User login
Three Anomalies and a Complication: Ruptured Noncoronary Sinus of Valsalva Aneurysm, Atrial Septal Aneurysm, and Patent Foramen Ovale
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
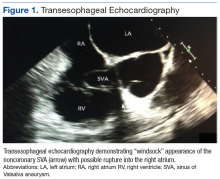
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
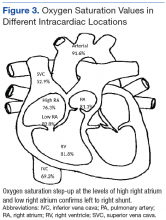
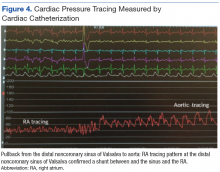
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
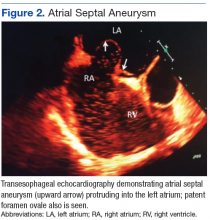
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).
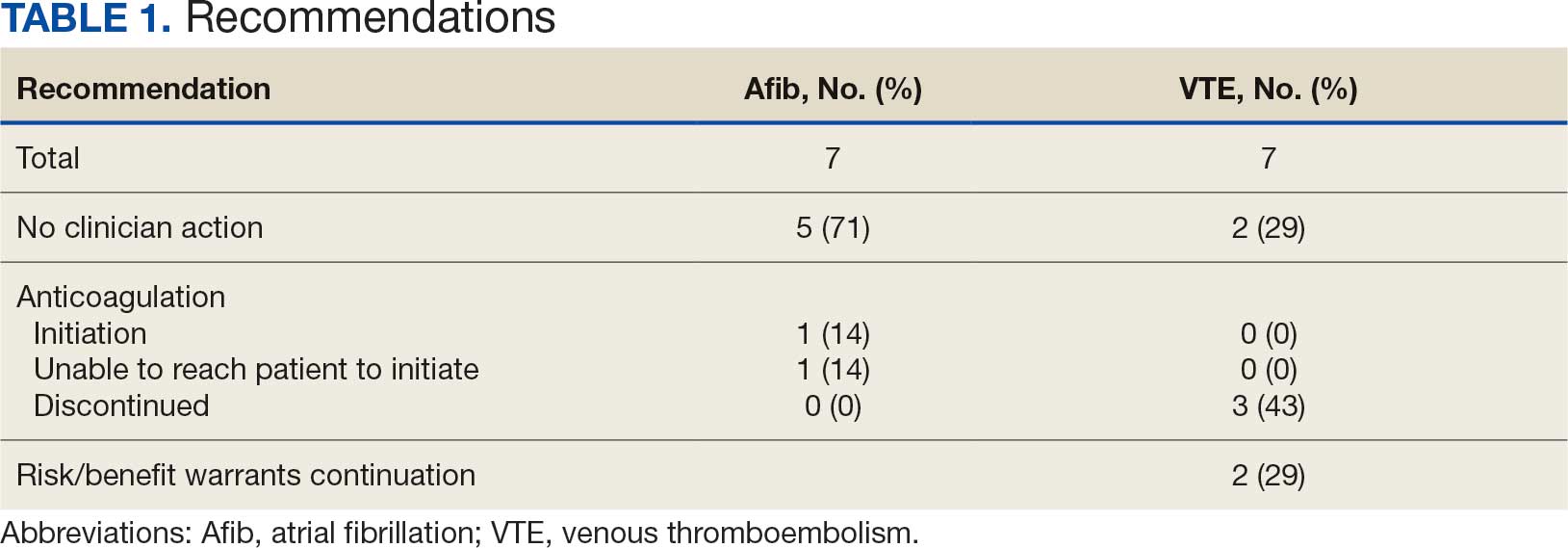
In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
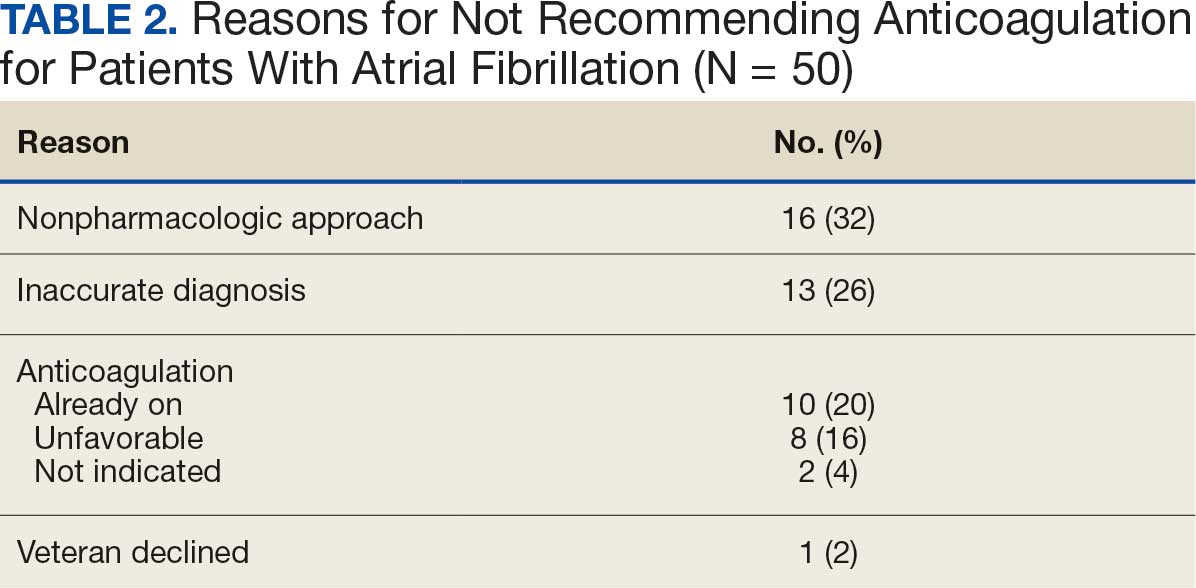
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).
