User login
For MD-IQ use only
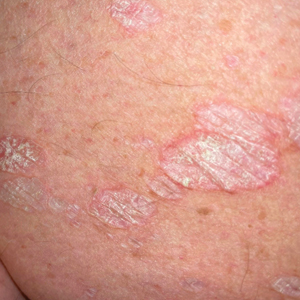
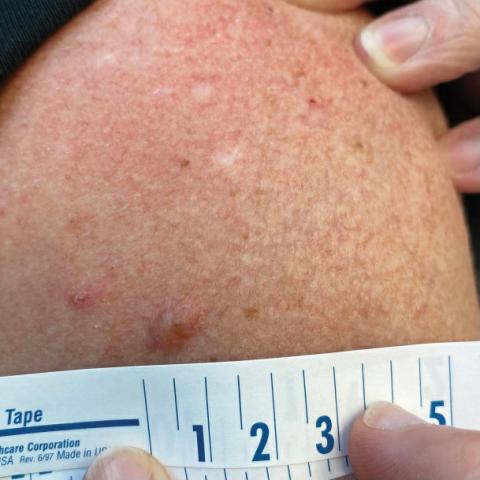
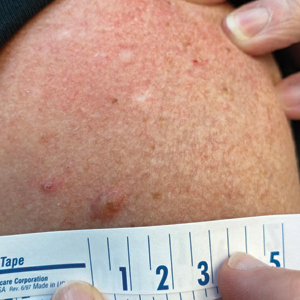
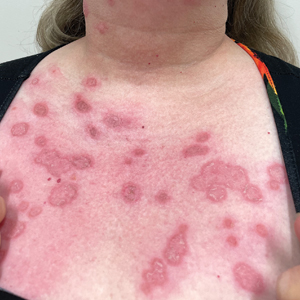
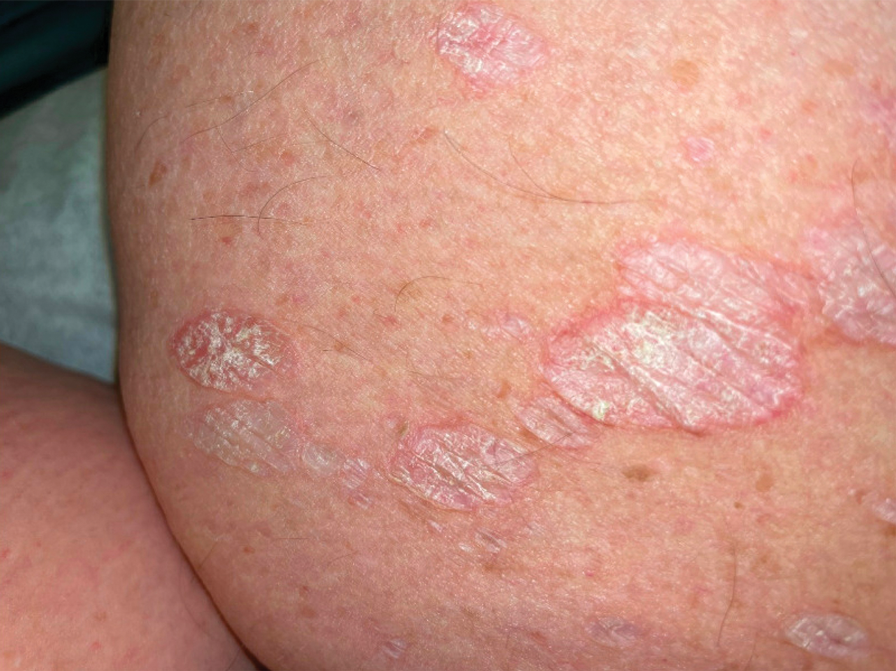
White Atrophic Plaques on the Thighs
White Atrophic Plaques on the Thighs
THE DIAGNOSIS: Lichen Sclerosus
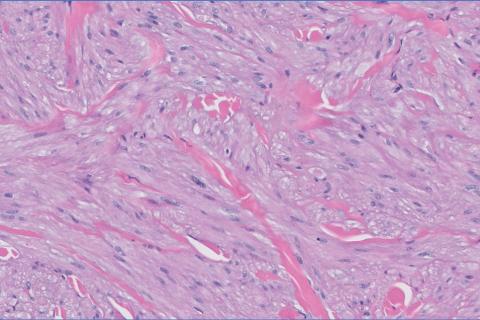
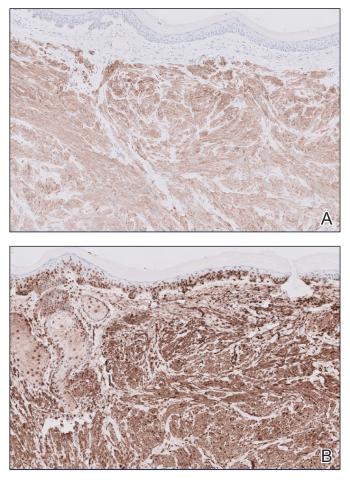
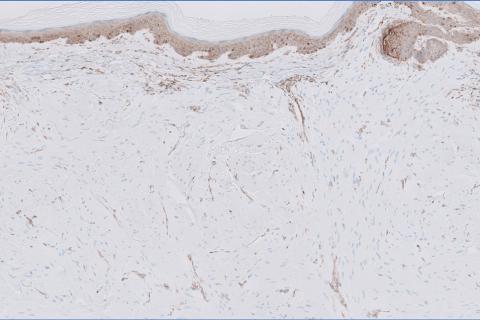
Given the clinical appearance of white atrophic plaques with characteristic wrinkling of the skin, a diagnosis of lichen sclerosus was strongly suspected. At the initial office visit, the patient was prescribed clobetasol 0.05% ointment twice daily for 6 weeks. Histopathology revealed hyperkeratosis, follicular plugging, papillary dermal pallor, and adjacent lymphocytic inflammation, confirming the clinical diagnosis of lichen sclerosus (Figure). The patient then was lost to follow-up.
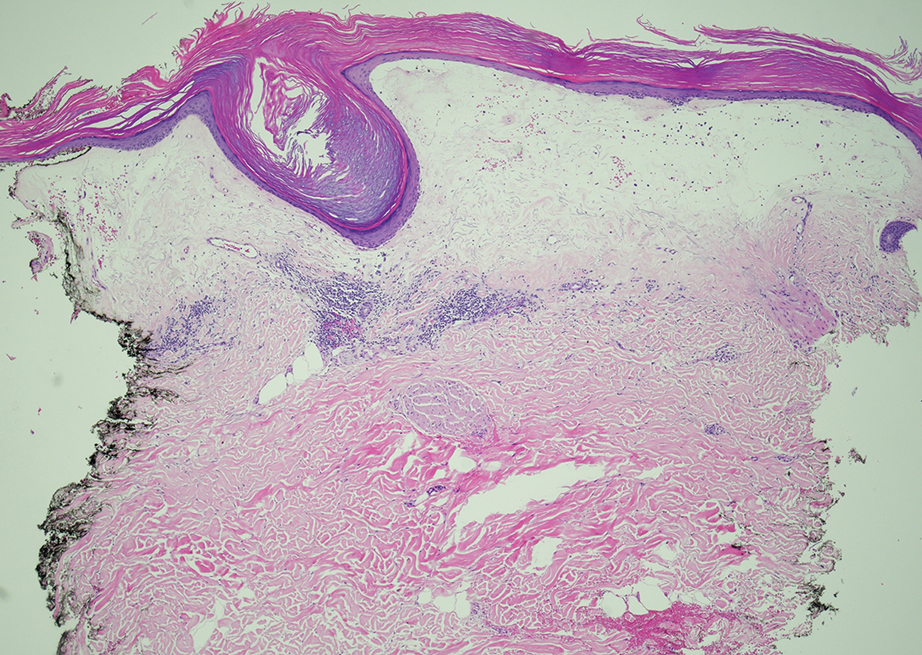
Lichen sclerosus is a chronic benign dermatologic condition of unknown etiology that is characterized by epidermal atrophy and inflammation and is common in postmenopausal women. It features pale, ivory-colored lesions with partially atrophic skin and a wrinkled cigarette paper appearance.1 The differential for lichen sclerosus is broad, and definitive diagnosis is made via biopsy to rule out potential malignancy and other inflammatory skin diseases.1 Lichen sclerosus is an immune-mediated disorder driven by type 1 T helper cells and regulated by miR-155. There has been an association with extracellular matrix protein 1, a glycoprotein that is found in the dermal-epidermal basement membrane zone, which provides structural integrity to the skin. Autoantibodies against extracellular matrix protein 1 and other antigens in the basement membrane generally are found in anogenital lichen sclerosus; however, their precise roles in the pathogenesis of lichen sclerosus remains unclear.1
The differential diagnoses for lichen sclerosus include psoriasis, tinea corporis, lichen simplex chronicus, and atopic dermatitis. Psoriasis typically manifests as pink plaques with silver scales on the elbows, knees, and scalp in adult patients.2 Our patient’s white plaques may have suggested psoriasis, but the partially atrophic skin with a wrinkled cigarette paper appearance was not compatible with that diagnosis.
Tinea corporis, a superficial fungal infection of the skin, manifests as circular or ovoid lesions with raised erythematous scaly borders, often with central clearing resembling a ring, that can occur anywhere on the body other than the feet, groin, face, scalp, or beard area.3 The fact that our patient previously had tried topical antifungal medications with no relief and that the skin lesions were atrophic rather than ring shaped made the diagnosis of tinea corporis unlikely.
Lichen simplex chronicus is a chronic condition caused by friction or scratching that is characterized by dry, patchy, scaly, and thickened areas of the skin. Typically affecting the head, arms, neck, scalp, and genital region, lichen simplex chronicus manifests with violaceous or hyperpigmented lesions.4 The nonpruritic atrophic plaques on the inner thighs and the presence of white patches on the vaginal area were not indicative of lichen simplex chronicus in our patient.
Atopic dermatitis manifests as pruritic erythematous scaly papules and plaques with secondary excoriation and possible lichenification. In adults, atopic dermatitis commonly appears on flexural surfaces.2 Atopic dermatitis does not manifest with atrophy and skin wrinkling as seen in our patient.
In the management of lichen sclerosus, the standard treatment is potent topical corticosteroids. Alternatively, topical calcineurin inhibitors can be employed; however, due to the unknown nature of the condition’s underlying cause, targeted treatment is challenging. Our case underscores how lichen sclerosus can be misdiagnosed, highlighting the need for more frequent reporting in the literature to enhance early recognition and reduce delays in patient treatment.
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389 /fmed.2023.1106318
- Chovatiya R, Silverberg JI. Pathophysiology of atopic dermatitis and psoriasis: implications for management in children. Children (Basel). 2019;6:108. doi:10.3390/children6100108
- Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291.
- Ju T, Vander Does A, Mohsin N, et al. Lichen simplex chronicus itch: an update. Acta Derm Venereol. 2022;102:adv00796. doi:10.2340 /actadv.v102.4367
THE DIAGNOSIS: Lichen Sclerosus
Given the clinical appearance of white atrophic plaques with characteristic wrinkling of the skin, a diagnosis of lichen sclerosus was strongly suspected. At the initial office visit, the patient was prescribed clobetasol 0.05% ointment twice daily for 6 weeks. Histopathology revealed hyperkeratosis, follicular plugging, papillary dermal pallor, and adjacent lymphocytic inflammation, confirming the clinical diagnosis of lichen sclerosus (Figure). The patient then was lost to follow-up.

Lichen sclerosus is a chronic benign dermatologic condition of unknown etiology that is characterized by epidermal atrophy and inflammation and is common in postmenopausal women. It features pale, ivory-colored lesions with partially atrophic skin and a wrinkled cigarette paper appearance.1 The differential for lichen sclerosus is broad, and definitive diagnosis is made via biopsy to rule out potential malignancy and other inflammatory skin diseases.1 Lichen sclerosus is an immune-mediated disorder driven by type 1 T helper cells and regulated by miR-155. There has been an association with extracellular matrix protein 1, a glycoprotein that is found in the dermal-epidermal basement membrane zone, which provides structural integrity to the skin. Autoantibodies against extracellular matrix protein 1 and other antigens in the basement membrane generally are found in anogenital lichen sclerosus; however, their precise roles in the pathogenesis of lichen sclerosus remains unclear.1
The differential diagnoses for lichen sclerosus include psoriasis, tinea corporis, lichen simplex chronicus, and atopic dermatitis. Psoriasis typically manifests as pink plaques with silver scales on the elbows, knees, and scalp in adult patients.2 Our patient’s white plaques may have suggested psoriasis, but the partially atrophic skin with a wrinkled cigarette paper appearance was not compatible with that diagnosis.
Tinea corporis, a superficial fungal infection of the skin, manifests as circular or ovoid lesions with raised erythematous scaly borders, often with central clearing resembling a ring, that can occur anywhere on the body other than the feet, groin, face, scalp, or beard area.3 The fact that our patient previously had tried topical antifungal medications with no relief and that the skin lesions were atrophic rather than ring shaped made the diagnosis of tinea corporis unlikely.
Lichen simplex chronicus is a chronic condition caused by friction or scratching that is characterized by dry, patchy, scaly, and thickened areas of the skin. Typically affecting the head, arms, neck, scalp, and genital region, lichen simplex chronicus manifests with violaceous or hyperpigmented lesions.4 The nonpruritic atrophic plaques on the inner thighs and the presence of white patches on the vaginal area were not indicative of lichen simplex chronicus in our patient.
Atopic dermatitis manifests as pruritic erythematous scaly papules and plaques with secondary excoriation and possible lichenification. In adults, atopic dermatitis commonly appears on flexural surfaces.2 Atopic dermatitis does not manifest with atrophy and skin wrinkling as seen in our patient.
In the management of lichen sclerosus, the standard treatment is potent topical corticosteroids. Alternatively, topical calcineurin inhibitors can be employed; however, due to the unknown nature of the condition’s underlying cause, targeted treatment is challenging. Our case underscores how lichen sclerosus can be misdiagnosed, highlighting the need for more frequent reporting in the literature to enhance early recognition and reduce delays in patient treatment.
THE DIAGNOSIS: Lichen Sclerosus
Given the clinical appearance of white atrophic plaques with characteristic wrinkling of the skin, a diagnosis of lichen sclerosus was strongly suspected. At the initial office visit, the patient was prescribed clobetasol 0.05% ointment twice daily for 6 weeks. Histopathology revealed hyperkeratosis, follicular plugging, papillary dermal pallor, and adjacent lymphocytic inflammation, confirming the clinical diagnosis of lichen sclerosus (Figure). The patient then was lost to follow-up.

