patients, though more trials will be necessary to generate a body of evidence to change these clinical recommendations.
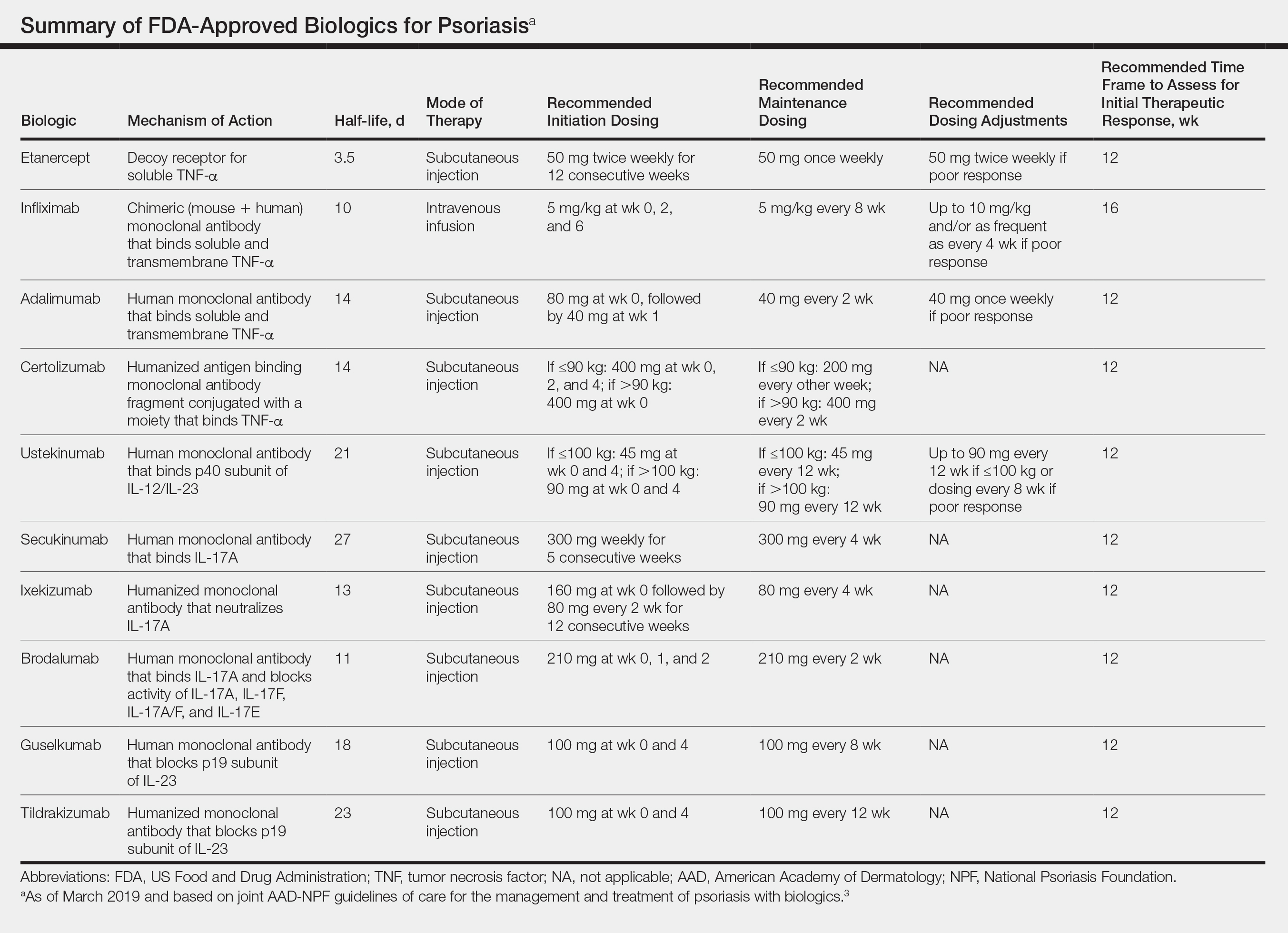
In pregnant women or those are anticipating pregnancy, certolizumab may be considered, as it is the only biologic shown to have minimal to no placental transfer. Other TNF-α inhibitors may undergo active placental transfer, particularly during the latter half of pregnancy,5 and the greatest theoretical risk of transfer occurs in the third trimester. Although these drugs may not directly harm the fetus, they do cause fetal immunosuppression for up to the first 3 months of life. All TNF-α inhibitors are considered safe during lactation. There are inadequate data regarding the safety of other classes of biologics during pregnancy and lactation.
Overweight and obese patients also require unique considerations when choosing a biologic. Infliximab is the only approved psoriasis biologic that utilizes proportional-to-weight dosing and hence may be particularly efficacious in patients with higher body mass. Ustekinumab dosing also takes patient weight into consideration; patients heavier than 100 kg should receive 90-mg doses at initiation and during maintenance compared to 45 mg for patients who weigh 100 kg or less. Other approved biologics also may be utilized in these patients but may require closer monitoring of treatment efficacy.
There are few serious contraindications for specific biologic therapies. Any history of allergic reaction to a particular therapy is an absolute contraindication to its use. In patients for whom IL-17 inhibitor treatment is being considered, inflammatory bowel disease (IBD) should be ruled out given the likelihood that IL-17 could reactivate or worsen IBD. Of note, TNF-α inhibitors and ustekinumab are approved therapies for patients with IBD and may be recommended in patients with comorbid psoriasis. Phase 2 and phase 3 trials have found no reactivation or worsening of IBD in patients with psoriasis who were treated with the IL-23 inhibitor tildrakizumab,6 and phase 2 trials of treatment of IBD with guselkumab are currently underway (ClinicalTrials.gov Identifier NCT03466411). In patients with New York Heart Association class III and class IV congestive heart failure or multiple sclerosis, initiation of TNF-α inhibitors should be avoided. Among 3 phase 3 trials encompassing nearly 3000 patients treated with the IL-17 inhibitor brodalumab, a total of 3 patients died by suicide7,8; hence, the FDA has issued a black box warning cautioning against use of this drug in patients with history of suicidal ideation or recent suicidal behavior. Although a causal relationship between brodalumab and suicide has not been well established,9 a thorough psychiatric history should be obtained in those initiating treatment with brodalumab.
Initiation of Therapy
Prior to initiating biologic therapy, it is important to obtain a complete blood cell count, complete metabolic panel, tuberculosis testing, and hepatitis B virus (HBV) and hepatitis C virus serologies. Testing for human immunodeficiency virus may be pursued at the clinician’s discretion. It is important to address any positive or concerning results prior to starting biologics. In patients with active infections, therapy may be initiated alongside guidance from an infectious disease specialist. Those with a positive purified protein derivative test, T-SPOT test, or QuantiFERON-TB Gold test must be referred for chest radiographs to rule out active tuberculosis. Patients with active HBV infection should receive appropriate referral to initiate antiviral therapy as well as core antibody testing, and those with active hepatitis C virus infection may only receive biologics under the combined discretion of a dermatologist and an appropriate specialist. Patients with human immunodeficiency virus must concurrently receive highly active antiretroviral therapy, show normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection.
Therapy should be commenced using specific dosing regimens, which are unique for each biologic (Table). Patients also must be educated on routine follow-up to assess treatment response and tolerability.
Assessment and Optimization of Treatment Response
Patients taking biologics may experience primary treatment failure, defined as lack of response to therapy from initiation. One predisposing factor may be increased body mass; patients who are overweight and obese are less likely to respond to standard regimens of TNF-α inhibitors and 45-mg dosing of ustekinumab. In most cases, however, the cause of primary nonresponse is unpredictable. For patients in whom therapy has failed within the recommended initial time frame (Table), dose escalation or shortening of dosing intervals may be pursued. Recommended dosing adjustments are outlined in the Table. Alternatively, patients may be switched to a different biologic.
If desired effectiveness is not reached with biologic monotherapy, topical corticosteroids, topical vitamin D analogues, or narrowband UVB light therapy may be concurrently used for difficult-to-treat areas. Evidence for safety and effectiveness of systemic adjuncts to biologics is moderate to low, warranting caution with their use. Methotrexate, cyclosporine, and apremilast have synergistic effects with biologics, though they may increase the risk for immunosuppression-related complications. Acitretin, an oral retinoid, likely is the most reasonable systemic adjunct to biologics because of its lack of immunosuppressive properties.
In patients with a suboptimal response to biologics, particularly those taking therapies that require frequent dosing, poor compliance should be considered.10 These patients may be switched to a biologic with less-frequent maintenance dosing (Table). Ustekinumab and tildrakizumab may be the best options for optimizing compliance, as they require dosing only once every 12 weeks after administration of loading doses.
Secondary treatment failure is diminished efficacy of treatment following successful initial response despite no changes in regimen. The best-known factor contributing to secondary nonresponse to biologics is the development of antidrug antibodies (ADAs), a phenomenon known as immunogenicity. The development of efficacy-limiting ADAs has been observed in response to most biologics, though ADAs against etanercept and guselkumab do not limit therapeutic response. Patients taking adalimumab and infliximab have particularly well-documented efficacy-limiting immunogenicity, and those who develop ADAs to infliximab are considered more prone to developing infusion reactions. Methotrexate, which limits antibody formation, may concomitantly be prescribed in patients who experience secondary treatment failure. It should be considered in all patients taking infliximab to increase efficacy and tolerability of therapy.
Considerations During Active Therapy
In addition to monitoring adherence and response to regimens, dermatologists must be heavily involved in counseling patients regarding the risks and adverse effects associated with these therapies. During maintenance therapy with biologics, patients must follow up with the prescriber at minimum every 3 to 6 months to evaluate for continued efficacy of treatment, extent of side effects, and effects of treatment on overall health and quality of life. Given the immunosuppressive effects of biologics, annual testing for tuberculosis should be considered in high-risk individuals. In those who are considered at low risk, tuberculosis testing may be done at the discretion of the dermatologist. In those with a history of HBV infection, HBV serologies should be pursued routinely given the risk for reactivation.
Annual screening for nonmelanoma skin cancer should be performed in all patients taking biologics. Tumor necrosis factor α inhibitor therapy in particular confers an elevated risk for cutaneous squamous cell carcinoma, especially in patients who are immunosuppressed at baseline and those with history of UV phototherapy. Use of acitretin alongside TNF-α inhibitors or ustekinumab may prevent squamous cell carcinoma formation in high-risk patients.
Because infliximab treatment poses an elevated risk of liver injury,11 liver function tests should be repeated 3 months following initiation of treatment and then every 6 to 12 months subsequently if results are normal. Periodic assessment of suicidal ideation is recommended in patients on brodalumab therapy, which may necessitate more frequent follow-up visits and potentially psychiatry referrals in certain patients. Patients taking IL-17 inhibitors, particularly those who are concurrently taking methotrexate, are at increased risk for developing mucocutaneous Candida infections; these patients should be monitored for such infections and treated appropriately.12
It is additionally important for prescribing dermatologists to ensure that patients on biologics are following up with their general providers to receive timely age-appropriate preventative screenings and vaccines. Inactivated vaccinations may be administered during therapy with any biologic; however, live vaccinations may induce systemic infection in those who are immunocompromised, which theoretically includes individuals taking biologic agents, though incidence data in this patient population are scarce.13 Some experts believe that administration of live vaccines warrants temporary discontinuation of biologic therapy for 2 to 3 half-lives before and after vaccination (Table). Others recommend stopping treatment at least 4 weeks before and until 2 weeks after vaccination. For patients taking biologics with half-lives greater than 20 days, which would theoretically require stopping the drug 2 months prior to vaccination, the benefit of vaccination should be weighed against the risk of prolonged discontinuation of therapy. Until recently, this recommendation was particularly important, as a live herpes zoster vaccination was recommended by the Centers for Disease Control and Prevention for adults older than 60 years. In 2017, a new inactivated herpes zoster vaccine was introduced and is now the preferred vaccine for all patients older than 50 years.14 It is especially important that patients on biologics receive this vaccine to avoid temporary drug discontinuation.