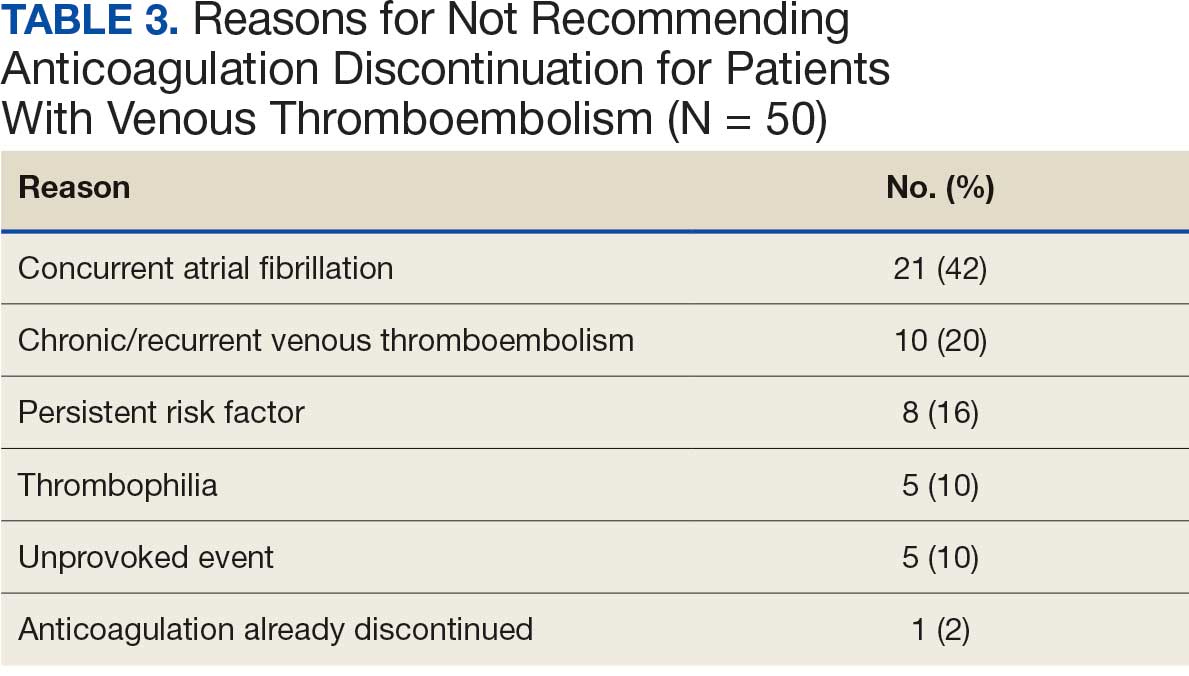
In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).
Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
Due to the underlying mechanism of atrial fibrillation (Afib), clots can form within the left atrial appendage. Clots that become dislodged may lead to ischemic stroke and possibly death. The 2023 guidelines for atrial fibrillation from the American College of Cardiology and American Heart Association recommend anticoagulation therapy for patients with an Afib diagnosis and a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes, stroke/vascular disease, age 65 to 74 years, and female sex) score pertinent for ≥ 1 non–sex-related factor (score ≥ 2 for women; ≥ 1 for men) to prevent stroke-related complications. The CHA2DS2-VASc score is a 9-point scoring tool based on comorbidities and conditions that increase risk of stroke in patients with Afib. Each value correlates to an annualized stroke risk percentage that increases as the score increases.
In clinical practice, patients meeting these thresholds are indicated for anticoagulation and are considered for indefinite use unless ≥ 1 of the following conditions are present: bleeding risk outweighs the stroke prevention benefit, Afib is episodic (< 48 hours) or a nonpharmacologic intervention, such as a left atrial appendage occlusion (LAAO) device is present.1
In patients with a diagnosed venous thromboembolism (VTE), such as deep vein thrombosis or pulmonary embolism, anticoagulation is used to treat the current thrombosis and prevent embolization that can ultimately lead to death. The 2021 guideline for VTE from the American College of Chest Physicians identifies certain risk factors that increase risk for VTE and categorizes them as transient or persistent. Transient risk factors include hospitalization > 3 days, major trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel > 8 hours. Persistent risk factors include malignancy, thrombophilia, and certain medications.
The guideline recommends therapy durations based on event frequency, the presence and classification of provoking risk factors, and bleeding risk. As the risk of recurrent thrombosis and other potential complications is greatest in the first 3 to 6 months after a diagnosed event, at least 3 months anticoagulation therapy is recommended following VTE diagnosis. At the 3-month mark, all regimens are suggested to be re-evaluated and considered for extended treatment duration if the event was unprovoked, recurrent, secondary to a persistent risk factor, or low bleed risk.2Anticoagulation is an important guideline-recommended pharmacologic intervention for various disease states, although its use is not without risks. The Institute for Safe Medication Practices has classified oral anticoagulants as high-alert medications. This designation was made because anticoagulant medications have the potential to cause harm when used or omitted in error and lead to life-threatening bleed or thrombotic complications.3Anticoagulation stewardship ensures that anticoagulation therapy is appropriately initiated, maintained, and discontinued when indicated. Because of the potential for harm, anticoagulation stewardship is an important part of Afib and VTE management. Pharmacists can help verify and evaluate anticoagulation therapies. Research suggests that pharmacist-led anticoagulation stewardship efforts may play a role in ensuring safer patient outcomes.4The purpose of this quality improvement (QI) study was to implement pharmacist-led anticoagulation stewardship practices at Veterans Affairs Phoenix Health Care System (VAPHCS) to identify veterans with Afib not currently on anticoagulation, as well as to identify veterans with a history of VTE events who have completed a sufficient treatment duration.
Methods
Anticoagulation stewardship efforts were implemented in 2 cohorts of patients: those with Afib who may be indicated to initiate anticoagulation, and those with a history of VTE events who may be indicated to consider anticoagulation discontinuation. Patient records were reviewed using a standardized note template, and recommendations to either initiate or discontinue anticoagulation therapy were documented. The VAPHCS Research Service reviewed this study and determined that it was not research and was exempt from institutional review board review.
Atrial Fibrillation Cohort
A population health dashboard created by the Stroke Prevention in Atrial Fibrillation/Flutter Targeting the uNTreated: a focus on health care disparities (SPAFF-TNT-D) national VA study team was used to identify veterans at VAPHCS with a diagnosis of Afib without an active VA prescription for an anticoagulant. The dashboard filtered and produced data points from the medical record that correlated to the components of the CHA2DS2-VASc score. All veterans identified by the dashboard with scores of 7 or 8 were included. No patients had a score of 9. Comprehensive chart reviews of available VA and non–VA-provided care records were conducted by the investigators, and a standardized note template designed by the SPAFF-TNT-D team (eAppendix 1) was used to document findings within the electronic health record (EHR). If anticoagulation was deemed to be indicated, the assigned primary care practitioner (PCP) as listed in the EHR was alerted to the note by the investigators for further evaluation and consideration of prescribing anticoagulation.
Venous Thromboembolism Cohort
VAPHCS pharmacy informatics pulled data that included veterans with documented VTE and an active VA anticoagulant prescription between November 2022 and November 2023. Veterans were reviewed in chronological order based on when the anticoagulant prescription was written. All veterans were included until an equal number of charts were reviewed in both the Afib and VTE cohorts. Comprehensive chart review of available VA- and non–VA-provided care records was conducted by the investigators, and a standardized note template as designed by the investigators (eAppendix 2) was used to document findings within the EHR. If the duration of anticoagulation therapy was deemed sufficient, the assigned anticoagulation clinical pharmacist practitioner (CPP) was alerted to the note by the investigators for further evaluation and consideration of discontinuing anticoagulation.