Lichen sclerosus is a chronic benign dermatologic condition of unknown etiology that is characterized by epidermal atrophy and inflammation and is common in postmenopausal women. It features pale, ivory-colored lesions with partially atrophic skin and a wrinkled cigarette paper appearance.1 The differential for lichen sclerosus is broad, and definitive diagnosis is made via biopsy to rule out potential malignancy and other inflammatory skin diseases.1 Lichen sclerosus is an immune-mediated disorder driven by type 1 T helper cells and regulated by miR-155. There has been an association with extracellular matrix protein 1, a glycoprotein that is found in the dermal-epidermal basement membrane zone, which provides structural integrity to the skin. Autoantibodies against extracellular matrix protein 1 and other antigens in the basement membrane generally are found in anogenital lichen sclerosus; however, their precise roles in the pathogenesis of lichen sclerosus remains unclear.1
The differential diagnoses for lichen sclerosus include psoriasis, tinea corporis, lichen simplex chronicus, and atopic dermatitis. Psoriasis typically manifests as pink plaques with silver scales on the elbows, knees, and scalp in adult patients.2 Our patient’s white plaques may have suggested psoriasis, but the partially atrophic skin with a wrinkled cigarette paper appearance was not compatible with that diagnosis.
Tinea corporis, a superficial fungal infection of the skin, manifests as circular or ovoid lesions with raised erythematous scaly borders, often with central clearing resembling a ring, that can occur anywhere on the body other than the feet, groin, face, scalp, or beard area.3 The fact that our patient previously had tried topical antifungal medications with no relief and that the skin lesions were atrophic rather than ring shaped made the diagnosis of tinea corporis unlikely.
Lichen simplex chronicus is a chronic condition caused by friction or scratching that is characterized by dry, patchy, scaly, and thickened areas of the skin. Typically affecting the head, arms, neck, scalp, and genital region, lichen simplex chronicus manifests with violaceous or hyperpigmented lesions.4 The nonpruritic atrophic plaques on the inner thighs and the presence of white patches on the vaginal area were not indicative of lichen simplex chronicus in our patient.
Atopic dermatitis manifests as pruritic erythematous scaly papules and plaques with secondary excoriation and possible lichenification. In adults, atopic dermatitis commonly appears on flexural surfaces.2 Atopic dermatitis does not manifest with atrophy and skin wrinkling as seen in our patient.
In the management of lichen sclerosus, the standard treatment is potent topical corticosteroids. Alternatively, topical calcineurin inhibitors can be employed; however, due to the unknown nature of the condition’s underlying cause, targeted treatment is challenging. Our case underscores how lichen sclerosus can be misdiagnosed, highlighting the need for more frequent reporting in the literature to enhance early recognition and reduce delays in patient treatment.
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389 /fmed.2023.1106318
- Chovatiya R, Silverberg JI. Pathophysiology of atopic dermatitis and psoriasis: implications for management in children. Children (Basel). 2019;6:108. doi:10.3390/children6100108
- Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291.
- Ju T, Vander Does A, Mohsin N, et al. Lichen simplex chronicus itch: an update. Acta Derm Venereol. 2022;102:adv00796. doi:10.2340 /actadv.v102.4367
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389 /fmed.2023.1106318
- Chovatiya R, Silverberg JI. Pathophysiology of atopic dermatitis and psoriasis: implications for management in children. Children (Basel). 2019;6:108. doi:10.3390/children6100108
- Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291.
- Ju T, Vander Does A, Mohsin N, et al. Lichen simplex chronicus itch: an update. Acta Derm Venereol. 2022;102:adv00796. doi:10.2340 /actadv.v102.4367
White Atrophic Plaques on the Thighs
White Atrophic Plaques on the Thighs
A 71-year-old woman presented to the dermatology clinic for evaluation of intense pruritus of the vaginal region and a nonpruritic rash on the inner thighs of 7 months’ duration. Physical examination revealed white atrophic plaques with scaling and a wrinkled appearance on the inner thighs. White atrophic patches also were noted on the vulva. The patient reported that she had tried over-the-counter antifungals with no improvement. A punch biopsy was performed.
Gastroenterology Knows No Country
The United States boasts one of the premier health care systems for medical education in the world. Indeed, institutions such as Johns Hopkins, Harvard, and the Mayo Clinic have storied reputations and are recognized names the world over. The United States also stands as a country of remarkable discovery in medicine with an abundance of enormously talented and productive medical scientists. This reputation draws physicians from every corner of the world who dream of studying medicine in our country.
Unfortunately, many US medical institutions, particularly the most prestigious medical centers, lean heavily toward preferential acceptance of US medical school graduates as an indicator of the highest-quality trainees. This historical bias is being further compounded by our current government’s pejorative view of immigrants in general. Will this affect the pool of tomorrow’s stars who will change the course of American medicine?
A glance at the list of recent AGA Presidents may yield some insight; over the past 10 years, three of our presidents trained internationally at universities in Malta, Libya, and Germany. This is a small snapshot of the multitude of international graduates in gastroenterology and hepatology who have served as division chiefs, AGA award winners, and journal editors, all now US citizens. This is not to mention the influence of varied insights and talents native to international study and culture that enhance our practice of medicine and biomedical research.
We live in time when “immigrant” has been assigned a negative and almost subhuman connotation, and diversity has become something to be demonized rather than celebrated. Yet, intuitively, should a top US medical graduate be any more intelligent or driven than a top graduate from the United Kingdom, India, China, or Syria? As American medical physicians, we place the utmost value on our traditions and high standards. We boast an unmatched depth of medical talent spread across our GI divisions and practices and take pride in the way we teach medicine, like no other nation. American medicine benefits from their talent and they inspire us to remember and care for diseases in our field that affect the world’s population, not just ours.
Over 100 years ago, Dr. William Mayo stated “American practice is too broad to be national. It had the scientific spirit, and science knows no country.” Dr. Mayo also said, “Democracy is safe only so long as culture is in the ascendancy.” These lessons apply more than ever today.
David Katzka, MD
Associate Editor
The United States boasts one of the premier health care systems for medical education in the world. Indeed, institutions such as Johns Hopkins, Harvard, and the Mayo Clinic have storied reputations and are recognized names the world over. The United States also stands as a country of remarkable discovery in medicine with an abundance of enormously talented and productive medical scientists. This reputation draws physicians from every corner of the world who dream of studying medicine in our country.
Unfortunately, many US medical institutions, particularly the most prestigious medical centers, lean heavily toward preferential acceptance of US medical school graduates as an indicator of the highest-quality trainees. This historical bias is being further compounded by our current government’s pejorative view of immigrants in general. Will this affect the pool of tomorrow’s stars who will change the course of American medicine?
A glance at the list of recent AGA Presidents may yield some insight; over the past 10 years, three of our presidents trained internationally at universities in Malta, Libya, and Germany. This is a small snapshot of the multitude of international graduates in gastroenterology and hepatology who have served as division chiefs, AGA award winners, and journal editors, all now US citizens. This is not to mention the influence of varied insights and talents native to international study and culture that enhance our practice of medicine and biomedical research.
We live in time when “immigrant” has been assigned a negative and almost subhuman connotation, and diversity has become something to be demonized rather than celebrated. Yet, intuitively, should a top US medical graduate be any more intelligent or driven than a top graduate from the United Kingdom, India, China, or Syria? As American medical physicians, we place the utmost value on our traditions and high standards. We boast an unmatched depth of medical talent spread across our GI divisions and practices and take pride in the way we teach medicine, like no other nation. American medicine benefits from their talent and they inspire us to remember and care for diseases in our field that affect the world’s population, not just ours.
Over 100 years ago, Dr. William Mayo stated “American practice is too broad to be national. It had the scientific spirit, and science knows no country.” Dr. Mayo also said, “Democracy is safe only so long as culture is in the ascendancy.” These lessons apply more than ever today.
David Katzka, MD
Associate Editor
The United States boasts one of the premier health care systems for medical education in the world. Indeed, institutions such as Johns Hopkins, Harvard, and the Mayo Clinic have storied reputations and are recognized names the world over. The United States also stands as a country of remarkable discovery in medicine with an abundance of enormously talented and productive medical scientists. This reputation draws physicians from every corner of the world who dream of studying medicine in our country.
Unfortunately, many US medical institutions, particularly the most prestigious medical centers, lean heavily toward preferential acceptance of US medical school graduates as an indicator of the highest-quality trainees. This historical bias is being further compounded by our current government’s pejorative view of immigrants in general. Will this affect the pool of tomorrow’s stars who will change the course of American medicine?
A glance at the list of recent AGA Presidents may yield some insight; over the past 10 years, three of our presidents trained internationally at universities in Malta, Libya, and Germany. This is a small snapshot of the multitude of international graduates in gastroenterology and hepatology who have served as division chiefs, AGA award winners, and journal editors, all now US citizens. This is not to mention the influence of varied insights and talents native to international study and culture that enhance our practice of medicine and biomedical research.
We live in time when “immigrant” has been assigned a negative and almost subhuman connotation, and diversity has become something to be demonized rather than celebrated. Yet, intuitively, should a top US medical graduate be any more intelligent or driven than a top graduate from the United Kingdom, India, China, or Syria? As American medical physicians, we place the utmost value on our traditions and high standards. We boast an unmatched depth of medical talent spread across our GI divisions and practices and take pride in the way we teach medicine, like no other nation. American medicine benefits from their talent and they inspire us to remember and care for diseases in our field that affect the world’s population, not just ours.
Over 100 years ago, Dr. William Mayo stated “American practice is too broad to be national. It had the scientific spirit, and science knows no country.” Dr. Mayo also said, “Democracy is safe only so long as culture is in the ascendancy.” These lessons apply more than ever today.
David Katzka, MD
Associate Editor
How Doctors Use Travel to Heal Themselves
Whatever’s ailing you, a vacation might just be the cure. Yes, getting away can improve your health, according to research published in in 2023. It might help combat symptoms of aging, suggested a 2024 study in Journal of Travel Research. But it could also have even more powerful psychological and physical benefits, transforming your life before you pack a bag and long after you return home.
This news organization spoke with two healthcare professionals who believe in the healing power of travel. They shared which personal “diagnoses” they have successfully treated with faraway places and how this therapy might work for you.
Stacey Funt, MD, NBC-HWC, a radiologist at Northwell Health in Long Island, New York, started the boutique wellness adventure travel company, LH Adventure Travel, in 2023. Funt curates and leads small groups to destinations like Peru, Guatemala, Morocco, and Italy. Each tour incorporates tenets of lifestyle medicine, including healthy eating, movement, stress management, and community building.
Kiya Thompson, RN, a surgical trauma nurse for 20 years, was similarly inspired to share her passion for travel. She is now a certified family travel coach who helps parents plan meaningful trips through her company, LuckyBucky, LLC.
Dx: Self-Esteem Deficiency / Rx: Vivaldi in Venice
In June 2015, Thompson found herself at an all-time low. As a nurse, she felt confident that she was “built for the adrenaline rush and could take on anything.” But outside the trauma center, Thompson felt inadequate, her self-esteem eroded by years of abusive relationships. “The daily hardships of my personal life, combined with the mental fortitude it took to endure the demands of caring for the sickest of the sick, were incredibly weighty,” she recalled.
To escape, Thompson booked her first solo trip: 3 weeks in Italy. But days after she arrived, she felt the need to “escape her escape.” On a bus in Naples, she was pick-pocketed. The man she had been dating before her trip stopped responding to her messages. In her hotel room in Venice, she felt “lost, alone, and helpless.”
One evening, Thompson attended a small orchestral performance of Vivaldi’s “The Four Seasons” in a centuries-old church. The music triggered memories of her Italian grandparents at whose home she’d listened to the same piece.
“A switch flipped, and I changed my whole outlook,” she remembers.
During the concert, she reflected on strangers who had shown her kindness and care. A Canadian man who gave her €50 after her wallet was stolen. A friend-of-a-friend who showed her around Rome. The clerk at her Venice hotel who had offered her a hug.
“In the wake of experiencing the worst of people, I’d experienced so much more of the best of people; strangers who were willing to go above and beyond to help me,” Thompson said.
When Thompson returned home, she brought her new mindset along. “ My ability to problem-solve my way through a solo trip that presented unexpected hardships empowered me,” she explained. “I learned I was much more capable than I’d thought.”
Dx: Wilderness Phobia / Rx: A Safari in Tanzania
On an evening in the mid-1990s, Funt was alone in a tent on a budget camping safari in Tanzania. Animals roared threateningly outside the thin walls. Earlier that day, a vulture had ripped a sandwich out of her hands. Funt was frightened to the core. Worrying that she’d be the next meal for the local wildlife, she started to sob. “This was as raw as I had ever gotten at that point in my life,” she said.
Suddenly, Funt said her brain shifted into problem-solving mode. She made one small decision: To switch to a different Jeep for the next day’s excursion. Having made a seemingly insignificant choice, she felt calmer and no longer like a victim. It brought control. Instead of worrying, she began looking forward to the wildlife she would see.
In the morning, in the new Jeep, she befriended a nurse from Canada. Together, they visited the Maasai Mara tribe and nearby pubs, meeting members of the community.
“It was the most exciting experience of my life,” Funt said. “And it had started with me crying.”
Dx: Parenting-itis / Rx: A Mountain Getaway
As Thompson pointed out, sometimes the destination is secondary to the intension behind a trip. And the quality of the time away matters more than how long you can stay. After becoming parents 4 years ago, Thompson and her husband hadn’t traveled alone together. Like many parents of young children, they were short on time to relax and reconnect as a couple.
So Thompson planned a weekend trip to an isolated cabin in the Massanutten Mountain Range within the George Washington National Forest, about a 2-hour drive from their Washington, DC, area home.
“We put our devices away and focused on being completely present with one another,” said Thompson. The couple took a walk in the woods, where “all we could hear were drops of water from the snowmelt, the crunch of the snow beneath our feet, and the occasional bird looking for food,” she recalled. “There were no cars, no other people. It was quiet, calm, and incredibly peaceful.”
Whether sitting by the fire, soaking in the outdoor hot tub, or playing card games, “our conversation didn’t surround what we’d have for dinner or who would do baths and bedtime with whom,” Thompson said. “We didn’t talk about work, upcoming commitments, or items on our to-do lists.” The getaway was so refreshing, the couple intend to repeat the trip each year.
Dx: Persistent Grief / Rx: Hiking and Hinduism in Nepal
Nearly 3 years ago, Funt experienced a 2-month period where both of her kids left for college and both her father and father-in-law passed away. Besieged by grief, she found herself questioning whether her best years were behind her. She was also grappling with her mortality, because she was then approaching 59, the age at which her own mother had died. So Funt decided to go trekking in Nepal. “I am a traveler — it’s what I do,” she said.
Having the trip to prepare for changed Funt’s whole outlook, she remembers. Throwing herself into the planning helped her transcend her grief. But being in Nepal was even more impactful. She and her husband spent hours trekking through majestic mountain ranges, which “touched their souls.” At a crematorium, they learned about Hindu beliefs on death, which helped them with the grieving process.
The trip “lifted me so high up on so many levels and brought me back to my authentic self,” Funt said. On her flight home from Kathmandu, she decided to start her travel business.
“I needed something else [in addition to radiology] to put my passion, heart, and creativity into, and it would be another way of doing service,” she explained.
Dx: Couch Potato Syndrome / Rx: Planning an Adventure
Like all of us, Funt knows exercise is important for health. But that knowledge alone doesn’t motivate her to move, she admitted. What does get her off the couch is scheduling an active trip — and then training for it. “When I have a goal tied to my values of adventure, connection, and community, fear will set in if I don’t start to move,” she said. It was after booking her Nepal trip (which included an 8-mile, 3000-foot trek) that Funt started getting in shape.
Travel has motivated Funt’s clients in similar ways. Last year, 8 months before one of her Morocco trips, Funt spoke over Zoom with a woman who’d just enrolled. This woman told her she’d signed up in order to commit to her health.
By the time Funt saw her again, on day 1 of the trip, the woman had lost 50 pounds. “It was the greatest transformation,” Funt recalled. “On the trip, she was the first one up the mountain and beamed the whole time. It was beautiful to watch her reclaim her power, body, and life.”
Getting Lost — Finding Inspiration
Since Thompson’s trip to Italy, she has traveled extensively, visiting nearly 25 countries. “Traveling inspired me to continue exploring the world and myself,” she said.
Since leading her first trip to Morocco in 2023, Funt said she’s received more letters of appreciation from her clients than her patients. The results from this type of travel therapy can be dramatic.
After a trip with Funt, one burned-out physician decided that she needed to find a job with a better work-life balance. An empty nester realized the “feeling of belonging and community” on the trip was what had been missing in her “regular” life. After returning home, she began rekindling relationships with old friends.
To many, a vacation is a treat. But, as Funt and Thompson have learned firsthand, it can also be a prescription — for ennui, sadness, loneliness, and all the physical issues that come with them. Sometimes, going far away helps you come home to yourself.
A version of this article first appeared on Medscape.com.
Whatever’s ailing you, a vacation might just be the cure. Yes, getting away can improve your health, according to research published in in 2023. It might help combat symptoms of aging, suggested a 2024 study in Journal of Travel Research. But it could also have even more powerful psychological and physical benefits, transforming your life before you pack a bag and long after you return home.
This news organization spoke with two healthcare professionals who believe in the healing power of travel. They shared which personal “diagnoses” they have successfully treated with faraway places and how this therapy might work for you.
Stacey Funt, MD, NBC-HWC, a radiologist at Northwell Health in Long Island, New York, started the boutique wellness adventure travel company, LH Adventure Travel, in 2023. Funt curates and leads small groups to destinations like Peru, Guatemala, Morocco, and Italy. Each tour incorporates tenets of lifestyle medicine, including healthy eating, movement, stress management, and community building.
Kiya Thompson, RN, a surgical trauma nurse for 20 years, was similarly inspired to share her passion for travel. She is now a certified family travel coach who helps parents plan meaningful trips through her company, LuckyBucky, LLC.
Dx: Self-Esteem Deficiency / Rx: Vivaldi in Venice
In June 2015, Thompson found herself at an all-time low. As a nurse, she felt confident that she was “built for the adrenaline rush and could take on anything.” But outside the trauma center, Thompson felt inadequate, her self-esteem eroded by years of abusive relationships. “The daily hardships of my personal life, combined with the mental fortitude it took to endure the demands of caring for the sickest of the sick, were incredibly weighty,” she recalled.
To escape, Thompson booked her first solo trip: 3 weeks in Italy. But days after she arrived, she felt the need to “escape her escape.” On a bus in Naples, she was pick-pocketed. The man she had been dating before her trip stopped responding to her messages. In her hotel room in Venice, she felt “lost, alone, and helpless.”
One evening, Thompson attended a small orchestral performance of Vivaldi’s “The Four Seasons” in a centuries-old church. The music triggered memories of her Italian grandparents at whose home she’d listened to the same piece.
“A switch flipped, and I changed my whole outlook,” she remembers.
During the concert, she reflected on strangers who had shown her kindness and care. A Canadian man who gave her €50 after her wallet was stolen. A friend-of-a-friend who showed her around Rome. The clerk at her Venice hotel who had offered her a hug.
“In the wake of experiencing the worst of people, I’d experienced so much more of the best of people; strangers who were willing to go above and beyond to help me,” Thompson said.
When Thompson returned home, she brought her new mindset along. “ My ability to problem-solve my way through a solo trip that presented unexpected hardships empowered me,” she explained. “I learned I was much more capable than I’d thought.”
Dx: Wilderness Phobia / Rx: A Safari in Tanzania
On an evening in the mid-1990s, Funt was alone in a tent on a budget camping safari in Tanzania. Animals roared threateningly outside the thin walls. Earlier that day, a vulture had ripped a sandwich out of her hands. Funt was frightened to the core. Worrying that she’d be the next meal for the local wildlife, she started to sob. “This was as raw as I had ever gotten at that point in my life,” she said.
Suddenly, Funt said her brain shifted into problem-solving mode. She made one small decision: To switch to a different Jeep for the next day’s excursion. Having made a seemingly insignificant choice, she felt calmer and no longer like a victim. It brought control. Instead of worrying, she began looking forward to the wildlife she would see.
In the morning, in the new Jeep, she befriended a nurse from Canada. Together, they visited the Maasai Mara tribe and nearby pubs, meeting members of the community.
“It was the most exciting experience of my life,” Funt said. “And it had started with me crying.”
Dx: Parenting-itis / Rx: A Mountain Getaway
As Thompson pointed out, sometimes the destination is secondary to the intension behind a trip. And the quality of the time away matters more than how long you can stay. After becoming parents 4 years ago, Thompson and her husband hadn’t traveled alone together. Like many parents of young children, they were short on time to relax and reconnect as a couple.
So Thompson planned a weekend trip to an isolated cabin in the Massanutten Mountain Range within the George Washington National Forest, about a 2-hour drive from their Washington, DC, area home.
“We put our devices away and focused on being completely present with one another,” said Thompson. The couple took a walk in the woods, where “all we could hear were drops of water from the snowmelt, the crunch of the snow beneath our feet, and the occasional bird looking for food,” she recalled. “There were no cars, no other people. It was quiet, calm, and incredibly peaceful.”
Whether sitting by the fire, soaking in the outdoor hot tub, or playing card games, “our conversation didn’t surround what we’d have for dinner or who would do baths and bedtime with whom,” Thompson said. “We didn’t talk about work, upcoming commitments, or items on our to-do lists.” The getaway was so refreshing, the couple intend to repeat the trip each year.
Dx: Persistent Grief / Rx: Hiking and Hinduism in Nepal
Nearly 3 years ago, Funt experienced a 2-month period where both of her kids left for college and both her father and father-in-law passed away. Besieged by grief, she found herself questioning whether her best years were behind her. She was also grappling with her mortality, because she was then approaching 59, the age at which her own mother had died. So Funt decided to go trekking in Nepal. “I am a traveler — it’s what I do,” she said.
Having the trip to prepare for changed Funt’s whole outlook, she remembers. Throwing herself into the planning helped her transcend her grief. But being in Nepal was even more impactful. She and her husband spent hours trekking through majestic mountain ranges, which “touched their souls.” At a crematorium, they learned about Hindu beliefs on death, which helped them with the grieving process.
The trip “lifted me so high up on so many levels and brought me back to my authentic self,” Funt said. On her flight home from Kathmandu, she decided to start her travel business.
“I needed something else [in addition to radiology] to put my passion, heart, and creativity into, and it would be another way of doing service,” she explained.
Dx: Couch Potato Syndrome / Rx: Planning an Adventure
Like all of us, Funt knows exercise is important for health. But that knowledge alone doesn’t motivate her to move, she admitted. What does get her off the couch is scheduling an active trip — and then training for it. “When I have a goal tied to my values of adventure, connection, and community, fear will set in if I don’t start to move,” she said. It was after booking her Nepal trip (which included an 8-mile, 3000-foot trek) that Funt started getting in shape.
Travel has motivated Funt’s clients in similar ways. Last year, 8 months before one of her Morocco trips, Funt spoke over Zoom with a woman who’d just enrolled. This woman told her she’d signed up in order to commit to her health.
By the time Funt saw her again, on day 1 of the trip, the woman had lost 50 pounds. “It was the greatest transformation,” Funt recalled. “On the trip, she was the first one up the mountain and beamed the whole time. It was beautiful to watch her reclaim her power, body, and life.”
Getting Lost — Finding Inspiration
Since Thompson’s trip to Italy, she has traveled extensively, visiting nearly 25 countries. “Traveling inspired me to continue exploring the world and myself,” she said.
Since leading her first trip to Morocco in 2023, Funt said she’s received more letters of appreciation from her clients than her patients. The results from this type of travel therapy can be dramatic.
After a trip with Funt, one burned-out physician decided that she needed to find a job with a better work-life balance. An empty nester realized the “feeling of belonging and community” on the trip was what had been missing in her “regular” life. After returning home, she began rekindling relationships with old friends.
To many, a vacation is a treat. But, as Funt and Thompson have learned firsthand, it can also be a prescription — for ennui, sadness, loneliness, and all the physical issues that come with them. Sometimes, going far away helps you come home to yourself.
A version of this article first appeared on Medscape.com.
Whatever’s ailing you, a vacation might just be the cure. Yes, getting away can improve your health, according to research published in in 2023. It might help combat symptoms of aging, suggested a 2024 study in Journal of Travel Research. But it could also have even more powerful psychological and physical benefits, transforming your life before you pack a bag and long after you return home.
This news organization spoke with two healthcare professionals who believe in the healing power of travel. They shared which personal “diagnoses” they have successfully treated with faraway places and how this therapy might work for you.
Stacey Funt, MD, NBC-HWC, a radiologist at Northwell Health in Long Island, New York, started the boutique wellness adventure travel company, LH Adventure Travel, in 2023. Funt curates and leads small groups to destinations like Peru, Guatemala, Morocco, and Italy. Each tour incorporates tenets of lifestyle medicine, including healthy eating, movement, stress management, and community building.
Kiya Thompson, RN, a surgical trauma nurse for 20 years, was similarly inspired to share her passion for travel. She is now a certified family travel coach who helps parents plan meaningful trips through her company, LuckyBucky, LLC.
Dx: Self-Esteem Deficiency / Rx: Vivaldi in Venice
In June 2015, Thompson found herself at an all-time low. As a nurse, she felt confident that she was “built for the adrenaline rush and could take on anything.” But outside the trauma center, Thompson felt inadequate, her self-esteem eroded by years of abusive relationships. “The daily hardships of my personal life, combined with the mental fortitude it took to endure the demands of caring for the sickest of the sick, were incredibly weighty,” she recalled.
To escape, Thompson booked her first solo trip: 3 weeks in Italy. But days after she arrived, she felt the need to “escape her escape.” On a bus in Naples, she was pick-pocketed. The man she had been dating before her trip stopped responding to her messages. In her hotel room in Venice, she felt “lost, alone, and helpless.”
One evening, Thompson attended a small orchestral performance of Vivaldi’s “The Four Seasons” in a centuries-old church. The music triggered memories of her Italian grandparents at whose home she’d listened to the same piece.
“A switch flipped, and I changed my whole outlook,” she remembers.
During the concert, she reflected on strangers who had shown her kindness and care. A Canadian man who gave her €50 after her wallet was stolen. A friend-of-a-friend who showed her around Rome. The clerk at her Venice hotel who had offered her a hug.
“In the wake of experiencing the worst of people, I’d experienced so much more of the best of people; strangers who were willing to go above and beyond to help me,” Thompson said.
When Thompson returned home, she brought her new mindset along. “ My ability to problem-solve my way through a solo trip that presented unexpected hardships empowered me,” she explained. “I learned I was much more capable than I’d thought.”
Dx: Wilderness Phobia / Rx: A Safari in Tanzania
On an evening in the mid-1990s, Funt was alone in a tent on a budget camping safari in Tanzania. Animals roared threateningly outside the thin walls. Earlier that day, a vulture had ripped a sandwich out of her hands. Funt was frightened to the core. Worrying that she’d be the next meal for the local wildlife, she started to sob. “This was as raw as I had ever gotten at that point in my life,” she said.
Suddenly, Funt said her brain shifted into problem-solving mode. She made one small decision: To switch to a different Jeep for the next day’s excursion. Having made a seemingly insignificant choice, she felt calmer and no longer like a victim. It brought control. Instead of worrying, she began looking forward to the wildlife she would see.
In the morning, in the new Jeep, she befriended a nurse from Canada. Together, they visited the Maasai Mara tribe and nearby pubs, meeting members of the community.
“It was the most exciting experience of my life,” Funt said. “And it had started with me crying.”
Dx: Parenting-itis / Rx: A Mountain Getaway
As Thompson pointed out, sometimes the destination is secondary to the intension behind a trip. And the quality of the time away matters more than how long you can stay. After becoming parents 4 years ago, Thompson and her husband hadn’t traveled alone together. Like many parents of young children, they were short on time to relax and reconnect as a couple.
So Thompson planned a weekend trip to an isolated cabin in the Massanutten Mountain Range within the George Washington National Forest, about a 2-hour drive from their Washington, DC, area home.
“We put our devices away and focused on being completely present with one another,” said Thompson. The couple took a walk in the woods, where “all we could hear were drops of water from the snowmelt, the crunch of the snow beneath our feet, and the occasional bird looking for food,” she recalled. “There were no cars, no other people. It was quiet, calm, and incredibly peaceful.”
Whether sitting by the fire, soaking in the outdoor hot tub, or playing card games, “our conversation didn’t surround what we’d have for dinner or who would do baths and bedtime with whom,” Thompson said. “We didn’t talk about work, upcoming commitments, or items on our to-do lists.” The getaway was so refreshing, the couple intend to repeat the trip each year.
Dx: Persistent Grief / Rx: Hiking and Hinduism in Nepal
Nearly 3 years ago, Funt experienced a 2-month period where both of her kids left for college and both her father and father-in-law passed away. Besieged by grief, she found herself questioning whether her best years were behind her. She was also grappling with her mortality, because she was then approaching 59, the age at which her own mother had died. So Funt decided to go trekking in Nepal. “I am a traveler — it’s what I do,” she said.
Having the trip to prepare for changed Funt’s whole outlook, she remembers. Throwing herself into the planning helped her transcend her grief. But being in Nepal was even more impactful. She and her husband spent hours trekking through majestic mountain ranges, which “touched their souls.” At a crematorium, they learned about Hindu beliefs on death, which helped them with the grieving process.
The trip “lifted me so high up on so many levels and brought me back to my authentic self,” Funt said. On her flight home from Kathmandu, she decided to start her travel business.
“I needed something else [in addition to radiology] to put my passion, heart, and creativity into, and it would be another way of doing service,” she explained.
Dx: Couch Potato Syndrome / Rx: Planning an Adventure
Like all of us, Funt knows exercise is important for health. But that knowledge alone doesn’t motivate her to move, she admitted. What does get her off the couch is scheduling an active trip — and then training for it. “When I have a goal tied to my values of adventure, connection, and community, fear will set in if I don’t start to move,” she said. It was after booking her Nepal trip (which included an 8-mile, 3000-foot trek) that Funt started getting in shape.
Travel has motivated Funt’s clients in similar ways. Last year, 8 months before one of her Morocco trips, Funt spoke over Zoom with a woman who’d just enrolled. This woman told her she’d signed up in order to commit to her health.
By the time Funt saw her again, on day 1 of the trip, the woman had lost 50 pounds. “It was the greatest transformation,” Funt recalled. “On the trip, she was the first one up the mountain and beamed the whole time. It was beautiful to watch her reclaim her power, body, and life.”
Getting Lost — Finding Inspiration
Since Thompson’s trip to Italy, she has traveled extensively, visiting nearly 25 countries. “Traveling inspired me to continue exploring the world and myself,” she said.
Since leading her first trip to Morocco in 2023, Funt said she’s received more letters of appreciation from her clients than her patients. The results from this type of travel therapy can be dramatic.
After a trip with Funt, one burned-out physician decided that she needed to find a job with a better work-life balance. An empty nester realized the “feeling of belonging and community” on the trip was what had been missing in her “regular” life. After returning home, she began rekindling relationships with old friends.
To many, a vacation is a treat. But, as Funt and Thompson have learned firsthand, it can also be a prescription — for ennui, sadness, loneliness, and all the physical issues that come with them. Sometimes, going far away helps you come home to yourself.
A version of this article first appeared on Medscape.com.
Scholarly Activity Among VA Podiatrists: A Cross-Sectional Study
Scholarly Activity Among VA Podiatrists: A Cross-Sectional Study
The US Department of Veterans Affairs (VA) delivers care to > 9 million veterans, including primary and specialty care.1 While clinical duties remain important across the health system, proposed productivity models have included clinician research activity, given that many hold roles in academia.2 Within this framework, research plays a pivotal role in advancing clinical practices and outcomes. Studies have found that physicians who participated in research report higher job satisfaction.3
As a specialty within the VA, podiatrists diagnose, treat, and prevent foot and ankle disorders. In addition to clinical practice, various scholarly activities are shared among these physicians.4 Reasons for scholarly pursuits among podiatrists vary, including participation in research for academic promotion or to establish expertise in a given area.4-7 Although research remains a component associated with promotion within the VA, little is known about the scholarly activity of VA podiatrists. Specifically, there remains a paucity of data concerning their expertise, as evidenced through peer-reviewed publications, among these physicians and surgeons. To date, no analysis of scholarly activity among VA podiatrists has been conducted.
The primary aim of this investigation was to describe the scholarly productivity among podiatrists employed by the VA through an analysis of the number of peer-reviewed publications and the respective h-index of each physician. The secondary aim of this investigation was to assess the effect of academic productivity on compensation. This study describes research activities pursued by VA physicians and provides the veteran patient population with the confidence that their foot health care remains in the hands of experts within the field.
MATERIALS AND METHODS
The Feds Data Center (www.fedsdatacenter.com) online database of employees was used to identify VA podiatrists on June 17, 2024. All GS-15 physicians and their respective salaries in fiscal year 2023 were recorded. Administratively determined employees, including residents, were excluded. The h-index and number of published documents from any point during a physician’s training or career were reported for each podiatrist using Scopus; podiatrists without an h-index or publication were excluded. 8 Among podiatrists with scholarly activity, this analysis collected academic appointment, sex, and region of practice.
Statistical Analysis
Descriptive statistics, presented as counts and frequencies, were used. The median and IQR were used to describe the number of publications and h-index due to their nonnormal distribution. A Kruskal-Wallis test was used to compare median publication counts and h-index values among for junior faculty (JF), which includes instructors and assistant professors; senior faculty (SF), which includes associate professors and professors; and those with no academic affiliation (NF). Salary was reported as mean (SD) as it remained normally distributed and was compared using analysis of variance with posthoc Tukey test to increase statistical power. Additionally, this analysis used linear regression to investigate the relationship between scholarly activity and salary. The threshold for statistical significance was set at P < .05.
RESULTS
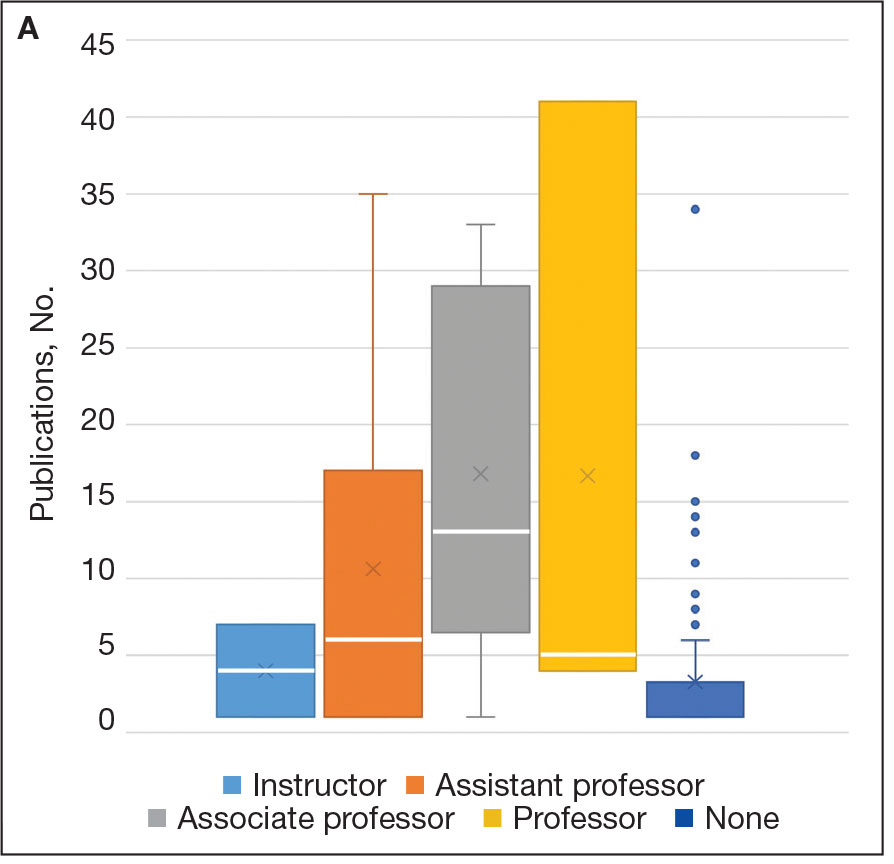
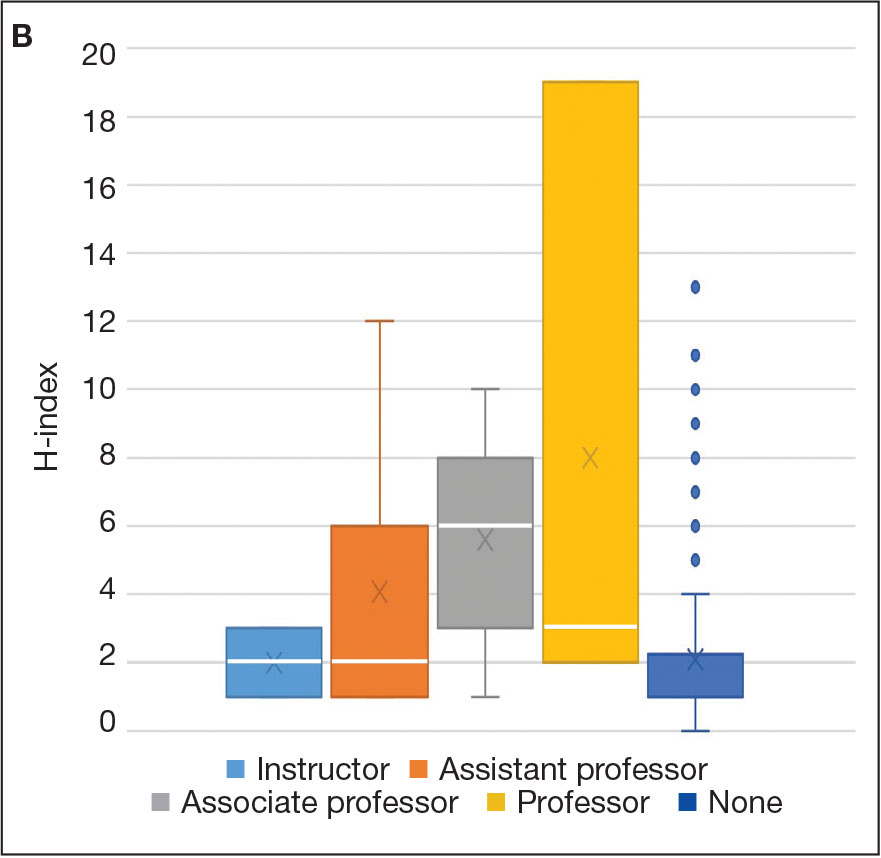
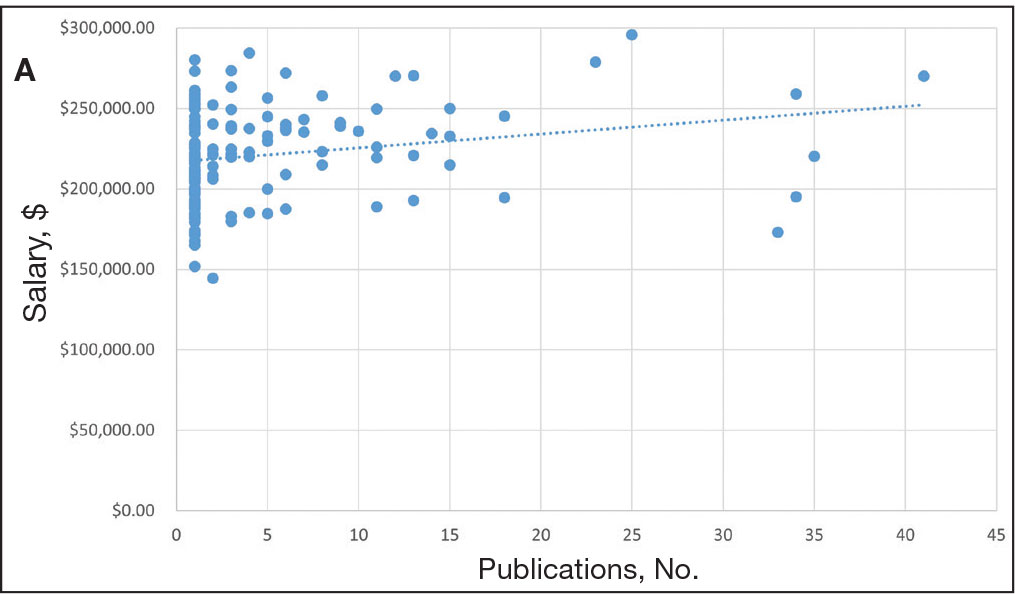
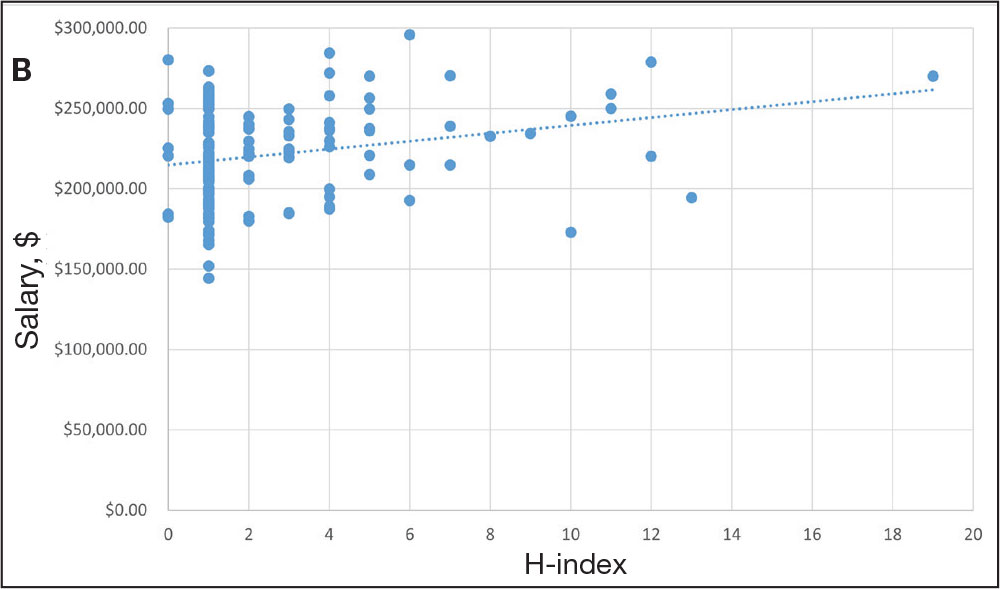
Among 819 VA podiatrists, 150 were administratively determined and excluded, and 512 were excluded for no history of publications, leaving 157 eligible for analysis (Table). A statistically significant difference was found in median (IQR) publication count by faculty appointment. JF had 6.0 (9.5), SF had 12.5 (22.3), and NF had 1.0 (2.0) publication(s) (P < .001) (Figure 1A). There was a statistically significant difference in h-index by faculty appointment. The median (IQR) h-index for JF was 2.0 (3.5), for SF was 5.5 (4.25), and for NF was 1.0 (2.0) (P = .002) (Figure 1B). Salary was not significantly associated with publication count (P = .20) or h-index (P = .62) (Figure 2). No statistically significant difference was found between academic appointment and mean (SD) salary. JF had a median (IQR) salary of $224,063 (27,989), SF of $234,260 (42,963), and NF of $219,811 (P = .35).
(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.
(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.
DISCUSSION
Focused on providing high-quality care, VA physicians use their expertise to practice comprehensive and specialized care.9,10 A cornerstone to this expertise is scholarly activity that contributes to the body of knowledge and, ultimately, the evidence-based medicine by which these physicians practice.11 With veterans considering VA care, it is important to highlight the commitment and dedication to the science and the practice of medicine. This analysis describes the scholarly activity of VA podiatrists and underscores the expertise veterans will receive for the diagnosis and treatment of their foot and ankle pathology.
were not part of an academic facility, a finding that may encourage further action to increase academic productivity in this specialty. For example, collaboration through academic affiliations has been seen throughout VA medical and surgical specialties and provides many benefits. Beginning with graduate medical education, the VA serves as a tremendous resource for resident training.12 Additionally, veterans who sought emergency care at the VA had a lower risk of death than those treated at non-VA hospitals.13 In podiatric medicine and surgery, scholarly activity has been linked to improved outcomes, particularly in the study of ulceration development and its role in either prolonging or preventing amputation.14
Beyond improving clinical outcomes and patient care, engagement in research and inquiry offers other benefits. A cross-sectional study of 7734 physicians within the VA found that research involvement was associated with more favorable job characteristics and job satisfaction perceptions. 3 While this analysis found that about 19% of podiatrists have published once in their career, it remains likely that more may continue to engage in research during their VA tenure. Although this finding shows that an appreciable number of VA podiatrists have published in their field of study, it also encourages departments to provide resources to engage in research. Similar to previous research among foot and ankle surgeons, this analysis also found an increase in publications and h-index as tenure increased.4 Unlike previous research, which found h-index and academic appointment to be contributors to VA dermatologists’ salaries, no significant difference in salary was found in this study associated with publications, h-index, or academic role.15 Although the increase was not statistically significant, salary tended to rise as these variables increased.
Limitations
This analysis was confined to the most recent year of available data, which may not fully capture the longitudinal academic contributions and trends of individual podiatrists. Academic productivity can fluctuate significantly over time due to various factors, including changes in research focus and administrative responsibilities. The study also relied on Scopus to identify and quantify academic productivity. This database may not include all publications relevant to podiatrists, particularly those in niche or nonindexed journals. Additionally, name variations and potential misspellings could lead to missing data for individual podiatrists’ publications. Furthermore, this study did not account for other significant contributors to salary and career advancement within the federal system. Factors such as clinical performance, administrative duties, patient satisfaction, and contributions to teaching and mentoring are critical elements that also influence career progression and compensation but were not captured in this analysis. The retrospective design of this study inherently limits the ability to establish causal relationships. While associations between academic productivity and certain outcomes may be identified, it is not possible to definitively determine the direction or causality of these relationships. Future research may examine how scholarly activity continues once a clinician is part of VA.
CONCLUSIONS
This study highlights the significant academic contributions of VA podiatrists to research and the medical literature. By fostering an active research environment, the VA can ensure veterans receive the highest quality of care from knowledgeable and expert clinicians. Future research should aim to provide a more comprehensive analysis, capturing long-term trends and considering all factors influencing career advancement in VA.
- Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
- Coleman DL, Moran E, Serfilippi D, et al. Measuring physicians’ productivity in a Veterans’ Affairs Medical Center. Acad Med. 2003;78(7):682-689. doi:10.1097/00001888-200307000-00007
- Mohr DC, Burgess JF Jr. Job characteristics and job satisfaction among physicians involved with research in the Veterans Health Administration. Acad Med. 2011;86(8):938-945. doi:10.1097/ACM.0b013e3182223b76
- Casciato DJ, Cravey KS, Barron IM. Scholarly productivity among academic foot and ankle surgeons affiliated with US podiatric medicine and surgery residency and fellowship training programs. J Foot Ankle Surg. 2021;60(6):1222-1226. doi:10.1053/j.jfas.2021.04.017
- Hyer CF, Casciato DJ, Rushing CJ, Schuberth JM. Incidence of scholarly publication by selected content experts presenting at national society foot and ankle meetings from 2016 to 2020. J Foot Ankle Surg. 2022;61(6):1317-1320. doi:10.1053/j.jfas.2022.04.011
- Casciato DJ, Thompson J, Yancovitz S, Chandra A, Prissel MA, Hyer CF. Research activity among foot and ankle surgery fellows: a systematic review. J Foot Ankle Surg. 2021;60(6):1227-1231. doi:10.1053/j.jfas.2021.04.018
- Casciato DJ, Thompson J, Hyer CF. Post-fellowship foot and ankle surgeon research productivity: a systematic review. J Foot Ankle Surg. 2022;61(4):896-899. doi:10.1053/j.jfas.2021.12.028
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA. 2005;102(46):16569-16572. doi:10.1073/pnas.0507655102
- US Department of Veterans Affairs. Veterans Health Administration. About VHA. Updated January 20, 2025. Accessed February 17, 2025. https://www.va.gov/health/aboutvha.asp
- US Department of Veterans Affairs. VHA National Center for Patient Safety. About Us. Updated November 29, 2023. Accessed February 17, 2025. https://www.patientsafety.va.gov/
- US Department of Veterans Affairs. VA/DoD Clinical Practice Guidelines. Updated February 7, 2025. Accessed February 17, 2025. https://www.healthquality.va.gov
- Ravin AG, Gottlieb NB, Wang HT, et al. Effect of the Veterans Affairs Medical System on plastic surgery residency training. Plast Reconstr Surg. 2006;117(2):656-660. doi:10.1097/01.prs.0000197216.95544.f7
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Gibson LW, Abbas A. Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2013;25(1):131-134. doi:10.1016/j.ccell.2012.11.004
- Do MH, Lipner SR. Contribution of gender on compensation of Veterans Affairs-affiliated dermatologists: a cross-sectional study. Int J Womens Dermatol. 2020;6(5):414-418. doi:10.1016/j.ijwd.2020.09.009
The US Department of Veterans Affairs (VA) delivers care to > 9 million veterans, including primary and specialty care.1 While clinical duties remain important across the health system, proposed productivity models have included clinician research activity, given that many hold roles in academia.2 Within this framework, research plays a pivotal role in advancing clinical practices and outcomes. Studies have found that physicians who participated in research report higher job satisfaction.3
As a specialty within the VA, podiatrists diagnose, treat, and prevent foot and ankle disorders. In addition to clinical practice, various scholarly activities are shared among these physicians.4 Reasons for scholarly pursuits among podiatrists vary, including participation in research for academic promotion or to establish expertise in a given area.4-7 Although research remains a component associated with promotion within the VA, little is known about the scholarly activity of VA podiatrists. Specifically, there remains a paucity of data concerning their expertise, as evidenced through peer-reviewed publications, among these physicians and surgeons. To date, no analysis of scholarly activity among VA podiatrists has been conducted.
The primary aim of this investigation was to describe the scholarly productivity among podiatrists employed by the VA through an analysis of the number of peer-reviewed publications and the respective h-index of each physician. The secondary aim of this investigation was to assess the effect of academic productivity on compensation. This study describes research activities pursued by VA physicians and provides the veteran patient population with the confidence that their foot health care remains in the hands of experts within the field.
MATERIALS AND METHODS
The Feds Data Center (www.fedsdatacenter.com) online database of employees was used to identify VA podiatrists on June 17, 2024. All GS-15 physicians and their respective salaries in fiscal year 2023 were recorded. Administratively determined employees, including residents, were excluded. The h-index and number of published documents from any point during a physician’s training or career were reported for each podiatrist using Scopus; podiatrists without an h-index or publication were excluded. 8 Among podiatrists with scholarly activity, this analysis collected academic appointment, sex, and region of practice.
Statistical Analysis
Descriptive statistics, presented as counts and frequencies, were used. The median and IQR were used to describe the number of publications and h-index due to their nonnormal distribution. A Kruskal-Wallis test was used to compare median publication counts and h-index values among for junior faculty (JF), which includes instructors and assistant professors; senior faculty (SF), which includes associate professors and professors; and those with no academic affiliation (NF). Salary was reported as mean (SD) as it remained normally distributed and was compared using analysis of variance with posthoc Tukey test to increase statistical power. Additionally, this analysis used linear regression to investigate the relationship between scholarly activity and salary. The threshold for statistical significance was set at P < .05.
RESULTS
Among 819 VA podiatrists, 150 were administratively determined and excluded, and 512 were excluded for no history of publications, leaving 157 eligible for analysis (Table). A statistically significant difference was found in median (IQR) publication count by faculty appointment. JF had 6.0 (9.5), SF had 12.5 (22.3), and NF had 1.0 (2.0) publication(s) (P < .001) (Figure 1A). There was a statistically significant difference in h-index by faculty appointment. The median (IQR) h-index for JF was 2.0 (3.5), for SF was 5.5 (4.25), and for NF was 1.0 (2.0) (P = .002) (Figure 1B). Salary was not significantly associated with publication count (P = .20) or h-index (P = .62) (Figure 2). No statistically significant difference was found between academic appointment and mean (SD) salary. JF had a median (IQR) salary of $224,063 (27,989), SF of $234,260 (42,963), and NF of $219,811 (P = .35).

