User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
IBS: Understanding a Common Yet Misunderstood Condition
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
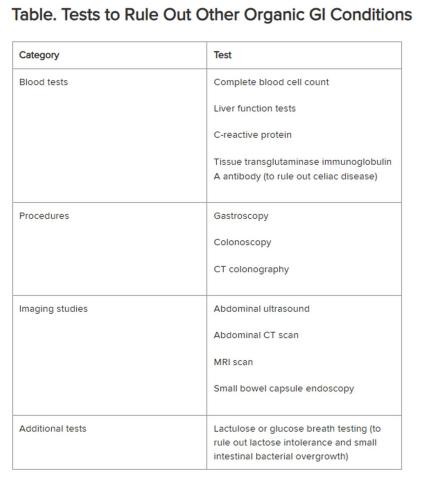
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
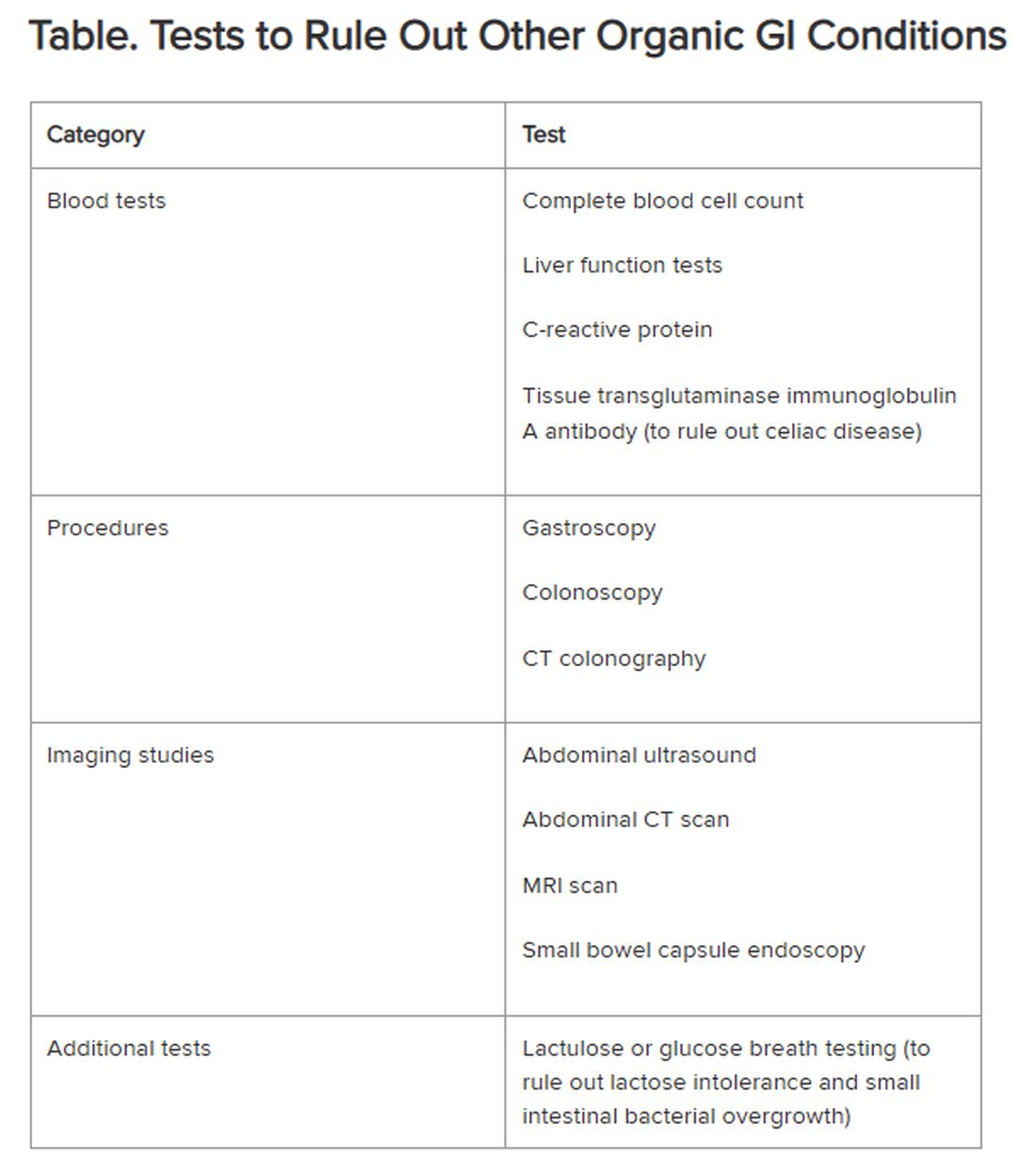
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Coming Soon: A New Disease Definition, ‘Clinical Obesity’
SAN ANTONIO, TEXAS —
The authors of the new framework are a Lancet Commission of 56 of the world’s leading obesity experts, including academic clinicians, scientists, public health experts, patient representatives, and officers from the World Health Organization. Following peer review, it will be launched via livestream and published in Lancet Diabetes & Endocrinology in mid-January 2025, with formal endorsement from more than 75 medical societies and other relevant stakeholder organizations.
On November 4, 2024, at the Obesity Society’s Obesity Week meeting, the publication’s lead author, Francesco Rubino, MD, Chair of Bariatric and Metabolic Surgery at King’s College London in England, gave a preview. He began by noting that, despite the declaration of obesity as a chronic disease over a decade ago, the concept is still debated and not widely accepted by the public or even by all in the medical community.
“The idea of obesity as a disease remains highly controversial,” Rubino noted, adding that the current body mass index (BMI)–based definition contributes to this because it doesn’t distinguish between people whose excess adiposity place them at excess risk for disease but they’re currently healthy vs those who already have undergone bodily harm from that adiposity.
“Having a framework that distinguishes at an individual level when you are in a condition of risk and when you have a condition of disease is fundamentally important. You don’t want to blur the picture in either direction, because obviously the consequence would be quite significant. ... So, the commission focused exactly on that point,” he said.
The new paper will propose a two-part clinical approach: First, assess whether the patient has excess adiposity, with methods that will be outlined. Next, assess on an organ-by-organ basis for the presence of abnormalities related to excess adiposity, or “clinical obesity.” The document will also provide those specific criteria, Rubino said, noting that those details are under embargo until January.
However, he did say that “We are going to propose a pragmatic approach to say that BMI alone is not enough in the clinic. It’s okay as a screening tool, but when somebody potentially has obesity, then you have to add additional measures of adiposity that makes sure you decrease the level of risk… Once you have obesity, then you need to establish if it’s clinical or nonclinical.”
Asked to comment, session moderator John D. Clark, MD, PhD, Chief Population Health Officer at Sharp Rees-Stealy Medical Group, San Diego, California, said in an interview, “I think it’ll help explain and move medicine as a whole in a direction to a greater understanding of obesity actually being a disease, how to define it, and how to identify it. And will, I think, lead to a greater understanding of the underlying disease.”