EHR reviews were conducted in October and November 2023 and lasted about 10 to 20 minutes per patient. To evaluate completeness and accuracy of the documented findings within the EHR, both investigators reviewed and cosigned the completed note template and verified the correct PCP was alerted to the recommendation for appropriate continuity of care. Results were reviewed in March 2024.
Outcomes
Atrial fibrillation cohort. The primary outcome was the number of veterans with Afib who were recommended to start anticoagulation therapy. Additional outcomes evaluated included the number of interventions completed, action taken by PCPs in response to the provided recommendation, and reasons provided by the investigators for not recommending initiation of anticoagulation therapy in specific veteran cases.
Venous thromboembolism cohort. The primary outcome was the number of veterans with a history of VTE events recommended to discontinue anticoagulation therapy. Additional outcomes included number of interventions completed, action taken by the anticoagulation CPP in response to the provided recommendation, and reasons provided by the investigators for not recommending discontinuation of anticoagulation therapy in specific veteran cases.
Analysis
Sample size was determined by the inclusion criteria and was not designed to attain statistical power. Data embedded in the Afib cohort standardized note template, also known as health factors, were later used for data analysis. Recommendations in the VTE cohort were manually tracked and recorded by the investigators. Results for this study were analyzed using descriptive statistics.
Results
A total of 114 veterans were reviewed and included in this study: 57 in each cohort. Seven recommendations were made regarding anticoagulation initiation for patients with Afib and 7 were made for anticoagulation discontinuation for patients with VTE (Table 1).

In the Afib cohort, 1 veteran was successfully initiated on anticoagulation therapy and 1 veteran was deemed appropriate for initiation of anticoagulation but was not reachable. Of the 5 recommendations with no action taken, 4 PCPs acknowledged the alert with no further documentation, and 1 PCP deferred the decision to cardiology with no further documentation. In the VTE cohort, 3 veterans successfully discontinued anticoagulation therapy and 2 veterans were further evaluated by the anticoagulation CPP and deemed appropriate to continue therapy based on potential for malignancy. Of the 2 recommendations with no action taken, 1 anticoagulation CPP acknowledged the alert with no further documentation and 1 anticoagulation CPP suggested further evaluation by PCP with no further documentation.
In the Afib cohort, a nonpharmacologic approach was defined as documentation of a LAAO device. An inaccurate diagnosis was defined as an Afib diagnosis being used in a previous visit, although there was no further confirmation of diagnosis via chart review. Veterans classified as already being on anticoagulation had documentation of non–VA-written anticoagulant prescriptions or receiving a supply of anticoagulants from a facility such as a nursing home. Anticoagulation was defined as unfavorable if a documented risk/benefit conversation was found via EHR review. Anticoagulation was defined as not indicated if the Afib was documented as transient, episodic, or historical (Table 2).

In the VTE cohort, no recommendations for discontinuation were made for veterans indicated to continue anticoagulation due to a concurrent Afib diagnosis. Chronic or recurrent events were defined as documentation of multiple VTE events and associated dates in the EHR. Persistent risk factors included malignancy or medications contributing to hypercoagulable states. Thrombophilia was defined as having documentation of a diagnosis in the EHR. An unprovoked event was defined as VTE without any documented transient risk factors (eg, hospitalization, trauma, surgery, cast immobilization, hormone therapy, pregnancy, or prolonged travel). Anticoagulation had already been discontinued in 1 veteran after the data were collected but before chart review occurred (Table 3).

Discussion
Pharmacy-led indication reviews resulted in appropriate recommendations for anticoagulation use in veterans with Afib and a history of VTE events. Overall, 12.3% of chart reviews in each cohort resulted in a recommendation being made, which was similar to the rate found by Koolian et al.5 In that study, 10% of recommendations were related to initiation or interruption of anticoagulation. This recommendation category consisted of several subcategories, including “suggesting therapeutic anticoagulation when none is currently ordered” and “suggesting anticoagulation cessation if no longer indicated,” but specific numerical prevalence was not provided.5
Online dashboard use allowed for greater population health management and identification of veterans with Afib who were not on active anticoagulation, providing opportunities to prevent stroke-related complications. Wang et al completed a similarly designed study that included a population health tool to identify patients with Afib who were not on anticoagulation and implemented pharmacist-led chart review and facilitation of recommendations to the responsible clinician. This study reviewed 1727 patients and recommended initiation of anticoagulation therapy for 75 (4.3%).6 The current study had a higher percentage of patients with recommendations for changes despite its smaller size.
Evaluating the duration of therapy for anticoagulation in veterans with a history of VTE events provided an opportunity to reduce unnecessary exposure to anticoagulation and minimize bleeding risks. Using a chart review process and standardized note template enabled the documentation of pertinent information that could be readily reviewed by the PCP. This process is a step toward ensuring VAPHCS PCPs provide guideline-recommended care and actively prevent stroke and bleeding complications. Adoption of this process into the current VAPHCS Anticoagulation Clinic workflow for review of veterans with either Afib or VTE could lead to more EHRs being reviewed and recommendations made, ultimately improving patient outcomes.
Therapeutic interventions based on the recommendations were completed for 1 of 7 veterans (14%) and 3 of 7 veterans (43%) in the Afib and VTE cohorts, respectively. The prevalence of completed interventions in this anticoagulation stewardship study was higher than those in Wang et al, who found only 9% of their recommendations resulted in PCPs considering action related to anticoagulation, and only 4% were successfully initiated.6
In the Afib cohort, veterans identified by the dashboard with a CHA2DS2-VASc of 7 or 8 were prioritized for review. Reviewing these veterans ensured that patients with the highest stroke risk were sufficiently evaluated and started on anticoagulation as needed to reduce stroke-related complications. In contrast, because these veterans had higher CHA2DS2-VASc scores, they may have already been evaluated for anticoagulation in the past and had a documented rationale for not being placed on anticoagulation (LAAO device placement was the most common rationale). Focusing on veterans with a lower CHA2DS2-VASc score such as 1 for men or 2 for women could potentially include more opportunities for recommendations. Although stroke risk may be lower in this population compared with those with higher CHA2DS2-VASc scores, guideline-recommended anticoagulation use may be missed for these patients.
In the VTE cohort, veterans with an anticoagulant prescription written 12 months before data collection were prioritized for review. Reviewing these veterans ensured that anticoagulation therapy met guideline recommendations of at least 3 months, with potential for extended duration upon further evaluation by a provider at that time. Based on collected results, most veterans were already reevaluated and had documented reasons why anticoagulation was still indicated; concurrent Afib was most common followed by chronic or recurrent VTE. Reviewing veterans with more recent prescriptions just over the recommended 3-month duration could potentially include more opportunities for recommendations to be made. It is more likely that by 3 months another PCP had not already weighed in on the duration of therapy, and the anticoagulation CPP could ensure a thorough review is conducted with guideline-based recommendations.