(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.

(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.


DISCUSSION
Focused on providing high-quality care, VA physicians use their expertise to practice comprehensive and specialized care.9,10 A cornerstone to this expertise is scholarly activity that contributes to the body of knowledge and, ultimately, the evidence-based medicine by which these physicians practice.11 With veterans considering VA care, it is important to highlight the commitment and dedication to the science and the practice of medicine. This analysis describes the scholarly activity of VA podiatrists and underscores the expertise veterans will receive for the diagnosis and treatment of their foot and ankle pathology.
were not part of an academic facility, a finding that may encourage further action to increase academic productivity in this specialty. For example, collaboration through academic affiliations has been seen throughout VA medical and surgical specialties and provides many benefits. Beginning with graduate medical education, the VA serves as a tremendous resource for resident training.12 Additionally, veterans who sought emergency care at the VA had a lower risk of death than those treated at non-VA hospitals.13 In podiatric medicine and surgery, scholarly activity has been linked to improved outcomes, particularly in the study of ulceration development and its role in either prolonging or preventing amputation.14
Beyond improving clinical outcomes and patient care, engagement in research and inquiry offers other benefits. A cross-sectional study of 7734 physicians within the VA found that research involvement was associated with more favorable job characteristics and job satisfaction perceptions. 3 While this analysis found that about 19% of podiatrists have published once in their career, it remains likely that more may continue to engage in research during their VA tenure. Although this finding shows that an appreciable number of VA podiatrists have published in their field of study, it also encourages departments to provide resources to engage in research. Similar to previous research among foot and ankle surgeons, this analysis also found an increase in publications and h-index as tenure increased.4 Unlike previous research, which found h-index and academic appointment to be contributors to VA dermatologists’ salaries, no significant difference in salary was found in this study associated with publications, h-index, or academic role.15 Although the increase was not statistically significant, salary tended to rise as these variables increased.
Limitations
This analysis was confined to the most recent year of available data, which may not fully capture the longitudinal academic contributions and trends of individual podiatrists. Academic productivity can fluctuate significantly over time due to various factors, including changes in research focus and administrative responsibilities. The study also relied on Scopus to identify and quantify academic productivity. This database may not include all publications relevant to podiatrists, particularly those in niche or nonindexed journals. Additionally, name variations and potential misspellings could lead to missing data for individual podiatrists’ publications. Furthermore, this study did not account for other significant contributors to salary and career advancement within the federal system. Factors such as clinical performance, administrative duties, patient satisfaction, and contributions to teaching and mentoring are critical elements that also influence career progression and compensation but were not captured in this analysis. The retrospective design of this study inherently limits the ability to establish causal relationships. While associations between academic productivity and certain outcomes may be identified, it is not possible to definitively determine the direction or causality of these relationships. Future research may examine how scholarly activity continues once a clinician is part of VA.
CONCLUSIONS
This study highlights the significant academic contributions of VA podiatrists to research and the medical literature. By fostering an active research environment, the VA can ensure veterans receive the highest quality of care from knowledgeable and expert clinicians. Future research should aim to provide a more comprehensive analysis, capturing long-term trends and considering all factors influencing career advancement in VA.
The US Department of Veterans Affairs (VA) delivers care to > 9 million veterans, including primary and specialty care.1 While clinical duties remain important across the health system, proposed productivity models have included clinician research activity, given that many hold roles in academia.2 Within this framework, research plays a pivotal role in advancing clinical practices and outcomes. Studies have found that physicians who participated in research report higher job satisfaction.3
As a specialty within the VA, podiatrists diagnose, treat, and prevent foot and ankle disorders. In addition to clinical practice, various scholarly activities are shared among these physicians.4 Reasons for scholarly pursuits among podiatrists vary, including participation in research for academic promotion or to establish expertise in a given area.4-7 Although research remains a component associated with promotion within the VA, little is known about the scholarly activity of VA podiatrists. Specifically, there remains a paucity of data concerning their expertise, as evidenced through peer-reviewed publications, among these physicians and surgeons. To date, no analysis of scholarly activity among VA podiatrists has been conducted.
The primary aim of this investigation was to describe the scholarly productivity among podiatrists employed by the VA through an analysis of the number of peer-reviewed publications and the respective h-index of each physician. The secondary aim of this investigation was to assess the effect of academic productivity on compensation. This study describes research activities pursued by VA physicians and provides the veteran patient population with the confidence that their foot health care remains in the hands of experts within the field.
MATERIALS AND METHODS
The Feds Data Center (www.fedsdatacenter.com) online database of employees was used to identify VA podiatrists on June 17, 2024. All GS-15 physicians and their respective salaries in fiscal year 2023 were recorded. Administratively determined employees, including residents, were excluded. The h-index and number of published documents from any point during a physician’s training or career were reported for each podiatrist using Scopus; podiatrists without an h-index or publication were excluded. 8 Among podiatrists with scholarly activity, this analysis collected academic appointment, sex, and region of practice.
Statistical Analysis
Descriptive statistics, presented as counts and frequencies, were used. The median and IQR were used to describe the number of publications and h-index due to their nonnormal distribution. A Kruskal-Wallis test was used to compare median publication counts and h-index values among for junior faculty (JF), which includes instructors and assistant professors; senior faculty (SF), which includes associate professors and professors; and those with no academic affiliation (NF). Salary was reported as mean (SD) as it remained normally distributed and was compared using analysis of variance with posthoc Tukey test to increase statistical power. Additionally, this analysis used linear regression to investigate the relationship between scholarly activity and salary. The threshold for statistical significance was set at P < .05.
RESULTS
Among 819 VA podiatrists, 150 were administratively determined and excluded, and 512 were excluded for no history of publications, leaving 157 eligible for analysis (Table). A statistically significant difference was found in median (IQR) publication count by faculty appointment. JF had 6.0 (9.5), SF had 12.5 (22.3), and NF had 1.0 (2.0) publication(s) (P < .001) (Figure 1A). There was a statistically significant difference in h-index by faculty appointment. The median (IQR) h-index for JF was 2.0 (3.5), for SF was 5.5 (4.25), and for NF was 1.0 (2.0) (P = .002) (Figure 1B). Salary was not significantly associated with publication count (P = .20) or h-index (P = .62) (Figure 2). No statistically significant difference was found between academic appointment and mean (SD) salary. JF had a median (IQR) salary of $224,063 (27,989), SF of $234,260 (42,963), and NF of $219,811 (P = .35).

(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.

(B) h-index.a
aBox sizes indicate IQR (bottom, IQR 1; top, IQR 3); whiskers indicate minimum and maximum within 1.5 x IQR; Xs indicate means; white
lines indicate medians; and dots indicate outliers.