Evidence that any particular class of biologics increases risk for solid tumors or lymphoreticular malignancy is limited. One case-control analysis reported that more than 12 months of treatment with TNF-α inhibitors may increase risk for malignancy; however, the confidence interval reported hardly allows for statistical significance.15 Another retrospective cohort study found no elevated incidence of cancer in patients on TNF-α inhibitors compared to nonbiologic comparators.16 Ustekinumab was shown to confer no increased risk for malignancy in 1 large study,15 but no large studies have been conducted for other classes of drugs. Given the limited and inconclusive evidence available, the guidelines recommend that age-appropriate cancer screenings recommended for the general population should be pursued in patients taking biologics.
Surgery while taking biologics may lead to stress-induced augmentation of immunosuppression, resulting in elevated risk of infection.17 Low-risk surgeries that do not warrant discontinuation of treatment include endoscopic, ophthalmologic, dermatologic, orthopedic, and breast procedures. In patients preparing for elective surgery in which respiratory, gastrointestinal, or genitourinary tracts will be entered, biologics may be discontinued at least 3 half-lives (Table) prior to surgery if the dermatologist and surgeon collaboratively deem that risk of infection outweighs benefit of continued therapy.18 Therapy may be resumed within 1 to 2 weeks postoperatively if there are no surgical complications.
Switching Biologics
Changing therapy to another biologic should be considered if there is no response to treatment or the patient experiences adverse effects while taking a particular biologic. Because evidence is limited regarding the ideal time frame between discontinuation of a prior medication and initiation of a new biologic, this interval should be determined at the discretion of the provider based on the patient’s disease severity and response to prior treatment. For individuals who experience primary or secondary treatment failure while maintaining appropriate dosing and treatment compliance, switching to a different biologic is recommended to maximize treatment response.19 Changing therapy to a biologic within the same class is generally effective,20 and switching to a biologic with another mechanism of action should be considered if a class-specific adverse effect is the major reason for altering the regimen. Nonetheless, some patients may be unresponsive to biologic changes. Further research is necessary to determine which biologics may be most effective when previously used biologics have failed and particular factors that may predispose patients to biologic unresponsiveness.
Resuming Biologic Treatment Following Cessation
In cases where therapy is discontinued for any reason, it may be necessary to repeat initiation dosing when resuming treatment. In patients with severe or flaring disease or if more than 3 to 4 half-lives have passed since the most recent dose, it may be necessary to restart therapy with the loading dose (Table). Unfortunately, restarting therapy may preclude some patients from experiencing the maximal response that they attained prior to cessation. In such cases, switching biologic therapy to a different class may prove beneficial.
Final Thoughts
These recommendations contain valuable information that will assist dermatologists when initiating biologics and managing outcomes of their psoriasis patients. It is, however, crucial to bear in mind that these guidelines serve as merely a tool. Given the paucity of comprehensive research, particularly regarding some of the more recently approved therapies, there are many questions that are unanswered within the guidelines. Their utility for each individual patient situation is therefore limited, and clinical judgement may outweigh the information presented. The recommendations nevertheless provide a pivotal and unprecedented framework that promotes discourse among patients, dermatologists, and other providers to optimize the efficacy of biologic therapy for psoriasis.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218-224.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol. 2016;12:937-944.
- Gooderham M, Elewski B, Pariser D, et al. Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: data from 3 large randomized clinical trials [abstract]. J Am Acad Dermatol. 2018;79(suppl 1):AB166.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(suppl 3):2-6.
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Huber F, Ehrensperger B, Hatz C, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics [published online January 1, 2018]. J Travel Med. doi:10.1093/jtm/tax082.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48-58.
- Fabiano A, De Simone C, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(suppl 1):S24-S26.
- Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75:798-805.e7.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44:1015-1019.
- Bracke S, Lambert J. Viewpoint on handling anti-TNF failure in psoriasis. Arch Dermatol Res. 2013;305:945-950.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 The disease is moderate to severe for approximately 1 in 6 individuals with psoriasis.2 These patients, particularly those with symptoms that are refractory to topical therapy and/or phototherapy, can benefit from the use of biologic agents, which are monoclonal antibodies and fusion proteins engineered to inhibit the action of cytokines that drive psoriatic inflammation.
In February 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of biologics in treating adult patients with psoriasis.3 The prior guidelines were released in 2008 when just 3 biologics—etanercept, infliximab, and adalimumab—were approved by the US Food and Drug Administration (FDA) for the management of psoriasis. These older recommendations were mostly based on studies of the efficacy and safety of biologics for patients with psoriatic arthritis.4 Over the last 11 years, 8 novel biologics have gained FDA approval, and numerous large phase 2 and phase 3 trials evaluating the risks and benefits of biologics have been conducted. The new guidelines contain considerably more detail and are based on evidence more specific to psoriasis rather than to psoriatic arthritis. Given the large repertoire of biologics available today and the increased amount of published research regarding each one, these guidelines may aid dermatologists in choosing the optimal biologic and managing therapy.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and adverse events of the 10 biologics that have been FDA approved for the treatment of psoriasis as of March 2019, plus risankizumab, which was pending FDA approval at the time of publication and was later approved in April 2019. They also address dosing regimens, potential to combine biologics with other therapies, and different forms of psoriasis for which each may be effective.3 The purpose of this discussion is to present these guidelines in a condensed form to prescribers of biologic therapies and review the most clinically significant considerations during each step of treatment. Of note, we highlight only treatment of adult patients and do not discuss information relevant to risankizumab, as it was not FDA approved when the AAD-NPF guidelines were released.
Choosing a Biologic
Biologic therapy may be considered for patients with psoriasis that affects more than 3% of the body’s surface and is recalcitrant to localized therapies. There is no particular first-line biologic recommended for all patients with psoriasis; rather, choice of therapy should be individualized to the patient, considering factors such as body parts affected, comorbidities, lifestyle, and drug cost.
All 10 FDA-approved biologics (Table) have been ranked by the AAD and NPF as having grade A evidence for efficacy as monotherapy in the treatment of moderate to severe plaque-type psoriasis. Involvement of difficult-to-treat areas may be considered when choosing a specific therapy. The tumor necrosis factor α (TNF-α) inhibitors etanercept and adalimumab, the IL-17 inhibitor secukinumab, and the IL-23 inhibitor guselkumab have the greatest evidence for efficacy in treatment of nail disease. For scalp involvement, etanercept and guselkumab have the highest-quality evidence, and for palmoplantar disease, adalimumab, secukinumab, and guselkumab are considered the most effective. The TNF-α inhibitors are considered the optimal treatment option for concurrent psoriatic arthritis, though the IL-12/IL-23 inhibitor ustekinumab and the IL-17 inhibitors secukinumab and ixekizumab also have shown grade A evidence of efficacy. Of note, because TNF-α inhibitors received the earliest FDA approval, there is most evidence available for this class. Therapies with lower evidence quality for certain forms of psoriasis may show real-world effectiveness in individual patients, though more trials will be necessary to generate a body of evidence to change these clinical recommendations.