Most published literature on anticoagulation stewardship efforts is focused on inpatient management and policy changes, or concentrate on attributes of therapy such as appropriate dosing and drug interactions. This study highlighted that gaps in care related to anticoagulation use and discontinuation are present in the VAPHCS population and can be appropriately addressed via pharmacist-led indication reviews. Future studies designed to focus on initiating anticoagulation where appropriate, and discontinuing where a sufficient treatment period has been completed, are warranted to minimize this gap in care and allow health systems to work toward process changes to ensure safe and optimized care is provided for the patients they serve.
Limitations
In the Afib cohort, 5 of 7 recommendations (71%) had no further action taken by the PCP, which may represent a barrier to care. In contrast, 2 of 7 recommendations (29%) had no further action in the VTE cohort. It is possible that the difference can be attributed to the anticoagulation CPP receiving VTE alerts and PCPs receiving Afib alerts. The anticoagulation CPP was familiar with this QI study and may have better understood the purpose of the chart review and the need to provide a timely response. PCPs may have been less likely to take action because they were unfamiliar with the anticoagulation stewardship initiative and standardized note template or overwhelmed by too many EHR alerts.
The lack of PCP response to a virtual alert or message also was observed by Wang et al, whereas Koolian et al reported higher intervention completion rates, with verbal recommendations being made to the responsible clinicians. To further ensure these pertinent recommendations for anticoagulation initiation in veterans with Afib are properly reviewed and evaluated, future research could include intentional follow-up with the PCP regarding the alert, PCP-specific education about the anticoagulation stewardship initiative and the role of the standardized note template, and collaboration with PCPs to identify alternative ways to relay recommendations in a way that would ensure the completion of appropriate and timely review.
Conclusions
This study identified gaps in care related to anticoagulation needs in the VAPHCS veteran population. Utilizing a standardized indication review process allows pharmacists to evaluate anticoagulant use for both appropriate indication and duration of therapy. Providing recommendations via chart review notes and alerting respective PCPs and CPPs results in veterans receiving safe and optimized care regarding their anticoagulation needs.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. doi:10.1161/CIR.0000000000001193
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi:10.1016/j.chest.2021.07.055
- Institute for Safe Medication Practices (ISMP). List of high-alert medications in community/ambulatory care settings. ISMP. September 30, 2021. Accessed September 11, 2025. https://home.ecri.org/blogs/ismp-resources/high-alert-medications-in-community-ambulatory-care-settings
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi:10.1002/rth2.12757
- Koolian M, Wiseman D, Mantzanis H, et al. Anticoagulation stewardship: descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. 2022;6:e12758. doi:10.1002/rth2.12758
- Wang SV, Rogers JR, Jin Y, et al. Stepped-wedge randomised trial to evaluate population health intervention designed to increase appropriate anticoagulation in patients with atrial fibrillation. BMJ Qual Saf. 2019;28:835-842. doi:10.1136/bmjqs-2019-009367
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Anticoagulation Stewardship Efforts Via Indication Reviews at a Veterans Affairs Health Care System
Quality of Life for Males With Abdominal Aortic Aneurysm
Quality of Life for Males With Abdominal Aortic Aneurysm
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.
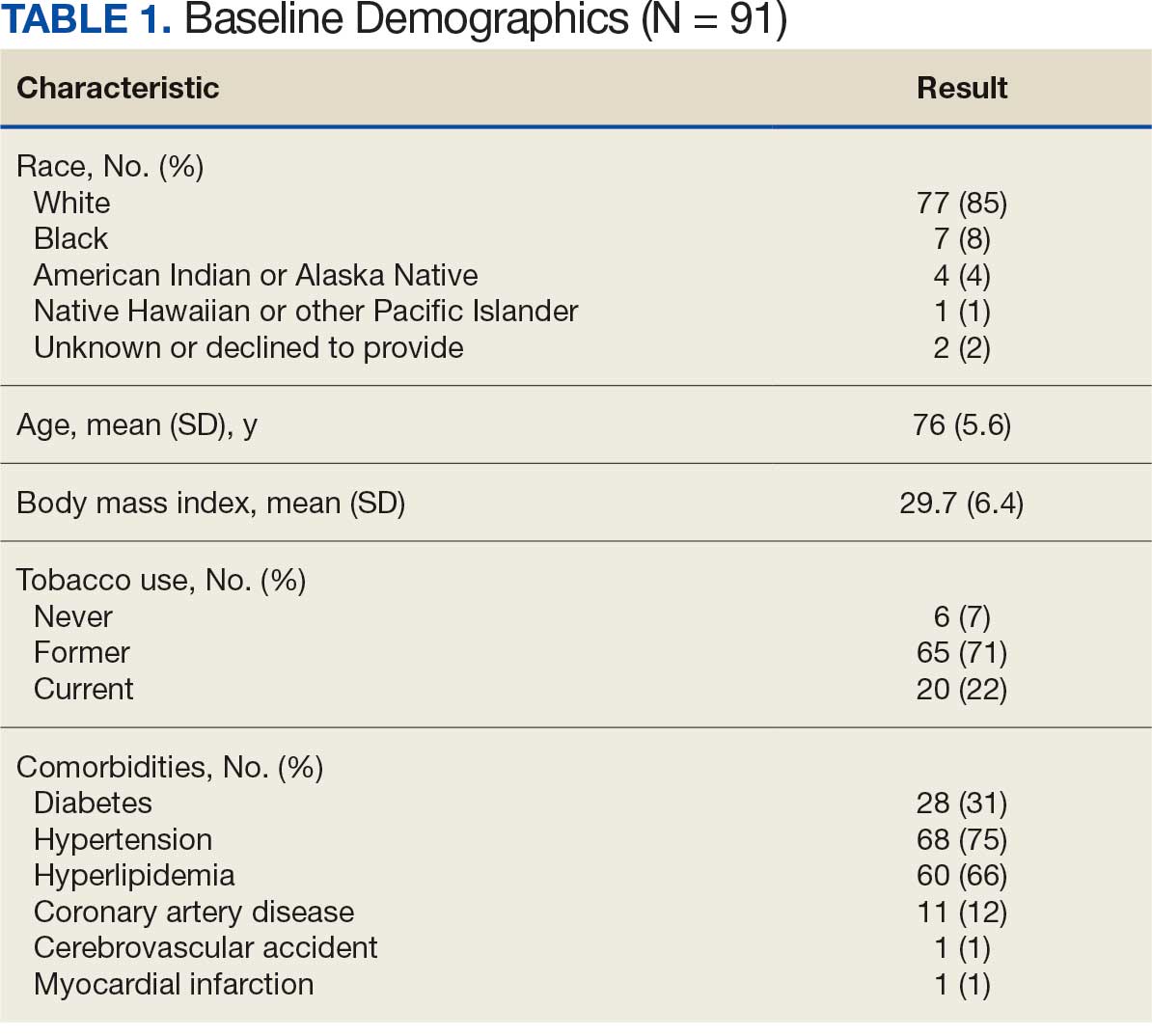
When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).