DISCUSSION
Focused on providing high-quality care, VA physicians use their expertise to practice comprehensive and specialized care.9,10 A cornerstone to this expertise is scholarly activity that contributes to the body of knowledge and, ultimately, the evidence-based medicine by which these physicians practice.11 With veterans considering VA care, it is important to highlight the commitment and dedication to the science and the practice of medicine. This analysis describes the scholarly activity of VA podiatrists and underscores the expertise veterans will receive for the diagnosis and treatment of their foot and ankle pathology.
were not part of an academic facility, a finding that may encourage further action to increase academic productivity in this specialty. For example, collaboration through academic affiliations has been seen throughout VA medical and surgical specialties and provides many benefits. Beginning with graduate medical education, the VA serves as a tremendous resource for resident training.12 Additionally, veterans who sought emergency care at the VA had a lower risk of death than those treated at non-VA hospitals.13 In podiatric medicine and surgery, scholarly activity has been linked to improved outcomes, particularly in the study of ulceration development and its role in either prolonging or preventing amputation.14
Beyond improving clinical outcomes and patient care, engagement in research and inquiry offers other benefits. A cross-sectional study of 7734 physicians within the VA found that research involvement was associated with more favorable job characteristics and job satisfaction perceptions. 3 While this analysis found that about 19% of podiatrists have published once in their career, it remains likely that more may continue to engage in research during their VA tenure. Although this finding shows that an appreciable number of VA podiatrists have published in their field of study, it also encourages departments to provide resources to engage in research. Similar to previous research among foot and ankle surgeons, this analysis also found an increase in publications and h-index as tenure increased.4 Unlike previous research, which found h-index and academic appointment to be contributors to VA dermatologists’ salaries, no significant difference in salary was found in this study associated with publications, h-index, or academic role.15 Although the increase was not statistically significant, salary tended to rise as these variables increased.
Limitations
This analysis was confined to the most recent year of available data, which may not fully capture the longitudinal academic contributions and trends of individual podiatrists. Academic productivity can fluctuate significantly over time due to various factors, including changes in research focus and administrative responsibilities. The study also relied on Scopus to identify and quantify academic productivity. This database may not include all publications relevant to podiatrists, particularly those in niche or nonindexed journals. Additionally, name variations and potential misspellings could lead to missing data for individual podiatrists’ publications. Furthermore, this study did not account for other significant contributors to salary and career advancement within the federal system. Factors such as clinical performance, administrative duties, patient satisfaction, and contributions to teaching and mentoring are critical elements that also influence career progression and compensation but were not captured in this analysis. The retrospective design of this study inherently limits the ability to establish causal relationships. While associations between academic productivity and certain outcomes may be identified, it is not possible to definitively determine the direction or causality of these relationships. Future research may examine how scholarly activity continues once a clinician is part of VA.
CONCLUSIONS
This study highlights the significant academic contributions of VA podiatrists to research and the medical literature. By fostering an active research environment, the VA can ensure veterans receive the highest quality of care from knowledgeable and expert clinicians. Future research should aim to provide a more comprehensive analysis, capturing long-term trends and considering all factors influencing career advancement in VA.
- Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
- Coleman DL, Moran E, Serfilippi D, et al. Measuring physicians’ productivity in a Veterans’ Affairs Medical Center. Acad Med. 2003;78(7):682-689. doi:10.1097/00001888-200307000-00007
- Mohr DC, Burgess JF Jr. Job characteristics and job satisfaction among physicians involved with research in the Veterans Health Administration. Acad Med. 2011;86(8):938-945. doi:10.1097/ACM.0b013e3182223b76
- Casciato DJ, Cravey KS, Barron IM. Scholarly productivity among academic foot and ankle surgeons affiliated with US podiatric medicine and surgery residency and fellowship training programs. J Foot Ankle Surg. 2021;60(6):1222-1226. doi:10.1053/j.jfas.2021.04.017
- Hyer CF, Casciato DJ, Rushing CJ, Schuberth JM. Incidence of scholarly publication by selected content experts presenting at national society foot and ankle meetings from 2016 to 2020. J Foot Ankle Surg. 2022;61(6):1317-1320. doi:10.1053/j.jfas.2022.04.011
- Casciato DJ, Thompson J, Yancovitz S, Chandra A, Prissel MA, Hyer CF. Research activity among foot and ankle surgery fellows: a systematic review. J Foot Ankle Surg. 2021;60(6):1227-1231. doi:10.1053/j.jfas.2021.04.018
- Casciato DJ, Thompson J, Hyer CF. Post-fellowship foot and ankle surgeon research productivity: a systematic review. J Foot Ankle Surg. 2022;61(4):896-899. doi:10.1053/j.jfas.2021.12.028
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA. 2005;102(46):16569-16572. doi:10.1073/pnas.0507655102
- US Department of Veterans Affairs. Veterans Health Administration. About VHA. Updated January 20, 2025. Accessed February 17, 2025. https://www.va.gov/health/aboutvha.asp
- US Department of Veterans Affairs. VHA National Center for Patient Safety. About Us. Updated November 29, 2023. Accessed February 17, 2025. https://www.patientsafety.va.gov/
- US Department of Veterans Affairs. VA/DoD Clinical Practice Guidelines. Updated February 7, 2025. Accessed February 17, 2025. https://www.healthquality.va.gov
- Ravin AG, Gottlieb NB, Wang HT, et al. Effect of the Veterans Affairs Medical System on plastic surgery residency training. Plast Reconstr Surg. 2006;117(2):656-660. doi:10.1097/01.prs.0000197216.95544.f7
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Gibson LW, Abbas A. Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2013;25(1):131-134. doi:10.1016/j.ccell.2012.11.004
- Do MH, Lipner SR. Contribution of gender on compensation of Veterans Affairs-affiliated dermatologists: a cross-sectional study. Int J Womens Dermatol. 2020;6(5):414-418. doi:10.1016/j.ijwd.2020.09.009
- Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
- Coleman DL, Moran E, Serfilippi D, et al. Measuring physicians’ productivity in a Veterans’ Affairs Medical Center. Acad Med. 2003;78(7):682-689. doi:10.1097/00001888-200307000-00007
- Mohr DC, Burgess JF Jr. Job characteristics and job satisfaction among physicians involved with research in the Veterans Health Administration. Acad Med. 2011;86(8):938-945. doi:10.1097/ACM.0b013e3182223b76
- Casciato DJ, Cravey KS, Barron IM. Scholarly productivity among academic foot and ankle surgeons affiliated with US podiatric medicine and surgery residency and fellowship training programs. J Foot Ankle Surg. 2021;60(6):1222-1226. doi:10.1053/j.jfas.2021.04.017
- Hyer CF, Casciato DJ, Rushing CJ, Schuberth JM. Incidence of scholarly publication by selected content experts presenting at national society foot and ankle meetings from 2016 to 2020. J Foot Ankle Surg. 2022;61(6):1317-1320. doi:10.1053/j.jfas.2022.04.011
- Casciato DJ, Thompson J, Yancovitz S, Chandra A, Prissel MA, Hyer CF. Research activity among foot and ankle surgery fellows: a systematic review. J Foot Ankle Surg. 2021;60(6):1227-1231. doi:10.1053/j.jfas.2021.04.018
- Casciato DJ, Thompson J, Hyer CF. Post-fellowship foot and ankle surgeon research productivity: a systematic review. J Foot Ankle Surg. 2022;61(4):896-899. doi:10.1053/j.jfas.2021.12.028
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA. 2005;102(46):16569-16572. doi:10.1073/pnas.0507655102
- US Department of Veterans Affairs. Veterans Health Administration. About VHA. Updated January 20, 2025. Accessed February 17, 2025. https://www.va.gov/health/aboutvha.asp
- US Department of Veterans Affairs. VHA National Center for Patient Safety. About Us. Updated November 29, 2023. Accessed February 17, 2025. https://www.patientsafety.va.gov/
- US Department of Veterans Affairs. VA/DoD Clinical Practice Guidelines. Updated February 7, 2025. Accessed February 17, 2025. https://www.healthquality.va.gov
- Ravin AG, Gottlieb NB, Wang HT, et al. Effect of the Veterans Affairs Medical System on plastic surgery residency training. Plast Reconstr Surg. 2006;117(2):656-660. doi:10.1097/01.prs.0000197216.95544.f7
- Chan DC, Danesh K, Costantini S, Card D, Taylor L, Studdert DM. Mortality among US veterans after emergency visits to Veterans Affairs and other hospitals: retrospective cohort study. BMJ. 2022;376:e068099. doi:10.1136/bmj-2021-068099
- Gibson LW, Abbas A. Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2013;25(1):131-134. doi:10.1016/j.ccell.2012.11.004
- Do MH, Lipner SR. Contribution of gender on compensation of Veterans Affairs-affiliated dermatologists: a cross-sectional study. Int J Womens Dermatol. 2020;6(5):414-418. doi:10.1016/j.ijwd.2020.09.009
Scholarly Activity Among VA Podiatrists: A Cross-Sectional Study
Scholarly Activity Among VA Podiatrists: A Cross-Sectional Study
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful recurrent abscesses. Several autoimmune and endocrine diseases are associated with HS, including inflammatory bowel disease and diabetes mellitus (DM).1 Notably, the association between HS and thyroid disorders is poorly characterized,2 and there are no known nationwide studies exploring this potential association in the hospital setting. In this cross-sectional matched cohort study, we aimed to characterize HS patients with comorbid thyroid disorders as well as to explore whether thyroid disease is associated with comorbidities and hospital outcome measures in these patients.
The 2019 National Inpatient Sample (NIS) was weighted in accordance with NIS-assigned weight variables and queried for HS, hypothyroidism, and hyperthyroidism cases using International Classification of Diseases, Tenth Revision, codes L73.2, E03, and E05, respectively. Propensity score matching based on age and sex was performed using a nearest-neighbor method in the MatchIt statistical R package. Patient demographics, comorbidities, and outcome variables were collected. Univariable analysis of HS patients with thyroid disease vs those without thyroid disease vs controls without HS were performed using X2 and t-test functions in SPSS statistical software (IBM). A series of multivariate analyses were performed using SPSS logistic and linear regression models to examine the effect of thyroid disease on hospital outcome measures and comorbidities in HS patients, with statistical significance set at P=.05.
A total of 1720 HS patients with comorbid thyroid disease (hyperthyroidism/hypothyroidism), 23,785 HS patients without thyroid disease, and 25,497 age- and sex-matched controls were included in the analysis. On average, HS patients with comorbid thyroid disease were older than HS patients without thyroid disease and controls (49.36 years vs 42.17 years vs 42.66 years [P<.001]), more likely to be female (75.58% vs 58.67% vs 59.81% [P<.001]), more likely to be in the highest income quartile (17.52% vs 12.18% vs 8.14% [P<.001]), and more likely to be Medicare insured (39.07% vs 27.47% vs 18.02% [P<.001])(eTable).
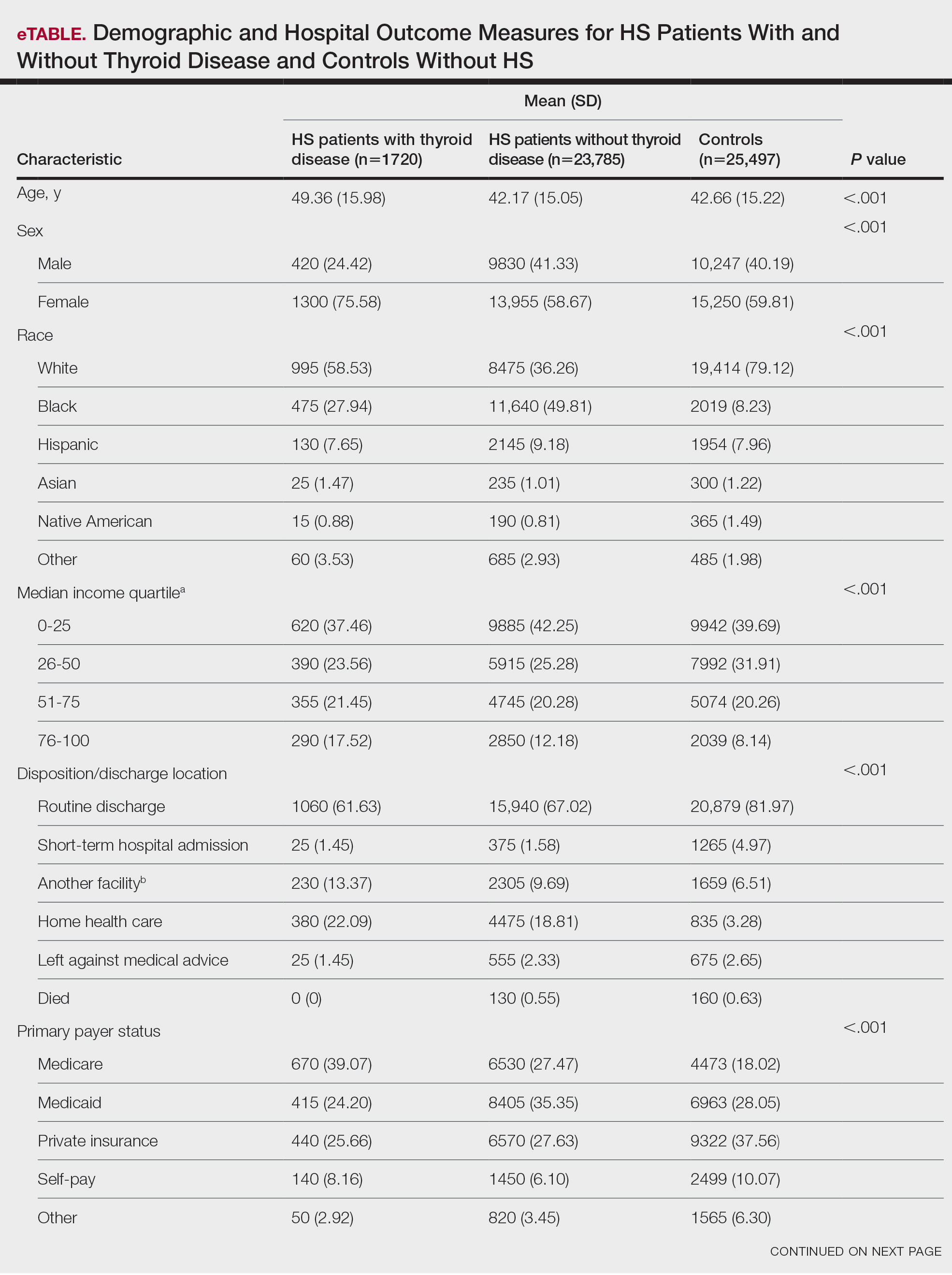
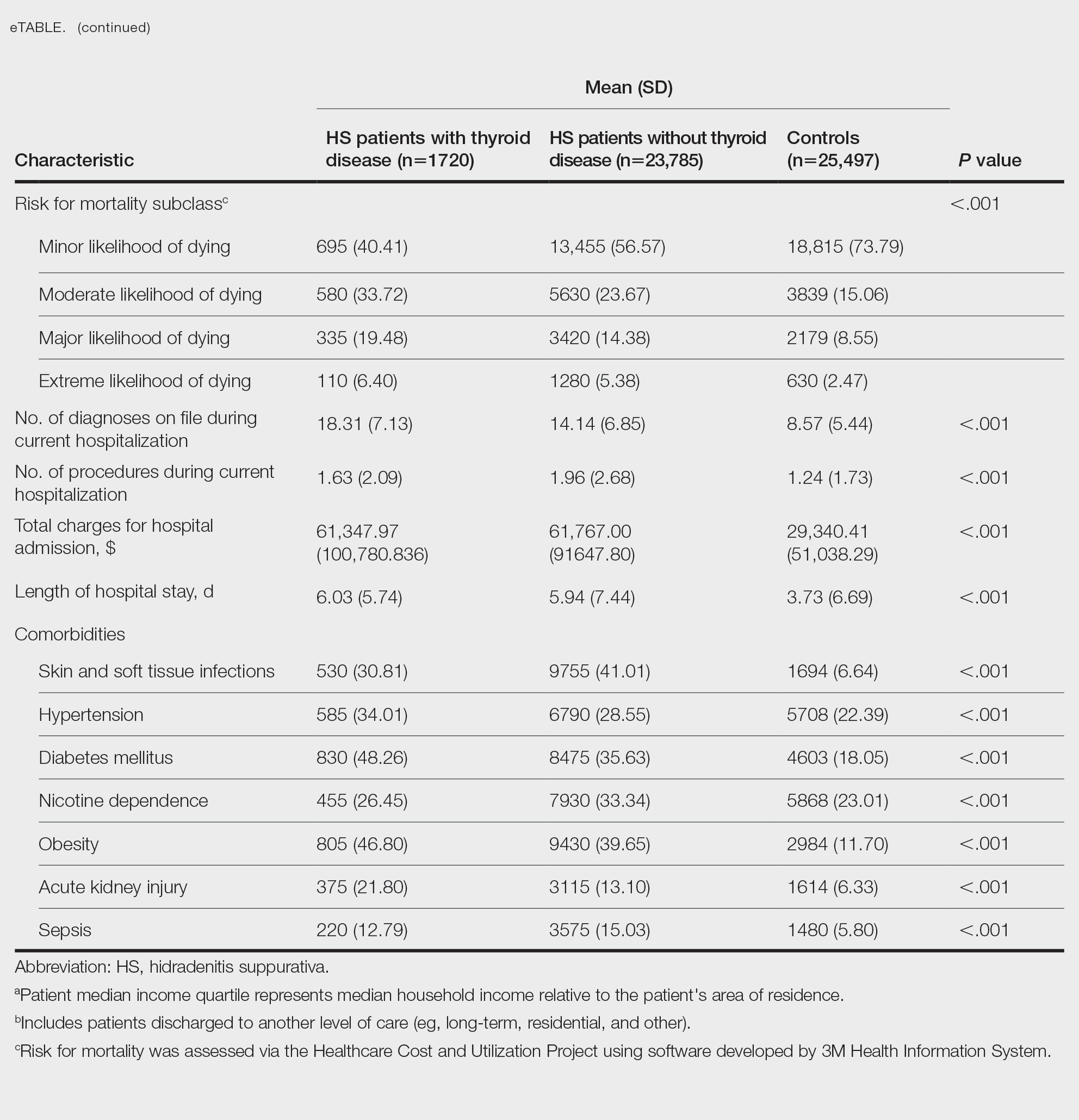
On univariate analysis of hospital outcome measures, HS patients with comorbid thyroid disease had the highest frequency of extreme likelihood of dying compared with HS patients without thyroid disease and with controls (6.40% vs 5.38% vs 2.47% [P<.001]), the highest mean number of diagnoses (18.31 vs 14.14 vs 8.57 [P<.001]), and the longest mean length of hospital stay (6.03 days vs 5.94 days vs 3.73 days [P<.001]). On univariate analysis of comorbidities, HS patients with thyroid disease had the highest incidence of the following comorbidities compared with HS patients without thyroid disease and controls: hypertension (34.01% vs 28.55% vs 22.39% [P<.001]), DM (48.26% vs 35.63% vs 18.05% [P<.001]), obesity (46.80% vs 39.65% vs 11.70% [P<.001]), and acute kidney injury (AKI)(21.80% vs 13.10% vs 6.33% [P<.001])(eTable).
A multivariate analysis adjusting for multiple potential confounders including age, sex, race, median income quartile, disposition/discharge location, and primary payer was performed for hospital outcome measures and comorbidities. There were no significant differences in hospital outcome measures between HS patients with comorbid thyroid disease vs those without thyroid disease (P>.05)(Table 1). Thyroid disease was associated with increased odds of comorbid DM (odds ratio [OR], 1.242 [95% CI, 1.113-1.386]), obesity (OR, 1.173 [95% CI, 1.057-1.302]), and AKI (OR, 1.623 [95% CI, 1.423-1.851]) and decreased odds of comorbid nicotine dependence (OR, 0.609 [95% CI, 0.540-0.687]), skin and soft tissue infections (OR, 0.712 [95% CI, 0.637-0.797]), and sepsis (OR, 0.836 [95% CI, 0.717-0.973]) in HS patients (Table 2).
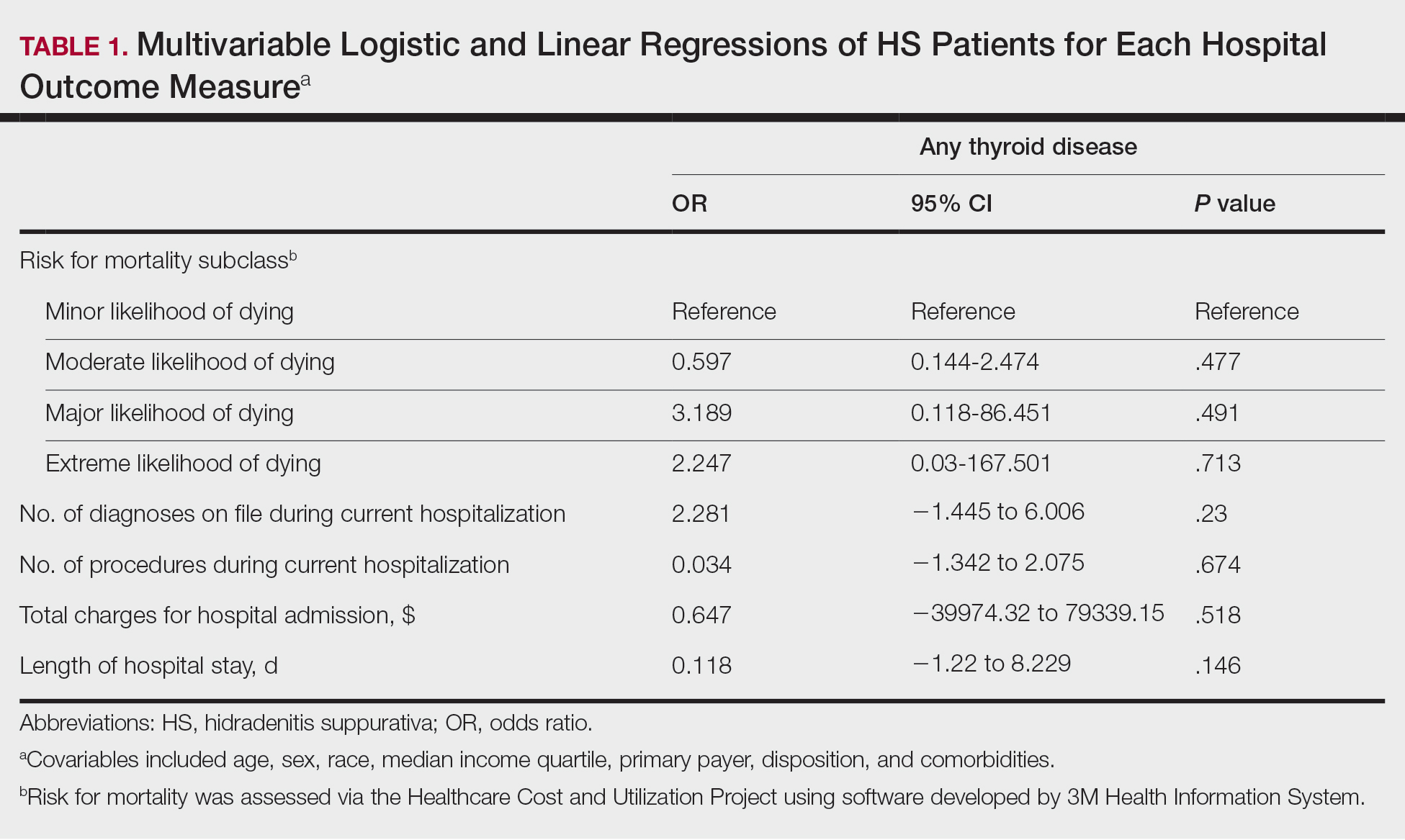