And, Clark said, it should also help target individuals with preventive vs therapeutic approaches. “I would describe it as matching the right tool to the right patient. If a person has clinical obesity, they likely can and would benefit from either different or additional tools, as opposed to otherwise healthy obesity.”
Rubino said he hopes the new framework will prompt improvements in reimbursement and public policy. “Policymakers scratch their heads when they have limited resources and you need to prioritize things. Having an obesity definition that is blurry doesn’t allow you to have a fair, human, and meaningful prioritization. ... Now that we have drugs that cannot be given to 100% of people, how do you decide who gets them first? I hope this will make it easier for people to access treatment. At the moment, it is not only difficult, but it’s also unfair. It’s random. Somebody gets access, while somebody else who is very, very sick has no access. I don’t think that’s what we want.”
A version of this article appeared on Medscape.com.
SAN ANTONIO, TEXAS —
The authors of the new framework are a Lancet Commission of 56 of the world’s leading obesity experts, including academic clinicians, scientists, public health experts, patient representatives, and officers from the World Health Organization. Following peer review, it will be launched via livestream and published in Lancet Diabetes & Endocrinology in mid-January 2025, with formal endorsement from more than 75 medical societies and other relevant stakeholder organizations.
On November 4, 2024, at the Obesity Society’s Obesity Week meeting, the publication’s lead author, Francesco Rubino, MD, Chair of Bariatric and Metabolic Surgery at King’s College London in England, gave a preview. He began by noting that, despite the declaration of obesity as a chronic disease over a decade ago, the concept is still debated and not widely accepted by the public or even by all in the medical community.
“The idea of obesity as a disease remains highly controversial,” Rubino noted, adding that the current body mass index (BMI)–based definition contributes to this because it doesn’t distinguish between people whose excess adiposity place them at excess risk for disease but they’re currently healthy vs those who already have undergone bodily harm from that adiposity.
“Having a framework that distinguishes at an individual level when you are in a condition of risk and when you have a condition of disease is fundamentally important. You don’t want to blur the picture in either direction, because obviously the consequence would be quite significant. ... So, the commission focused exactly on that point,” he said.
The new paper will propose a two-part clinical approach: First, assess whether the patient has excess adiposity, with methods that will be outlined. Next, assess on an organ-by-organ basis for the presence of abnormalities related to excess adiposity, or “clinical obesity.” The document will also provide those specific criteria, Rubino said, noting that those details are under embargo until January.
However, he did say that “We are going to propose a pragmatic approach to say that BMI alone is not enough in the clinic. It’s okay as a screening tool, but when somebody potentially has obesity, then you have to add additional measures of adiposity that makes sure you decrease the level of risk… Once you have obesity, then you need to establish if it’s clinical or nonclinical.”
Asked to comment, session moderator John D. Clark, MD, PhD, Chief Population Health Officer at Sharp Rees-Stealy Medical Group, San Diego, California, said in an interview, “I think it’ll help explain and move medicine as a whole in a direction to a greater understanding of obesity actually being a disease, how to define it, and how to identify it. And will, I think, lead to a greater understanding of the underlying disease.”
And, Clark said, it should also help target individuals with preventive vs therapeutic approaches. “I would describe it as matching the right tool to the right patient. If a person has clinical obesity, they likely can and would benefit from either different or additional tools, as opposed to otherwise healthy obesity.”
Rubino said he hopes the new framework will prompt improvements in reimbursement and public policy. “Policymakers scratch their heads when they have limited resources and you need to prioritize things. Having an obesity definition that is blurry doesn’t allow you to have a fair, human, and meaningful prioritization. ... Now that we have drugs that cannot be given to 100% of people, how do you decide who gets them first? I hope this will make it easier for people to access treatment. At the moment, it is not only difficult, but it’s also unfair. It’s random. Somebody gets access, while somebody else who is very, very sick has no access. I don’t think that’s what we want.”
A version of this article appeared on Medscape.com.
SAN ANTONIO, TEXAS —
The authors of the new framework are a Lancet Commission of 56 of the world’s leading obesity experts, including academic clinicians, scientists, public health experts, patient representatives, and officers from the World Health Organization. Following peer review, it will be launched via livestream and published in Lancet Diabetes & Endocrinology in mid-January 2025, with formal endorsement from more than 75 medical societies and other relevant stakeholder organizations.
On November 4, 2024, at the Obesity Society’s Obesity Week meeting, the publication’s lead author, Francesco Rubino, MD, Chair of Bariatric and Metabolic Surgery at King’s College London in England, gave a preview. He began by noting that, despite the declaration of obesity as a chronic disease over a decade ago, the concept is still debated and not widely accepted by the public or even by all in the medical community.
“The idea of obesity as a disease remains highly controversial,” Rubino noted, adding that the current body mass index (BMI)–based definition contributes to this because it doesn’t distinguish between people whose excess adiposity place them at excess risk for disease but they’re currently healthy vs those who already have undergone bodily harm from that adiposity.
“Having a framework that distinguishes at an individual level when you are in a condition of risk and when you have a condition of disease is fundamentally important. You don’t want to blur the picture in either direction, because obviously the consequence would be quite significant. ... So, the commission focused exactly on that point,” he said.
The new paper will propose a two-part clinical approach: First, assess whether the patient has excess adiposity, with methods that will be outlined. Next, assess on an organ-by-organ basis for the presence of abnormalities related to excess adiposity, or “clinical obesity.” The document will also provide those specific criteria, Rubino said, noting that those details are under embargo until January.
However, he did say that “We are going to propose a pragmatic approach to say that BMI alone is not enough in the clinic. It’s okay as a screening tool, but when somebody potentially has obesity, then you have to add additional measures of adiposity that makes sure you decrease the level of risk… Once you have obesity, then you need to establish if it’s clinical or nonclinical.”
Asked to comment, session moderator John D. Clark, MD, PhD, Chief Population Health Officer at Sharp Rees-Stealy Medical Group, San Diego, California, said in an interview, “I think it’ll help explain and move medicine as a whole in a direction to a greater understanding of obesity actually being a disease, how to define it, and how to identify it. And will, I think, lead to a greater understanding of the underlying disease.”
And, Clark said, it should also help target individuals with preventive vs therapeutic approaches. “I would describe it as matching the right tool to the right patient. If a person has clinical obesity, they likely can and would benefit from either different or additional tools, as opposed to otherwise healthy obesity.”
Rubino said he hopes the new framework will prompt improvements in reimbursement and public policy. “Policymakers scratch their heads when they have limited resources and you need to prioritize things. Having an obesity definition that is blurry doesn’t allow you to have a fair, human, and meaningful prioritization. ... Now that we have drugs that cannot be given to 100% of people, how do you decide who gets them first? I hope this will make it easier for people to access treatment. At the moment, it is not only difficult, but it’s also unfair. It’s random. Somebody gets access, while somebody else who is very, very sick has no access. I don’t think that’s what we want.”
A version of this article appeared on Medscape.com.
FROM OBESITY WEEK
Men Wanted: New Efforts to Attract Male Nurses
Only 12% of the nurses providing patient care at hospitals and health clinics today are men. Although the percentage of nurses has increased — men made up just 2.7% of nurses in 1970 — nursing is still considered a “pink collar” profession, a female-dominated field.
“We’ve made strides over the last couple of decades, but [the number of men pursuing nursing careers] is leveling out,” said Jason Dunne, DNP, MSN, RN, chief academic officer at the Arizona College of Nursing, Phoenix. “There continues to be persistent gender stereotypes that [have] discouraged men from entering the profession.”
“The nursing shortage is very real,” Dunne said. “We need to be highly focused on the shortage and look at opportunities to bring diversity into the profession, and one big way to solve it is bringing more men into nursing.”
Representation Matters
Colleges recognize the need to diversify their nursing student population and have turned their attention to increasing the number of men attending informational sessions and career days. Dunne believes, “There is a general lack of awareness of nursing as a career choice [for men].”
The Nursing Consortium of Florida hosts a “Day in the Life of a Nurse” program to introduce high school students to nursing careers, and the University of Virginia School of Nursing invites male nursing students to speak at educational events to promote workforce diversity.
“When I was growing up, the males wouldn’t have been included in those sessions,” said Melissa Gilbert Gomes, PhD, APRN, PMHNP-BC, FNAP, FAAN, associate dean for diversity, equity, and inclusion at the University of Virginia School of Nursing, Charlottesville, Virginia. “It was nice to see their interest and to have a male student there for them to ask questions and to help them see that this could be a place for them.”
Nursing schools have also engaged in other efforts to encourage more men to consider nursing careers, from highlighting male nurses in marketing materials and engaging with men at career fairs to updating course curriculum to include content on men’s health and connecting male nursing students with men in nursing faculty or clinical settings.
Focusing on nursing as a lucrative career choice could also attract more men to the profession. On average, male registered nurses (RNs) make $7300 per year more than their female counterparts due to the gender pay gap. The median wage for male RNs in acute care, cardiology, and perioperative specialties is $90,000 annually.
At the University of Virginia School of Nursing, which the American Association for Men in Nursing (AAMN) named “Best School for Men in Nursing” in 2023, 20% of nursing students are men.
The school has a Men Advancing Nursing club and is in the process of chartering a new AAMN chapter. The goal, according to Gomes, is to create an environment where male nursing students feel represented and supported.
“Valuing the perspective that men bring [to nursing] is important,” she said. “Coming together [and] having that camaraderie and intrinsic motivation to specifically speak to areas that impact men ... is important.”
Promoting Patient Care
Highlighting the diversity of career options within the nursing profession is also essential. RNs can pursue careers in specialties ranging from pediatrics, orthopedics, and occupational health to anesthesia, cardiology, and nephrology. The specialty with the highest number of male RNs tends to be acute care, which encompasses emergency/trauma and medical-surgical.
John Schmidt, DNP, MSN, BSN, faculty member and program lead for the acute care nurse practitioner program at Purdue Global School of Nursing, refers to these specialties as having a high excitement factor.
“Men gravitate to nursing to help people,” he said. “In critical care, there is instant gratification. You see patients get better. It’s the same in the [intensive care unit] and the emergency department. We take care of them and can see how we made a difference.”
When hospitals and health systems create environments that support men in nursing, patients also benefit. Research shows that patients often prefer nurses of the same gender, and a more diverse healthcare workforce has been linked to improved patient outcomes. Reducing gender inequities among nursing staff could also improve job satisfaction and retention rates for men in nursing.
“When you’re in a vulnerable space as a patient ... it’s important to know that your care provider understands you [and] having men as nurses is a part of that,” said Gomes. “Even though patients might not be used to having a male nurse at the bedside, once they have the experience, it challenges preconceived notions [and] that connection is important.”
Hospitals must proactively support men in nursing to achieve the benefits of greater gender diversity in the nursing workforce. Male nurses have fewer role models and report higher levels of loneliness, isolation, and role strain.
Groups such as NYC Men in Nursing and mentorship programs such as Men in Nursing at RUSH University College of Nursing and RUSH University Medical Center, and the North Carolina Healthcare Association Diverse Healthcare Leaders Mentorship Program were designed to provide coaching, education, and networking opportunities and connect men in nursing.
Male nurses, Dunne added, must be role models and must take the lead in changing the conversations about gender roles in nursing. Establishing support systems and mentorship opportunities is instrumental in inspiring men to pursue nursing careers and creating visibility into the profession and “would create a level of parity for men in the profession and encourage them to want to stay in nursing as a long-term career.”
He told this news organization that creating scholarships for men enrolled in nursing school, increasing the involvement of male nurse leaders in recruitment efforts, and updating curriculum to ensure men are reflected in the materials is also essential.
“We’ve got to be willing and open to having the conversations to end the stereotypes that have plagued the profession,” said Dunne. “And we’ve got to push men in nursing to be front and center so folks see that there are opportunities for men in nursing.”
A version of this article appeared on Medscape.com.
Only 12% of the nurses providing patient care at hospitals and health clinics today are men. Although the percentage of nurses has increased — men made up just 2.7% of nurses in 1970 — nursing is still considered a “pink collar” profession, a female-dominated field.
“We’ve made strides over the last couple of decades, but [the number of men pursuing nursing careers] is leveling out,” said Jason Dunne, DNP, MSN, RN, chief academic officer at the Arizona College of Nursing, Phoenix. “There continues to be persistent gender stereotypes that [have] discouraged men from entering the profession.”
“The nursing shortage is very real,” Dunne said. “We need to be highly focused on the shortage and look at opportunities to bring diversity into the profession, and one big way to solve it is bringing more men into nursing.”
Representation Matters
Colleges recognize the need to diversify their nursing student population and have turned their attention to increasing the number of men attending informational sessions and career days. Dunne believes, “There is a general lack of awareness of nursing as a career choice [for men].”
The Nursing Consortium of Florida hosts a “Day in the Life of a Nurse” program to introduce high school students to nursing careers, and the University of Virginia School of Nursing invites male nursing students to speak at educational events to promote workforce diversity.
“When I was growing up, the males wouldn’t have been included in those sessions,” said Melissa Gilbert Gomes, PhD, APRN, PMHNP-BC, FNAP, FAAN, associate dean for diversity, equity, and inclusion at the University of Virginia School of Nursing, Charlottesville, Virginia. “It was nice to see their interest and to have a male student there for them to ask questions and to help them see that this could be a place for them.”
Nursing schools have also engaged in other efforts to encourage more men to consider nursing careers, from highlighting male nurses in marketing materials and engaging with men at career fairs to updating course curriculum to include content on men’s health and connecting male nursing students with men in nursing faculty or clinical settings.
Focusing on nursing as a lucrative career choice could also attract more men to the profession. On average, male registered nurses (RNs) make $7300 per year more than their female counterparts due to the gender pay gap. The median wage for male RNs in acute care, cardiology, and perioperative specialties is $90,000 annually.
At the University of Virginia School of Nursing, which the American Association for Men in Nursing (AAMN) named “Best School for Men in Nursing” in 2023, 20% of nursing students are men.
The school has a Men Advancing Nursing club and is in the process of chartering a new AAMN chapter. The goal, according to Gomes, is to create an environment where male nursing students feel represented and supported.
“Valuing the perspective that men bring [to nursing] is important,” she said. “Coming together [and] having that camaraderie and intrinsic motivation to specifically speak to areas that impact men ... is important.”
Promoting Patient Care
Highlighting the diversity of career options within the nursing profession is also essential. RNs can pursue careers in specialties ranging from pediatrics, orthopedics, and occupational health to anesthesia, cardiology, and nephrology. The specialty with the highest number of male RNs tends to be acute care, which encompasses emergency/trauma and medical-surgical.
John Schmidt, DNP, MSN, BSN, faculty member and program lead for the acute care nurse practitioner program at Purdue Global School of Nursing, refers to these specialties as having a high excitement factor.
“Men gravitate to nursing to help people,” he said. “In critical care, there is instant gratification. You see patients get better. It’s the same in the [intensive care unit] and the emergency department. We take care of them and can see how we made a difference.”
When hospitals and health systems create environments that support men in nursing, patients also benefit. Research shows that patients often prefer nurses of the same gender, and a more diverse healthcare workforce has been linked to improved patient outcomes. Reducing gender inequities among nursing staff could also improve job satisfaction and retention rates for men in nursing.
“When you’re in a vulnerable space as a patient ... it’s important to know that your care provider understands you [and] having men as nurses is a part of that,” said Gomes. “Even though patients might not be used to having a male nurse at the bedside, once they have the experience, it challenges preconceived notions [and] that connection is important.”
Hospitals must proactively support men in nursing to achieve the benefits of greater gender diversity in the nursing workforce. Male nurses have fewer role models and report higher levels of loneliness, isolation, and role strain.
Groups such as NYC Men in Nursing and mentorship programs such as Men in Nursing at RUSH University College of Nursing and RUSH University Medical Center, and the North Carolina Healthcare Association Diverse Healthcare Leaders Mentorship Program were designed to provide coaching, education, and networking opportunities and connect men in nursing.
Male nurses, Dunne added, must be role models and must take the lead in changing the conversations about gender roles in nursing. Establishing support systems and mentorship opportunities is instrumental in inspiring men to pursue nursing careers and creating visibility into the profession and “would create a level of parity for men in the profession and encourage them to want to stay in nursing as a long-term career.”
He told this news organization that creating scholarships for men enrolled in nursing school, increasing the involvement of male nurse leaders in recruitment efforts, and updating curriculum to ensure men are reflected in the materials is also essential.
“We’ve got to be willing and open to having the conversations to end the stereotypes that have plagued the profession,” said Dunne. “And we’ve got to push men in nursing to be front and center so folks see that there are opportunities for men in nursing.”
A version of this article appeared on Medscape.com.
Only 12% of the nurses providing patient care at hospitals and health clinics today are men. Although the percentage of nurses has increased — men made up just 2.7% of nurses in 1970 — nursing is still considered a “pink collar” profession, a female-dominated field.
“We’ve made strides over the last couple of decades, but [the number of men pursuing nursing careers] is leveling out,” said Jason Dunne, DNP, MSN, RN, chief academic officer at the Arizona College of Nursing, Phoenix. “There continues to be persistent gender stereotypes that [have] discouraged men from entering the profession.”
“The nursing shortage is very real,” Dunne said. “We need to be highly focused on the shortage and look at opportunities to bring diversity into the profession, and one big way to solve it is bringing more men into nursing.”
Representation Matters
Colleges recognize the need to diversify their nursing student population and have turned their attention to increasing the number of men attending informational sessions and career days. Dunne believes, “There is a general lack of awareness of nursing as a career choice [for men].”
The Nursing Consortium of Florida hosts a “Day in the Life of a Nurse” program to introduce high school students to nursing careers, and the University of Virginia School of Nursing invites male nursing students to speak at educational events to promote workforce diversity.
“When I was growing up, the males wouldn’t have been included in those sessions,” said Melissa Gilbert Gomes, PhD, APRN, PMHNP-BC, FNAP, FAAN, associate dean for diversity, equity, and inclusion at the University of Virginia School of Nursing, Charlottesville, Virginia. “It was nice to see their interest and to have a male student there for them to ask questions and to help them see that this could be a place for them.”
Nursing schools have also engaged in other efforts to encourage more men to consider nursing careers, from highlighting male nurses in marketing materials and engaging with men at career fairs to updating course curriculum to include content on men’s health and connecting male nursing students with men in nursing faculty or clinical settings.
Focusing on nursing as a lucrative career choice could also attract more men to the profession. On average, male registered nurses (RNs) make $7300 per year more than their female counterparts due to the gender pay gap. The median wage for male RNs in acute care, cardiology, and perioperative specialties is $90,000 annually.
At the University of Virginia School of Nursing, which the American Association for Men in Nursing (AAMN) named “Best School for Men in Nursing” in 2023, 20% of nursing students are men.
The school has a Men Advancing Nursing club and is in the process of chartering a new AAMN chapter. The goal, according to Gomes, is to create an environment where male nursing students feel represented and supported.
“Valuing the perspective that men bring [to nursing] is important,” she said. “Coming together [and] having that camaraderie and intrinsic motivation to specifically speak to areas that impact men ... is important.”
Promoting Patient Care
Highlighting the diversity of career options within the nursing profession is also essential. RNs can pursue careers in specialties ranging from pediatrics, orthopedics, and occupational health to anesthesia, cardiology, and nephrology. The specialty with the highest number of male RNs tends to be acute care, which encompasses emergency/trauma and medical-surgical.
John Schmidt, DNP, MSN, BSN, faculty member and program lead for the acute care nurse practitioner program at Purdue Global School of Nursing, refers to these specialties as having a high excitement factor.
“Men gravitate to nursing to help people,” he said. “In critical care, there is instant gratification. You see patients get better. It’s the same in the [intensive care unit] and the emergency department. We take care of them and can see how we made a difference.”
When hospitals and health systems create environments that support men in nursing, patients also benefit. Research shows that patients often prefer nurses of the same gender, and a more diverse healthcare workforce has been linked to improved patient outcomes. Reducing gender inequities among nursing staff could also improve job satisfaction and retention rates for men in nursing.
“When you’re in a vulnerable space as a patient ... it’s important to know that your care provider understands you [and] having men as nurses is a part of that,” said Gomes. “Even though patients might not be used to having a male nurse at the bedside, once they have the experience, it challenges preconceived notions [and] that connection is important.”
Hospitals must proactively support men in nursing to achieve the benefits of greater gender diversity in the nursing workforce. Male nurses have fewer role models and report higher levels of loneliness, isolation, and role strain.
Groups such as NYC Men in Nursing and mentorship programs such as Men in Nursing at RUSH University College of Nursing and RUSH University Medical Center, and the North Carolina Healthcare Association Diverse Healthcare Leaders Mentorship Program were designed to provide coaching, education, and networking opportunities and connect men in nursing.
Male nurses, Dunne added, must be role models and must take the lead in changing the conversations about gender roles in nursing. Establishing support systems and mentorship opportunities is instrumental in inspiring men to pursue nursing careers and creating visibility into the profession and “would create a level of parity for men in the profession and encourage them to want to stay in nursing as a long-term career.”
He told this news organization that creating scholarships for men enrolled in nursing school, increasing the involvement of male nurse leaders in recruitment efforts, and updating curriculum to ensure men are reflected in the materials is also essential.
“We’ve got to be willing and open to having the conversations to end the stereotypes that have plagued the profession,” said Dunne. “And we’ve got to push men in nursing to be front and center so folks see that there are opportunities for men in nursing.”
A version of this article appeared on Medscape.com.
Lawmakers Rush to Stave Off Doctor Pay Cuts as Medicare Finalizes 2025 Rates
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Being a Weekend Warrior Linked to Lower Dementia Risk
TOPLINE:
, a new study shows. Investigators say the findings suggest even limited physical activity may offer protective cognitive benefits.
METHODOLOGY:
- Researchers analyzed the data of 10,033 participants in the Mexico City Prospective Study who were aged 35 years or older.
- Physical activity patterns were categorized into four groups: No exercise, weekend warriors (one or two sessions per week), regularly active (three or more sessions per week), and a combined group.
- Cognitive function was assessed using the Mini-Mental State Examination (MMSE).
- The analysis adjusted for confounders such as age, sex, education, income, blood pressure, smoking status, body mass index, civil status, sleep duration, diet, and alcohol intake.
- The mean follow-up duration was 16 years.
TAKEAWAY:
- When mild dementia was defined as an MMSE score ≤ 22, dementia prevalence was 26% in those who did not exercise, 14% in weekend warriors, and 18.5% in the regularly active group.
- When mild dementia was defined as an MMSE score ≤ 23, dementia prevalence was 30% in those who did not exercise, 20% in weekend warriors, and 22% in the regularly active group.
- Compared with people who did not exercise and after adjusting for confounding factors, risk for mild dementia was 13%-25% lower in weekend warriors, 11%-12% lower in the regular activity group, and 12%-16% lower in the two groups combined.
- The findings were consistent in men and women.
IN PRACTICE:
“To the best of our knowledge, this is the first prospective cohort study to show that the weekend warrior physical activity pattern and the regularly active physical activity pattern are associated with similar reductions in the risk of mild dementia. This study has important implications for policy and practice because the weekend warrior physical activity pattern may be a more convenient option for busy people around the world,” the authors wrote.
SOURCE:
The study was led by Gary O’Donovan, Faculty of Medicine, University of the Andes, Bogotá, Colombia. It was published online in the British Journal of Sports Medicine.
LIMITATIONS:
The survey respondents may not have been truly representative of middle-aged adults. Further, there were no objective measures of physical activity. The observational nature of the study does not provide insights into causality.
DISCLOSURES:
The study was funded by the Mexican Health Ministry, the National Council of Science and Technology for Mexico, Wellcome, and the UK Medical Research Council. No conflicts of interest were disclosed.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study shows. Investigators say the findings suggest even limited physical activity may offer protective cognitive benefits.
METHODOLOGY:
- Researchers analyzed the data of 10,033 participants in the Mexico City Prospective Study who were aged 35 years or older.
- Physical activity patterns were categorized into four groups: No exercise, weekend warriors (one or two sessions per week), regularly active (three or more sessions per week), and a combined group.
- Cognitive function was assessed using the Mini-Mental State Examination (MMSE).
- The analysis adjusted for confounders such as age, sex, education, income, blood pressure, smoking status, body mass index, civil status, sleep duration, diet, and alcohol intake.
- The mean follow-up duration was 16 years.
TAKEAWAY:
- When mild dementia was defined as an MMSE score ≤ 22, dementia prevalence was 26% in those who did not exercise, 14% in weekend warriors, and 18.5% in the regularly active group.
- When mild dementia was defined as an MMSE score ≤ 23, dementia prevalence was 30% in those who did not exercise, 20% in weekend warriors, and 22% in the regularly active group.
- Compared with people who did not exercise and after adjusting for confounding factors, risk for mild dementia was 13%-25% lower in weekend warriors, 11%-12% lower in the regular activity group, and 12%-16% lower in the two groups combined.
- The findings were consistent in men and women.
IN PRACTICE:
“To the best of our knowledge, this is the first prospective cohort study to show that the weekend warrior physical activity pattern and the regularly active physical activity pattern are associated with similar reductions in the risk of mild dementia. This study has important implications for policy and practice because the weekend warrior physical activity pattern may be a more convenient option for busy people around the world,” the authors wrote.
SOURCE:
The study was led by Gary O’Donovan, Faculty of Medicine, University of the Andes, Bogotá, Colombia. It was published online in the British Journal of Sports Medicine.
LIMITATIONS:
The survey respondents may not have been truly representative of middle-aged adults. Further, there were no objective measures of physical activity. The observational nature of the study does not provide insights into causality.
DISCLOSURES:
The study was funded by the Mexican Health Ministry, the National Council of Science and Technology for Mexico, Wellcome, and the UK Medical Research Council. No conflicts of interest were disclosed.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study shows. Investigators say the findings suggest even limited physical activity may offer protective cognitive benefits.
METHODOLOGY:
- Researchers analyzed the data of 10,033 participants in the Mexico City Prospective Study who were aged 35 years or older.
- Physical activity patterns were categorized into four groups: No exercise, weekend warriors (one or two sessions per week), regularly active (three or more sessions per week), and a combined group.
- Cognitive function was assessed using the Mini-Mental State Examination (MMSE).
- The analysis adjusted for confounders such as age, sex, education, income, blood pressure, smoking status, body mass index, civil status, sleep duration, diet, and alcohol intake.
- The mean follow-up duration was 16 years.
TAKEAWAY:
- When mild dementia was defined as an MMSE score ≤ 22, dementia prevalence was 26% in those who did not exercise, 14% in weekend warriors, and 18.5% in the regularly active group.
- When mild dementia was defined as an MMSE score ≤ 23, dementia prevalence was 30% in those who did not exercise, 20% in weekend warriors, and 22% in the regularly active group.
- Compared with people who did not exercise and after adjusting for confounding factors, risk for mild dementia was 13%-25% lower in weekend warriors, 11%-12% lower in the regular activity group, and 12%-16% lower in the two groups combined.
- The findings were consistent in men and women.
IN PRACTICE:
“To the best of our knowledge, this is the first prospective cohort study to show that the weekend warrior physical activity pattern and the regularly active physical activity pattern are associated with similar reductions in the risk of mild dementia. This study has important implications for policy and practice because the weekend warrior physical activity pattern may be a more convenient option for busy people around the world,” the authors wrote.
SOURCE:
The study was led by Gary O’Donovan, Faculty of Medicine, University of the Andes, Bogotá, Colombia. It was published online in the British Journal of Sports Medicine.
LIMITATIONS:
The survey respondents may not have been truly representative of middle-aged adults. Further, there were no objective measures of physical activity. The observational nature of the study does not provide insights into causality.
DISCLOSURES:
The study was funded by the Mexican Health Ministry, the National Council of Science and Technology for Mexico, Wellcome, and the UK Medical Research Council. No conflicts of interest were disclosed.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Can We Repurpose Obesity Drugs to Reverse Liver Disease?
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Anaphylaxis Treatment Uncertainty Persists for Patients and Professionals
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Gardasil 9 at 10 Years: Vaccine Protects Against Multiple Cancers
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Cannabis Use Linked to Brain Thinning in Adolescents
, research in mice and humans suggested.
The multilevel study demonstrated that tetrahydrocannabinol (THC), an active substance in cannabis, causes shrinkage of dendritic arborization — the neurons’ network of antennae that play a critical role in communication between brain cells.
The connection between dendritic arborization and cortical thickness was hinted at in an earlier study by Tomáš Paus, MD, PhD, professor of psychiatry and addictology at the University of Montreal, Quebec, Canada, and colleagues, who found that cannabis use in early adolescence was associated with lower cortical thickness in boys with a high genetic risk for schizophrenia.
“We speculated at that time that the differences in cortical thickness might be related to differences in dendritic arborization, and our current study confirmed it,” Paus said.
That confirmation came in the mouse part of the study, when coauthor Graciela Piñeyro, MD, PhD, also of the University of Montreal, counted the dendritic branches of mice exposed to THC and compared the total with the number of dendritic branches in unexposed mice. “What surprised me was finding that THC in the mice was targeting the same type of cells and structures that Dr. Paus had predicted would be affected from the human studies,” she said. “Structurally, they were mostly the neurons that contribute to synapses in the cortex, and their branching was reduced.”
Paus explained that in humans, a decrease in input from the affected dendrites “makes it harder for the brain to learn new things, interact with people, cope with new situations, et cetera. In other words, it makes the brain more vulnerable to everything that can happen in a young person’s life.”
The study was published online on October 9 in the Journal of Neuroscience.
Of Mice, Men, and Cannabis
Although associations between cannabis use by teenagers and variations in brain maturation have been well studied, the cellular and molecular underpinnings of these associations were unclear, according to the authors.
To investigate further, they conducted this three-step study. First, they exposed adolescent male mice to THC or a synthetic cannabinoid (WIN 55,212-2) and assessed differentially expressed genes, spine numbers, and the extent of dendritic complexity in the frontal cortex of each mouse.
Next, using MRI, they examined differences in cortical thickness in 34 brain regions in 140 male adolescents who experimented with cannabis before age 16 years and 327 who did not.
Then, they again conducted experiments in mice and found that 13 THC-related genes correlated with variations in cortical thickness. Virtual histology revealed that these 13 genes were coexpressed with cell markers of astrocytes, microglia, and a type of pyramidal cell enriched in genes that regulate dendritic expression.
Similarly, the WIN-related genes correlated with differences in cortical thickness and showed coexpression patterns with the same three cell types.
Furthermore, the affected genes were also found in humans, particularly in the thinner cortical regions of the adolescents who experimented with cannabis.
By acting on microglia, THC seems to promote the removal of synapses and, eventually, the reduction of the dendritic tree in mice, Piñeyro explained. That’s important not only because a similar mechanism may be at work in humans but also because “we now might have a model to test different types of cannabis products to see which ones are producing the greatest effect on neurons and therefore greater removal of synapses through the microglia. This could be a way of testing drugs that are out in the street to see which would be the most or least dangerous to the synapses in the brain.”
‘Significant Implications’
Commenting on the study, Yasmin Hurd, PhD, Ward-Coleman chair of translational neuroscience at the Icahn School of Medicine at Mount Sinai and director of the Addiction Institute of Mount Sinai in New York City, said, “These findings are in line with previous results, so they are feasible. This study adds more depth by showing that cortical genes that were differentially altered by adolescent THC correlated with cannabis-related changes in cortical thickness based on human neuroimaging data.” Hurd did not participate in the research.
“The results emphasize that consumption of potent cannabis products during adolescence can impact cortical function, which has significant implications for decision-making and risky behavior as well. It also can increase vulnerability to psychiatric disorders such as schizophrenia.”
Although a mouse model is “not truly the same as the human condition, the fact that the animal model also showed evidence of the morphological changes indicative of reduced cortical thickness, [like] the humans, is strong,” she said.
Additional research could include women and assess potential sex differences, she added.
Ronald Ellis, MD, PhD, an investigator in the Center for Medicinal Cannabis Research at the University of California, San Diego School of Medicine, said, “The findings are plausible and extend prior work showing evidence of increased risk for psychotic disorders later in life in adolescents who use cannabis.” Ellis did not participate in the research.
“Future studies should explore how these findings might vary across different demographic groups, which could provide a more inclusive understanding of how cannabis impacts the brain,” he said. “Additionally, longitudinal studies to track changes in the brain over time could help to establish causal relationships more robustly.
“The take-home message to clinicians at this point is to discuss cannabis use history carefully and confidentially with adolescent patients to better provide advice on its potential risks,” he concluded.
Paus added that he would tell patients, “If you’re going to use cannabis, don’t start early. If you have to, then do so in moderation. And if you have family history of mental illness, be very careful.”
No funding for the study was reported. Paus, Piñeyro, Hurd, and Ellis declared having no relevant financial relationships.
A version of this article appeared on Medscape.com.
, research in mice and humans suggested.
The multilevel study demonstrated that tetrahydrocannabinol (THC), an active substance in cannabis, causes shrinkage of dendritic arborization — the neurons’ network of antennae that play a critical role in communication between brain cells.
The connection between dendritic arborization and cortical thickness was hinted at in an earlier study by Tomáš Paus, MD, PhD, professor of psychiatry and addictology at the University of Montreal, Quebec, Canada, and colleagues, who found that cannabis use in early adolescence was associated with lower cortical thickness in boys with a high genetic risk for schizophrenia.
“We speculated at that time that the differences in cortical thickness might be related to differences in dendritic arborization, and our current study confirmed it,” Paus said.
That confirmation came in the mouse part of the study, when coauthor Graciela Piñeyro, MD, PhD, also of the University of Montreal, counted the dendritic branches of mice exposed to THC and compared the total with the number of dendritic branches in unexposed mice. “What surprised me was finding that THC in the mice was targeting the same type of cells and structures that Dr. Paus had predicted would be affected from the human studies,” she said. “Structurally, they were mostly the neurons that contribute to synapses in the cortex, and their branching was reduced.”
Paus explained that in humans, a decrease in input from the affected dendrites “makes it harder for the brain to learn new things, interact with people, cope with new situations, et cetera. In other words, it makes the brain more vulnerable to everything that can happen in a young person’s life.”
The study was published online on October 9 in the Journal of Neuroscience.
Of Mice, Men, and Cannabis
Although associations between cannabis use by teenagers and variations in brain maturation have been well studied, the cellular and molecular underpinnings of these associations were unclear, according to the authors.
To investigate further, they conducted this three-step study. First, they exposed adolescent male mice to THC or a synthetic cannabinoid (WIN 55,212-2) and assessed differentially expressed genes, spine numbers, and the extent of dendritic complexity in the frontal cortex of each mouse.
Next, using MRI, they examined differences in cortical thickness in 34 brain regions in 140 male adolescents who experimented with cannabis before age 16 years and 327 who did not.
Then, they again conducted experiments in mice and found that 13 THC-related genes correlated with variations in cortical thickness. Virtual histology revealed that these 13 genes were coexpressed with cell markers of astrocytes, microglia, and a type of pyramidal cell enriched in genes that regulate dendritic expression.
Similarly, the WIN-related genes correlated with differences in cortical thickness and showed coexpression patterns with the same three cell types.
Furthermore, the affected genes were also found in humans, particularly in the thinner cortical regions of the adolescents who experimented with cannabis.
By acting on microglia, THC seems to promote the removal of synapses and, eventually, the reduction of the dendritic tree in mice, Piñeyro explained. That’s important not only because a similar mechanism may be at work in humans but also because “we now might have a model to test different types of cannabis products to see which ones are producing the greatest effect on neurons and therefore greater removal of synapses through the microglia. This could be a way of testing drugs that are out in the street to see which would be the most or least dangerous to the synapses in the brain.”
‘Significant Implications’
Commenting on the study, Yasmin Hurd, PhD, Ward-Coleman chair of translational neuroscience at the Icahn School of Medicine at Mount Sinai and director of the Addiction Institute of Mount Sinai in New York City, said, “These findings are in line with previous results, so they are feasible. This study adds more depth by showing that cortical genes that were differentially altered by adolescent THC correlated with cannabis-related changes in cortical thickness based on human neuroimaging data.” Hurd did not participate in the research.
“The results emphasize that consumption of potent cannabis products during adolescence can impact cortical function, which has significant implications for decision-making and risky behavior as well. It also can increase vulnerability to psychiatric disorders such as schizophrenia.”
Although a mouse model is “not truly the same as the human condition, the fact that the animal model also showed evidence of the morphological changes indicative of reduced cortical thickness, [like] the humans, is strong,” she said.
Additional research could include women and assess potential sex differences, she added.
Ronald Ellis, MD, PhD, an investigator in the Center for Medicinal Cannabis Research at the University of California, San Diego School of Medicine, said, “The findings are plausible and extend prior work showing evidence of increased risk for psychotic disorders later in life in adolescents who use cannabis.” Ellis did not participate in the research.
“Future studies should explore how these findings might vary across different demographic groups, which could provide a more inclusive understanding of how cannabis impacts the brain,” he said. “Additionally, longitudinal studies to track changes in the brain over time could help to establish causal relationships more robustly.
“The take-home message to clinicians at this point is to discuss cannabis use history carefully and confidentially with adolescent patients to better provide advice on its potential risks,” he concluded.
Paus added that he would tell patients, “If you’re going to use cannabis, don’t start early. If you have to, then do so in moderation. And if you have family history of mental illness, be very careful.”
No funding for the study was reported. Paus, Piñeyro, Hurd, and Ellis declared having no relevant financial relationships.
A version of this article appeared on Medscape.com.
, research in mice and humans suggested.
The multilevel study demonstrated that tetrahydrocannabinol (THC), an active substance in cannabis, causes shrinkage of dendritic arborization — the neurons’ network of antennae that play a critical role in communication between brain cells.
The connection between dendritic arborization and cortical thickness was hinted at in an earlier study by Tomáš Paus, MD, PhD, professor of psychiatry and addictology at the University of Montreal, Quebec, Canada, and colleagues, who found that cannabis use in early adolescence was associated with lower cortical thickness in boys with a high genetic risk for schizophrenia.
“We speculated at that time that the differences in cortical thickness might be related to differences in dendritic arborization, and our current study confirmed it,” Paus said.
That confirmation came in the mouse part of the study, when coauthor Graciela Piñeyro, MD, PhD, also of the University of Montreal, counted the dendritic branches of mice exposed to THC and compared the total with the number of dendritic branches in unexposed mice. “What surprised me was finding that THC in the mice was targeting the same type of cells and structures that Dr. Paus had predicted would be affected from the human studies,” she said. “Structurally, they were mostly the neurons that contribute to synapses in the cortex, and their branching was reduced.”
Paus explained that in humans, a decrease in input from the affected dendrites “makes it harder for the brain to learn new things, interact with people, cope with new situations, et cetera. In other words, it makes the brain more vulnerable to everything that can happen in a young person’s life.”
The study was published online on October 9 in the Journal of Neuroscience.
Of Mice, Men, and Cannabis
Although associations between cannabis use by teenagers and variations in brain maturation have been well studied, the cellular and molecular underpinnings of these associations were unclear, according to the authors.
To investigate further, they conducted this three-step study. First, they exposed adolescent male mice to THC or a synthetic cannabinoid (WIN 55,212-2) and assessed differentially expressed genes, spine numbers, and the extent of dendritic complexity in the frontal cortex of each mouse.
Next, using MRI, they examined differences in cortical thickness in 34 brain regions in 140 male adolescents who experimented with cannabis before age 16 years and 327 who did not.
Then, they again conducted experiments in mice and found that 13 THC-related genes correlated with variations in cortical thickness. Virtual histology revealed that these 13 genes were coexpressed with cell markers of astrocytes, microglia, and a type of pyramidal cell enriched in genes that regulate dendritic expression.
Similarly, the WIN-related genes correlated with differences in cortical thickness and showed coexpression patterns with the same three cell types.
Furthermore, the affected genes were also found in humans, particularly in the thinner cortical regions of the adolescents who experimented with cannabis.
By acting on microglia, THC seems to promote the removal of synapses and, eventually, the reduction of the dendritic tree in mice, Piñeyro explained. That’s important not only because a similar mechanism may be at work in humans but also because “we now might have a model to test different types of cannabis products to see which ones are producing the greatest effect on neurons and therefore greater removal of synapses through the microglia. This could be a way of testing drugs that are out in the street to see which would be the most or least dangerous to the synapses in the brain.”
‘Significant Implications’
Commenting on the study, Yasmin Hurd, PhD, Ward-Coleman chair of translational neuroscience at the Icahn School of Medicine at Mount Sinai and director of the Addiction Institute of Mount Sinai in New York City, said, “These findings are in line with previous results, so they are feasible. This study adds more depth by showing that cortical genes that were differentially altered by adolescent THC correlated with cannabis-related changes in cortical thickness based on human neuroimaging data.” Hurd did not participate in the research.
“The results emphasize that consumption of potent cannabis products during adolescence can impact cortical function, which has significant implications for decision-making and risky behavior as well. It also can increase vulnerability to psychiatric disorders such as schizophrenia.”
Although a mouse model is “not truly the same as the human condition, the fact that the animal model also showed evidence of the morphological changes indicative of reduced cortical thickness, [like] the humans, is strong,” she said.
Additional research could include women and assess potential sex differences, she added.
Ronald Ellis, MD, PhD, an investigator in the Center for Medicinal Cannabis Research at the University of California, San Diego School of Medicine, said, “The findings are plausible and extend prior work showing evidence of increased risk for psychotic disorders later in life in adolescents who use cannabis.” Ellis did not participate in the research.
“Future studies should explore how these findings might vary across different demographic groups, which could provide a more inclusive understanding of how cannabis impacts the brain,” he said. “Additionally, longitudinal studies to track changes in the brain over time could help to establish causal relationships more robustly.
“The take-home message to clinicians at this point is to discuss cannabis use history carefully and confidentially with adolescent patients to better provide advice on its potential risks,” he concluded.
Paus added that he would tell patients, “If you’re going to use cannabis, don’t start early. If you have to, then do so in moderation. And if you have family history of mental illness, be very careful.”
No funding for the study was reported. Paus, Piñeyro, Hurd, and Ellis declared having no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF NEUROSCIENCE
ATA: Updates on Risk, Diagnosis, and Treatment of Thyroid Cancer
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
The study, presented by Juan Brito Campana, MBBS, of the Mayo Clinic in Rochester, Minnesota, used Medicare records to perform a secondary analysis of 41,000 adults with type 2 diabetes and moderate cardiovascular risk who were new users of GLP-1 receptor agonists, compared to users of other diabetes medications.
“We took the innovative approach of applying the methodological rigor of a randomized clinical trial to the very large dataset of observational studies,” said Brito Campana.
The results showed a low absolute risk of thyroid cancer, with only 0.17% of patients in the GLP-1 group developing the disease. However, the data also showed a potential relative increase in risk during the first year of GLP-1 receptor agonist use.
“This is likely due to increased detection rather than true incidence, as the latency period for thyroid cancer development is typically longer,” Brito Campana said.
“We also note the limitations of the observational study design, including the short follow-up period and lack of detailed histological data. However, we believe the benefits of GLP-1 receptor agonists likely outweigh the risk of thyroid cancer.”
Malignancy in Bethesda III and IV Thyroid Nodules
At the same ATA session, Sapir Nachum Goldberg, MD, of the University of Pennsylvania, Philadelphia, presented the results of a retrospective record review that examined the prevalence of malignancy in Bethesda III and IV thyroid nodules with negative Thyrogen Receptor Signaling (ThyroSeq) version 3 molecular testing results.
Goldberg reported that 87% of patients with ThyroSeq negative subtype results were managed nonoperatively. “Based on our data, the true prevalence of malignancy likely lies between our low and high estimates of 3% and 23%,” she said. “We believe that the prevalence of malignancy may be higher in real-world practice than validation studies.”
Additionally, nodules with “currently negative” or “negative but limited” ThyroSeq results had a higher prevalence of malignancy (7%), compared with those with a “negative” result (2%). Factors like immediate vs delayed surgery, nodule size, and ultrasound pattern did not significantly impact malignancy prevalence.
The study results also indicated that surveillance ultrasonography is not routinely performed in up to one-third of patients, Goldberg said.
She closed by suggesting that colleagues consider the negative subtype in clinical decision-making. For “negative but limited” nodules, repeat the fine needle aspiration and, for “negative” and “currently negative” nodules, consider ultrasound follow-up as per ATA guidelines for Bethesda II cytology, she said.
RET-Mutated Medullary Thyroid Cancer
For patients with RET-mutated medullary thyroid cancer, Julien Hadoux, MD, PhD, of Institut de Cancérologie Gustave Roussy, Villejuif, France, presented a combined analysis of the efficacy of the RET inhibitor selpercatinib from the phase 1/2 LIBRETTO-001 and phase 3 LIBRETTO-531 trials.
This post hoc analysis used a combined cohort of 509 patients with RET-mutated advanced or metastatic medullary thyroid cancer who had received selpercatinib in the two trials.
Hadoux reported that robust and durable responses were seen across all mutation groups, including M918T, extracellular cysteine, and an “other” group composed of various uncommon RET mutations. “The median [progression-free survival] PFS was not reached for either the M918T or extracellular groups and it was 51.4 months for the Other group,” he said.
“Selpercatinib showed superior median PFS vs control, regardless of the RET mutation. This analysis constitutes the largest catalog of RET mutations in medullary thyroid cancers treated with RET-specific inhibitors.”
TRK-Fusion Differentiated Thyroid Cancer
Steven Waguespack, MD, of the University of Texas MD Anderson Cancer Center, Houston, shared updated efficacy and safety data from three phase 1/2 pooled clinical trials of the tropomyosin kinase receptor (TRK) inhibitor larotrectinib in thyroid cancer. These data updated results initially published in 2022.
“Larotrectinib continues to demonstrate rapid and durable responses, extended survival, and offers a favorable safety profile in patients with TRK fusion differentiated thyroid cancer, with limited activity in anaplastic thyroid cancer,” Waguespack said.
“Additionally, in a subset of patients, we identified some acquired on-target NTRK mutations and off-target GNAS and TP53 mutations that may give further insight into mechanisms of resistance.”
The primary endpoint was the investigator-assessed objective response rate (ORR); at 48 months, the ORR was 79% by independent review. The median PFS in patients with TRK fusion differentiated thyroid cancer was 44 months, while the median duration of response was 41 months. The 4-year overall survival rate was 86%.
Waguespack closed with a cautionary note to colleagues: “While circulating tumor DNA next-generation sequencing (NGS) analysis can be used to test for NTRK gene fusions, negative results should be followed up with tissue-based NGS,” he said.
Brito Campana and Goldberg disclosed no relevant financial relationships. Hadoux reported receiving honoraria for speaker engagements, advisory roles, or funding for CME from Eli Lilly, AAA, IPSEN, Roche, Pharma Mar, and EISAI, and research grants from Novartis, Sanofi, and Eli Lilly.
A version of this article appeared on Medscape.com.
FROM ATA 2024