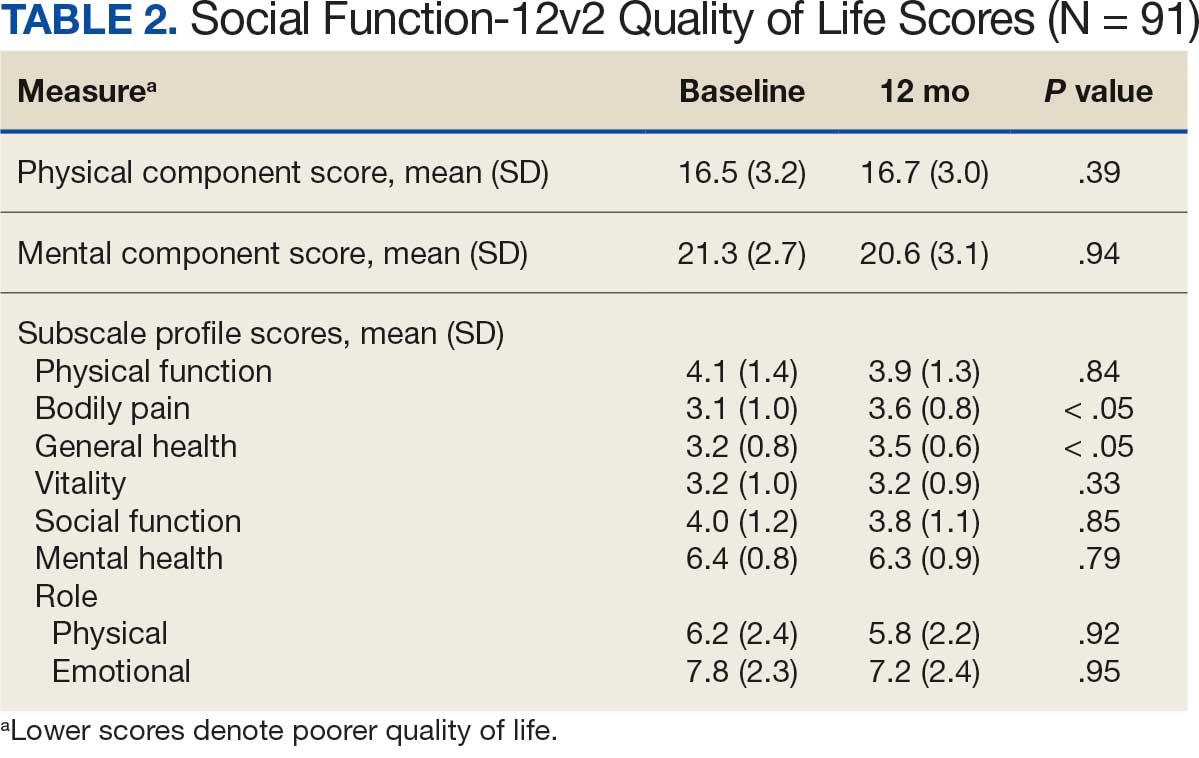
Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.

When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).

Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.

When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).

Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
Quality of Life for Males With Abdominal Aortic Aneurysm
Quality of Life for Males With Abdominal Aortic Aneurysm
Targeted Syncope Workup in Hypercoagulable Patients
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Hypereosinophilic Syndrome With Eosinophilic Endomyocarditis: A Rare Cardiac Manifestation
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
The Immune Heartache: Pericarditis Following Checkpoint Inhibition
Background
Immune checkpoint inhibitors (ICI) have changed the landscape of cancer therapy. Pembrolizumab is an ICI which targets programmed cell death protein-1 on T-cells and acts to release inhibition of the T-cell antitumor response. Pembrolizumab is approved for the treatment of multiple malignancies. However, ICI therapy may precipitate immune-related adverse events (IRAEs). We describe a unique presentation of irAE-cardiotoxicity.
Case Discussion
A 70-year-old female with a history of uterine cancer previously treated with pembrolizumab (discontinued in January) presented to the emergency department with acute onset nausea and vomiting. On arrival, she was afebrile, tachycardic, normotensive, and saturated well in room air. Labs were notable for troponin of 20, normal TSH, elevated proBNP, ESR and CRP. EKG revealed atrial fibrillation with rapid ventricular response and subtle ST changes in leads II, aVF, V4-V6. She was started on diltiazem infusion for rate control and was subsequently transitioned to oral amiodarone. Given the concern for pericarditis, NSAIDs were initiated. Transthoracic echocardiogram was notable for an ejection fraction of 58% with moderate circumferential pericardial effusion without tamponade. Given her recent ICI exposure and evolving clinical course, she was diagnosed with pembrolizumab- induced pericarditis with associated atrial fibrillation and pericardial effusion. High-dose corticosteroids and colchicine were initiated for stabilization and symptomatic improvement.
Discussion
IRAEs usually occur within 3 months of therapy but may develop later. They are classified as low-grade (1-2), high-grade (3-4), or lethal (5). Anti- PD1 therapy is frequently associated with minor IRAEs, which develop in ~70% of patients; dermatologic IRAEs are most common. Major IRAEs develop in 10-15% of patients, and lethal IRAEs may develop in up to 3%. Cardiac IRAEs are infrequent but significant. Presentation is variable and may involve the myocardium, pericardium, or conductive system. In the case of pericardial disease, high-dose IV methylprednisolone with oral steroid taper should be considered. Re-challenge with ICI therapy should only be considered if clinically stable and pericarditis or myocarditis are excluded.
Conclusions
Our patient illustrates a rare but significant IRAE associated with ICI with improvement following immunosuppressive and rate-control therapy.
Background
Immune checkpoint inhibitors (ICI) have changed the landscape of cancer therapy. Pembrolizumab is an ICI which targets programmed cell death protein-1 on T-cells and acts to release inhibition of the T-cell antitumor response. Pembrolizumab is approved for the treatment of multiple malignancies. However, ICI therapy may precipitate immune-related adverse events (IRAEs). We describe a unique presentation of irAE-cardiotoxicity.