We found that HS patients with thyroid disease had increased odds of comorbid obesity, DM, and AKI compared with HS patients without thyroid disease when adjusting for potential confounders on multivariate analysis. A 2019 nationwide cross-sectional study of 18,224 patients with thyroid disease and 72,896 controls in Taiwan showed a higher prevalence of obesity (1.26% vs 0.57% [P<.0001]) and a higher hazard ratio (HR) of type 2 DM (HR, 1.23 [95% CI, 1.16-1.31]) in the thyroid disease group vs the controls.3 In a 2024 claims-based national cohort study of 4,152,830 patients with 2 or more consecutive thyroid-stimulating hormone measurements in the United States, patients with hypothyroidism and hyperthyroidism had a higher incidence risk for kidney dysfunction vs patients with euthyroidism (HRs, 1.37 [95% CI, 1.34–1.40] and 1.42 [95% CI, 1.39-1.45]).4 In addition, patients with and without DM and thyroid disease had increased risk for kidney disease compared to patients with and without DM and euthyroidism (hypothyroidism: HRs, 1.17 [95% CI, 1.13-1.22] and 1.52 [95% CI, 1.49-1.56]; hyperthyroidism: HRs, 1.34 [95% CI, 1.29-1.38] and 1.36 [95% CI, 1.33-1.39]). Furthermore, patients with and without obesity and thyroid disease had increased risk for kidney disease compared to patients with and without obesity and with euthyroidism (hypothyroidism: HRs, 1.40 [95% CI, 1.36-1.45] and 1.26 [95% CI, 1.21-1.32]; hyperthyroidism: HRs, 1.34 [95% CI, 1.30-1.39] and 1.35 [95% CI, 1.30-1.40]).4 However, these studies did not focus on HS patients.5
Hidradenitis suppurativa has a major comorbidity burden, including obesity, DM, and kidney disease.5 Our findings suggest a potential additive risk for these conditions in HS patients with comorbid thyroid disease; therefore, heightened surveillance for obesity, DM, and AKI in this population is encouraged. Prospective and retrospective studies in HS patients assessing the risk for each comorbidity while controlling for the others may help to better characterize these relationships.
Using multivariate analysis, we found that HS patients with comorbid thyroid disease had no significant differences in hospital outcome measures compared with HS patients without thyroid disease despite significant differences on univariate analysis (P<.05). Similarly, in a 2018 cross-sectional study of 430 HS patients and 20,780 controls in Denmark, the HS group had 10% lower thyroid-stimulating hormone levels vs the control group, but this did not significantly affect HS severity and thyroid function on multivariate analysis.6 In a 2020 cross-sectional analysis of 290 Greek HS patients, thyroid disease was associated with higher HS severity using Hurley classification (OR, 1.19 [95% CI, 1.03-1.51]) and International Hidradenitis Suppurativa Severity Score System 4 classification (OR, 1.29 [95% CI, 1.13-1.62]); however, this analysis was univariate and did not account for confounders.7 Taken together, our study and previous research suggest that thyroid disease is not an independent prognostic indicator for hospital outcome measures in HS patients when cofounders are considered and therefore may not warrant extra caution when treating hospitalized HS patients.
Nicotine dependence was an important potential confounder with regard to the effects of comorbid thyroid disease on outcomes of HS patients in our study. While we found that the prevalence of nicotine dependence was higher in HS patients vs matched controls, HS patients with comorbid thyroid disease had a lower prevalence of nicotine dependence than HS patients without thyroid disease. Furthermore, thyroid disease was associated with decreased odds of nicotine dependence in HS patients when adjusting for confounders. Previous studies have shown an association between cigarette smoking and HS. Smoking also may affect thyroid function via thiocyanate, sympathetic activation, or immunologic disturbances. Smoking may have both prothyroid and antithyroid effects.6 In a 2023 cross-sectional study of 108 HS patients and 52 age- and sex-matched controls in Germany, HS patients had higher thyroid antibody (TRAb) levels compared with controls (median TRAb level, 15.4 vs 14.2 [P=.026]), with even greater increases in TRAb in HS patients who were smokers or former smokers vs never smokers (median TRAb level, 1.18 vs 1.08 [P=.042]).2
There was a lower frequency of thyroid disease in our HS cohort compared with our matched controls cohort. While there are conflicting reports on the association between HS and thyroid disease in the literature, 2 recent meta-analyses of 5 and 6 case-control studies, respectively, found an association between HS and thyroid disease (OR, 1.36 [95% CI, 1.13-1.64] and 1.88 [95% CI, 1.25-2.81]).1,8 Notably, these studies were either claims or survey based, included outpatients, or were unspecified. One potential explanation for the difference in our findings vs those of other studies could be underdiagnosis of thyroid disease in hospitalized HS patients. We found that HS patients were most frequently Medicaid or Medicare insured compared to controls, who most frequently were privately insured. Increased availability and ease of access to outpatient medical care through private health insurance may be a possible contributor to the higher frequency of diagnosed thyroid disease in control patients in our study; therefore, awareness of potential underdiagnosis of thyroid disease in hospitalized HS patients is recommended.
Limitations of our study included those inherent to the NIS database, including potential miscoding and lack of data on pharmacologic treatments. Outcome measures assessed were limited by inclusion of both primary and secondary diagnoses of HS and thyroid disease in our cohort and may have been affected by other conditions. As with any observational study, there was a possibility of unidentified confounders unaccounted for in our study.
In conclusion, in this national inpatient-matched cohort study, thyroid disease was associated with increased odds of obesity, DM, and AKI in HS inpatients but was not an independent risk factor for worse hospital outcome measures. Therefore, while increased surveillance of associated comorbidities is appropriate, thyroid disease may not be a cause for increased concern for dermatologists treating hospitalized HS patients. Prospective studies are necessary to better characterize these findings.
- Phan K, Huo YR, Charlton O, et al. Hidradenitis suppurativa and thyroid disease: systematic review and meta-analysis. J Cutan Med Surg. 2020;24:23-27. doi:10.1177/1203475419874411
- Abu Rached N, Dietrich JW, Ocker L, et al. Primary thyroid dysfunction is prevalent in hidradenitis suppurativa and marked by a signature of hypothyroid Graves’ disease: a case-control study. J Clin Med. 2023;12:7490. doi:10.3390/jcm12237490
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine (Baltimore). 2019;98:E15631. doi:10.1097/md.0000000000015631
- You AS, Kalantar-Zadeh K, Brent GA, et al. Impact of thyroid status on incident kidney dysfunction and chronic kidney disease progression in a nationally representative cohort. Mayo Clin Proc. 2024;99:39-56. doi:10.1016/j.mayocp.2023.08.028
- Almuhanna N, Tobe SW, Alhusayen R. Risk of chronic kidney disease in hospitalized patients with hidradenitis suppurativa. Dermatology. 2023;239:912-918. doi:10.1159/000531960
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population]based cross]sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905. doi:10.1111/ced.13606
- Liakou AI, Kontochristopoulos G, Marnelakis I, et al. Thyroid disease and active smoking may be associated with more severe hidradenitis suppurativa: data from a prospective cross sectional single-center study. Dermatology. 2021;237:125-130. doi:10.1159/000508528
- Acharya P, Mathur M. Thyroid disorders in patients with hidradenitis suppurativa: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:491-493. doi:10.1016/j.jaad.2019.07.025
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful recurrent abscesses. Several autoimmune and endocrine diseases are associated with HS, including inflammatory bowel disease and diabetes mellitus (DM).1 Notably, the association between HS and thyroid disorders is poorly characterized,2 and there are no known nationwide studies exploring this potential association in the hospital setting. In this cross-sectional matched cohort study, we aimed to characterize HS patients with comorbid thyroid disorders as well as to explore whether thyroid disease is associated with comorbidities and hospital outcome measures in these patients.
The 2019 National Inpatient Sample (NIS) was weighted in accordance with NIS-assigned weight variables and queried for HS, hypothyroidism, and hyperthyroidism cases using International Classification of Diseases, Tenth Revision, codes L73.2, E03, and E05, respectively. Propensity score matching based on age and sex was performed using a nearest-neighbor method in the MatchIt statistical R package. Patient demographics, comorbidities, and outcome variables were collected. Univariable analysis of HS patients with thyroid disease vs those without thyroid disease vs controls without HS were performed using X2 and t-test functions in SPSS statistical software (IBM). A series of multivariate analyses were performed using SPSS logistic and linear regression models to examine the effect of thyroid disease on hospital outcome measures and comorbidities in HS patients, with statistical significance set at P=.05.
A total of 1720 HS patients with comorbid thyroid disease (hyperthyroidism/hypothyroidism), 23,785 HS patients without thyroid disease, and 25,497 age- and sex-matched controls were included in the analysis. On average, HS patients with comorbid thyroid disease were older than HS patients without thyroid disease and controls (49.36 years vs 42.17 years vs 42.66 years [P<.001]), more likely to be female (75.58% vs 58.67% vs 59.81% [P<.001]), more likely to be in the highest income quartile (17.52% vs 12.18% vs 8.14% [P<.001]), and more likely to be Medicare insured (39.07% vs 27.47% vs 18.02% [P<.001])(eTable).


On univariate analysis of hospital outcome measures, HS patients with comorbid thyroid disease had the highest frequency of extreme likelihood of dying compared with HS patients without thyroid disease and with controls (6.40% vs 5.38% vs 2.47% [P<.001]), the highest mean number of diagnoses (18.31 vs 14.14 vs 8.57 [P<.001]), and the longest mean length of hospital stay (6.03 days vs 5.94 days vs 3.73 days [P<.001]). On univariate analysis of comorbidities, HS patients with thyroid disease had the highest incidence of the following comorbidities compared with HS patients without thyroid disease and controls: hypertension (34.01% vs 28.55% vs 22.39% [P<.001]), DM (48.26% vs 35.63% vs 18.05% [P<.001]), obesity (46.80% vs 39.65% vs 11.70% [P<.001]), and acute kidney injury (AKI)(21.80% vs 13.10% vs 6.33% [P<.001])(eTable).
A multivariate analysis adjusting for multiple potential confounders including age, sex, race, median income quartile, disposition/discharge location, and primary payer was performed for hospital outcome measures and comorbidities. There were no significant differences in hospital outcome measures between HS patients with comorbid thyroid disease vs those without thyroid disease (P>.05)(Table 1). Thyroid disease was associated with increased odds of comorbid DM (odds ratio [OR], 1.242 [95% CI, 1.113-1.386]), obesity (OR, 1.173 [95% CI, 1.057-1.302]), and AKI (OR, 1.623 [95% CI, 1.423-1.851]) and decreased odds of comorbid nicotine dependence (OR, 0.609 [95% CI, 0.540-0.687]), skin and soft tissue infections (OR, 0.712 [95% CI, 0.637-0.797]), and sepsis (OR, 0.836 [95% CI, 0.717-0.973]) in HS patients (Table 2).


We found that HS patients with thyroid disease had increased odds of comorbid obesity, DM, and AKI compared with HS patients without thyroid disease when adjusting for potential confounders on multivariate analysis. A 2019 nationwide cross-sectional study of 18,224 patients with thyroid disease and 72,896 controls in Taiwan showed a higher prevalence of obesity (1.26% vs 0.57% [P<.0001]) and a higher hazard ratio (HR) of type 2 DM (HR, 1.23 [95% CI, 1.16-1.31]) in the thyroid disease group vs the controls.3 In a 2024 claims-based national cohort study of 4,152,830 patients with 2 or more consecutive thyroid-stimulating hormone measurements in the United States, patients with hypothyroidism and hyperthyroidism had a higher incidence risk for kidney dysfunction vs patients with euthyroidism (HRs, 1.37 [95% CI, 1.34–1.40] and 1.42 [95% CI, 1.39-1.45]).4 In addition, patients with and without DM and thyroid disease had increased risk for kidney disease compared to patients with and without DM and euthyroidism (hypothyroidism: HRs, 1.17 [95% CI, 1.13-1.22] and 1.52 [95% CI, 1.49-1.56]; hyperthyroidism: HRs, 1.34 [95% CI, 1.29-1.38] and 1.36 [95% CI, 1.33-1.39]). Furthermore, patients with and without obesity and thyroid disease had increased risk for kidney disease compared to patients with and without obesity and with euthyroidism (hypothyroidism: HRs, 1.40 [95% CI, 1.36-1.45] and 1.26 [95% CI, 1.21-1.32]; hyperthyroidism: HRs, 1.34 [95% CI, 1.30-1.39] and 1.35 [95% CI, 1.30-1.40]).4 However, these studies did not focus on HS patients.5
Hidradenitis suppurativa has a major comorbidity burden, including obesity, DM, and kidney disease.5 Our findings suggest a potential additive risk for these conditions in HS patients with comorbid thyroid disease; therefore, heightened surveillance for obesity, DM, and AKI in this population is encouraged. Prospective and retrospective studies in HS patients assessing the risk for each comorbidity while controlling for the others may help to better characterize these relationships.
Using multivariate analysis, we found that HS patients with comorbid thyroid disease had no significant differences in hospital outcome measures compared with HS patients without thyroid disease despite significant differences on univariate analysis (P<.05). Similarly, in a 2018 cross-sectional study of 430 HS patients and 20,780 controls in Denmark, the HS group had 10% lower thyroid-stimulating hormone levels vs the control group, but this did not significantly affect HS severity and thyroid function on multivariate analysis.6 In a 2020 cross-sectional analysis of 290 Greek HS patients, thyroid disease was associated with higher HS severity using Hurley classification (OR, 1.19 [95% CI, 1.03-1.51]) and International Hidradenitis Suppurativa Severity Score System 4 classification (OR, 1.29 [95% CI, 1.13-1.62]); however, this analysis was univariate and did not account for confounders.7 Taken together, our study and previous research suggest that thyroid disease is not an independent prognostic indicator for hospital outcome measures in HS patients when cofounders are considered and therefore may not warrant extra caution when treating hospitalized HS patients.
Nicotine dependence was an important potential confounder with regard to the effects of comorbid thyroid disease on outcomes of HS patients in our study. While we found that the prevalence of nicotine dependence was higher in HS patients vs matched controls, HS patients with comorbid thyroid disease had a lower prevalence of nicotine dependence than HS patients without thyroid disease. Furthermore, thyroid disease was associated with decreased odds of nicotine dependence in HS patients when adjusting for confounders. Previous studies have shown an association between cigarette smoking and HS. Smoking also may affect thyroid function via thiocyanate, sympathetic activation, or immunologic disturbances. Smoking may have both prothyroid and antithyroid effects.6 In a 2023 cross-sectional study of 108 HS patients and 52 age- and sex-matched controls in Germany, HS patients had higher thyroid antibody (TRAb) levels compared with controls (median TRAb level, 15.4 vs 14.2 [P=.026]), with even greater increases in TRAb in HS patients who were smokers or former smokers vs never smokers (median TRAb level, 1.18 vs 1.08 [P=.042]).2
There was a lower frequency of thyroid disease in our HS cohort compared with our matched controls cohort. While there are conflicting reports on the association between HS and thyroid disease in the literature, 2 recent meta-analyses of 5 and 6 case-control studies, respectively, found an association between HS and thyroid disease (OR, 1.36 [95% CI, 1.13-1.64] and 1.88 [95% CI, 1.25-2.81]).1,8 Notably, these studies were either claims or survey based, included outpatients, or were unspecified. One potential explanation for the difference in our findings vs those of other studies could be underdiagnosis of thyroid disease in hospitalized HS patients. We found that HS patients were most frequently Medicaid or Medicare insured compared to controls, who most frequently were privately insured. Increased availability and ease of access to outpatient medical care through private health insurance may be a possible contributor to the higher frequency of diagnosed thyroid disease in control patients in our study; therefore, awareness of potential underdiagnosis of thyroid disease in hospitalized HS patients is recommended.
Limitations of our study included those inherent to the NIS database, including potential miscoding and lack of data on pharmacologic treatments. Outcome measures assessed were limited by inclusion of both primary and secondary diagnoses of HS and thyroid disease in our cohort and may have been affected by other conditions. As with any observational study, there was a possibility of unidentified confounders unaccounted for in our study.
In conclusion, in this national inpatient-matched cohort study, thyroid disease was associated with increased odds of obesity, DM, and AKI in HS inpatients but was not an independent risk factor for worse hospital outcome measures. Therefore, while increased surveillance of associated comorbidities is appropriate, thyroid disease may not be a cause for increased concern for dermatologists treating hospitalized HS patients. Prospective studies are necessary to better characterize these findings.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful recurrent abscesses. Several autoimmune and endocrine diseases are associated with HS, including inflammatory bowel disease and diabetes mellitus (DM).1 Notably, the association between HS and thyroid disorders is poorly characterized,2 and there are no known nationwide studies exploring this potential association in the hospital setting. In this cross-sectional matched cohort study, we aimed to characterize HS patients with comorbid thyroid disorders as well as to explore whether thyroid disease is associated with comorbidities and hospital outcome measures in these patients.
The 2019 National Inpatient Sample (NIS) was weighted in accordance with NIS-assigned weight variables and queried for HS, hypothyroidism, and hyperthyroidism cases using International Classification of Diseases, Tenth Revision, codes L73.2, E03, and E05, respectively. Propensity score matching based on age and sex was performed using a nearest-neighbor method in the MatchIt statistical R package. Patient demographics, comorbidities, and outcome variables were collected. Univariable analysis of HS patients with thyroid disease vs those without thyroid disease vs controls without HS were performed using X2 and t-test functions in SPSS statistical software (IBM). A series of multivariate analyses were performed using SPSS logistic and linear regression models to examine the effect of thyroid disease on hospital outcome measures and comorbidities in HS patients, with statistical significance set at P=.05.
A total of 1720 HS patients with comorbid thyroid disease (hyperthyroidism/hypothyroidism), 23,785 HS patients without thyroid disease, and 25,497 age- and sex-matched controls were included in the analysis. On average, HS patients with comorbid thyroid disease were older than HS patients without thyroid disease and controls (49.36 years vs 42.17 years vs 42.66 years [P<.001]), more likely to be female (75.58% vs 58.67% vs 59.81% [P<.001]), more likely to be in the highest income quartile (17.52% vs 12.18% vs 8.14% [P<.001]), and more likely to be Medicare insured (39.07% vs 27.47% vs 18.02% [P<.001])(eTable).


On univariate analysis of hospital outcome measures, HS patients with comorbid thyroid disease had the highest frequency of extreme likelihood of dying compared with HS patients without thyroid disease and with controls (6.40% vs 5.38% vs 2.47% [P<.001]), the highest mean number of diagnoses (18.31 vs 14.14 vs 8.57 [P<.001]), and the longest mean length of hospital stay (6.03 days vs 5.94 days vs 3.73 days [P<.001]). On univariate analysis of comorbidities, HS patients with thyroid disease had the highest incidence of the following comorbidities compared with HS patients without thyroid disease and controls: hypertension (34.01% vs 28.55% vs 22.39% [P<.001]), DM (48.26% vs 35.63% vs 18.05% [P<.001]), obesity (46.80% vs 39.65% vs 11.70% [P<.001]), and acute kidney injury (AKI)(21.80% vs 13.10% vs 6.33% [P<.001])(eTable).
A multivariate analysis adjusting for multiple potential confounders including age, sex, race, median income quartile, disposition/discharge location, and primary payer was performed for hospital outcome measures and comorbidities. There were no significant differences in hospital outcome measures between HS patients with comorbid thyroid disease vs those without thyroid disease (P>.05)(Table 1). Thyroid disease was associated with increased odds of comorbid DM (odds ratio [OR], 1.242 [95% CI, 1.113-1.386]), obesity (OR, 1.173 [95% CI, 1.057-1.302]), and AKI (OR, 1.623 [95% CI, 1.423-1.851]) and decreased odds of comorbid nicotine dependence (OR, 0.609 [95% CI, 0.540-0.687]), skin and soft tissue infections (OR, 0.712 [95% CI, 0.637-0.797]), and sepsis (OR, 0.836 [95% CI, 0.717-0.973]) in HS patients (Table 2).