In pregnant women or those are anticipating pregnancy, certolizumab may be considered, as it is the only biologic shown to have minimal to no placental transfer. Other TNF-α inhibitors may undergo active placental transfer, particularly during the latter half of pregnancy,5 and the greatest theoretical risk of transfer occurs in the third trimester. Although these drugs may not directly harm the fetus, they do cause fetal immunosuppression for up to the first 3 months of life. All TNF-α inhibitors are considered safe during lactation. There are inadequate data regarding the safety of other classes of biologics during pregnancy and lactation.
Overweight and obese patients also require unique considerations when choosing a biologic. Infliximab is the only approved psoriasis biologic that utilizes proportional-to-weight dosing and hence may be particularly efficacious in patients with higher body mass. Ustekinumab dosing also takes patient weight into consideration; patients heavier than 100 kg should receive 90-mg doses at initiation and during maintenance compared to 45 mg for patients who weigh 100 kg or less. Other approved biologics also may be utilized in these patients but may require closer monitoring of treatment efficacy.
There are few serious contraindications for specific biologic therapies. Any history of allergic reaction to a particular therapy is an absolute contraindication to its use. In patients for whom IL-17 inhibitor treatment is being considered, inflammatory bowel disease (IBD) should be ruled out given the likelihood that IL-17 could reactivate or worsen IBD. Of note, TNF-α inhibitors and ustekinumab are approved therapies for patients with IBD and may be recommended in patients with comorbid psoriasis. Phase 2 and phase 3 trials have found no reactivation or worsening of IBD in patients with psoriasis who were treated with the IL-23 inhibitor tildrakizumab,6 and phase 2 trials of treatment of IBD with guselkumab are currently underway (ClinicalTrials.gov Identifier NCT03466411). In patients with New York Heart Association class III and class IV congestive heart failure or multiple sclerosis, initiation of TNF-α inhibitors should be avoided. Among 3 phase 3 trials encompassing nearly 3000 patients treated with the IL-17 inhibitor brodalumab, a total of 3 patients died by suicide7,8; hence, the FDA has issued a black box warning cautioning against use of this drug in patients with history of suicidal ideation or recent suicidal behavior. Although a causal relationship between brodalumab and suicide has not been well established,9 a thorough psychiatric history should be obtained in those initiating treatment with brodalumab.
Initiation of Therapy
Prior to initiating biologic therapy, it is important to obtain a complete blood cell count, complete metabolic panel, tuberculosis testing, and hepatitis B virus (HBV) and hepatitis C virus serologies. Testing for human immunodeficiency virus may be pursued at the clinician’s discretion. It is important to address any positive or concerning results prior to starting biologics. In patients with active infections, therapy may be initiated alongside guidance from an infectious disease specialist. Those with a positive purified protein derivative test, T-SPOT test, or QuantiFERON-TB Gold test must be referred for chest radiographs to rule out active tuberculosis. Patients with active HBV infection should receive appropriate referral to initiate antiviral therapy as well as core antibody testing, and those with active hepatitis C virus infection may only receive biologics under the combined discretion of a dermatologist and an appropriate specialist. Patients with human immunodeficiency virus must concurrently receive highly active antiretroviral therapy, show normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection.
Therapy should be commenced using specific dosing regimens, which are unique for each biologic (Table). Patients also must be educated on routine follow-up to assess treatment response and tolerability.
Assessment and Optimization of Treatment Response
Patients taking biologics may experience primary treatment failure, defined as lack of response to therapy from initiation. One predisposing factor may be increased body mass; patients who are overweight and obese are less likely to respond to standard regimens of TNF-α inhibitors and 45-mg dosing of ustekinumab. In most cases, however, the cause of primary nonresponse is unpredictable. For patients in whom therapy has failed within the recommended initial time frame (Table), dose escalation or shortening of dosing intervals may be pursued. Recommended dosing adjustments are outlined in the Table. Alternatively, patients may be switched to a different biologic.
If desired effectiveness is not reached with biologic monotherapy, topical corticosteroids, topical vitamin D analogues, or narrowband UVB light therapy may be concurrently used for difficult-to-treat areas. Evidence for safety and effectiveness of systemic adjuncts to biologics is moderate to low, warranting caution with their use. Methotrexate, cyclosporine, and apremilast have synergistic effects with biologics, though they may increase the risk for immunosuppression-related complications. Acitretin, an oral retinoid, likely is the most reasonable systemic adjunct to biologics because of its lack of immunosuppressive properties.
In patients with a suboptimal response to biologics, particularly those taking therapies that require frequent dosing, poor compliance should be considered.10 These patients may be switched to a biologic with less-frequent maintenance dosing (Table). Ustekinumab and tildrakizumab may be the best options for optimizing compliance, as they require dosing only once every 12 weeks after administration of loading doses.
Secondary treatment failure is diminished efficacy of treatment following successful initial response despite no changes in regimen. The best-known factor contributing to secondary nonresponse to biologics is the development of antidrug antibodies (ADAs), a phenomenon known as immunogenicity. The development of efficacy-limiting ADAs has been observed in response to most biologics, though ADAs against etanercept and guselkumab do not limit therapeutic response. Patients taking adalimumab and infliximab have particularly well-documented efficacy-limiting immunogenicity, and those who develop ADAs to infliximab are considered more prone to developing infusion reactions. Methotrexate, which limits antibody formation, may concomitantly be prescribed in patients who experience secondary treatment failure. It should be considered in all patients taking infliximab to increase efficacy and tolerability of therapy.
Considerations During Active Therapy
In addition to monitoring adherence and response to regimens, dermatologists must be heavily involved in counseling patients regarding the risks and adverse effects associated with these therapies. During maintenance therapy with biologics, patients must follow up with the prescriber at minimum every 3 to 6 months to evaluate for continued efficacy of treatment, extent of side effects, and effects of treatment on overall health and quality of life. Given the immunosuppressive effects of biologics, annual testing for tuberculosis should be considered in high-risk individuals. In those who are considered at low risk, tuberculosis testing may be done at the discretion of the dermatologist. In those with a history of HBV infection, HBV serologies should be pursued routinely given the risk for reactivation.
Annual screening for nonmelanoma skin cancer should be performed in all patients taking biologics. Tumor necrosis factor α inhibitor therapy in particular confers an elevated risk for cutaneous squamous cell carcinoma, especially in patients who are immunosuppressed at baseline and those with history of UV phototherapy. Use of acitretin alongside TNF-α inhibitors or ustekinumab may prevent squamous cell carcinoma formation in high-risk patients.
Because infliximab treatment poses an elevated risk of liver injury,11 liver function tests should be repeated 3 months following initiation of treatment and then every 6 to 12 months subsequently if results are normal. Periodic assessment of suicidal ideation is recommended in patients on brodalumab therapy, which may necessitate more frequent follow-up visits and potentially psychiatry referrals in certain patients. Patients taking IL-17 inhibitors, particularly those who are concurrently taking methotrexate, are at increased risk for developing mucocutaneous Candida infections; these patients should be monitored for such infections and treated appropriately.12
It is additionally important for prescribing dermatologists to ensure that patients on biologics are following up with their general providers to receive timely age-appropriate preventative screenings and vaccines. Inactivated vaccinations may be administered during therapy with any biologic; however, live vaccinations may induce systemic infection in those who are immunocompromised, which theoretically includes individuals taking biologic agents, though incidence data in this patient population are scarce.13 Some experts believe that administration of live vaccines warrants temporary discontinuation of biologic therapy for 2 to 3 half-lives before and after vaccination (Table). Others recommend stopping treatment at least 4 weeks before and until 2 weeks after vaccination. For patients taking biologics with half-lives greater than 20 days, which would theoretically require stopping the drug 2 months prior to vaccination, the benefit of vaccination should be weighed against the risk of prolonged discontinuation of therapy. Until recently, this recommendation was particularly important, as a live herpes zoster vaccination was recommended by the Centers for Disease Control and Prevention for adults older than 60 years. In 2017, a new inactivated herpes zoster vaccine was introduced and is now the preferred vaccine for all patients older than 50 years.14 It is especially important that patients on biologics receive this vaccine to avoid temporary drug discontinuation.
Evidence that any particular class of biologics increases risk for solid tumors or lymphoreticular malignancy is limited. One case-control analysis reported that more than 12 months of treatment with TNF-α inhibitors may increase risk for malignancy; however, the confidence interval reported hardly allows for statistical significance.15 Another retrospective cohort study found no elevated incidence of cancer in patients on TNF-α inhibitors compared to nonbiologic comparators.16 Ustekinumab was shown to confer no increased risk for malignancy in 1 large study,15 but no large studies have been conducted for other classes of drugs. Given the limited and inconclusive evidence available, the guidelines recommend that age-appropriate cancer screenings recommended for the general population should be pursued in patients taking biologics.
Surgery while taking biologics may lead to stress-induced augmentation of immunosuppression, resulting in elevated risk of infection.17 Low-risk surgeries that do not warrant discontinuation of treatment include endoscopic, ophthalmologic, dermatologic, orthopedic, and breast procedures. In patients preparing for elective surgery in which respiratory, gastrointestinal, or genitourinary tracts will be entered, biologics may be discontinued at least 3 half-lives (Table) prior to surgery if the dermatologist and surgeon collaboratively deem that risk of infection outweighs benefit of continued therapy.18 Therapy may be resumed within 1 to 2 weeks postoperatively if there are no surgical complications.
Switching Biologics
Changing therapy to another biologic should be considered if there is no response to treatment or the patient experiences adverse effects while taking a particular biologic. Because evidence is limited regarding the ideal time frame between discontinuation of a prior medication and initiation of a new biologic, this interval should be determined at the discretion of the provider based on the patient’s disease severity and response to prior treatment. For individuals who experience primary or secondary treatment failure while maintaining appropriate dosing and treatment compliance, switching to a different biologic is recommended to maximize treatment response.19 Changing therapy to a biologic within the same class is generally effective,20 and switching to a biologic with another mechanism of action should be considered if a class-specific adverse effect is the major reason for altering the regimen. Nonetheless, some patients may be unresponsive to biologic changes. Further research is necessary to determine which biologics may be most effective when previously used biologics have failed and particular factors that may predispose patients to biologic unresponsiveness.
Resuming Biologic Treatment Following Cessation
In cases where therapy is discontinued for any reason, it may be necessary to repeat initiation dosing when resuming treatment. In patients with severe or flaring disease or if more than 3 to 4 half-lives have passed since the most recent dose, it may be necessary to restart therapy with the loading dose (Table). Unfortunately, restarting therapy may preclude some patients from experiencing the maximal response that they attained prior to cessation. In such cases, switching biologic therapy to a different class may prove beneficial.
Final Thoughts
These recommendations contain valuable information that will assist dermatologists when initiating biologics and managing outcomes of their psoriasis patients. It is, however, crucial to bear in mind that these guidelines serve as merely a tool. Given the paucity of comprehensive research, particularly regarding some of the more recently approved therapies, there are many questions that are unanswered within the guidelines. Their utility for each individual patient situation is therefore limited, and clinical judgement may outweigh the information presented. The recommendations nevertheless provide a pivotal and unprecedented framework that promotes discourse among patients, dermatologists, and other providers to optimize the efficacy of biologic therapy for psoriasis.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 The disease is moderate to severe for approximately 1 in 6 individuals with psoriasis.2 These patients, particularly those with symptoms that are refractory to topical therapy and/or phototherapy, can benefit from the use of biologic agents, which are monoclonal antibodies and fusion proteins engineered to inhibit the action of cytokines that drive psoriatic inflammation.
In February 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of biologics in treating adult patients with psoriasis.3 The prior guidelines were released in 2008 when just 3 biologics—etanercept, infliximab, and adalimumab—were approved by the US Food and Drug Administration (FDA) for the management of psoriasis. These older recommendations were mostly based on studies of the efficacy and safety of biologics for patients with psoriatic arthritis.4 Over the last 11 years, 8 novel biologics have gained FDA approval, and numerous large phase 2 and phase 3 trials evaluating the risks and benefits of biologics have been conducted. The new guidelines contain considerably more detail and are based on evidence more specific to psoriasis rather than to psoriatic arthritis. Given the large repertoire of biologics available today and the increased amount of published research regarding each one, these guidelines may aid dermatologists in choosing the optimal biologic and managing therapy.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and adverse events of the 10 biologics that have been FDA approved for the treatment of psoriasis as of March 2019, plus risankizumab, which was pending FDA approval at the time of publication and was later approved in April 2019. They also address dosing regimens, potential to combine biologics with other therapies, and different forms of psoriasis for which each may be effective.3 The purpose of this discussion is to present these guidelines in a condensed form to prescribers of biologic therapies and review the most clinically significant considerations during each step of treatment. Of note, we highlight only treatment of adult patients and do not discuss information relevant to risankizumab, as it was not FDA approved when the AAD-NPF guidelines were released.
Choosing a Biologic
Biologic therapy may be considered for patients with psoriasis that affects more than 3% of the body’s surface and is recalcitrant to localized therapies. There is no particular first-line biologic recommended for all patients with psoriasis; rather, choice of therapy should be individualized to the patient, considering factors such as body parts affected, comorbidities, lifestyle, and drug cost.
All 10 FDA-approved biologics (Table) have been ranked by the AAD and NPF as having grade A evidence for efficacy as monotherapy in the treatment of moderate to severe plaque-type psoriasis. Involvement of difficult-to-treat areas may be considered when choosing a specific therapy. The tumor necrosis factor α (TNF-α) inhibitors etanercept and adalimumab, the IL-17 inhibitor secukinumab, and the IL-23 inhibitor guselkumab have the greatest evidence for efficacy in treatment of nail disease. For scalp involvement, etanercept and guselkumab have the highest-quality evidence, and for palmoplantar disease, adalimumab, secukinumab, and guselkumab are considered the most effective. The TNF-α inhibitors are considered the optimal treatment option for concurrent psoriatic arthritis, though the IL-12/IL-23 inhibitor ustekinumab and the IL-17 inhibitors secukinumab and ixekizumab also have shown grade A evidence of efficacy. Of note, because TNF-α inhibitors received the earliest FDA approval, there is most evidence available for this class. Therapies with lower evidence quality for certain forms of psoriasis may show real-world effectiveness in individual patients, though more trials will be necessary to generate a body of evidence to change these clinical recommendations.

In pregnant women or those are anticipating pregnancy, certolizumab may be considered, as it is the only biologic shown to have minimal to no placental transfer. Other TNF-α inhibitors may undergo active placental transfer, particularly during the latter half of pregnancy,5 and the greatest theoretical risk of transfer occurs in the third trimester. Although these drugs may not directly harm the fetus, they do cause fetal immunosuppression for up to the first 3 months of life. All TNF-α inhibitors are considered safe during lactation. There are inadequate data regarding the safety of other classes of biologics during pregnancy and lactation.
Overweight and obese patients also require unique considerations when choosing a biologic. Infliximab is the only approved psoriasis biologic that utilizes proportional-to-weight dosing and hence may be particularly efficacious in patients with higher body mass. Ustekinumab dosing also takes patient weight into consideration; patients heavier than 100 kg should receive 90-mg doses at initiation and during maintenance compared to 45 mg for patients who weigh 100 kg or less. Other approved biologics also may be utilized in these patients but may require closer monitoring of treatment efficacy.
There are few serious contraindications for specific biologic therapies. Any history of allergic reaction to a particular therapy is an absolute contraindication to its use. In patients for whom IL-17 inhibitor treatment is being considered, inflammatory bowel disease (IBD) should be ruled out given the likelihood that IL-17 could reactivate or worsen IBD. Of note, TNF-α inhibitors and ustekinumab are approved therapies for patients with IBD and may be recommended in patients with comorbid psoriasis. Phase 2 and phase 3 trials have found no reactivation or worsening of IBD in patients with psoriasis who were treated with the IL-23 inhibitor tildrakizumab,6 and phase 2 trials of treatment of IBD with guselkumab are currently underway (ClinicalTrials.gov Identifier NCT03466411). In patients with New York Heart Association class III and class IV congestive heart failure or multiple sclerosis, initiation of TNF-α inhibitors should be avoided. Among 3 phase 3 trials encompassing nearly 3000 patients treated with the IL-17 inhibitor brodalumab, a total of 3 patients died by suicide7,8; hence, the FDA has issued a black box warning cautioning against use of this drug in patients with history of suicidal ideation or recent suicidal behavior. Although a causal relationship between brodalumab and suicide has not been well established,9 a thorough psychiatric history should be obtained in those initiating treatment with brodalumab.
Initiation of Therapy
Prior to initiating biologic therapy, it is important to obtain a complete blood cell count, complete metabolic panel, tuberculosis testing, and hepatitis B virus (HBV) and hepatitis C virus serologies. Testing for human immunodeficiency virus may be pursued at the clinician’s discretion. It is important to address any positive or concerning results prior to starting biologics. In patients with active infections, therapy may be initiated alongside guidance from an infectious disease specialist. Those with a positive purified protein derivative test, T-SPOT test, or QuantiFERON-TB Gold test must be referred for chest radiographs to rule out active tuberculosis. Patients with active HBV infection should receive appropriate referral to initiate antiviral therapy as well as core antibody testing, and those with active hepatitis C virus infection may only receive biologics under the combined discretion of a dermatologist and an appropriate specialist. Patients with human immunodeficiency virus must concurrently receive highly active antiretroviral therapy, show normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection.
Therapy should be commenced using specific dosing regimens, which are unique for each biologic (Table). Patients also must be educated on routine follow-up to assess treatment response and tolerability.
Assessment and Optimization of Treatment Response
Patients taking biologics may experience primary treatment failure, defined as lack of response to therapy from initiation. One predisposing factor may be increased body mass; patients who are overweight and obese are less likely to respond to standard regimens of TNF-α inhibitors and 45-mg dosing of ustekinumab. In most cases, however, the cause of primary nonresponse is unpredictable. For patients in whom therapy has failed within the recommended initial time frame (Table), dose escalation or shortening of dosing intervals may be pursued. Recommended dosing adjustments are outlined in the Table. Alternatively, patients may be switched to a different biologic.
If desired effectiveness is not reached with biologic monotherapy, topical corticosteroids, topical vitamin D analogues, or narrowband UVB light therapy may be concurrently used for difficult-to-treat areas. Evidence for safety and effectiveness of systemic adjuncts to biologics is moderate to low, warranting caution with their use. Methotrexate, cyclosporine, and apremilast have synergistic effects with biologics, though they may increase the risk for immunosuppression-related complications. Acitretin, an oral retinoid, likely is the most reasonable systemic adjunct to biologics because of its lack of immunosuppressive properties.
In patients with a suboptimal response to biologics, particularly those taking therapies that require frequent dosing, poor compliance should be considered.10 These patients may be switched to a biologic with less-frequent maintenance dosing (Table). Ustekinumab and tildrakizumab may be the best options for optimizing compliance, as they require dosing only once every 12 weeks after administration of loading doses.
Secondary treatment failure is diminished efficacy of treatment following successful initial response despite no changes in regimen. The best-known factor contributing to secondary nonresponse to biologics is the development of antidrug antibodies (ADAs), a phenomenon known as immunogenicity. The development of efficacy-limiting ADAs has been observed in response to most biologics, though ADAs against etanercept and guselkumab do not limit therapeutic response. Patients taking adalimumab and infliximab have particularly well-documented efficacy-limiting immunogenicity, and those who develop ADAs to infliximab are considered more prone to developing infusion reactions. Methotrexate, which limits antibody formation, may concomitantly be prescribed in patients who experience secondary treatment failure. It should be considered in all patients taking infliximab to increase efficacy and tolerability of therapy.
Considerations During Active Therapy
In addition to monitoring adherence and response to regimens, dermatologists must be heavily involved in counseling patients regarding the risks and adverse effects associated with these therapies. During maintenance therapy with biologics, patients must follow up with the prescriber at minimum every 3 to 6 months to evaluate for continued efficacy of treatment, extent of side effects, and effects of treatment on overall health and quality of life. Given the immunosuppressive effects of biologics, annual testing for tuberculosis should be considered in high-risk individuals. In those who are considered at low risk, tuberculosis testing may be done at the discretion of the dermatologist. In those with a history of HBV infection, HBV serologies should be pursued routinely given the risk for reactivation.
Annual screening for nonmelanoma skin cancer should be performed in all patients taking biologics. Tumor necrosis factor α inhibitor therapy in particular confers an elevated risk for cutaneous squamous cell carcinoma, especially in patients who are immunosuppressed at baseline and those with history of UV phototherapy. Use of acitretin alongside TNF-α inhibitors or ustekinumab may prevent squamous cell carcinoma formation in high-risk patients.
Because infliximab treatment poses an elevated risk of liver injury,11 liver function tests should be repeated 3 months following initiation of treatment and then every 6 to 12 months subsequently if results are normal. Periodic assessment of suicidal ideation is recommended in patients on brodalumab therapy, which may necessitate more frequent follow-up visits and potentially psychiatry referrals in certain patients. Patients taking IL-17 inhibitors, particularly those who are concurrently taking methotrexate, are at increased risk for developing mucocutaneous Candida infections; these patients should be monitored for such infections and treated appropriately.12
It is additionally important for prescribing dermatologists to ensure that patients on biologics are following up with their general providers to receive timely age-appropriate preventative screenings and vaccines. Inactivated vaccinations may be administered during therapy with any biologic; however, live vaccinations may induce systemic infection in those who are immunocompromised, which theoretically includes individuals taking biologic agents, though incidence data in this patient population are scarce.13 Some experts believe that administration of live vaccines warrants temporary discontinuation of biologic therapy for 2 to 3 half-lives before and after vaccination (Table). Others recommend stopping treatment at least 4 weeks before and until 2 weeks after vaccination. For patients taking biologics with half-lives greater than 20 days, which would theoretically require stopping the drug 2 months prior to vaccination, the benefit of vaccination should be weighed against the risk of prolonged discontinuation of therapy. Until recently, this recommendation was particularly important, as a live herpes zoster vaccination was recommended by the Centers for Disease Control and Prevention for adults older than 60 years. In 2017, a new inactivated herpes zoster vaccine was introduced and is now the preferred vaccine for all patients older than 50 years.14 It is especially important that patients on biologics receive this vaccine to avoid temporary drug discontinuation.
Evidence that any particular class of biologics increases risk for solid tumors or lymphoreticular malignancy is limited. One case-control analysis reported that more than 12 months of treatment with TNF-α inhibitors may increase risk for malignancy; however, the confidence interval reported hardly allows for statistical significance.15 Another retrospective cohort study found no elevated incidence of cancer in patients on TNF-α inhibitors compared to nonbiologic comparators.16 Ustekinumab was shown to confer no increased risk for malignancy in 1 large study,15 but no large studies have been conducted for other classes of drugs. Given the limited and inconclusive evidence available, the guidelines recommend that age-appropriate cancer screenings recommended for the general population should be pursued in patients taking biologics.
Surgery while taking biologics may lead to stress-induced augmentation of immunosuppression, resulting in elevated risk of infection.17 Low-risk surgeries that do not warrant discontinuation of treatment include endoscopic, ophthalmologic, dermatologic, orthopedic, and breast procedures. In patients preparing for elective surgery in which respiratory, gastrointestinal, or genitourinary tracts will be entered, biologics may be discontinued at least 3 half-lives (Table) prior to surgery if the dermatologist and surgeon collaboratively deem that risk of infection outweighs benefit of continued therapy.18 Therapy may be resumed within 1 to 2 weeks postoperatively if there are no surgical complications.
Switching Biologics
Changing therapy to another biologic should be considered if there is no response to treatment or the patient experiences adverse effects while taking a particular biologic. Because evidence is limited regarding the ideal time frame between discontinuation of a prior medication and initiation of a new biologic, this interval should be determined at the discretion of the provider based on the patient’s disease severity and response to prior treatment. For individuals who experience primary or secondary treatment failure while maintaining appropriate dosing and treatment compliance, switching to a different biologic is recommended to maximize treatment response.19 Changing therapy to a biologic within the same class is generally effective,20 and switching to a biologic with another mechanism of action should be considered if a class-specific adverse effect is the major reason for altering the regimen. Nonetheless, some patients may be unresponsive to biologic changes. Further research is necessary to determine which biologics may be most effective when previously used biologics have failed and particular factors that may predispose patients to biologic unresponsiveness.
Resuming Biologic Treatment Following Cessation
In cases where therapy is discontinued for any reason, it may be necessary to repeat initiation dosing when resuming treatment. In patients with severe or flaring disease or if more than 3 to 4 half-lives have passed since the most recent dose, it may be necessary to restart therapy with the loading dose (Table). Unfortunately, restarting therapy may preclude some patients from experiencing the maximal response that they attained prior to cessation. In such cases, switching biologic therapy to a different class may prove beneficial.
Final Thoughts
These recommendations contain valuable information that will assist dermatologists when initiating biologics and managing outcomes of their psoriasis patients. It is, however, crucial to bear in mind that these guidelines serve as merely a tool. Given the paucity of comprehensive research, particularly regarding some of the more recently approved therapies, there are many questions that are unanswered within the guidelines. Their utility for each individual patient situation is therefore limited, and clinical judgement may outweigh the information presented. The recommendations nevertheless provide a pivotal and unprecedented framework that promotes discourse among patients, dermatologists, and other providers to optimize the efficacy of biologic therapy for psoriasis.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218-224.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol. 2016;12:937-944.
- Gooderham M, Elewski B, Pariser D, et al. Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: data from 3 large randomized clinical trials [abstract]. J Am Acad Dermatol. 2018;79(suppl 1):AB166.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(suppl 3):2-6.
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Huber F, Ehrensperger B, Hatz C, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics [published online January 1, 2018]. J Travel Med. doi:10.1093/jtm/tax082.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48-58.
- Fabiano A, De Simone C, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(suppl 1):S24-S26.
- Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75:798-805.e7.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44:1015-1019.
- Bracke S, Lambert J. Viewpoint on handling anti-TNF failure in psoriasis. Arch Dermatol Res. 2013;305:945-950.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218-224.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol. 2016;12:937-944.
- Gooderham M, Elewski B, Pariser D, et al. Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: data from 3 large randomized clinical trials [abstract]. J Am Acad Dermatol. 2018;79(suppl 1):AB166.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(suppl 3):2-6.
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Huber F, Ehrensperger B, Hatz C, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics [published online January 1, 2018]. J Travel Med. doi:10.1093/jtm/tax082.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48-58.
- Fabiano A, De Simone C, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(suppl 1):S24-S26.
- Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75:798-805.e7.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44:1015-1019.
- Bracke S, Lambert J. Viewpoint on handling anti-TNF failure in psoriasis. Arch Dermatol Res. 2013;305:945-950.
Practice Points
- There are currently 11 biologics approved for psoriasis, but there is no first-line or optimalbiologic. The choice must be made using clinical judgment based on a variety of medical and social factors.
- Frequent assessment for efficacy of and adverse events due to biologic therapy is warranted, as lack of response, loss of response, or severe side effects may warrant addition of concurrent therapies or switching to a different biologic.
- There are important considerations to make when immunizing and planning for surgery in patients on biologics.
Psoriasis Treatment in Patients With HIV
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
Psoriasis Treatment in Patients With Human Immunodeficiency Virus

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8
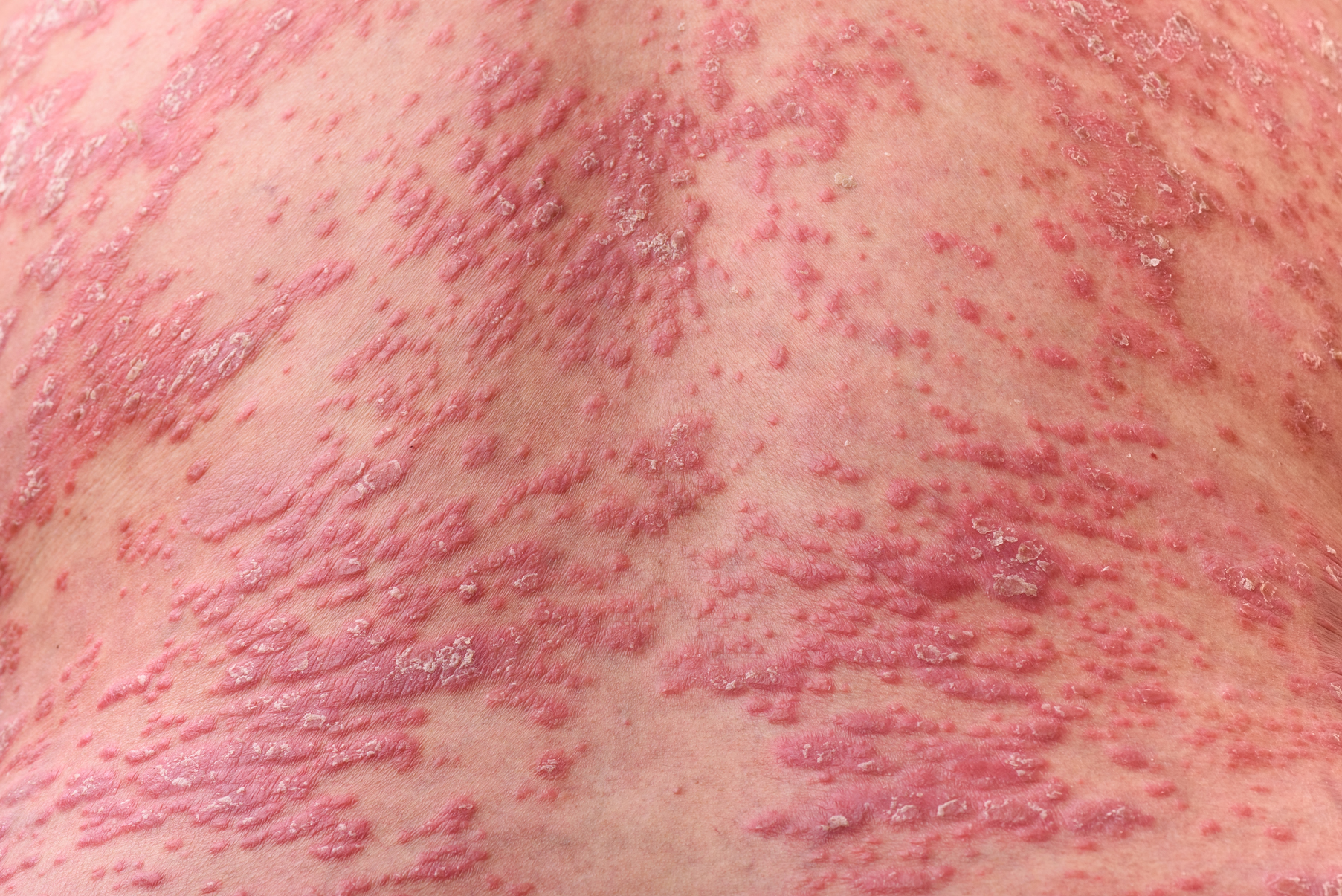
Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1
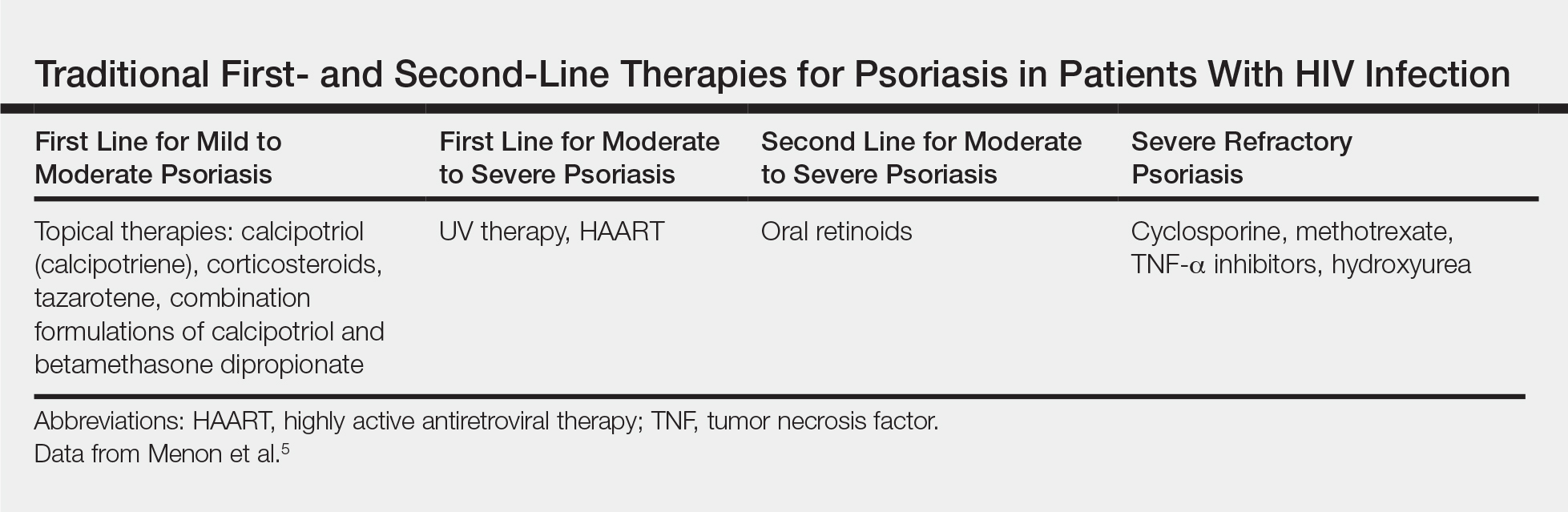
Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16
Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8

Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1

Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16

Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.

The treatment of psoriasis in patients with HIV infection represents a clinical challenge.1,2 Up to 3% of patients with HIV infection are estimated to have psoriasis. Although this prevalence is similar to the general population, psoriatic disease in patients with HIV tends to be more severe, refractory, and more difficult to treat.3-5 Additionally, up to half of patients with comorbid HIV and psoriasis also have substantial psoriatic arthritis (PsA).1,6
Drug treatments for psoriasis and PsA often are immunosuppressive; as such, the treatment of psoriasis in this patient population requires careful consideration of the potential risks and benefits of treatment as well as fastidious monitoring for the emergence of potentially adverse treatment effects.1 A careful diagnostic process to determine the severity of HIV-associated psoriasis and to select the appropriate treatment relative to the patient’s immunologic status is of critical importance.3
Presentation of Psoriasis in Patients With HIV Infection
The presentation and severity of psoriasis in patients with HIV infection is highly variable and is often related to the degree of immune suppression experienced by the patient.3,7 In some individuals, psoriasis may be the first outward manifestation of HIV, whereas in others, it only manifests after HIV has progressed to AIDS.7

Recognition of the atypical presentations of psoriasis that are frequently seen in patients with HIV infection can help to facilitate early diagnosis and treatment to improve patient outcomes.3,8 Psoriasis vulgaris, for example, typically presents as erythematous plaques with silvery-white scales on extensor surfaces of the body such as the knees and elbows. However, in patients with HIV, psoriasis vulgaris may present with scales that appear thick and oyster shell–like instead of silvery-white; these lesions also may occur on flexural areas rather than extensor surfaces.8 Similarly, the sudden onset of widespread psoriasis in otherwise healthy persons should trigger suspicion for HIV infection and recommendations for appropriate testing, even when no risk factors are present.8

Guttate, inverse, and erythrodermic psoriasis are the most common subtypes in patients with HIV infection, though all clinical subtypes may occur. Overlapping of psoriasis subtypes often occurs in individuals with HIV infection and should serve as a red flag to recommend screening for HIV.5,8 Acral involvement, frequently with pustules and occasionally with severe destructive nail changes, is commonly seen in patients with HIV-associated psoriasis.7,9 In cases involving severe psoriatic exacerbations among individuals with AIDS, there is a heightened risk of developing systemic infections, including superinfection of Staphylococcus aureus, which is a rare occurrence in immunocompetent patients with psoriasis.7,10,11
Therapeutic Options
Because the clinical course of psoriasis in patients with HIV infection is frequently progressive and refractory to treatment, traditional first- and second-line therapies (Table) including topical agents, phototherapy, and oral retinoids may be unable to achieve lasting control of both skin and joint manifestations.1

Topical Therapy
As in the general population, targeted therapies such as topical agents are recommended as first-line treatment of mild HIV-associated psoriasis.12 Topical corticosteroids, calcipotriol, tazarotene, and formulations combining 2 of these medications form the cornerstone of topical therapies for mild psoriasis in patients with HIV infection. These agents have the advantage of possessing limited and localized effects, making it unlikely for them to increase immunosuppression in patients with HIV infection. They generally can be safely used in patients with HIV infection, and their side-effect profile in patients with HIV infection is similar to the general population.12 However, calcipotriol is the least desirable for use in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4
UV Phototherapy
Topical therapy is limited by its lack of potency; limited field coverage; and the inconvenience of application, particularly in patients with more widespread disease.12 Therefore, UV phototherapy is preferred as first-line treatment of moderate to severe psoriasis. UV phototherapy has been shown to inhibit cell proliferation and inflammation and result in clinical improvement of HIV-associated psoriasis; moreover, most of the reports in the literature support it as an option that will not increase immunocompromise in patients with HIV infection.12
Caution is warranted, however, regarding the immunomodulatory effects of UV therapies, which may result in an increased risk for skin cancer and diminished resistance to infection, which can be of particular concern in immunocompromised patients who are already at risk.7,13,14 In patients who are candidates for phototherapy, HIV serology and close monitoring of viral load and CD4 lymphocyte count before treatment, at monthly interludes throughout treatment, and 3 months following the cessation of treatment have been recommended.7,15 Careful consideration of the risk-benefit ratio of phototherapy for individual patients, including the patient’s stage of HIV disease, the degree of discomfort, disfigurement, and disability caused by the psoriasis (or other dermatologic condition), as well as the availability of alternative treatment options is essential.7,16

Systemic Agents
In patients who are intolerant of or unresponsive to antiretroviral therapy, topical therapies, and phototherapy, traditional systemic agents may be considered,12 including acitretin, methotrexate, and cyclosporine. However, updated guidelines indicate that methotrexate and cyclosporine should be avoided in this population given the risk for increased immunosuppression with these agents.4,17
Oral retinoids, such as acitretin, continue to be important options for second-line psoriasis treatment in patients with comorbid HIV infection, either as monotherapy or in association with phototherapy.3 Acitretin has the notable benefit of not causing or worsening immune compromise; however, its use is less than desirable in patients with hypertriglyceridemia, which can be a side effect of antiretroviral drugs.4,12 Providers also must be aware of the possible association between acitretin (and other antiretrovirals) and pancreatitis, remaining vigilant in monitoring patients for this adverse effect.3
Biologics
The relatively recent addition of cytokine-suppressive biologic agents to the treatment armamentarium has transformed the management of psoriasis in otherwise healthy individuals. These agents have been shown to possess an excellent safety and efficacy profile.12 However, their use in patients with HIV infection has been mired in concerns regarding a potential increase in the risk for opportunistic infections, sepsis, and HIV disease progression in this patient population.7,12
Case reports have detailed the safe treatment of recalcitrant HIV-associated psoriasis with tumor necrosis factor (TNF) blockers, such as etanercept.7,12 In most of these case reports, no harm to CD4 lymphocyte counts, serum viral loads, overall immune status, and susceptibility to infection have been noted; on the contrary, CD4 count increased in most patients following treatment with biologic agents.12 Because patients with HIV infection tend to be excluded from clinical trials, anecdotal evidence derived from case reports and case series often provides clinically relevant information and often forms the basis for treatment recommendations in this patient population.12 Indeed, in the wake of positive case reports, TNF-α inhibitors are now recommended for highly selected patients with refractory chronic psoriatic disease, including those with incapacitating joint pain.7,18
When TNF-α inhibitors are used in patients with HIV infection and psoriasis, optimal antiretroviral therapy and exceedingly close monitoring of clinical and laboratory parameters are of the utmost importance; Pneumocystis jiroveci prophylaxis also is recommended in patients with low CD4 counts.7,18
In 2014, the oral phosphodiesterase 4 inhibitor apremilast was approved for the treatment of moderate to severe plaque psoriasis and PsA. Recent case reports have described its successful use in patients with HIV infection and psoriasis, including the case reported herein, with no reports of opportunistic infections.4,19 Furthermore, HIV infection is not listed as a contraindication on its label.20
Apremilast is thought to increase intracellular cyclic adenosine monophosphate, thereby helping to attain improved homeostasis between proinflammatory and anti-inflammatory mediators.4,19 Several of the proinflammatory mediators that are indirectly targeted by apremilast, including TNF-α and IL-23, are explicitly inhibited by other biologics. It is this equilibrium between proinflammatory and anti-inflammatory mediators that most markedly differentiates apremilast from most other available biologic therapies for psoriasis, which typically have a specific proinflammatory target.4,21 As with other systemic therapies, close monitoring of CD4 levels and viral loads, as well as use of relevant prophylactic agents, is essential when apremilast is used in the setting of HIV infection, making coordination with infectious disease specialists essential.19

Bottom Line
Management of psoriasis in patients with HIV infection represents a clinical challenge. Case reports suggest a role for apremilast as an adjuvant to first-line therapy such as UV phototherapy in the setting of HIV infection in a patient with moderate to severe psoriasis, but close monitoring of CD4 count and viral load in these patients is needed in collaboration with infectious disease specialists. Updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population are needed.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
A 50-year-old man with Fitzpatrick skin type IV presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. The patient’s medical history was positive for human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected), for which clobetasol spray and calcitriol ointment had been prescribed. The patient’s CD4 count was 460 at presentation, and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to presentation. For the last 5 months, the patient had been undergoing phototherapy 3 times weekly for treatment of psoriasis.
An apremilast starter pack was initiated with the dosage titrated from 10 mg to 30 mg over the course of 1 week. The patient was maintained on a dose of 30 mg twice daily after 1 week, while continuing clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the patient’s affected BSA was 0%. Apremilast was continued, and phototherapy was reduced to once weekly. After 7 months of concomitant treatment with apremilast, phototherapy was discontinued after clearance was maintained. Phototherapy was reinitiated twice weekly after a mild flare (3% BSA affected).
The patient continued apremilast for a total of 20 months until it became cost prohibitive. After discontinuing apremilast for 4 months, he presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with intention to apply for an apremilast financial assistance program.
This case was adapted from Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7
Apremilast and Phototherapy for Treatment of Psoriasis in a Patient With Human Immunodeficiency Virus
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
Practice Point
- Apremilast may be considered as a first-line therapy in the human immunodeficiency virus population due to decreased immunosuppression.
Risk for Appendicitis, Cholecystitis, or Diverticulitis in Patients With Psoriasis
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
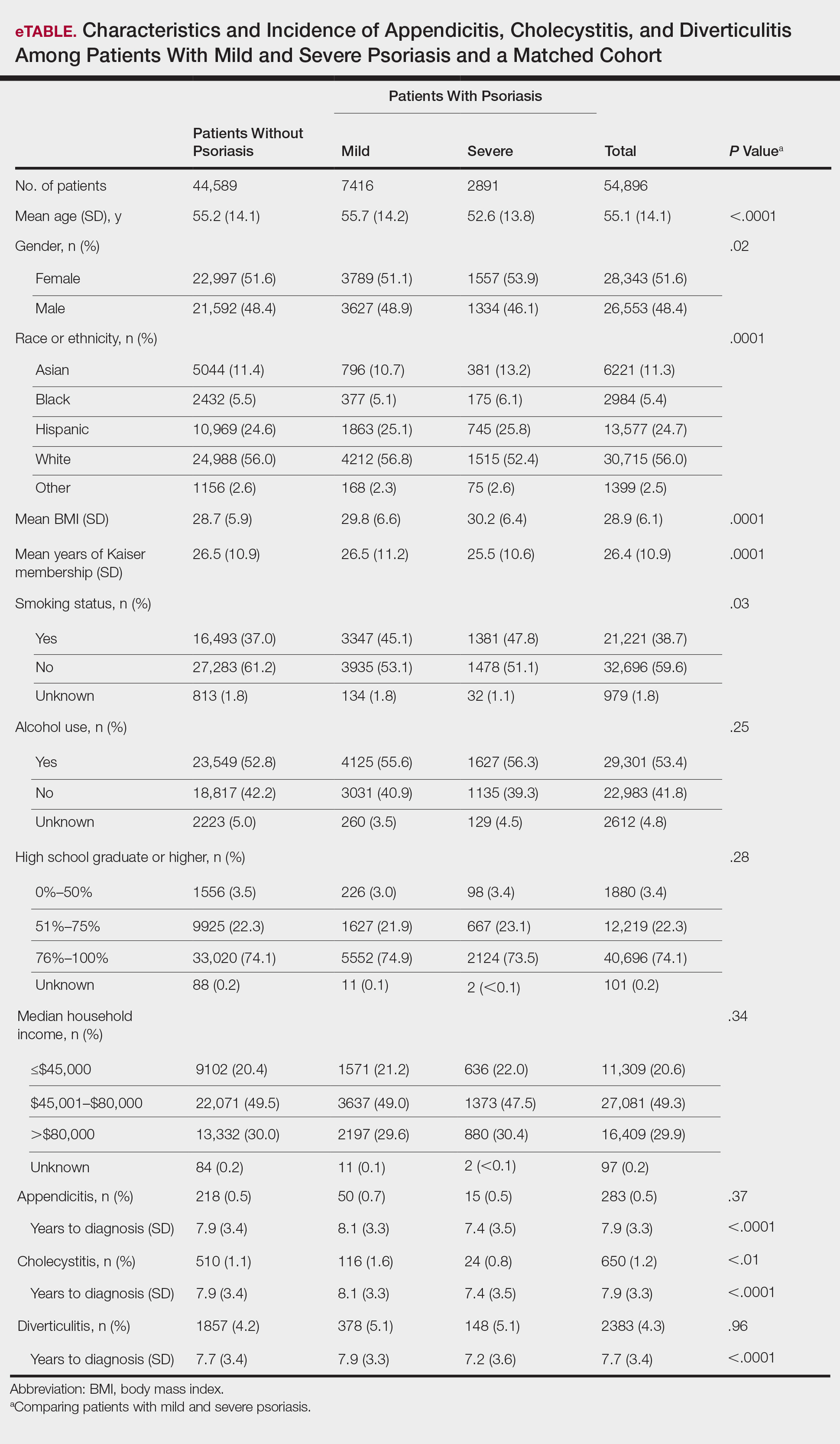
Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
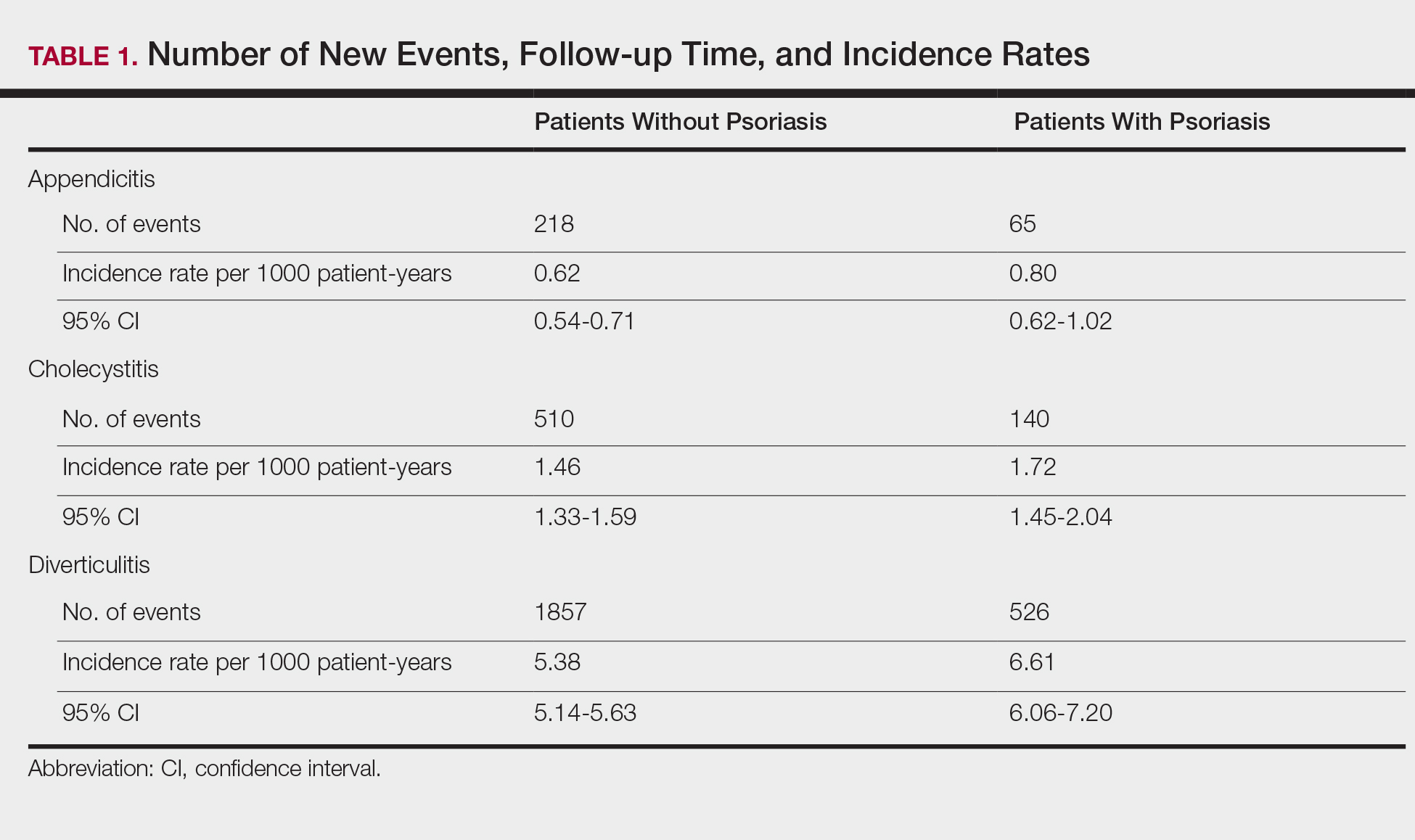
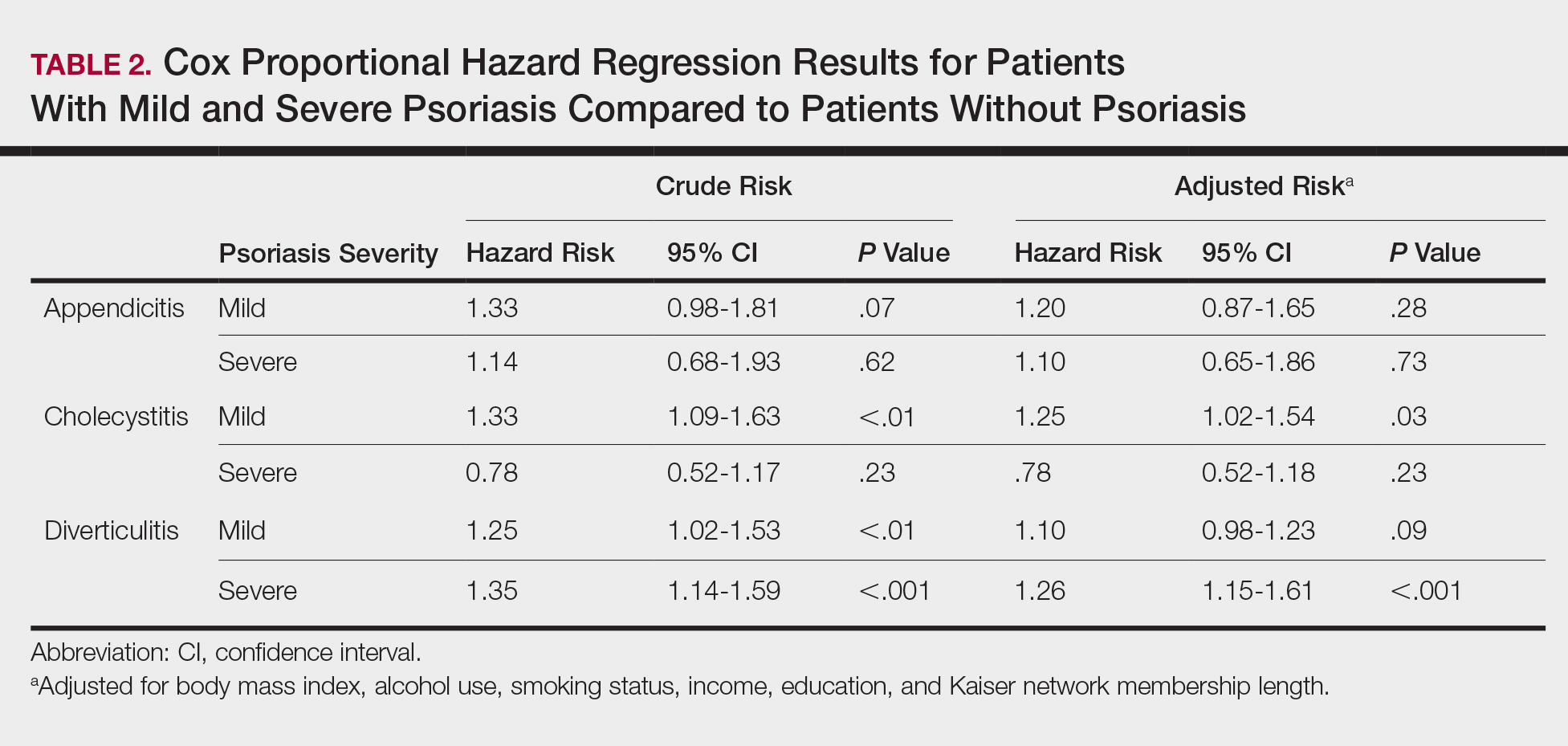
Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.

Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).


Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).

Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.

Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).


Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).

Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Practice Points
- Patients with psoriasis may have elevated risk of diverticulitis compared to healthy patients. However, psoriasis patients do not appear to have increased risk of appendicitis or cholecystitis.
- Clinicians treating psoriasis patients should consider assessing for other risk factors of diverticulitis at regular intervals.
Clearance of Psoriasis After Ischemic Stroke
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
Practice Points
- Psoriasis is exacerbated in the presence of stress, and psoriatic lesions often have a symmetric distribution, which is evidence that the nervous system is involved in the pathophysiology of the condition.
- Various neuropeptides are involved in the pathophysiology of psoriasis, including substance P, nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide.
- Peripheral nerve damage results in decreased secretion of neuropeptides, which can lead to remission of psoriasis.