Case Discussion
A 70-year-old female with a history of uterine cancer previously treated with pembrolizumab (discontinued in January) presented to the emergency department with acute onset nausea and vomiting. On arrival, she was afebrile, tachycardic, normotensive, and saturated well in room air. Labs were notable for troponin of 20, normal TSH, elevated proBNP, ESR and CRP. EKG revealed atrial fibrillation with rapid ventricular response and subtle ST changes in leads II, aVF, V4-V6. She was started on diltiazem infusion for rate control and was subsequently transitioned to oral amiodarone. Given the concern for pericarditis, NSAIDs were initiated. Transthoracic echocardiogram was notable for an ejection fraction of 58% with moderate circumferential pericardial effusion without tamponade. Given her recent ICI exposure and evolving clinical course, she was diagnosed with pembrolizumab- induced pericarditis with associated atrial fibrillation and pericardial effusion. High-dose corticosteroids and colchicine were initiated for stabilization and symptomatic improvement.
Discussion
IRAEs usually occur within 3 months of therapy but may develop later. They are classified as low-grade (1-2), high-grade (3-4), or lethal (5). Anti- PD1 therapy is frequently associated with minor IRAEs, which develop in ~70% of patients; dermatologic IRAEs are most common. Major IRAEs develop in 10-15% of patients, and lethal IRAEs may develop in up to 3%. Cardiac IRAEs are infrequent but significant. Presentation is variable and may involve the myocardium, pericardium, or conductive system. In the case of pericardial disease, high-dose IV methylprednisolone with oral steroid taper should be considered. Re-challenge with ICI therapy should only be considered if clinically stable and pericarditis or myocarditis are excluded.
Conclusions
Our patient illustrates a rare but significant IRAE associated with ICI with improvement following immunosuppressive and rate-control therapy.
Background
Immune checkpoint inhibitors (ICI) have changed the landscape of cancer therapy. Pembrolizumab is an ICI which targets programmed cell death protein-1 on T-cells and acts to release inhibition of the T-cell antitumor response. Pembrolizumab is approved for the treatment of multiple malignancies. However, ICI therapy may precipitate immune-related adverse events (IRAEs). We describe a unique presentation of irAE-cardiotoxicity.
Case Discussion