We found that HS patients with thyroid disease had increased odds of comorbid obesity, DM, and AKI compared with HS patients without thyroid disease when adjusting for potential confounders on multivariate analysis. A 2019 nationwide cross-sectional study of 18,224 patients with thyroid disease and 72,896 controls in Taiwan showed a higher prevalence of obesity (1.26% vs 0.57% [P<.0001]) and a higher hazard ratio (HR) of type 2 DM (HR, 1.23 [95% CI, 1.16-1.31]) in the thyroid disease group vs the controls.3 In a 2024 claims-based national cohort study of 4,152,830 patients with 2 or more consecutive thyroid-stimulating hormone measurements in the United States, patients with hypothyroidism and hyperthyroidism had a higher incidence risk for kidney dysfunction vs patients with euthyroidism (HRs, 1.37 [95% CI, 1.34–1.40] and 1.42 [95% CI, 1.39-1.45]).4 In addition, patients with and without DM and thyroid disease had increased risk for kidney disease compared to patients with and without DM and euthyroidism (hypothyroidism: HRs, 1.17 [95% CI, 1.13-1.22] and 1.52 [95% CI, 1.49-1.56]; hyperthyroidism: HRs, 1.34 [95% CI, 1.29-1.38] and 1.36 [95% CI, 1.33-1.39]). Furthermore, patients with and without obesity and thyroid disease had increased risk for kidney disease compared to patients with and without obesity and with euthyroidism (hypothyroidism: HRs, 1.40 [95% CI, 1.36-1.45] and 1.26 [95% CI, 1.21-1.32]; hyperthyroidism: HRs, 1.34 [95% CI, 1.30-1.39] and 1.35 [95% CI, 1.30-1.40]).4 However, these studies did not focus on HS patients.5
Hidradenitis suppurativa has a major comorbidity burden, including obesity, DM, and kidney disease.5 Our findings suggest a potential additive risk for these conditions in HS patients with comorbid thyroid disease; therefore, heightened surveillance for obesity, DM, and AKI in this population is encouraged. Prospective and retrospective studies in HS patients assessing the risk for each comorbidity while controlling for the others may help to better characterize these relationships.
Using multivariate analysis, we found that HS patients with comorbid thyroid disease had no significant differences in hospital outcome measures compared with HS patients without thyroid disease despite significant differences on univariate analysis (P<.05). Similarly, in a 2018 cross-sectional study of 430 HS patients and 20,780 controls in Denmark, the HS group had 10% lower thyroid-stimulating hormone levels vs the control group, but this did not significantly affect HS severity and thyroid function on multivariate analysis.6 In a 2020 cross-sectional analysis of 290 Greek HS patients, thyroid disease was associated with higher HS severity using Hurley classification (OR, 1.19 [95% CI, 1.03-1.51]) and International Hidradenitis Suppurativa Severity Score System 4 classification (OR, 1.29 [95% CI, 1.13-1.62]); however, this analysis was univariate and did not account for confounders.7 Taken together, our study and previous research suggest that thyroid disease is not an independent prognostic indicator for hospital outcome measures in HS patients when cofounders are considered and therefore may not warrant extra caution when treating hospitalized HS patients.
Nicotine dependence was an important potential confounder with regard to the effects of comorbid thyroid disease on outcomes of HS patients in our study. While we found that the prevalence of nicotine dependence was higher in HS patients vs matched controls, HS patients with comorbid thyroid disease had a lower prevalence of nicotine dependence than HS patients without thyroid disease. Furthermore, thyroid disease was associated with decreased odds of nicotine dependence in HS patients when adjusting for confounders. Previous studies have shown an association between cigarette smoking and HS. Smoking also may affect thyroid function via thiocyanate, sympathetic activation, or immunologic disturbances. Smoking may have both prothyroid and antithyroid effects.6 In a 2023 cross-sectional study of 108 HS patients and 52 age- and sex-matched controls in Germany, HS patients had higher thyroid antibody (TRAb) levels compared with controls (median TRAb level, 15.4 vs 14.2 [P=.026]), with even greater increases in TRAb in HS patients who were smokers or former smokers vs never smokers (median TRAb level, 1.18 vs 1.08 [P=.042]).2
There was a lower frequency of thyroid disease in our HS cohort compared with our matched controls cohort. While there are conflicting reports on the association between HS and thyroid disease in the literature, 2 recent meta-analyses of 5 and 6 case-control studies, respectively, found an association between HS and thyroid disease (OR, 1.36 [95% CI, 1.13-1.64] and 1.88 [95% CI, 1.25-2.81]).1,8 Notably, these studies were either claims or survey based, included outpatients, or were unspecified. One potential explanation for the difference in our findings vs those of other studies could be underdiagnosis of thyroid disease in hospitalized HS patients. We found that HS patients were most frequently Medicaid or Medicare insured compared to controls, who most frequently were privately insured. Increased availability and ease of access to outpatient medical care through private health insurance may be a possible contributor to the higher frequency of diagnosed thyroid disease in control patients in our study; therefore, awareness of potential underdiagnosis of thyroid disease in hospitalized HS patients is recommended.
Limitations of our study included those inherent to the NIS database, including potential miscoding and lack of data on pharmacologic treatments. Outcome measures assessed were limited by inclusion of both primary and secondary diagnoses of HS and thyroid disease in our cohort and may have been affected by other conditions. As with any observational study, there was a possibility of unidentified confounders unaccounted for in our study.
In conclusion, in this national inpatient-matched cohort study, thyroid disease was associated with increased odds of obesity, DM, and AKI in HS inpatients but was not an independent risk factor for worse hospital outcome measures. Therefore, while increased surveillance of associated comorbidities is appropriate, thyroid disease may not be a cause for increased concern for dermatologists treating hospitalized HS patients. Prospective studies are necessary to better characterize these findings.
- Phan K, Huo YR, Charlton O, et al. Hidradenitis suppurativa and thyroid disease: systematic review and meta-analysis. J Cutan Med Surg. 2020;24:23-27. doi:10.1177/1203475419874411
- Abu Rached N, Dietrich JW, Ocker L, et al. Primary thyroid dysfunction is prevalent in hidradenitis suppurativa and marked by a signature of hypothyroid Graves’ disease: a case-control study. J Clin Med. 2023;12:7490. doi:10.3390/jcm12237490
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine (Baltimore). 2019;98:E15631. doi:10.1097/md.0000000000015631
- You AS, Kalantar-Zadeh K, Brent GA, et al. Impact of thyroid status on incident kidney dysfunction and chronic kidney disease progression in a nationally representative cohort. Mayo Clin Proc. 2024;99:39-56. doi:10.1016/j.mayocp.2023.08.028
- Almuhanna N, Tobe SW, Alhusayen R. Risk of chronic kidney disease in hospitalized patients with hidradenitis suppurativa. Dermatology. 2023;239:912-918. doi:10.1159/000531960
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population]based cross]sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905. doi:10.1111/ced.13606
- Liakou AI, Kontochristopoulos G, Marnelakis I, et al. Thyroid disease and active smoking may be associated with more severe hidradenitis suppurativa: data from a prospective cross sectional single-center study. Dermatology. 2021;237:125-130. doi:10.1159/000508528
- Acharya P, Mathur M. Thyroid disorders in patients with hidradenitis suppurativa: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:491-493. doi:10.1016/j.jaad.2019.07.025
- Phan K, Huo YR, Charlton O, et al. Hidradenitis suppurativa and thyroid disease: systematic review and meta-analysis. J Cutan Med Surg. 2020;24:23-27. doi:10.1177/1203475419874411
- Abu Rached N, Dietrich JW, Ocker L, et al. Primary thyroid dysfunction is prevalent in hidradenitis suppurativa and marked by a signature of hypothyroid Graves’ disease: a case-control study. J Clin Med. 2023;12:7490. doi:10.3390/jcm12237490
- Chen RH, Chen HY, Man KM, et al. Thyroid diseases increased the risk of type 2 diabetes mellitus: a nation-wide cohort study. Medicine (Baltimore). 2019;98:E15631. doi:10.1097/md.0000000000015631
- You AS, Kalantar-Zadeh K, Brent GA, et al. Impact of thyroid status on incident kidney dysfunction and chronic kidney disease progression in a nationally representative cohort. Mayo Clin Proc. 2024;99:39-56. doi:10.1016/j.mayocp.2023.08.028
- Almuhanna N, Tobe SW, Alhusayen R. Risk of chronic kidney disease in hospitalized patients with hidradenitis suppurativa. Dermatology. 2023;239:912-918. doi:10.1159/000531960
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population]based cross]sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905. doi:10.1111/ced.13606
- Liakou AI, Kontochristopoulos G, Marnelakis I, et al. Thyroid disease and active smoking may be associated with more severe hidradenitis suppurativa: data from a prospective cross sectional single-center study. Dermatology. 2021;237:125-130. doi:10.1159/000508528
- Acharya P, Mathur M. Thyroid disorders in patients with hidradenitis suppurativa: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:491-493. doi:10.1016/j.jaad.2019.07.025
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
Implications of Thyroid Disease in Hospitalized Patients With Hidradenitis Suppurativa
PRACTICE
- Hidradenitis suppurativa (HS) is associated with autoimmune and endocrine conditions, but the association between HS and thyroid disorders is poorly characterized.
Not as Bland as You May Think: Celery (Apium graveolens) Commonly Induces Phytophotodermatitis
Not as Bland as You May Think: Celery (Apium graveolens) Commonly Induces Phytophotodermatitis
Celery (Apium graveolens)—that lowly vegetable that often languishes in the refrigerator crisper and apparently supplies fewer calories than are required to consume it—contains a myriad of photosensitizing chemicals known as furocoumarins and psoralens that can cause phytophotodermatitis (PPD) when handled prior to exposure to UV light.1 Individuals who are most likely to develop PPD caused by repeated contact with celery include food industry workers (eg, grocery store workers, farmers) who pick, handle, or prepare celery for consumption. While eating celery as part of a standard diet is highly unlikely to cause PPD, celery infected with Sclerotinia sclerotiorum (known as pink rot) causes more severe generalized sun sensitivity due to an increased amount of furocoumarins produced in response to the fungus.2 Contact with celery also can induce cutaneous manifestations unrelated to sun exposure in some individuals, including urticaria, allergic contact dermatitis, and anaphylaxis.3 In this article, we provide an overview of the life cycle and origin of celery as well as its irritant and allergic properties. We also describe cutaneous rashes associated with PPD caused by exposure to celery and highlight treatment options.
Morphology and Distribution
The Apiaceae family features aromatic flowering plants that comprise more than 3500 species, including many economically important vegetables, herbs, and spices.4 It also includes many alkaloid-containing species that are known to be poisonous to humans, such as poison hemlock (Conium maculatum) and water hemlock (Cicuta maculate). Most Apiaceae plants that are consumed by humans originate from the Mediterranean region.5 While known for their diversity of flavor and aroma, most of the plants from this family have low caloric value and provide minimal amounts of energy.
Members of the Apiaceae family have flowers that create a classic umbel shape mimicking the appearance of an upside-down umbrella (thus the former name for this family, Umbelliferae). The pedicles—the small stems attached to the base of each flower—spread from a common center to form the umbel.5 The Apiaceae family also includes the greatest number of plants that cause PPD due to their high concentration of furocoumarins, which deter fungus from harming the plants.6
A biennial plant, celery completes its life cycle in 2 years. During the first season, the stems, roots, and leaves sprout; in the second and final year, the flowers, fruits, and seeds proliferate, followed by decomposition. Apium graveolens approaches heights of 2 to 3 ft, growing upright and displaying grooved stems. Each stem terminates in a basal rosette of leaves. The second season brings white flower blooms in terminal or axillary umbels.7
Celery originated in the temperate Mediterranean regions of Europe, but farmers now cultivate it globally.8 It grows best in rich moist soil with full exposure to sunlight. Plants multiply their numbers through self-seeding. Celery commonly is found in suburban and rural homes, both in refrigerators for consumption as well as in medicine cabinets in capsule form for the treatment of arthritis.4
Irritant and Allergenic Properties
Despite the potential health benefits of celery, the Apiaceae family, which includes hogweed, dill, and fennel, prevails as the most common culprit for phytotoxic reactions. The Rutaceae family, including citrus plants and rue, remains runner-up for causes of PPD.9 Phytophotodermatitis is not an immunologic reaction, making anyone susceptible to formation of the cutaneous lesions when exposed to UV light after handling celery. Pruritis rarely occurs, unlike in allergic phytodermatitis.10 Upon photoexcitation from exposure to UVA light, individual psoralen molecules covalently bind to pyrimidine bases, causing interstrand cross-linking that prevents DNA replication and triggering a cascade leading to apoptosis of the cell. Apoptosis induces cell membrane edema, which manifests as cutaneous vesicles and bullae on the skin.10 Regardless of plant species, PPD reactions have similar appearance.
Celery roots contain the greatest concentration of psoralens, making it the most likely part of the plant to induce PPD.6 Phytophotodermatitis caused by celery can occur at any time of the year, but most eruptions occur during the summer months due to increased sunlight exposure and intensity. Among 320 randomly selected Michigan celery harvesters, 163 (51%) displayed evidence of vesicular and bullous dermatitis on the fingers, hands, and forearms.11 In this study, celery infected with pink rot fungus induced an erythematous eruption with vesicles and bullae within 48 hours of contact after just 30 seconds of summer sunlight exposure; however, eruptions are not limited to summer months, as the cutaneous presentation depends solely on exposure to UVA light, which can occur year-round.
Use of tanning beds is a major risk factor for PPD.12 Tanning beds utilize fluorescent bulbs that primarily emit UVA light, with UVB light emitted to a lesser degree. The UVA radiation produced by tanning beds is more than 3 times as intense as natural sunlight.12 Among grocery store employees, the combination of these 2 risk factors—regular contact with celery and tanning bed use—resulted in a prevalence ratio for PPD more than 40 times greater than that of individuals with neither risk factor.13
Cutaneous Manifestations of PPD
Phytophotodermatitis is a nonimmunologic dermatitis that forms via the interaction between UV light exposure and the photosensitizing chemicals inherent to some plant species. Development of PPD following contact with celery may be caused by the photoactive substances in celery, including the psoralens 8-methoxypsoralen and 5-methoxypsoralen.14 The psoralens must become activated by UV light with wavelengths between 320 nm and 400 nm (UVA) to initiate biologic effects.15
Once chemically activated, the photoactive mediators cause an erythematous and edematous sunburnlike reaction. Current hypotheses state that psoralen plus UVA generates reactive oxygen species, which damage the DNA within cells and alter receptors on cell membranes within the epidermis.14 The cutaneous eruption usually appears between 12 and 36 hours after sun exposure. Although they generally are not pruritic, the eruptions may induce pain. Within 7 to 10 days following development of the rash, hyperpigmentation occurs in the affected area and often persists for months to years.16 Ingestion of large amounts of celery has been cited to cause generalized phototoxic reactions; however, PPD rarely arises solely after ingestion, unless excessive amounts are consumed with concomitant exposure to psoralen plus UVA or tanning beds.17 In these cases, patients develop diffuse redness with superficial scaling, pain, and blistering if severe.
Treatment of PPD
Prevention remains the best form of treatment for PPD caused by exposure to celery. Postcontact management includes washing the affected area with soap and water and changing clothes promptly. Topical corticosteroids have mild utility in treatment of PPD.18 Oral steroid tapers, which reduce acute inflammation, also are an option for treatment. Alternatively, intramuscular triamcinolone acetonide 1 mg/kg mixed with budesonide 0.1 mg/kg is an option and is associated with a reduced risk for adverse effects compared to oral steroids. The resulting hyperpigmentation develops 1 to 2 weeks postepithelialization.19 Hyperpigmentation often fades slowly over several months in lighter-skinned individuals but may last for years or indefinitely in darker-skinned patients.
Final Thoughts
Dermatologists should be knowledgeable about the various plant culprits that can induce PPD. Understanding the mechanism and pathophysiology can help guide both therapeutic interventions and preventive counseling. Understanding that even readily available vegetables such as celery can induce cutaneous eruptions should put PPD in the differential diagnosis more commonly when unspecified dermatitides are present.
- Walansky A. Study finally confirms eating celery burns more calories than it contains. Food & Wine. June 22, 2017. Accessed January 17, 2025. https://www.foodandwine.com/news/study-finally-confirms-eating-celery-burns-more-caloriesit-contains
- Puig L. Enhancement of PUVA phototoxic effects following celery ingestion: cool broth also can burn. Arch Dermatol. 1994;130:809-810. doi:10.1001/archderm.130.6.809
- Perez-Pimiento AJ, Moneo I, Santaolalla M, et al. Anaphylactic reaction to young garlic. Allergy. 1999;54:626-629.
- The Editors of Encyclopaedia Britannica. Apiaceae. Britannica. Updated November 25, 2024. Accessed January 17, 2025. https://www.britannica.com/plant/Apiaceae
- Smith R. Celery. In: Geoffriau E, Simon PW, eds. Carrots and Related Apiaceae Crops. 2nd ed. CABI; 2021:272-282.
- Dijkstra JWE, Chang L. Severe phototoxic burn following celery ingestion. Arch Dermatol. 1992;128:1277.
- Tobyn G, Denham A, Whitelegg M. Apium graveolens, wild celery. The Western Herbal Tradition: 2000 years of Medicinal Plant Knowledge. Elsevier. 2011:79-89. doi:10.1016/b978-0-443-10344-5.00014-8
- Rademaker M. Celery. DermNet. Accessed January 17, 2025. https://dermnetnz.org/topics/celery
- Sasseville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
- Jin Goon AT, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710. doi:10.4103/0019-5154.91833
- Birmingham DJ, Key MM, Tublich GE. Phototoxic bullae among celery harvesters. Arch Dermatol. 1961;83:73-87.
- Robb-Nicholson C. By the way, doctor: is a tanning bed safer than sunlight? Harvard Health Publishing. Harvard Medical School. September 1, 2009. Accessed January 17, 2025. https://www.health.harvard.edu/staying-healthy/is-a-tanning-bed-saferthan-sunlight
- Vester L, Thyssen JP, Menne T, et al. Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermatitis. 2012;67:328-333.
- Ling TC, Clayton TH, Crawley J, et al. British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen-ultraviolet A therapy 2015. Br J Dermatol. 2016;174:24-55.
- Laskin JD. Cellular and molecular mechanisms in photochemical sensitization: studies on the mechanism of action of psoralens. Food Chem Toxicol. 1994;32:119-127. doi:10.1016/0278-6915(94)90172-4
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UpToDate. Updated February 23, 2023. Accessed January 17, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Boffa, MJ, Gilmour E, Ead RD. Celery soup causing severe phototoxity during PUVA therapy. Br J Dermatol. 1996;135:334. doi:10.1111/j.1365-2133.1996.tb01182.x
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Mosby; 2018:286-303.
Celery (Apium graveolens)—that lowly vegetable that often languishes in the refrigerator crisper and apparently supplies fewer calories than are required to consume it—contains a myriad of photosensitizing chemicals known as furocoumarins and psoralens that can cause phytophotodermatitis (PPD) when handled prior to exposure to UV light.1 Individuals who are most likely to develop PPD caused by repeated contact with celery include food industry workers (eg, grocery store workers, farmers) who pick, handle, or prepare celery for consumption. While eating celery as part of a standard diet is highly unlikely to cause PPD, celery infected with Sclerotinia sclerotiorum (known as pink rot) causes more severe generalized sun sensitivity due to an increased amount of furocoumarins produced in response to the fungus.2 Contact with celery also can induce cutaneous manifestations unrelated to sun exposure in some individuals, including urticaria, allergic contact dermatitis, and anaphylaxis.3 In this article, we provide an overview of the life cycle and origin of celery as well as its irritant and allergic properties. We also describe cutaneous rashes associated with PPD caused by exposure to celery and highlight treatment options.
Morphology and Distribution
The Apiaceae family features aromatic flowering plants that comprise more than 3500 species, including many economically important vegetables, herbs, and spices.4 It also includes many alkaloid-containing species that are known to be poisonous to humans, such as poison hemlock (Conium maculatum) and water hemlock (Cicuta maculate). Most Apiaceae plants that are consumed by humans originate from the Mediterranean region.5 While known for their diversity of flavor and aroma, most of the plants from this family have low caloric value and provide minimal amounts of energy.
Members of the Apiaceae family have flowers that create a classic umbel shape mimicking the appearance of an upside-down umbrella (thus the former name for this family, Umbelliferae). The pedicles—the small stems attached to the base of each flower—spread from a common center to form the umbel.5 The Apiaceae family also includes the greatest number of plants that cause PPD due to their high concentration of furocoumarins, which deter fungus from harming the plants.6
A biennial plant, celery completes its life cycle in 2 years. During the first season, the stems, roots, and leaves sprout; in the second and final year, the flowers, fruits, and seeds proliferate, followed by decomposition. Apium graveolens approaches heights of 2 to 3 ft, growing upright and displaying grooved stems. Each stem terminates in a basal rosette of leaves. The second season brings white flower blooms in terminal or axillary umbels.7
Celery originated in the temperate Mediterranean regions of Europe, but farmers now cultivate it globally.8 It grows best in rich moist soil with full exposure to sunlight. Plants multiply their numbers through self-seeding. Celery commonly is found in suburban and rural homes, both in refrigerators for consumption as well as in medicine cabinets in capsule form for the treatment of arthritis.4
Irritant and Allergenic Properties
Despite the potential health benefits of celery, the Apiaceae family, which includes hogweed, dill, and fennel, prevails as the most common culprit for phytotoxic reactions. The Rutaceae family, including citrus plants and rue, remains runner-up for causes of PPD.9 Phytophotodermatitis is not an immunologic reaction, making anyone susceptible to formation of the cutaneous lesions when exposed to UV light after handling celery. Pruritis rarely occurs, unlike in allergic phytodermatitis.10 Upon photoexcitation from exposure to UVA light, individual psoralen molecules covalently bind to pyrimidine bases, causing interstrand cross-linking that prevents DNA replication and triggering a cascade leading to apoptosis of the cell. Apoptosis induces cell membrane edema, which manifests as cutaneous vesicles and bullae on the skin.10 Regardless of plant species, PPD reactions have similar appearance.
Celery roots contain the greatest concentration of psoralens, making it the most likely part of the plant to induce PPD.6 Phytophotodermatitis caused by celery can occur at any time of the year, but most eruptions occur during the summer months due to increased sunlight exposure and intensity. Among 320 randomly selected Michigan celery harvesters, 163 (51%) displayed evidence of vesicular and bullous dermatitis on the fingers, hands, and forearms.11 In this study, celery infected with pink rot fungus induced an erythematous eruption with vesicles and bullae within 48 hours of contact after just 30 seconds of summer sunlight exposure; however, eruptions are not limited to summer months, as the cutaneous presentation depends solely on exposure to UVA light, which can occur year-round.
Use of tanning beds is a major risk factor for PPD.12 Tanning beds utilize fluorescent bulbs that primarily emit UVA light, with UVB light emitted to a lesser degree. The UVA radiation produced by tanning beds is more than 3 times as intense as natural sunlight.12 Among grocery store employees, the combination of these 2 risk factors—regular contact with celery and tanning bed use—resulted in a prevalence ratio for PPD more than 40 times greater than that of individuals with neither risk factor.13
Cutaneous Manifestations of PPD
Phytophotodermatitis is a nonimmunologic dermatitis that forms via the interaction between UV light exposure and the photosensitizing chemicals inherent to some plant species. Development of PPD following contact with celery may be caused by the photoactive substances in celery, including the psoralens 8-methoxypsoralen and 5-methoxypsoralen.14 The psoralens must become activated by UV light with wavelengths between 320 nm and 400 nm (UVA) to initiate biologic effects.15
Once chemically activated, the photoactive mediators cause an erythematous and edematous sunburnlike reaction. Current hypotheses state that psoralen plus UVA generates reactive oxygen species, which damage the DNA within cells and alter receptors on cell membranes within the epidermis.14 The cutaneous eruption usually appears between 12 and 36 hours after sun exposure. Although they generally are not pruritic, the eruptions may induce pain. Within 7 to 10 days following development of the rash, hyperpigmentation occurs in the affected area and often persists for months to years.16 Ingestion of large amounts of celery has been cited to cause generalized phototoxic reactions; however, PPD rarely arises solely after ingestion, unless excessive amounts are consumed with concomitant exposure to psoralen plus UVA or tanning beds.17 In these cases, patients develop diffuse redness with superficial scaling, pain, and blistering if severe.
Treatment of PPD
Prevention remains the best form of treatment for PPD caused by exposure to celery. Postcontact management includes washing the affected area with soap and water and changing clothes promptly. Topical corticosteroids have mild utility in treatment of PPD.18 Oral steroid tapers, which reduce acute inflammation, also are an option for treatment. Alternatively, intramuscular triamcinolone acetonide 1 mg/kg mixed with budesonide 0.1 mg/kg is an option and is associated with a reduced risk for adverse effects compared to oral steroids. The resulting hyperpigmentation develops 1 to 2 weeks postepithelialization.19 Hyperpigmentation often fades slowly over several months in lighter-skinned individuals but may last for years or indefinitely in darker-skinned patients.
Final Thoughts
Dermatologists should be knowledgeable about the various plant culprits that can induce PPD. Understanding the mechanism and pathophysiology can help guide both therapeutic interventions and preventive counseling. Understanding that even readily available vegetables such as celery can induce cutaneous eruptions should put PPD in the differential diagnosis more commonly when unspecified dermatitides are present.
Celery (Apium graveolens)—that lowly vegetable that often languishes in the refrigerator crisper and apparently supplies fewer calories than are required to consume it—contains a myriad of photosensitizing chemicals known as furocoumarins and psoralens that can cause phytophotodermatitis (PPD) when handled prior to exposure to UV light.1 Individuals who are most likely to develop PPD caused by repeated contact with celery include food industry workers (eg, grocery store workers, farmers) who pick, handle, or prepare celery for consumption. While eating celery as part of a standard diet is highly unlikely to cause PPD, celery infected with Sclerotinia sclerotiorum (known as pink rot) causes more severe generalized sun sensitivity due to an increased amount of furocoumarins produced in response to the fungus.2 Contact with celery also can induce cutaneous manifestations unrelated to sun exposure in some individuals, including urticaria, allergic contact dermatitis, and anaphylaxis.3 In this article, we provide an overview of the life cycle and origin of celery as well as its irritant and allergic properties. We also describe cutaneous rashes associated with PPD caused by exposure to celery and highlight treatment options.
Morphology and Distribution
The Apiaceae family features aromatic flowering plants that comprise more than 3500 species, including many economically important vegetables, herbs, and spices.4 It also includes many alkaloid-containing species that are known to be poisonous to humans, such as poison hemlock (Conium maculatum) and water hemlock (Cicuta maculate). Most Apiaceae plants that are consumed by humans originate from the Mediterranean region.5 While known for their diversity of flavor and aroma, most of the plants from this family have low caloric value and provide minimal amounts of energy.
Members of the Apiaceae family have flowers that create a classic umbel shape mimicking the appearance of an upside-down umbrella (thus the former name for this family, Umbelliferae). The pedicles—the small stems attached to the base of each flower—spread from a common center to form the umbel.5 The Apiaceae family also includes the greatest number of plants that cause PPD due to their high concentration of furocoumarins, which deter fungus from harming the plants.6
A biennial plant, celery completes its life cycle in 2 years. During the first season, the stems, roots, and leaves sprout; in the second and final year, the flowers, fruits, and seeds proliferate, followed by decomposition. Apium graveolens approaches heights of 2 to 3 ft, growing upright and displaying grooved stems. Each stem terminates in a basal rosette of leaves. The second season brings white flower blooms in terminal or axillary umbels.7
Celery originated in the temperate Mediterranean regions of Europe, but farmers now cultivate it globally.8 It grows best in rich moist soil with full exposure to sunlight. Plants multiply their numbers through self-seeding. Celery commonly is found in suburban and rural homes, both in refrigerators for consumption as well as in medicine cabinets in capsule form for the treatment of arthritis.4
Irritant and Allergenic Properties
Despite the potential health benefits of celery, the Apiaceae family, which includes hogweed, dill, and fennel, prevails as the most common culprit for phytotoxic reactions. The Rutaceae family, including citrus plants and rue, remains runner-up for causes of PPD.9 Phytophotodermatitis is not an immunologic reaction, making anyone susceptible to formation of the cutaneous lesions when exposed to UV light after handling celery. Pruritis rarely occurs, unlike in allergic phytodermatitis.10 Upon photoexcitation from exposure to UVA light, individual psoralen molecules covalently bind to pyrimidine bases, causing interstrand cross-linking that prevents DNA replication and triggering a cascade leading to apoptosis of the cell. Apoptosis induces cell membrane edema, which manifests as cutaneous vesicles and bullae on the skin.10 Regardless of plant species, PPD reactions have similar appearance.
Celery roots contain the greatest concentration of psoralens, making it the most likely part of the plant to induce PPD.6 Phytophotodermatitis caused by celery can occur at any time of the year, but most eruptions occur during the summer months due to increased sunlight exposure and intensity. Among 320 randomly selected Michigan celery harvesters, 163 (51%) displayed evidence of vesicular and bullous dermatitis on the fingers, hands, and forearms.11 In this study, celery infected with pink rot fungus induced an erythematous eruption with vesicles and bullae within 48 hours of contact after just 30 seconds of summer sunlight exposure; however, eruptions are not limited to summer months, as the cutaneous presentation depends solely on exposure to UVA light, which can occur year-round.
Use of tanning beds is a major risk factor for PPD.12 Tanning beds utilize fluorescent bulbs that primarily emit UVA light, with UVB light emitted to a lesser degree. The UVA radiation produced by tanning beds is more than 3 times as intense as natural sunlight.12 Among grocery store employees, the combination of these 2 risk factors—regular contact with celery and tanning bed use—resulted in a prevalence ratio for PPD more than 40 times greater than that of individuals with neither risk factor.13
Cutaneous Manifestations of PPD
Phytophotodermatitis is a nonimmunologic dermatitis that forms via the interaction between UV light exposure and the photosensitizing chemicals inherent to some plant species. Development of PPD following contact with celery may be caused by the photoactive substances in celery, including the psoralens 8-methoxypsoralen and 5-methoxypsoralen.14 The psoralens must become activated by UV light with wavelengths between 320 nm and 400 nm (UVA) to initiate biologic effects.15
Once chemically activated, the photoactive mediators cause an erythematous and edematous sunburnlike reaction. Current hypotheses state that psoralen plus UVA generates reactive oxygen species, which damage the DNA within cells and alter receptors on cell membranes within the epidermis.14 The cutaneous eruption usually appears between 12 and 36 hours after sun exposure. Although they generally are not pruritic, the eruptions may induce pain. Within 7 to 10 days following development of the rash, hyperpigmentation occurs in the affected area and often persists for months to years.16 Ingestion of large amounts of celery has been cited to cause generalized phototoxic reactions; however, PPD rarely arises solely after ingestion, unless excessive amounts are consumed with concomitant exposure to psoralen plus UVA or tanning beds.17 In these cases, patients develop diffuse redness with superficial scaling, pain, and blistering if severe.
Treatment of PPD
Prevention remains the best form of treatment for PPD caused by exposure to celery. Postcontact management includes washing the affected area with soap and water and changing clothes promptly. Topical corticosteroids have mild utility in treatment of PPD.18 Oral steroid tapers, which reduce acute inflammation, also are an option for treatment. Alternatively, intramuscular triamcinolone acetonide 1 mg/kg mixed with budesonide 0.1 mg/kg is an option and is associated with a reduced risk for adverse effects compared to oral steroids. The resulting hyperpigmentation develops 1 to 2 weeks postepithelialization.19 Hyperpigmentation often fades slowly over several months in lighter-skinned individuals but may last for years or indefinitely in darker-skinned patients.
Final Thoughts
Dermatologists should be knowledgeable about the various plant culprits that can induce PPD. Understanding the mechanism and pathophysiology can help guide both therapeutic interventions and preventive counseling. Understanding that even readily available vegetables such as celery can induce cutaneous eruptions should put PPD in the differential diagnosis more commonly when unspecified dermatitides are present.
- Walansky A. Study finally confirms eating celery burns more calories than it contains. Food & Wine. June 22, 2017. Accessed January 17, 2025. https://www.foodandwine.com/news/study-finally-confirms-eating-celery-burns-more-caloriesit-contains
- Puig L. Enhancement of PUVA phototoxic effects following celery ingestion: cool broth also can burn. Arch Dermatol. 1994;130:809-810. doi:10.1001/archderm.130.6.809
- Perez-Pimiento AJ, Moneo I, Santaolalla M, et al. Anaphylactic reaction to young garlic. Allergy. 1999;54:626-629.
- The Editors of Encyclopaedia Britannica. Apiaceae. Britannica. Updated November 25, 2024. Accessed January 17, 2025. https://www.britannica.com/plant/Apiaceae
- Smith R. Celery. In: Geoffriau E, Simon PW, eds. Carrots and Related Apiaceae Crops. 2nd ed. CABI; 2021:272-282.
- Dijkstra JWE, Chang L. Severe phototoxic burn following celery ingestion. Arch Dermatol. 1992;128:1277.
- Tobyn G, Denham A, Whitelegg M. Apium graveolens, wild celery. The Western Herbal Tradition: 2000 years of Medicinal Plant Knowledge. Elsevier. 2011:79-89. doi:10.1016/b978-0-443-10344-5.00014-8
- Rademaker M. Celery. DermNet. Accessed January 17, 2025. https://dermnetnz.org/topics/celery
- Sasseville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
- Jin Goon AT, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710. doi:10.4103/0019-5154.91833
- Birmingham DJ, Key MM, Tublich GE. Phototoxic bullae among celery harvesters. Arch Dermatol. 1961;83:73-87.
- Robb-Nicholson C. By the way, doctor: is a tanning bed safer than sunlight? Harvard Health Publishing. Harvard Medical School. September 1, 2009. Accessed January 17, 2025. https://www.health.harvard.edu/staying-healthy/is-a-tanning-bed-saferthan-sunlight
- Vester L, Thyssen JP, Menne T, et al. Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermatitis. 2012;67:328-333.
- Ling TC, Clayton TH, Crawley J, et al. British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen-ultraviolet A therapy 2015. Br J Dermatol. 2016;174:24-55.
- Laskin JD. Cellular and molecular mechanisms in photochemical sensitization: studies on the mechanism of action of psoralens. Food Chem Toxicol. 1994;32:119-127. doi:10.1016/0278-6915(94)90172-4
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UpToDate. Updated February 23, 2023. Accessed January 17, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Boffa, MJ, Gilmour E, Ead RD. Celery soup causing severe phototoxity during PUVA therapy. Br J Dermatol. 1996;135:334. doi:10.1111/j.1365-2133.1996.tb01182.x
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Mosby; 2018:286-303.
- Walansky A. Study finally confirms eating celery burns more calories than it contains. Food & Wine. June 22, 2017. Accessed January 17, 2025. https://www.foodandwine.com/news/study-finally-confirms-eating-celery-burns-more-caloriesit-contains
- Puig L. Enhancement of PUVA phototoxic effects following celery ingestion: cool broth also can burn. Arch Dermatol. 1994;130:809-810. doi:10.1001/archderm.130.6.809
- Perez-Pimiento AJ, Moneo I, Santaolalla M, et al. Anaphylactic reaction to young garlic. Allergy. 1999;54:626-629.
- The Editors of Encyclopaedia Britannica. Apiaceae. Britannica. Updated November 25, 2024. Accessed January 17, 2025. https://www.britannica.com/plant/Apiaceae
- Smith R. Celery. In: Geoffriau E, Simon PW, eds. Carrots and Related Apiaceae Crops. 2nd ed. CABI; 2021:272-282.
- Dijkstra JWE, Chang L. Severe phototoxic burn following celery ingestion. Arch Dermatol. 1992;128:1277.
- Tobyn G, Denham A, Whitelegg M. Apium graveolens, wild celery. The Western Herbal Tradition: 2000 years of Medicinal Plant Knowledge. Elsevier. 2011:79-89. doi:10.1016/b978-0-443-10344-5.00014-8
- Rademaker M. Celery. DermNet. Accessed January 17, 2025. https://dermnetnz.org/topics/celery
- Sasseville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
- Jin Goon AT, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710. doi:10.4103/0019-5154.91833
- Birmingham DJ, Key MM, Tublich GE. Phototoxic bullae among celery harvesters. Arch Dermatol. 1961;83:73-87.
- Robb-Nicholson C. By the way, doctor: is a tanning bed safer than sunlight? Harvard Health Publishing. Harvard Medical School. September 1, 2009. Accessed January 17, 2025. https://www.health.harvard.edu/staying-healthy/is-a-tanning-bed-saferthan-sunlight
- Vester L, Thyssen JP, Menne T, et al. Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermatitis. 2012;67:328-333.
- Ling TC, Clayton TH, Crawley J, et al. British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen-ultraviolet A therapy 2015. Br J Dermatol. 2016;174:24-55.
- Laskin JD. Cellular and molecular mechanisms in photochemical sensitization: studies on the mechanism of action of psoralens. Food Chem Toxicol. 1994;32:119-127. doi:10.1016/0278-6915(94)90172-4
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UpToDate. Updated February 23, 2023. Accessed January 17, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Boffa, MJ, Gilmour E, Ead RD. Celery soup causing severe phototoxity during PUVA therapy. Br J Dermatol. 1996;135:334. doi:10.1111/j.1365-2133.1996.tb01182.x
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Mosby; 2018:286-303.
Not as Bland as You May Think: Celery (Apium graveolens) Commonly Induces Phytophotodermatitis
Not as Bland as You May Think: Celery (Apium graveolens) Commonly Induces Phytophotodermatitis
PRACTICE POINTS
- Clinicians should consider phytophotodermatitis (PPD) in the differential diagnosis for erythematous eruptions with bullae and vesicles manifesting in sun-exposed distributions.
- A clinical history that includes the patient’s occupation, diet, and history of treatment with psoralen plus UVA and use of tanning beds may help diagnose PPD.
- It is important to educate patients who regularly handle celery and other plants containing furocoumarins and psoralens on how to prevent PPD and utilize effective photoprotection.
A Threat to Scientific Progress
The United States has long been recognized as a global leader in biomedical research and scientific discovery, with federal research and development (R&D) funding serving as the bedrock of national innovation. Substantial federal investment in biomedical research has stemmed from a recognition of its importance in fueling critical discoveries that improve patient care and the health of our communities.
In the United States, academic institutions play a key role in conducting research in the national interest and collaborating with industry, with most of the federal research funding distributed by the National Institutes of Health, National Science Foundation, and other agencies awarded to university-based academic investigators. In a 2014 report, the National Academies of Sciences, Engineering and Medicine identified three pillars of a highly productive research system: a talented and interconnected workforce, adequate and dependable resources, and world-class basic research in all major areas of science.
A series of recent, short-sighted federal policy decisions threaten the future of scientific discovery by eroding these pillars. Decisions to freeze previously awarded federal grant funding, delay grant review panels, fire federal scientists, and propose crippling cuts to indirect cost rates (among others) have sent shock waves through the research community and already have led some prominent research institutions to cut staff and divert resources away from groundbreaking research. While the acute effects of these changes are just beginning to be felt, it is the long-term effects of these decisions on future medical and scientific discovery that will be most devastating to society.
In our April issue, we highlight important research advancements in inflammatory bowel disease presented at February’s Congress of the European Crohn’s and Colitis Organisation (ECCO) in Berlin. In this month’s Member Spotlight, Abigail Meyers, MPAS, PA-C, outlines her impactful work as a member of AGA’s newly formed Nurse Practitioner and Physician Assistant Task Force and shares how her personal journey as a patient with inflammatory bowel disease allows her to be a more powerful advocate for important issues impacting other patients with this condition.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The United States has long been recognized as a global leader in biomedical research and scientific discovery, with federal research and development (R&D) funding serving as the bedrock of national innovation. Substantial federal investment in biomedical research has stemmed from a recognition of its importance in fueling critical discoveries that improve patient care and the health of our communities.
In the United States, academic institutions play a key role in conducting research in the national interest and collaborating with industry, with most of the federal research funding distributed by the National Institutes of Health, National Science Foundation, and other agencies awarded to university-based academic investigators. In a 2014 report, the National Academies of Sciences, Engineering and Medicine identified three pillars of a highly productive research system: a talented and interconnected workforce, adequate and dependable resources, and world-class basic research in all major areas of science.
A series of recent, short-sighted federal policy decisions threaten the future of scientific discovery by eroding these pillars. Decisions to freeze previously awarded federal grant funding, delay grant review panels, fire federal scientists, and propose crippling cuts to indirect cost rates (among others) have sent shock waves through the research community and already have led some prominent research institutions to cut staff and divert resources away from groundbreaking research. While the acute effects of these changes are just beginning to be felt, it is the long-term effects of these decisions on future medical and scientific discovery that will be most devastating to society.
In our April issue, we highlight important research advancements in inflammatory bowel disease presented at February’s Congress of the European Crohn’s and Colitis Organisation (ECCO) in Berlin. In this month’s Member Spotlight, Abigail Meyers, MPAS, PA-C, outlines her impactful work as a member of AGA’s newly formed Nurse Practitioner and Physician Assistant Task Force and shares how her personal journey as a patient with inflammatory bowel disease allows her to be a more powerful advocate for important issues impacting other patients with this condition.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The United States has long been recognized as a global leader in biomedical research and scientific discovery, with federal research and development (R&D) funding serving as the bedrock of national innovation. Substantial federal investment in biomedical research has stemmed from a recognition of its importance in fueling critical discoveries that improve patient care and the health of our communities.
In the United States, academic institutions play a key role in conducting research in the national interest and collaborating with industry, with most of the federal research funding distributed by the National Institutes of Health, National Science Foundation, and other agencies awarded to university-based academic investigators. In a 2014 report, the National Academies of Sciences, Engineering and Medicine identified three pillars of a highly productive research system: a talented and interconnected workforce, adequate and dependable resources, and world-class basic research in all major areas of science.
A series of recent, short-sighted federal policy decisions threaten the future of scientific discovery by eroding these pillars. Decisions to freeze previously awarded federal grant funding, delay grant review panels, fire federal scientists, and propose crippling cuts to indirect cost rates (among others) have sent shock waves through the research community and already have led some prominent research institutions to cut staff and divert resources away from groundbreaking research. While the acute effects of these changes are just beginning to be felt, it is the long-term effects of these decisions on future medical and scientific discovery that will be most devastating to society.
In our April issue, we highlight important research advancements in inflammatory bowel disease presented at February’s Congress of the European Crohn’s and Colitis Organisation (ECCO) in Berlin. In this month’s Member Spotlight, Abigail Meyers, MPAS, PA-C, outlines her impactful work as a member of AGA’s newly formed Nurse Practitioner and Physician Assistant Task Force and shares how her personal journey as a patient with inflammatory bowel disease allows her to be a more powerful advocate for important issues impacting other patients with this condition.
Megan A. Adams, MD, JD, MSc
Editor in Chief
A Painful Flesh-Colored Papule on the Shoulder
A Painful Flesh-Colored Papule on the Shoulder
The Diagnosis: Leiomyoma
Histopathology revealed a dermal mesenchymal tumor composed of fascicles of bland spindle cells with tapered nuclei, perinuclear vacuoles, eosinophilic cytoplasm, and low cellularity (Figure 1). Immunohistochemical studies of the cells stained strongly positive for smooth muscle actin and desmin, consistent with a smooth muscle neoplasm (Figure 2). Fumarate hydratase (FH) staining revealed loss of expression in tumor cells, consistent with FH deficiency (Figure 3). A diagnosis of cutaneous leiomyoma was made, and although the clinical and histologic findings suggested hereditary leiomyomatosis and renal cell cancer (HLRCC), genetic testing was negative for an FH gene mutation. This negative result indicated that HLRCC was unlikely despite the initial concerns based on the findings.
Leiomyomas are benign neoplasms that are challenging to diagnose based on the clinical picture alone. Leiomyomas most commonly are found in the genitourinary and gastrointestinal systems, with cutaneous manifestation being the second most common presentation.1 These benign smooth muscle tumors manifest as tender, firm, flesh-colored, pink or reddish-brown nodules that are subcategorized based on the derivation of the smooth muscle within the tumor.2 Angioleiomyomas, the most common type, arise from the tunica media of blood vessels, whereas piloleiomyomas and genital leiomyomas arise from the arrector pili musculature of the hair follicle and the smooth muscle found in the scrotum, labia, or nipple.2 Rare cases of cutaneous leiomyosarcomas and angioleiomyosarcomas have been reported in the literature.3,4 Solitary leiomyomas tend to develop on the lower extremities, whereas multiple lesions frequently manifest on the extensor surfaces of extremities and the trunk. Lesions often are painful, either spontaneously or in association with applied pressure, emotional stress, or exposure to cold temperatures.2
Although leiomyomas themselves are benign, patients with multiple cutaneous leiomyomas may have an underlying genetic mutation that increases their risk of developing HLRCC, an autosomal-dominant syndrome.5 Referral should be considered for individuals with a personal history of or a first-degree relative with cutaneous leiomyomas or renal cell carcinoma (RCC) with histology typical of hereditary leiomyomatosis and RCC, as recommended by the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors.6 In this case, the decision to refer the patient for genetic testing was based on her family history, specifically her paternal uncle having multiple similar lesions, which, while not a first-degree relative, still raised concerns about potential hereditary risks and warranted further evaluation. A germline mutation in the FH gene, which encodes an enzyme that converts fumarate to malate in the Krebs cycle and plays a role in tumor suppression, is the cause of HLRCC.2,7 When part of this genetic condition, cutaneous leiomyomas tend to occur around 25 years of age (range, 10-50 years).2 A diagnosis of HLRCC should be strongly considered if a patient displays multiple cutaneous leiomyomas with at least 1 histologically confirmed lesion or at least 2 of the following: solitary cutaneous leiomyoma with family history of HLRCC, onset of severely symptomatic uterine fibroids before age 40 years, type II papillary or collecting duct renal cell cancer before age 40 years, or a first-degree family member who meets 1 of these criteria.5,8
Diagnosis of cutaneous leiomyoma may be accomplished by microscopic examination of a tissue sample; however, further diagnostic workup is warranted due to the strong correlation with HLRCC.2 A definitive diagnosis of HLRCC is confirmed with a germline mutation in the FH gene, and genetic screening should be offered to patients before renal cancer surveillance to avoid unwarranted investigations.8 Timely clinical diagnosis enables early genetic testing and enhanced outcomes for patients with confirmed HLRCC who may need a multidisciplinary approach of dermatologists, gynecologists, and urologic oncologists.5,8
Cutaneous leiomyomas can be excised, and this typically is the gold standard of care for small and localized lesions, although the use of cryosurgery and carbon dioxide lasers has been reported as well.2,9,10 For more widespread lesions or for patients who are not appropriate candidates for surgery, pharmacologic therapies (α-blockers, calcium channel blockers, nitroglycerin), intralesional corticosteroids, and/or botulinum toxin injections can be utilized.2,11
The acronym BLEND AN EGG encompasses the clinical differential diagnosis for painful skin tumors: blue rubber bleb nevus, leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomangioma, and granular cell tumor. Blue rubber bleb nevi are deep blue in color, and angiolipomas sit under the skin and present as subcutaneous swellings. Dermatofibromas and neurofibromas also are included in the differential.12 Dermatofibromas are firm solitary lesions that have a pathognomonic pinch sign. Neurofibromas are soft and rubbery, have a buttonhole sign, and stain positively for S-100 protein and SOX-10 but negatively for actin and desmin.12
- Malhotra P, Walia H, Singh A, et al. Leiomyoma cutis: a clinicopathological series of 37 cases. Indian J Dermatol. 2010;55:337-341.
- Bernett CN, Mammino JJ. Cutaneous leiomyomas. In: StatPearls. StatPearls Publishing; 2023.
- Chayed Z, Kristensen LK, Ousager LB, et al. Hereditary leiomyomatosis and renal cell carcinoma: a case series and literature review. Orphanet J Rare Dis. 2021;16:34. doi:10.1186/s13023-020-01653-9
- Perkins J, Scarbrough C, Sammons D, et al. Reed syndrome: an atypical presentation of a rare disease. Dermatol Online J. 2014;21: 13030/qt5k35r5pn.
- Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis. 2014;7:253-260. doi:10.2147 /IJNRD.S42097
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17:70-87. doi:10.1038/gim.2014.147
- Alam NA, Barclay E, Rowan AJ, et al. Clinical features of multiple cutaneous and uterine leiomyomatosis: an underdiagnosed tumor syndrome. Arch Dermatol. 2005;141:199-206. doi:10.1001 /archderm.141.2.199
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637-644. doi:10.1007/s10689-014-9735-2
- Uyar B, Acar EM, Subas¸ıog˘lu A. Treatment of three hereditary leiomyomatosis patients with cryotherapy. Dermatol Ther. 2020;33:e13226. doi:10.1111/dth.13226
- Christenson LJ, Smith K, Arpey CJ. Treatment of multiple cutaneous leiomyomas with CO2 laser ablation. Dermatol Surg. 2000;26:319-322. doi:10.1046/j.1524-4725.2000.99250.x
- Onder M, Adis¸en E. A new indication of botulinum toxin: leiomyoma- related pain. J Am Acad Dermatol. 2009;60:325-328. doi:10.1016 /j.jaad.2008.05.044
- Clarey DD, Lauer SR, Adams JL. Painful papules on the arms. Cutis. 2020;106:232-249. doi:10.12788/cutis.0109
The Diagnosis: Leiomyoma
Histopathology revealed a dermal mesenchymal tumor composed of fascicles of bland spindle cells with tapered nuclei, perinuclear vacuoles, eosinophilic cytoplasm, and low cellularity (Figure 1). Immunohistochemical studies of the cells stained strongly positive for smooth muscle actin and desmin, consistent with a smooth muscle neoplasm (Figure 2). Fumarate hydratase (FH) staining revealed loss of expression in tumor cells, consistent with FH deficiency (Figure 3). A diagnosis of cutaneous leiomyoma was made, and although the clinical and histologic findings suggested hereditary leiomyomatosis and renal cell cancer (HLRCC), genetic testing was negative for an FH gene mutation. This negative result indicated that HLRCC was unlikely despite the initial concerns based on the findings.
Leiomyomas are benign neoplasms that are challenging to diagnose based on the clinical picture alone. Leiomyomas most commonly are found in the genitourinary and gastrointestinal systems, with cutaneous manifestation being the second most common presentation.1 These benign smooth muscle tumors manifest as tender, firm, flesh-colored, pink or reddish-brown nodules that are subcategorized based on the derivation of the smooth muscle within the tumor.2 Angioleiomyomas, the most common type, arise from the tunica media of blood vessels, whereas piloleiomyomas and genital leiomyomas arise from the arrector pili musculature of the hair follicle and the smooth muscle found in the scrotum, labia, or nipple.2 Rare cases of cutaneous leiomyosarcomas and angioleiomyosarcomas have been reported in the literature.3,4 Solitary leiomyomas tend to develop on the lower extremities, whereas multiple lesions frequently manifest on the extensor surfaces of extremities and the trunk. Lesions often are painful, either spontaneously or in association with applied pressure, emotional stress, or exposure to cold temperatures.2
Although leiomyomas themselves are benign, patients with multiple cutaneous leiomyomas may have an underlying genetic mutation that increases their risk of developing HLRCC, an autosomal-dominant syndrome.5 Referral should be considered for individuals with a personal history of or a first-degree relative with cutaneous leiomyomas or renal cell carcinoma (RCC) with histology typical of hereditary leiomyomatosis and RCC, as recommended by the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors.6 In this case, the decision to refer the patient for genetic testing was based on her family history, specifically her paternal uncle having multiple similar lesions, which, while not a first-degree relative, still raised concerns about potential hereditary risks and warranted further evaluation. A germline mutation in the FH gene, which encodes an enzyme that converts fumarate to malate in the Krebs cycle and plays a role in tumor suppression, is the cause of HLRCC.2,7 When part of this genetic condition, cutaneous leiomyomas tend to occur around 25 years of age (range, 10-50 years).2 A diagnosis of HLRCC should be strongly considered if a patient displays multiple cutaneous leiomyomas with at least 1 histologically confirmed lesion or at least 2 of the following: solitary cutaneous leiomyoma with family history of HLRCC, onset of severely symptomatic uterine fibroids before age 40 years, type II papillary or collecting duct renal cell cancer before age 40 years, or a first-degree family member who meets 1 of these criteria.5,8
Diagnosis of cutaneous leiomyoma may be accomplished by microscopic examination of a tissue sample; however, further diagnostic workup is warranted due to the strong correlation with HLRCC.2 A definitive diagnosis of HLRCC is confirmed with a germline mutation in the FH gene, and genetic screening should be offered to patients before renal cancer surveillance to avoid unwarranted investigations.8 Timely clinical diagnosis enables early genetic testing and enhanced outcomes for patients with confirmed HLRCC who may need a multidisciplinary approach of dermatologists, gynecologists, and urologic oncologists.5,8
Cutaneous leiomyomas can be excised, and this typically is the gold standard of care for small and localized lesions, although the use of cryosurgery and carbon dioxide lasers has been reported as well.2,9,10 For more widespread lesions or for patients who are not appropriate candidates for surgery, pharmacologic therapies (α-blockers, calcium channel blockers, nitroglycerin), intralesional corticosteroids, and/or botulinum toxin injections can be utilized.2,11
The acronym BLEND AN EGG encompasses the clinical differential diagnosis for painful skin tumors: blue rubber bleb nevus, leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomangioma, and granular cell tumor. Blue rubber bleb nevi are deep blue in color, and angiolipomas sit under the skin and present as subcutaneous swellings. Dermatofibromas and neurofibromas also are included in the differential.12 Dermatofibromas are firm solitary lesions that have a pathognomonic pinch sign. Neurofibromas are soft and rubbery, have a buttonhole sign, and stain positively for S-100 protein and SOX-10 but negatively for actin and desmin.12
The Diagnosis: Leiomyoma
Histopathology revealed a dermal mesenchymal tumor composed of fascicles of bland spindle cells with tapered nuclei, perinuclear vacuoles, eosinophilic cytoplasm, and low cellularity (Figure 1). Immunohistochemical studies of the cells stained strongly positive for smooth muscle actin and desmin, consistent with a smooth muscle neoplasm (Figure 2). Fumarate hydratase (FH) staining revealed loss of expression in tumor cells, consistent with FH deficiency (Figure 3). A diagnosis of cutaneous leiomyoma was made, and although the clinical and histologic findings suggested hereditary leiomyomatosis and renal cell cancer (HLRCC), genetic testing was negative for an FH gene mutation. This negative result indicated that HLRCC was unlikely despite the initial concerns based on the findings.
Leiomyomas are benign neoplasms that are challenging to diagnose based on the clinical picture alone. Leiomyomas most commonly are found in the genitourinary and gastrointestinal systems, with cutaneous manifestation being the second most common presentation.1 These benign smooth muscle tumors manifest as tender, firm, flesh-colored, pink or reddish-brown nodules that are subcategorized based on the derivation of the smooth muscle within the tumor.2 Angioleiomyomas, the most common type, arise from the tunica media of blood vessels, whereas piloleiomyomas and genital leiomyomas arise from the arrector pili musculature of the hair follicle and the smooth muscle found in the scrotum, labia, or nipple.2 Rare cases of cutaneous leiomyosarcomas and angioleiomyosarcomas have been reported in the literature.3,4 Solitary leiomyomas tend to develop on the lower extremities, whereas multiple lesions frequently manifest on the extensor surfaces of extremities and the trunk. Lesions often are painful, either spontaneously or in association with applied pressure, emotional stress, or exposure to cold temperatures.2
Although leiomyomas themselves are benign, patients with multiple cutaneous leiomyomas may have an underlying genetic mutation that increases their risk of developing HLRCC, an autosomal-dominant syndrome.5 Referral should be considered for individuals with a personal history of or a first-degree relative with cutaneous leiomyomas or renal cell carcinoma (RCC) with histology typical of hereditary leiomyomatosis and RCC, as recommended by the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors.6 In this case, the decision to refer the patient for genetic testing was based on her family history, specifically her paternal uncle having multiple similar lesions, which, while not a first-degree relative, still raised concerns about potential hereditary risks and warranted further evaluation. A germline mutation in the FH gene, which encodes an enzyme that converts fumarate to malate in the Krebs cycle and plays a role in tumor suppression, is the cause of HLRCC.2,7 When part of this genetic condition, cutaneous leiomyomas tend to occur around 25 years of age (range, 10-50 years).2 A diagnosis of HLRCC should be strongly considered if a patient displays multiple cutaneous leiomyomas with at least 1 histologically confirmed lesion or at least 2 of the following: solitary cutaneous leiomyoma with family history of HLRCC, onset of severely symptomatic uterine fibroids before age 40 years, type II papillary or collecting duct renal cell cancer before age 40 years, or a first-degree family member who meets 1 of these criteria.5,8
Diagnosis of cutaneous leiomyoma may be accomplished by microscopic examination of a tissue sample; however, further diagnostic workup is warranted due to the strong correlation with HLRCC.2 A definitive diagnosis of HLRCC is confirmed with a germline mutation in the FH gene, and genetic screening should be offered to patients before renal cancer surveillance to avoid unwarranted investigations.8 Timely clinical diagnosis enables early genetic testing and enhanced outcomes for patients with confirmed HLRCC who may need a multidisciplinary approach of dermatologists, gynecologists, and urologic oncologists.5,8
Cutaneous leiomyomas can be excised, and this typically is the gold standard of care for small and localized lesions, although the use of cryosurgery and carbon dioxide lasers has been reported as well.2,9,10 For more widespread lesions or for patients who are not appropriate candidates for surgery, pharmacologic therapies (α-blockers, calcium channel blockers, nitroglycerin), intralesional corticosteroids, and/or botulinum toxin injections can be utilized.2,11
The acronym BLEND AN EGG encompasses the clinical differential diagnosis for painful skin tumors: blue rubber bleb nevus, leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomangioma, and granular cell tumor. Blue rubber bleb nevi are deep blue in color, and angiolipomas sit under the skin and present as subcutaneous swellings. Dermatofibromas and neurofibromas also are included in the differential.12 Dermatofibromas are firm solitary lesions that have a pathognomonic pinch sign. Neurofibromas are soft and rubbery, have a buttonhole sign, and stain positively for S-100 protein and SOX-10 but negatively for actin and desmin.12
- Malhotra P, Walia H, Singh A, et al. Leiomyoma cutis: a clinicopathological series of 37 cases. Indian J Dermatol. 2010;55:337-341.
- Bernett CN, Mammino JJ. Cutaneous leiomyomas. In: StatPearls. StatPearls Publishing; 2023.
- Chayed Z, Kristensen LK, Ousager LB, et al. Hereditary leiomyomatosis and renal cell carcinoma: a case series and literature review. Orphanet J Rare Dis. 2021;16:34. doi:10.1186/s13023-020-01653-9
- Perkins J, Scarbrough C, Sammons D, et al. Reed syndrome: an atypical presentation of a rare disease. Dermatol Online J. 2014;21: 13030/qt5k35r5pn.
- Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis. 2014;7:253-260. doi:10.2147 /IJNRD.S42097
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17:70-87. doi:10.1038/gim.2014.147
- Alam NA, Barclay E, Rowan AJ, et al. Clinical features of multiple cutaneous and uterine leiomyomatosis: an underdiagnosed tumor syndrome. Arch Dermatol. 2005;141:199-206. doi:10.1001 /archderm.141.2.199
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637-644. doi:10.1007/s10689-014-9735-2
- Uyar B, Acar EM, Subas¸ıog˘lu A. Treatment of three hereditary leiomyomatosis patients with cryotherapy. Dermatol Ther. 2020;33:e13226. doi:10.1111/dth.13226
- Christenson LJ, Smith K, Arpey CJ. Treatment of multiple cutaneous leiomyomas with CO2 laser ablation. Dermatol Surg. 2000;26:319-322. doi:10.1046/j.1524-4725.2000.99250.x
- Onder M, Adis¸en E. A new indication of botulinum toxin: leiomyoma- related pain. J Am Acad Dermatol. 2009;60:325-328. doi:10.1016 /j.jaad.2008.05.044
- Clarey DD, Lauer SR, Adams JL. Painful papules on the arms. Cutis. 2020;106:232-249. doi:10.12788/cutis.0109
- Malhotra P, Walia H, Singh A, et al. Leiomyoma cutis: a clinicopathological series of 37 cases. Indian J Dermatol. 2010;55:337-341.
- Bernett CN, Mammino JJ. Cutaneous leiomyomas. In: StatPearls. StatPearls Publishing; 2023.
- Chayed Z, Kristensen LK, Ousager LB, et al. Hereditary leiomyomatosis and renal cell carcinoma: a case series and literature review. Orphanet J Rare Dis. 2021;16:34. doi:10.1186/s13023-020-01653-9
- Perkins J, Scarbrough C, Sammons D, et al. Reed syndrome: an atypical presentation of a rare disease. Dermatol Online J. 2014;21: 13030/qt5k35r5pn.
- Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis. 2014;7:253-260. doi:10.2147 /IJNRD.S42097
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17:70-87. doi:10.1038/gim.2014.147
- Alam NA, Barclay E, Rowan AJ, et al. Clinical features of multiple cutaneous and uterine leiomyomatosis: an underdiagnosed tumor syndrome. Arch Dermatol. 2005;141:199-206. doi:10.1001 /archderm.141.2.199
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637-644. doi:10.1007/s10689-014-9735-2
- Uyar B, Acar EM, Subas¸ıog˘lu A. Treatment of three hereditary leiomyomatosis patients with cryotherapy. Dermatol Ther. 2020;33:e13226. doi:10.1111/dth.13226
- Christenson LJ, Smith K, Arpey CJ. Treatment of multiple cutaneous leiomyomas with CO2 laser ablation. Dermatol Surg. 2000;26:319-322. doi:10.1046/j.1524-4725.2000.99250.x
- Onder M, Adis¸en E. A new indication of botulinum toxin: leiomyoma- related pain. J Am Acad Dermatol. 2009;60:325-328. doi:10.1016 /j.jaad.2008.05.044
- Clarey DD, Lauer SR, Adams JL. Painful papules on the arms. Cutis. 2020;106:232-249. doi:10.12788/cutis.0109
A Painful Flesh-Colored Papule on the Shoulder
A Painful Flesh-Colored Papule on the Shoulder
A 65-year-old woman with a history of metabolic syndrome presented to the family medicine clinic for evaluation of a papule on the right shoulder that had started small and increased in size over the past 3 years. Physical examination revealed a 1.0×0.8×0.1-cm, smooth, flesh-colored to light brown papule on the right shoulder that was notably tender to palpation. The patient reported that her paternal uncle had multiple skin lesions of similar morphology dispersed on the bilateral upper extremities. A shave biopsy of the lesion was performed.
Erythematous Annular Scaly Plaques on the Upper Chest
Erythematous Annular Scaly Plaques on the Upper Chest
THE DIAGNOSIS: Tinea Corporis
Due to the scaly and acute nature of the rash, a potassium hydroxide (KOH) preparation was performed, and hyphal elements were floridly present. After further questioning, the patient reported finding a stray kitten a few weeks before the onset of the eruption and shared a picture of it lying on her chest in the area corresponding with the main distribution of the rash (Figure). Based on the patient’s personal history and the positive KOH preparation, a diagnosis of tinea corporis was made. She was immediately started on fluconazole 300 mg once weekly for 4 weeks and naftifine gel 1%, which she used for 6 to 8 weeks with complete resolution of the eruption.

Tinea corporis is a dermatophyte infection that typically affects exposed areas of the skin such as the chest, arms, and legs. Spread via human-to-human contact, Trichophyton rubrum is the most common cause worldwide. The second most common is Trichophyton mentagrophytes, which is spread through animal-to-human contact.1,2
Symptoms of tinea corporis usually appear 1 to 3 weeks after exposure and manifest as itchy scaly papules that spread outward, forming annular, circinate, and petaloid erythematous plaques with central clearing. The condition most commonly is diagnosed through the examination of scale from the affected area using a KOH preparation, which will reveal hyphae when positive.2-4 Cultures are the gold standard for identifying dermatophyte species,5 but results can take several weeks. Biopsy also can confirm the diagnosis by showing the presence of hyphae in the stratum corneum, which can be highlighted using periodic acid–Schiff or silver stains.3
Topical antifungals are the first-line treatment for cutaneous dermatophyte infections.3-5 The most effective topical therapies are allylamines and azoles, which work by inhibiting the growth of the fungus. Allylamines are more effective than azoles due to their fungicidal properties and ability to penetrate the skin more effectively.6,7 Topical medications should be applied at least 2 cm beyond the infected area for 2 to 4 weeks or until the infection has cleared.3 Systemic antifungals may be necessary in more complicated cases.
It is important to consider a broad differential and take into consideration the distribution of the plaques, the patient’s history, and other clinical features when differentiating tinea corporis from other conditions. Erythema annulare centrifugum more often presents as nonpruritic annular plaques with a trailing scale instead of a leading scale seen in tinea corporis. Biopsy exhibits a dense, perivascular, lymphocytic infiltrate in superficial vessels, resembling a coat sleeve.3,8 Pemphigus foliaceous can manifest with painful crusted scaly plaques and vesicles in a seborrheic distribution. Biopsy reveals subcorneal acantholytic vesicles and can be confirmed on direct immunofluorescence.3,8 Subacute cutaneous lupus erythematosus presents with annular plaques that often are symmetric and most prominent in sun-exposed areas, sparing the face.3,9,10 It can be associated with other autoimmune conditions as well as medications such as thiazides, terbinafine, and calcium channel blockers. Additionally, 76% to 90% of patients are Ro/SSA antibody positive.3 Biopsy often demonstrates follicular plugging, perivascular and periadnexal lymphocytic infiltrates, and mucin.3,10 Lastly, pityriasis rosea typically begins with a herald patch, followed by a widespread rash that often appears in a Christmas tree distribution.3
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51 (suppl 4):2-15. doi: 10.1111 /j.1439-0507.2008.01606.x
- Yee G, Al Aboud AM. Tinea corporis. 2022 Aug 8. In: StatPearls [Internet]. StatPearls Publishing; 2023
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Elsevier; 2018.
- Diseases resulting from fungal and yeast. In: James WD, Berger TG, Elston DM, et al, eds. Andrews’ Diseases of The Skin: Clinical Dermatology. 12th ed. Elsevier; 2016: 289-290.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6 . doi:10.7573/dic.2020-5-6
- El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014;2014:CD009992. doi:10.1002/14651858 .CD009992.pub2
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2018.
- Burgdorf W. Erythema annulare centrifugum and other figurate erythemas. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. McGraw-Hill; 2008: 366-368.
- Modi GM, Maender JL, Coleman N, et al. Tinea corporis masquerading as subacute cutaneous lupus erythematosus. Dermatol Online J. 2008;14:8.
- Stavropoulos PG, Goules AV, Avgerinou G, et al. Pathogenesis of subacute cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2008;22:1281.
THE DIAGNOSIS: Tinea Corporis
Due to the scaly and acute nature of the rash, a potassium hydroxide (KOH) preparation was performed, and hyphal elements were floridly present. After further questioning, the patient reported finding a stray kitten a few weeks before the onset of the eruption and shared a picture of it lying on her chest in the area corresponding with the main distribution of the rash (Figure). Based on the patient’s personal history and the positive KOH preparation, a diagnosis of tinea corporis was made. She was immediately started on fluconazole 300 mg once weekly for 4 weeks and naftifine gel 1%, which she used for 6 to 8 weeks with complete resolution of the eruption.

Tinea corporis is a dermatophyte infection that typically affects exposed areas of the skin such as the chest, arms, and legs. Spread via human-to-human contact, Trichophyton rubrum is the most common cause worldwide. The second most common is Trichophyton mentagrophytes, which is spread through animal-to-human contact.1,2
Symptoms of tinea corporis usually appear 1 to 3 weeks after exposure and manifest as itchy scaly papules that spread outward, forming annular, circinate, and petaloid erythematous plaques with central clearing. The condition most commonly is diagnosed through the examination of scale from the affected area using a KOH preparation, which will reveal hyphae when positive.2-4 Cultures are the gold standard for identifying dermatophyte species,5 but results can take several weeks. Biopsy also can confirm the diagnosis by showing the presence of hyphae in the stratum corneum, which can be highlighted using periodic acid–Schiff or silver stains.3
Topical antifungals are the first-line treatment for cutaneous dermatophyte infections.3-5 The most effective topical therapies are allylamines and azoles, which work by inhibiting the growth of the fungus. Allylamines are more effective than azoles due to their fungicidal properties and ability to penetrate the skin more effectively.6,7 Topical medications should be applied at least 2 cm beyond the infected area for 2 to 4 weeks or until the infection has cleared.3 Systemic antifungals may be necessary in more complicated cases.
It is important to consider a broad differential and take into consideration the distribution of the plaques, the patient’s history, and other clinical features when differentiating tinea corporis from other conditions. Erythema annulare centrifugum more often presents as nonpruritic annular plaques with a trailing scale instead of a leading scale seen in tinea corporis. Biopsy exhibits a dense, perivascular, lymphocytic infiltrate in superficial vessels, resembling a coat sleeve.3,8 Pemphigus foliaceous can manifest with painful crusted scaly plaques and vesicles in a seborrheic distribution. Biopsy reveals subcorneal acantholytic vesicles and can be confirmed on direct immunofluorescence.3,8 Subacute cutaneous lupus erythematosus presents with annular plaques that often are symmetric and most prominent in sun-exposed areas, sparing the face.3,9,10 It can be associated with other autoimmune conditions as well as medications such as thiazides, terbinafine, and calcium channel blockers. Additionally, 76% to 90% of patients are Ro/SSA antibody positive.3 Biopsy often demonstrates follicular plugging, perivascular and periadnexal lymphocytic infiltrates, and mucin.3,10 Lastly, pityriasis rosea typically begins with a herald patch, followed by a widespread rash that often appears in a Christmas tree distribution.3
THE DIAGNOSIS: Tinea Corporis
Due to the scaly and acute nature of the rash, a potassium hydroxide (KOH) preparation was performed, and hyphal elements were floridly present. After further questioning, the patient reported finding a stray kitten a few weeks before the onset of the eruption and shared a picture of it lying on her chest in the area corresponding with the main distribution of the rash (Figure). Based on the patient’s personal history and the positive KOH preparation, a diagnosis of tinea corporis was made. She was immediately started on fluconazole 300 mg once weekly for 4 weeks and naftifine gel 1%, which she used for 6 to 8 weeks with complete resolution of the eruption.

Tinea corporis is a dermatophyte infection that typically affects exposed areas of the skin such as the chest, arms, and legs. Spread via human-to-human contact, Trichophyton rubrum is the most common cause worldwide. The second most common is Trichophyton mentagrophytes, which is spread through animal-to-human contact.1,2
Symptoms of tinea corporis usually appear 1 to 3 weeks after exposure and manifest as itchy scaly papules that spread outward, forming annular, circinate, and petaloid erythematous plaques with central clearing. The condition most commonly is diagnosed through the examination of scale from the affected area using a KOH preparation, which will reveal hyphae when positive.2-4 Cultures are the gold standard for identifying dermatophyte species,5 but results can take several weeks. Biopsy also can confirm the diagnosis by showing the presence of hyphae in the stratum corneum, which can be highlighted using periodic acid–Schiff or silver stains.3
Topical antifungals are the first-line treatment for cutaneous dermatophyte infections.3-5 The most effective topical therapies are allylamines and azoles, which work by inhibiting the growth of the fungus. Allylamines are more effective than azoles due to their fungicidal properties and ability to penetrate the skin more effectively.6,7 Topical medications should be applied at least 2 cm beyond the infected area for 2 to 4 weeks or until the infection has cleared.3 Systemic antifungals may be necessary in more complicated cases.
It is important to consider a broad differential and take into consideration the distribution of the plaques, the patient’s history, and other clinical features when differentiating tinea corporis from other conditions. Erythema annulare centrifugum more often presents as nonpruritic annular plaques with a trailing scale instead of a leading scale seen in tinea corporis. Biopsy exhibits a dense, perivascular, lymphocytic infiltrate in superficial vessels, resembling a coat sleeve.3,8 Pemphigus foliaceous can manifest with painful crusted scaly plaques and vesicles in a seborrheic distribution. Biopsy reveals subcorneal acantholytic vesicles and can be confirmed on direct immunofluorescence.3,8 Subacute cutaneous lupus erythematosus presents with annular plaques that often are symmetric and most prominent in sun-exposed areas, sparing the face.3,9,10 It can be associated with other autoimmune conditions as well as medications such as thiazides, terbinafine, and calcium channel blockers. Additionally, 76% to 90% of patients are Ro/SSA antibody positive.3 Biopsy often demonstrates follicular plugging, perivascular and periadnexal lymphocytic infiltrates, and mucin.3,10 Lastly, pityriasis rosea typically begins with a herald patch, followed by a widespread rash that often appears in a Christmas tree distribution.3
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51 (suppl 4):2-15. doi: 10.1111 /j.1439-0507.2008.01606.x
- Yee G, Al Aboud AM. Tinea corporis. 2022 Aug 8. In: StatPearls [Internet]. StatPearls Publishing; 2023
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Elsevier; 2018.
- Diseases resulting from fungal and yeast. In: James WD, Berger TG, Elston DM, et al, eds. Andrews’ Diseases of The Skin: Clinical Dermatology. 12th ed. Elsevier; 2016: 289-290.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6 . doi:10.7573/dic.2020-5-6
- El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014;2014:CD009992. doi:10.1002/14651858 .CD009992.pub2
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2018.
- Burgdorf W. Erythema annulare centrifugum and other figurate erythemas. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. McGraw-Hill; 2008: 366-368.
- Modi GM, Maender JL, Coleman N, et al. Tinea corporis masquerading as subacute cutaneous lupus erythematosus. Dermatol Online J. 2008;14:8.
- Stavropoulos PG, Goules AV, Avgerinou G, et al. Pathogenesis of subacute cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2008;22:1281.
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51 (suppl 4):2-15. doi: 10.1111 /j.1439-0507.2008.01606.x
- Yee G, Al Aboud AM. Tinea corporis. 2022 Aug 8. In: StatPearls [Internet]. StatPearls Publishing; 2023
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Elsevier; 2018.
- Diseases resulting from fungal and yeast. In: James WD, Berger TG, Elston DM, et al, eds. Andrews’ Diseases of The Skin: Clinical Dermatology. 12th ed. Elsevier; 2016: 289-290.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6 . doi:10.7573/dic.2020-5-6
- El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014;2014:CD009992. doi:10.1002/14651858 .CD009992.pub2
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier; 2018.
- Burgdorf W. Erythema annulare centrifugum and other figurate erythemas. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. McGraw-Hill; 2008: 366-368.
- Modi GM, Maender JL, Coleman N, et al. Tinea corporis masquerading as subacute cutaneous lupus erythematosus. Dermatol Online J. 2008;14:8.
- Stavropoulos PG, Goules AV, Avgerinou G, et al. Pathogenesis of subacute cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2008;22:1281.
Erythematous Annular Scaly Plaques on the Upper Chest
Erythematous Annular Scaly Plaques on the Upper Chest
A 60-year-old woman with a history of keratinocyte carcinomas, hypertension, diabetes mellitus, and anxiety presented to the dermatology department with a widespread rash of more than 2 weeks’ duration. The patient had tried 1 to 2 days of self-treatment with triamcinolone cream that she had previously been prescribed for an unknown dermatitis and zinc oxide cream, which caused considerable inflammation of the rash and prompted her to discontinue use. She could not recall any recent use of new personal care products or medications or eating any new foods. She also denied any recent yard work, known arthropod bites, illnesses, prolonged sun exposure, or constitutional symptoms. Her medications included metformin, hydrochlorothiazide, losartan, and sertraline. She also reported taking daily supplements of vitamins D, K, and C as well as acetaminophen and ibuprofen as needed. Physical examination revealed several 2- to 4-cm, erythematous, annular, circinate, petaloid plaques with scale mostly on photodistributed areas of the central anterior chest, neck, lower cheeks, and chin as well as a few scattered lesions with similar morphology on the arms, lower abdomen, left buttock, and back.
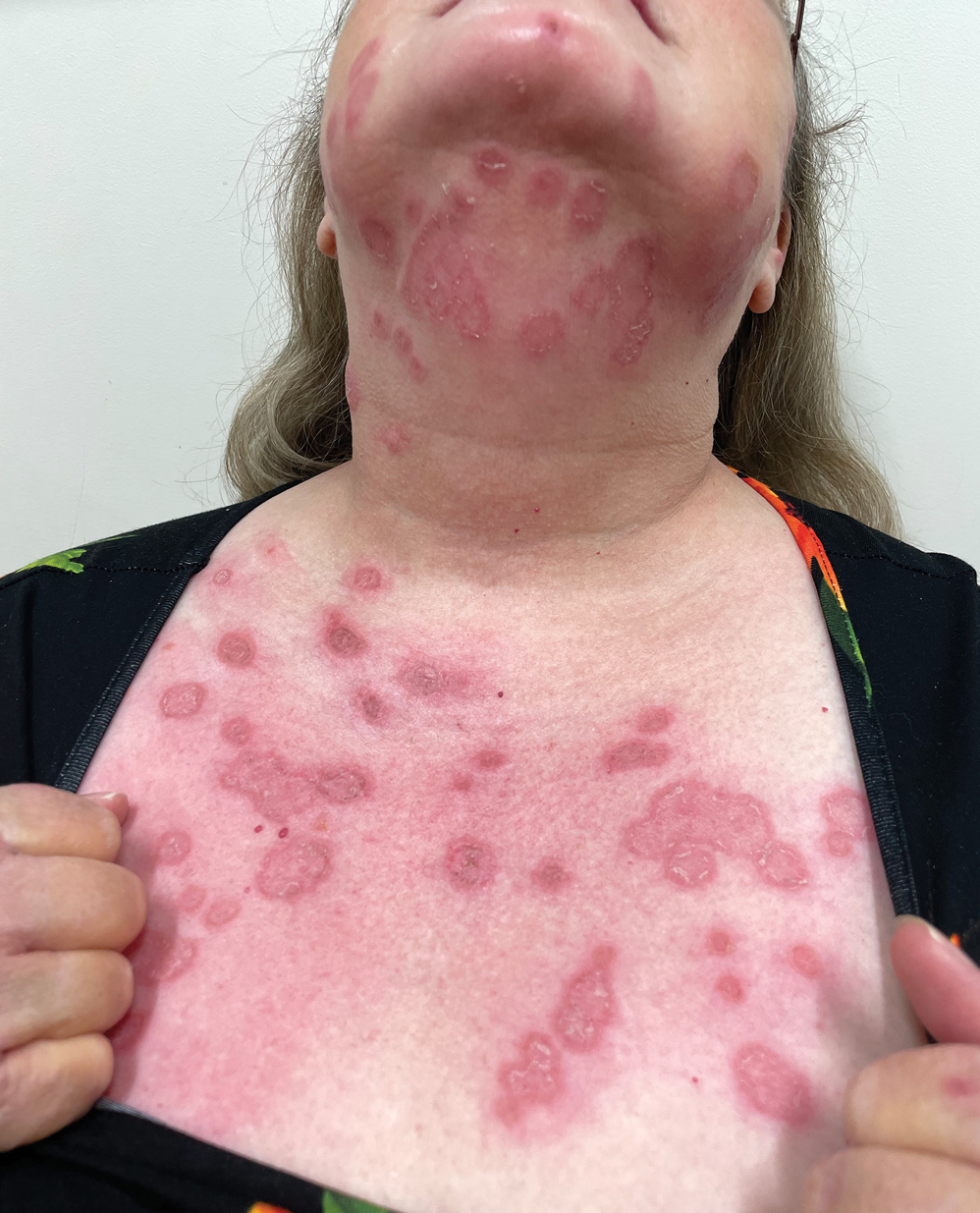
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4 C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).
Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13 C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4 C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).


Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13 C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4 C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).


Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13 C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review