A 70-year-old female with a history of uterine cancer previously treated with pembrolizumab (discontinued in January) presented to the emergency department with acute onset nausea and vomiting. On arrival, she was afebrile, tachycardic, normotensive, and saturated well in room air. Labs were notable for troponin of 20, normal TSH, elevated proBNP, ESR and CRP. EKG revealed atrial fibrillation with rapid ventricular response and subtle ST changes in leads II, aVF, V4-V6. She was started on diltiazem infusion for rate control and was subsequently transitioned to oral amiodarone. Given the concern for pericarditis, NSAIDs were initiated. Transthoracic echocardiogram was notable for an ejection fraction of 58% with moderate circumferential pericardial effusion without tamponade. Given her recent ICI exposure and evolving clinical course, she was diagnosed with pembrolizumab- induced pericarditis with associated atrial fibrillation and pericardial effusion. High-dose corticosteroids and colchicine were initiated for stabilization and symptomatic improvement.
Discussion
IRAEs usually occur within 3 months of therapy but may develop later. They are classified as low-grade (1-2), high-grade (3-4), or lethal (5). Anti- PD1 therapy is frequently associated with minor IRAEs, which develop in ~70% of patients; dermatologic IRAEs are most common. Major IRAEs develop in 10-15% of patients, and lethal IRAEs may develop in up to 3%. Cardiac IRAEs are infrequent but significant. Presentation is variable and may involve the myocardium, pericardium, or conductive system. In the case of pericardial disease, high-dose IV methylprednisolone with oral steroid taper should be considered. Re-challenge with ICI therapy should only be considered if clinically stable and pericarditis or myocarditis are excluded.
Conclusions
Our patient illustrates a rare but significant IRAE associated with ICI with improvement following immunosuppressive and rate-control therapy.
Data Trends 2025: Cardiology
Data Trends 2025: Cardiology
Click here to view more from Federal Health Care Data Trends 2025.
- Almuwaqqat Z, et al. JAMA Netw Open. 2024;7(3):e243062. doi:10.1001/jamanetworkopen.2024.3062
- Carrico M, et al. Telemed J E Health. 2024;30(4):1006-1012. doi:10.1089/tmj.2023.0269
- US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. National veteran health equity report 2021. September 2022:177-179. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf
- New program for veterans with cholesterol, associated cardiovascular disease [press release]. American Heart Association. March 21, 2023. Accessed April 11, 2025. https://newsroom.heart.org/news/new-program-for-veterans-with-high-cholesterol-associated-cardiovascular-disease
Washington DL, et al. US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. February 2024. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/Rates_of_Hypertension_by_Race_or_Ethnicity.pdf
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
- Almuwaqqat Z, et al. JAMA Netw Open. 2024;7(3):e243062. doi:10.1001/jamanetworkopen.2024.3062
- Carrico M, et al. Telemed J E Health. 2024;30(4):1006-1012. doi:10.1089/tmj.2023.0269
- US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. National veteran health equity report 2021. September 2022:177-179. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf
- New program for veterans with cholesterol, associated cardiovascular disease [press release]. American Heart Association. March 21, 2023. Accessed April 11, 2025. https://newsroom.heart.org/news/new-program-for-veterans-with-high-cholesterol-associated-cardiovascular-disease
Washington DL, et al. US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. February 2024. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/Rates_of_Hypertension_by_Race_or_Ethnicity.pdf
- Almuwaqqat Z, et al. JAMA Netw Open. 2024;7(3):e243062. doi:10.1001/jamanetworkopen.2024.3062
- Carrico M, et al. Telemed J E Health. 2024;30(4):1006-1012. doi:10.1089/tmj.2023.0269
- US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. National veteran health equity report 2021. September 2022:177-179. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf
- New program for veterans with cholesterol, associated cardiovascular disease [press release]. American Heart Association. March 21, 2023. Accessed April 11, 2025. https://newsroom.heart.org/news/new-program-for-veterans-with-high-cholesterol-associated-cardiovascular-disease
Washington DL, et al. US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. February 2024. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/Rates_of_Hypertension_by_Race_or_Ethnicity.pdf
Data Trends 2025: Cardiology
Data Trends 2025: Cardiology
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12
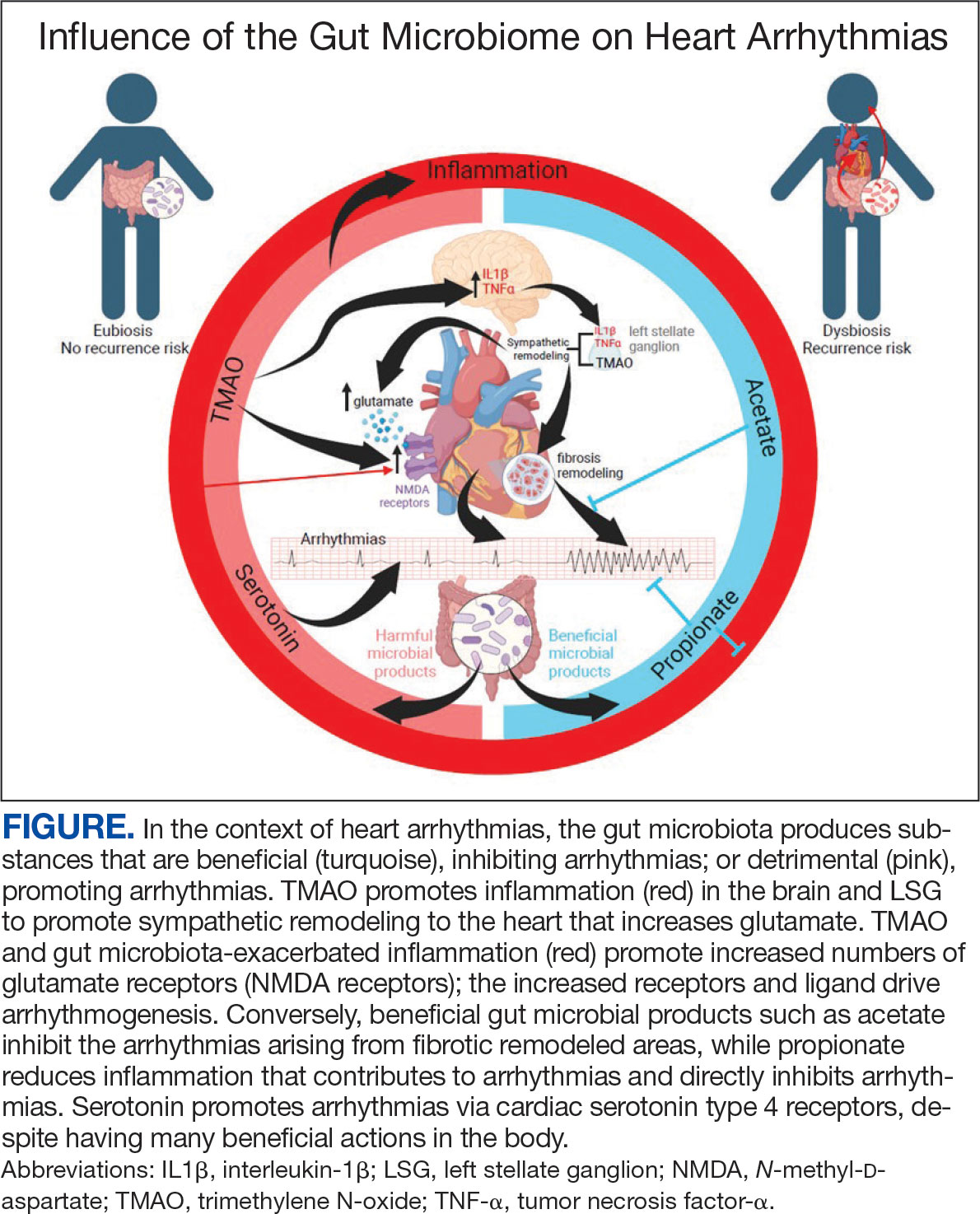
Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
Safety and Efficacy of Ezetimibe in Patients With and Without Chronic Kidney Disease at a Pharmacist-Managed Clinic
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.
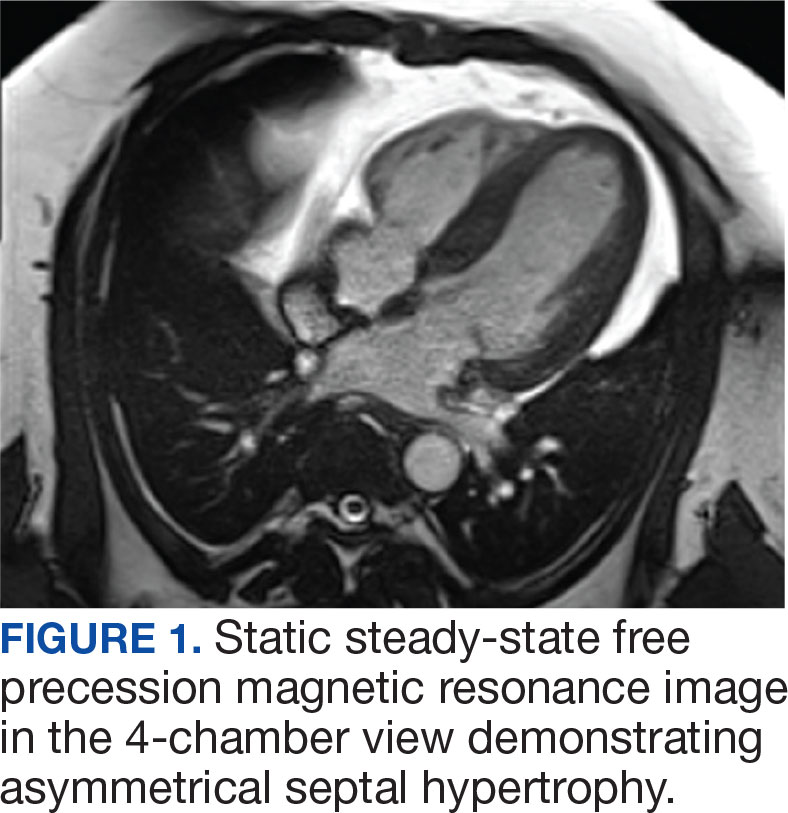
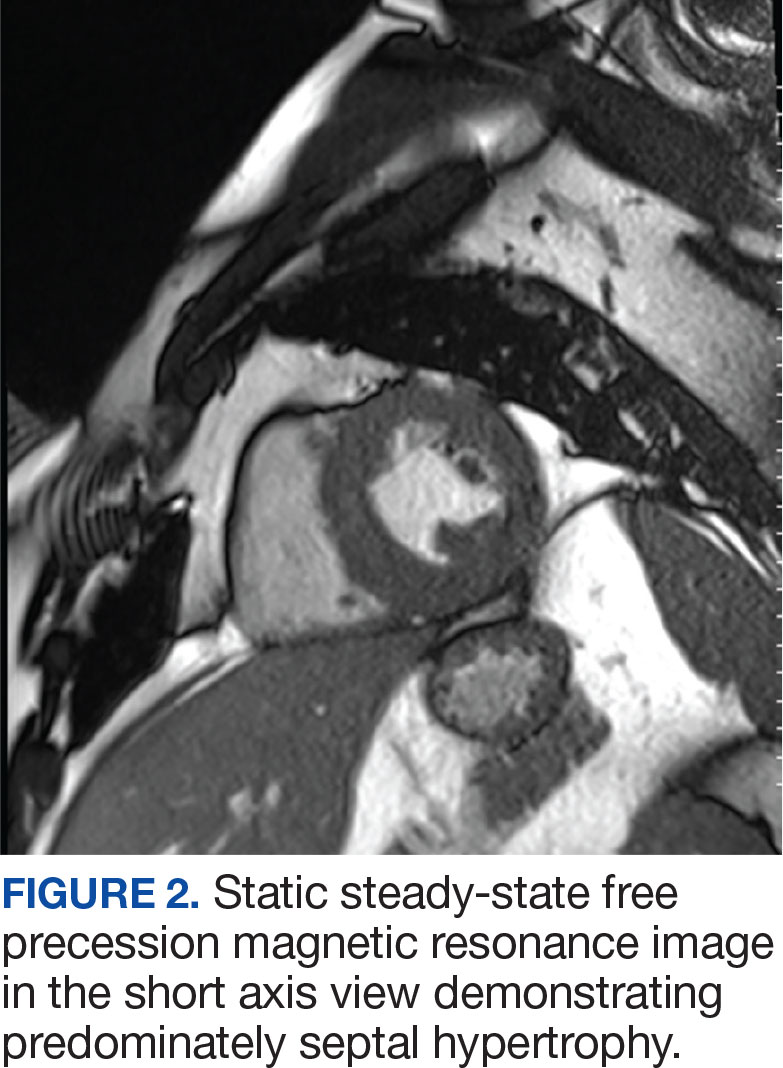
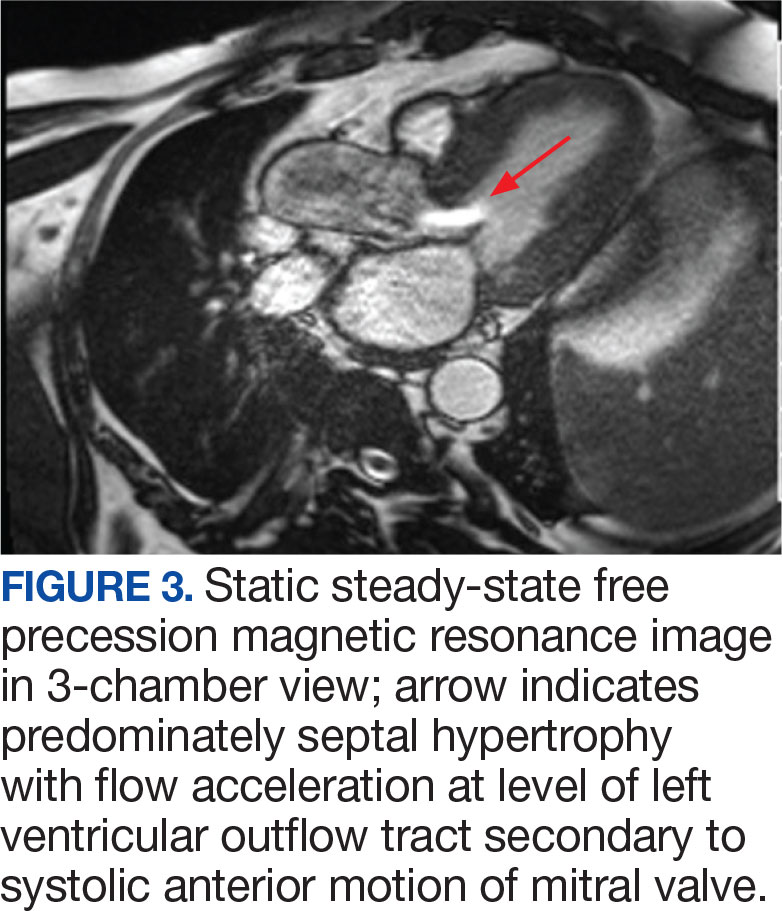
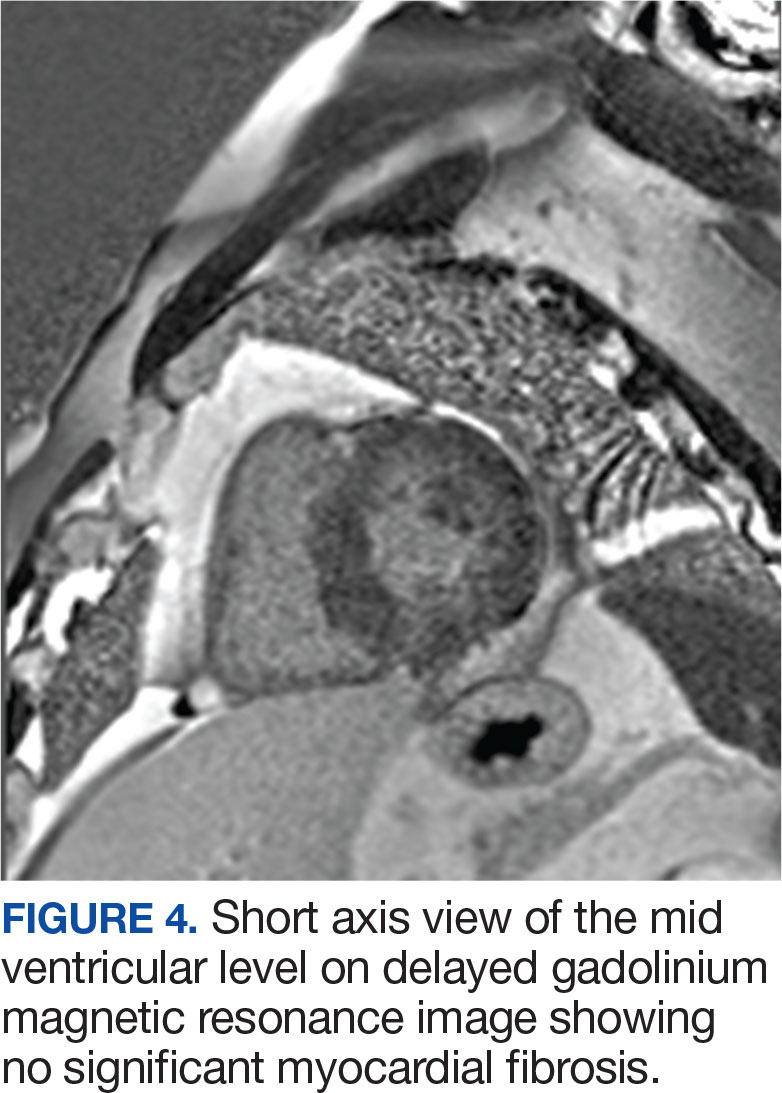
DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic