User login
Pectoralis Major Rupture in a 49-Year-Old Woman
Approach to the Limping Child
The child with limp represents a common scenario in the pediatric ED. Evaluation of such patients may be difficult due to vague clinical histories and nonspecific physical examination findings. The differential diagnosis is broad and includes mild self-limited processes (eg, toxic synovitis), as well as potentially limb and life-threatening etiologies (eg, osteomyelitis, malignancy). Careful attention to historical clues and a focused physical examination are often suggestive of a diagnosis, but laboratory and radiographic studies are necessary in most cases.
While trauma is the most common source of limp in children, infectious, inflammatory, and anatomic causes are also frequently encountered. This review focuses on several of the most important etiologies of limp in children: toxic synovitis, septic arthritis, osteomyelitis, Perthes disease, slipped capital femoral epiphysis (SCFE), and Osgood-Schlatter disease.
Case Presentation
Kailey, a 28-month-old girl, was brought to the ED by her parents, who stated that their child “isn’t walking right.” They noted that their daughter’s right leg had been bothering her for about a week, but that the limp had become more noticeable. Aside from a cold a week before presentation, both parents stated that the child has been healthy; they also denied any trauma or systemic symptoms such as rash, weight loss, vomiting, or diarrhea. The mother believed Kailey may have had a low-grade fever earlier in the week. There were no sick contacts at home, but the child did attend daycare.
On examination, Kailey was well-appearing on her mother’s lap. Her vital signs were unremarkable and she was afebrile. She was able to bear weight on the right leg but walked with a slightly antalgic gait, which became more apparent when she was asked to run across the room to her mother. Her right leg was normal appearing on examination with normal reflexes; however, the child appeared to wince when passively ranging her right hip and right knee joint. The emergency physician (EP) was not able to determine any particular areas of point tenderness. The remainder of the examination, including neurological and musculoskeletal examinations, was normal.
Regarding further history, the child’s parents informed the EP that they had taken their daughter to an urgent care clinic earlier in the week. X-rays taken of the girl’s right knee and hip at this visit were both normal; however, the girl’s limp had been getting worse.
Overview
Limp is a common presenting complaint in the pediatric ED, and its evaluation is often challenging as the clinical course of limp in a child varies from benign and self-limited to serious and limb-threatening. However, with careful attention to the history and physical examination, appropriate laboratory evaluation, and focused imaging studies, a diagnosis can be made in most cases and treatment initiated. Of paramount importance for clinicians is to remember that limp in a child nearly always represents an organic disease.1,2
While trauma is the most common cause of limp in children, infectious, inflammatory, and anatomic processes are other potential etiologies. A clue to the diagnosis may also be inferred from the patient’s age, as certain conditions such as fracture, Perthes disease, and transient synovitis are seen in younger children, while SCFE and Osgood-Schlatter are more common in children older than 10 years of age. Other serious conditions such as septic hip or osteomyelitis may be encountered at any age.
Initial Management
A thorough history and physical examination provide the basis for subsequent laboratory and radiographic testing of children presenting with a limp. The duration and localization of symptoms should be elicited; traumatic or infectious causes are more common among those presenting with acute (<2 weeks) complaints. The presence of systemic symptoms (eg, fever, weight loss, chills, rashes, recurrent arthralgias) increases the likelihood of underlying oncologic or rheumatologic process. Examination of the child begins with a full physical examination to uncover other possible etiologic clues such as other involved joints (juvenile idiopathic arthritis), signs of old bruising (nonaccidental trauma), firm lymph nodes (malignancy), abdominal pain (eg, appendicitis, psoas abscess, constipation), or limb-length discrepancy (developmental dysplasia of the hip).
Focused assessment of the limp itself involves watching the child walk or run; different variations of limp may also offer a clue to the diagnosis. An antalgic gait simply refers to one in which the affected leg spends less time in the weight-bearing stage, and it is most commonly seen with infection and trauma. Trendelenburg gait, frequently seen with SCFE and Perthes disease, is characterized by a downward tilt of the pelvis away from the affected side while the affected leg is bearing weight.
In many cases, it may be difficult to accurately characterize a limp due to a patient’s pain or lack of compliance. Evaluation of any limp should also focus on the joint above and below the child’s apparent main source of pain. This is particularly true of knee complaints as referred pain from the hip may often present as isolated thigh or knee pain. Areas of point tenderness, erythema, joint effusion, and warmth strongly point to an infectious source but are frequently absent early in disease presentation. While swelling and severe pain with passive movement of a joint indicate septic arthritis, limitation of joint movement at the hip can be seen with SCFE and Perthes disease.
Laboratory Studies
In most children presenting with limp, extensive laboratory testing is not needed for the diagnosis but is helpful when infectious, oncologic, and rheumatologic causes are considered. Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in most acute infectious processes. A complete blood count (CBC) should also be obtained in cases of suspected infection to evaluate the white blood cell count (WBC). Due to the high prevalence of joint infections and postinfectious processes caused by group A streptococcus (GAS), an antistreptolysin titer (ASO), throat culture, and rapid streptococcal antigen swab should be considered. If infection is suspected and a joint effusion is present, cell counts, gram stain, and culture from the synovial fluid should be sent to assist with diagnosis and antibiotic management. If a child appears septic, a blood culture should be obtained as well. With suspected osteomyelitis, cultures of the bone should be taken in the operating room in addition to a peripheral blood culture. Ideally, antibiotics should be withheld until cultures are sent unless the child appears acutely ill.
Diagnostic Imaging
Toxic Synovitis and Septic Arthritis
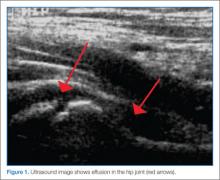
Clinicians may have difficulty differentiating between toxic synovitis (also known as transient synovitis) and septic arthritis of the hip in pediatric patients. In both toxic synovitis and septic arthritis, the child is unable to bear weight on the affected side, and ultrasound may demonstrate effusion.3,4
Toxic synovitis is thought to be a viral or postviral phenomenon, though the exact agent responsible for initiating the inflammatory cascade is not known. It has a relatively benign course and generally responds over 3 to 10 days to rest and nonsteroidal anti-inflammatory drugs (NSAIDs).5,6
Septic arthritis, however, is a serious infection with the capacity to cause permanent joint damage as well as spread into the blood or bone. Staphylococcal and streptococcal bacteria, including GAS and methicillin-resistant staphylococcus aureus (MRSA) are the most common causes of septic arthritis in the pediatric population.7 However, Kingella kingae, a gram-negative organism, is an increasingly recognized cause of septic arthritis in children younger than 3 years of age. Commonly found in the posterior pharynx, Kingella is very difficult to culture but may be detected by polymerase chain reaction (PCR) assays.8 The spectrum of GAS-related joint infections ranges from a postinfectious type that may be indistinguishable from toxic synovitis (but with evidence of recent GAS pharyngitis) to typical bacterial septic arthritis.9
Multiple clinical prediction rules and algorithms have been developed to assist with the management of these cases. The Kocher criteria, which include history of fever >101.3˚F, nonweight-bearing status, ESR >40 mm/hour, and WBC >12,000 cells/mm3 were found to be independent clinical predictors for the differentiation between transient arthritis and septic arthritis. Children with none of the risk factors had a 0.2% chance of septic arthritis while those with two risk factors had a 40% chance of septic arthritis. Subsequent validation studies among pediatric populations with a lower prevalence of septic arthritis found the positive predictive values to be lower; nevertheless, the Kocher criteria remain useful in guiding management.3,4
If a diagnosis of septic arthritis remains a consideration after the history, physical examination, and plain radiography, laboratory studies should be obtained, including a CBC, ESR, CRP, blood culture, rapid streptococcal assay, throat culture, and ASO titer. An ultrasound should also be performed to assess for the presence of a hip effusion.
Elevated inflammatory markers (ESR >40 mm/hour, CRP >20 mg/L, WBC >12,000 cells/mm3) and an effusion should prompt orthopedic consultation and arthrocentesis for synovial fluid-cell counts, gram stain, and culture. Synovial fluid WBC counts >50,000 cells/mm3 or a positive gram stain or culture is diagnostic of septic arthritis and requires treatment with intravenous (IV) antibiotics and likely operative intervention. The absence of an effusion on ultrasound, synovial fluid WBC counts <50,000 cells/mm3, and inflammatory markers that are not significantly elevated indicate an alternative diagnosis such as toxic synovitis. However, there may be cases in which the diagnosis is not clear due to ambiguous laboratory values from the blood or synovial fluid; in these cases the decision to proceed with IV antibiotics must be made in consultation with an orthopedist.10
Empiric antibiotic therapy in patients with septic arthritis should take into account the prevalence of MRSA in the community. Clindamycin is a popular choice due to its high oral bioavailability, which facilitates transition to a home regimen. Vancomycin is another option and can be reserved for more severely ill children. Empiric coverage for suspected septic arthritis in children younger than 3 years of age should also include an anti-gram negative agent, such as a cephalosporin, to treat Kingella.7
Osteomyelitis
Acute osteomyelitis is a common cause of limp among children and is usually caused by the hematogenous spread of bacteria. Staphylococcus aureus, including MRSA, is responsible for up to 90% of cases of osteomyelitis, though recent studies have identified Kingella as an etiologic agent among children younger age 3 years.7 The highly vascular metaphysis of the tibia and femur in children can become infected during times of otherwise asymptomatic bacteremia in healthy patients.8 Among younger children with less well-developed anatomic separation between the bone and joint space, it is possible for infection to spread into the joint space. Younger children with osteomyelitis may present with poorly localized pain with or without systemic symptoms; older children and those with more advanced disease may describe point tenderness on the bone. In many cases it may be difficult clinically to distinguish osteomyelitis from septic arthritis; excessive pain with passive range of motion of the joint is more indicative of septic arthritis.
Ideally, antibiotic therapy should be deferred until cultures from the site of infection are obtained operatively or via aspiration. The local staphylococcus aureus resistance pattern should guide empiric antibiotic therapy. With the rise of MRSA, clindamycin has become first-line therapy with vancomycin as an alternative for severely-ill or clindamycin-allergic patients. For children younger than 3 years of age with a more subacute presentation, the possibility of Kingella infection should be considered and treated with a cephalosporin. Some children with osteomyelitis will require operative debridement, though
many can be treated with antibiotics alone. The standard duration of antibiotic treatment is 4 to 6 weeks, with transition to an oral regimen once the patient is afebrile with downtrending inflammatory markers.7,8,11
Perthes Disease
Perthes disease (also called Legg-Calve-Perthes disease) is an idiopathic process that involves avascular necrosis and revascularization of the blood supply of the femoral head. The condition is most common in children ages 3 to 12 years and has a 4:1 male to female predominance. The remodeling takes place over the course of 2 to 4 years. During this process, the epiphysis of the femoral head is weakened and undergoes irreversible deformation that, if uncorrected, will persist throughout life. Severe degenerative arthritis may result and ultimately require hip replacement.12
Perthes disease typically presents as a subacute limp, sometimes with referred pain to the groin, thigh, or knee of the affected side. Range of motion of the hip may be limited, particularly in abduction and internal rotation. Plain films demonstrate necrotic avascular areas of the distal femoral head during active disease; after the remodeling process is complete, the femoral head often shows residual deformities. Magnetic resonance imaging is useful in cases in which plain film findings are subtle, particularly early in the course of the disease.12,13
Management of Perthes disease depends on the age of the child and clinical factors such as radiographic progression and range of motion of the hip. The goal of therapy is to limit damage to the femoral head during the revascularization process. In children younger than 5 years of age, nonsurgical management with an abduction splint to keep the femoral head contained and protected within the acetabulum may be used. Surgical osteotomies are used in older and more severely affected children to artificially contain the femoral head during the healing process.13
Slipped Capital Femoral Epiphysis
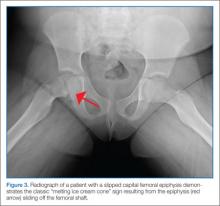
A common cause of limp among older children, the average age of presentation for SCFE is 13.5 years for boys and 12 years for girls. This condition occurs when the proximal femoral epiphysis slides posteriorly and inferiorly relative to the metaphysis. Epidemiological studies have established a connection between SCFE and obesity. Although the precise pathogenesis remains unknown, it is hypothesized that increased mechanical forces during a time of rapid pubertal growth lead to weakness at the physis. Among children who develop SCFE outside of the usual age distribution, endocrinopathies such as hypothyroidism, hypogonadism, and panhypopituitarism are often discovered.14
As with Perthes disease, SCFE may present as a chronic, subacute, or acute limp with referred pain to the groin, thigh, or knee. The patient with SCFE often has severe pain with internal rotation of the affected hip and will hold the hip in obligatory external rotation if it is flexed. Patients unable to bear weight on the affected side have unstable SCFE.
All patients with SCFE should be made nonweight bearing on the affected hip to prevent further slippage of the epiphysis. Definitive treatment involves in-situ fixation of the femoral neck with the proximal femoral epiphysis. Close orthopedic follow up is essential as roughly 50% of children with unilateral SCFE will go on to develop SCFE in the contralateral hip. Long-term complications of SCFE include osteonecrosis, joint space narrowing, and osteoarthritis.14,15
Osgood-Schlatter Disease
Osgood-Schlatter disease is a relatively benign cause of limp that is thought to occur due to repetitive trauma to the secondary ossification center tibial tubercle. Over time, the strong pull of the quadriceps muscle group on the patellar tendon causes a chronic avulsion at the site of the patellar tendon insertion on the tibial tuberosity. It most commonly develops in early puberty from ages 9 to 14 years. Affected children develop tenderness over the tibial tuberosity that is made worse with activity. On examination, pain can be elicited by having the child extend the knee against resistance or kneel. Lateral radiographs of the knee may be normal or demonstrate swelling, irregularity, or elevation of the tibial tubercle. Treatment consists of NSAIDs, ice, and physical therapy; activity restriction is generally unnecessary. Most cases resolve over 6 to 18 months as the growth plate at the secondary ossification center ossifies.16
Case Conclusion
Initial examination confirmed the presence of a limp; the differential in this age group includes toxic synovitis, septic arthritis, and osteomyelitis. Although Kailey appeared relatively well on examination, her history of recent fever and the worsening symptoms over the past week were concerning. The workup began with plain films of the pelvis and the right knee because of the possibility of referred pain and the lack of localizing signs on examination. In addition, laboratory evaluation was performed, including CBC, CRP, and ESR. Due to her young age, a rapid streptococcal assay, throat culture, or an ASO titer was not necessary.
Plain films of the pelvis and the right knee were normal. The patient’s WBC was unremarkable, but her ESR was 50 mm/hour and CRP was 25 mg/L. Given these elevated inflammatory markers, ultrasound of the right hip was ordered, which revealed a small effusion. An orthopedic specialist was consulted, who performed a sedated joint aspiration. Cell counts from the joint aspirate were sent for evaluation, as well as culture, gram stain, and a PCR for Kingella.
After joint aspiration, Kailey was admitted to the hospital overnight and was started on empiric treatment with IV clindamycin and ceftriaxone. The synovial fluid gram stain was negative, but the WBC was 65,000 cells/mm3. Over the next several days, her inflammatory markers trended downward, she remained afebrile, and her gait slowly improved. The synovial fluid culture remained negative, but the PCR was positive for Kingella. Kailey was discharged on hospital day 3 with a 21-day course of oral cephalexin.
Dr Kane is a fellow in the department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee. Dr McMorrow is an assistant professor of emergency medicine and assistant professor of pediatrics, department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee.
- Smith E, Anderson M, Foster H. The child with a limp: a symptom and not a diagnosis. Arch Dis Child Educ Pract Ed. 2012;97(5):185-193.
- Leung AK, Lemay JF. The limping child. J Pediatr Health Care. 2004;18(5):219-223.
- Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94(2):167,168.
- Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956-962.
- Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12(1):48-51.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23(1):32-36.
- Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13(5):451-460.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013;25(1):58-63.
- Mignemi ME, Martus JE, Bracikowski AC, Lovejoy SA, Mencio GA, Schoenecker JG. The spectrum of group A streptococcal joint pathology in the acute care setting. Pediatr Emerg Care. 2012;28(11):
1185-1189. - Rutz E, Spoerri M. Septic arthritis of the paediatric hip - A review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg. 2013;79(2):123-134.
- Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175-181.
- Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45(1):87-97.
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: current principles of diagnosis
and treatment. Dtsch Arztebl Int. 2009;106(31-32):517-523. - Novais EN, Millis MB. Slipped capital femoral epiphysis: prevalence, pathogenesis, and natural history. Clin Orthop Relat Res. 2012;470(12):3432-3438.
- Peck D. Slipped capital femoral epiphysis: diagnosis and management. Am Fam Physician. 2010;82(3):258-262.
- Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44-50.
The child with limp represents a common scenario in the pediatric ED. Evaluation of such patients may be difficult due to vague clinical histories and nonspecific physical examination findings. The differential diagnosis is broad and includes mild self-limited processes (eg, toxic synovitis), as well as potentially limb and life-threatening etiologies (eg, osteomyelitis, malignancy). Careful attention to historical clues and a focused physical examination are often suggestive of a diagnosis, but laboratory and radiographic studies are necessary in most cases.
While trauma is the most common source of limp in children, infectious, inflammatory, and anatomic causes are also frequently encountered. This review focuses on several of the most important etiologies of limp in children: toxic synovitis, septic arthritis, osteomyelitis, Perthes disease, slipped capital femoral epiphysis (SCFE), and Osgood-Schlatter disease.
Case Presentation
Kailey, a 28-month-old girl, was brought to the ED by her parents, who stated that their child “isn’t walking right.” They noted that their daughter’s right leg had been bothering her for about a week, but that the limp had become more noticeable. Aside from a cold a week before presentation, both parents stated that the child has been healthy; they also denied any trauma or systemic symptoms such as rash, weight loss, vomiting, or diarrhea. The mother believed Kailey may have had a low-grade fever earlier in the week. There were no sick contacts at home, but the child did attend daycare.
On examination, Kailey was well-appearing on her mother’s lap. Her vital signs were unremarkable and she was afebrile. She was able to bear weight on the right leg but walked with a slightly antalgic gait, which became more apparent when she was asked to run across the room to her mother. Her right leg was normal appearing on examination with normal reflexes; however, the child appeared to wince when passively ranging her right hip and right knee joint. The emergency physician (EP) was not able to determine any particular areas of point tenderness. The remainder of the examination, including neurological and musculoskeletal examinations, was normal.
Regarding further history, the child’s parents informed the EP that they had taken their daughter to an urgent care clinic earlier in the week. X-rays taken of the girl’s right knee and hip at this visit were both normal; however, the girl’s limp had been getting worse.
Overview
Limp is a common presenting complaint in the pediatric ED, and its evaluation is often challenging as the clinical course of limp in a child varies from benign and self-limited to serious and limb-threatening. However, with careful attention to the history and physical examination, appropriate laboratory evaluation, and focused imaging studies, a diagnosis can be made in most cases and treatment initiated. Of paramount importance for clinicians is to remember that limp in a child nearly always represents an organic disease.1,2
While trauma is the most common cause of limp in children, infectious, inflammatory, and anatomic processes are other potential etiologies. A clue to the diagnosis may also be inferred from the patient’s age, as certain conditions such as fracture, Perthes disease, and transient synovitis are seen in younger children, while SCFE and Osgood-Schlatter are more common in children older than 10 years of age. Other serious conditions such as septic hip or osteomyelitis may be encountered at any age.
Initial Management
A thorough history and physical examination provide the basis for subsequent laboratory and radiographic testing of children presenting with a limp. The duration and localization of symptoms should be elicited; traumatic or infectious causes are more common among those presenting with acute (<2 weeks) complaints. The presence of systemic symptoms (eg, fever, weight loss, chills, rashes, recurrent arthralgias) increases the likelihood of underlying oncologic or rheumatologic process. Examination of the child begins with a full physical examination to uncover other possible etiologic clues such as other involved joints (juvenile idiopathic arthritis), signs of old bruising (nonaccidental trauma), firm lymph nodes (malignancy), abdominal pain (eg, appendicitis, psoas abscess, constipation), or limb-length discrepancy (developmental dysplasia of the hip).
Focused assessment of the limp itself involves watching the child walk or run; different variations of limp may also offer a clue to the diagnosis. An antalgic gait simply refers to one in which the affected leg spends less time in the weight-bearing stage, and it is most commonly seen with infection and trauma. Trendelenburg gait, frequently seen with SCFE and Perthes disease, is characterized by a downward tilt of the pelvis away from the affected side while the affected leg is bearing weight.
In many cases, it may be difficult to accurately characterize a limp due to a patient’s pain or lack of compliance. Evaluation of any limp should also focus on the joint above and below the child’s apparent main source of pain. This is particularly true of knee complaints as referred pain from the hip may often present as isolated thigh or knee pain. Areas of point tenderness, erythema, joint effusion, and warmth strongly point to an infectious source but are frequently absent early in disease presentation. While swelling and severe pain with passive movement of a joint indicate septic arthritis, limitation of joint movement at the hip can be seen with SCFE and Perthes disease.
Laboratory Studies
In most children presenting with limp, extensive laboratory testing is not needed for the diagnosis but is helpful when infectious, oncologic, and rheumatologic causes are considered. Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in most acute infectious processes. A complete blood count (CBC) should also be obtained in cases of suspected infection to evaluate the white blood cell count (WBC). Due to the high prevalence of joint infections and postinfectious processes caused by group A streptococcus (GAS), an antistreptolysin titer (ASO), throat culture, and rapid streptococcal antigen swab should be considered. If infection is suspected and a joint effusion is present, cell counts, gram stain, and culture from the synovial fluid should be sent to assist with diagnosis and antibiotic management. If a child appears septic, a blood culture should be obtained as well. With suspected osteomyelitis, cultures of the bone should be taken in the operating room in addition to a peripheral blood culture. Ideally, antibiotics should be withheld until cultures are sent unless the child appears acutely ill.
Diagnostic Imaging
Toxic Synovitis and Septic Arthritis
Clinicians may have difficulty differentiating between toxic synovitis (also known as transient synovitis) and septic arthritis of the hip in pediatric patients. In both toxic synovitis and septic arthritis, the child is unable to bear weight on the affected side, and ultrasound may demonstrate effusion.3,4
Toxic synovitis is thought to be a viral or postviral phenomenon, though the exact agent responsible for initiating the inflammatory cascade is not known. It has a relatively benign course and generally responds over 3 to 10 days to rest and nonsteroidal anti-inflammatory drugs (NSAIDs).5,6
Septic arthritis, however, is a serious infection with the capacity to cause permanent joint damage as well as spread into the blood or bone. Staphylococcal and streptococcal bacteria, including GAS and methicillin-resistant staphylococcus aureus (MRSA) are the most common causes of septic arthritis in the pediatric population.7 However, Kingella kingae, a gram-negative organism, is an increasingly recognized cause of septic arthritis in children younger than 3 years of age. Commonly found in the posterior pharynx, Kingella is very difficult to culture but may be detected by polymerase chain reaction (PCR) assays.8 The spectrum of GAS-related joint infections ranges from a postinfectious type that may be indistinguishable from toxic synovitis (but with evidence of recent GAS pharyngitis) to typical bacterial septic arthritis.9
Multiple clinical prediction rules and algorithms have been developed to assist with the management of these cases. The Kocher criteria, which include history of fever >101.3˚F, nonweight-bearing status, ESR >40 mm/hour, and WBC >12,000 cells/mm3 were found to be independent clinical predictors for the differentiation between transient arthritis and septic arthritis. Children with none of the risk factors had a 0.2% chance of septic arthritis while those with two risk factors had a 40% chance of septic arthritis. Subsequent validation studies among pediatric populations with a lower prevalence of septic arthritis found the positive predictive values to be lower; nevertheless, the Kocher criteria remain useful in guiding management.3,4
If a diagnosis of septic arthritis remains a consideration after the history, physical examination, and plain radiography, laboratory studies should be obtained, including a CBC, ESR, CRP, blood culture, rapid streptococcal assay, throat culture, and ASO titer. An ultrasound should also be performed to assess for the presence of a hip effusion.
Elevated inflammatory markers (ESR >40 mm/hour, CRP >20 mg/L, WBC >12,000 cells/mm3) and an effusion should prompt orthopedic consultation and arthrocentesis for synovial fluid-cell counts, gram stain, and culture. Synovial fluid WBC counts >50,000 cells/mm3 or a positive gram stain or culture is diagnostic of septic arthritis and requires treatment with intravenous (IV) antibiotics and likely operative intervention. The absence of an effusion on ultrasound, synovial fluid WBC counts <50,000 cells/mm3, and inflammatory markers that are not significantly elevated indicate an alternative diagnosis such as toxic synovitis. However, there may be cases in which the diagnosis is not clear due to ambiguous laboratory values from the blood or synovial fluid; in these cases the decision to proceed with IV antibiotics must be made in consultation with an orthopedist.10
Empiric antibiotic therapy in patients with septic arthritis should take into account the prevalence of MRSA in the community. Clindamycin is a popular choice due to its high oral bioavailability, which facilitates transition to a home regimen. Vancomycin is another option and can be reserved for more severely ill children. Empiric coverage for suspected septic arthritis in children younger than 3 years of age should also include an anti-gram negative agent, such as a cephalosporin, to treat Kingella.7
Osteomyelitis
Acute osteomyelitis is a common cause of limp among children and is usually caused by the hematogenous spread of bacteria. Staphylococcus aureus, including MRSA, is responsible for up to 90% of cases of osteomyelitis, though recent studies have identified Kingella as an etiologic agent among children younger age 3 years.7 The highly vascular metaphysis of the tibia and femur in children can become infected during times of otherwise asymptomatic bacteremia in healthy patients.8 Among younger children with less well-developed anatomic separation between the bone and joint space, it is possible for infection to spread into the joint space. Younger children with osteomyelitis may present with poorly localized pain with or without systemic symptoms; older children and those with more advanced disease may describe point tenderness on the bone. In many cases it may be difficult clinically to distinguish osteomyelitis from septic arthritis; excessive pain with passive range of motion of the joint is more indicative of septic arthritis.
Ideally, antibiotic therapy should be deferred until cultures from the site of infection are obtained operatively or via aspiration. The local staphylococcus aureus resistance pattern should guide empiric antibiotic therapy. With the rise of MRSA, clindamycin has become first-line therapy with vancomycin as an alternative for severely-ill or clindamycin-allergic patients. For children younger than 3 years of age with a more subacute presentation, the possibility of Kingella infection should be considered and treated with a cephalosporin. Some children with osteomyelitis will require operative debridement, though
many can be treated with antibiotics alone. The standard duration of antibiotic treatment is 4 to 6 weeks, with transition to an oral regimen once the patient is afebrile with downtrending inflammatory markers.7,8,11
Perthes Disease
Perthes disease (also called Legg-Calve-Perthes disease) is an idiopathic process that involves avascular necrosis and revascularization of the blood supply of the femoral head. The condition is most common in children ages 3 to 12 years and has a 4:1 male to female predominance. The remodeling takes place over the course of 2 to 4 years. During this process, the epiphysis of the femoral head is weakened and undergoes irreversible deformation that, if uncorrected, will persist throughout life. Severe degenerative arthritis may result and ultimately require hip replacement.12
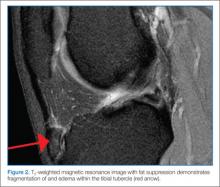
Perthes disease typically presents as a subacute limp, sometimes with referred pain to the groin, thigh, or knee of the affected side. Range of motion of the hip may be limited, particularly in abduction and internal rotation. Plain films demonstrate necrotic avascular areas of the distal femoral head during active disease; after the remodeling process is complete, the femoral head often shows residual deformities. Magnetic resonance imaging is useful in cases in which plain film findings are subtle, particularly early in the course of the disease.12,13
Management of Perthes disease depends on the age of the child and clinical factors such as radiographic progression and range of motion of the hip. The goal of therapy is to limit damage to the femoral head during the revascularization process. In children younger than 5 years of age, nonsurgical management with an abduction splint to keep the femoral head contained and protected within the acetabulum may be used. Surgical osteotomies are used in older and more severely affected children to artificially contain the femoral head during the healing process.13
Slipped Capital Femoral Epiphysis
A common cause of limp among older children, the average age of presentation for SCFE is 13.5 years for boys and 12 years for girls. This condition occurs when the proximal femoral epiphysis slides posteriorly and inferiorly relative to the metaphysis. Epidemiological studies have established a connection between SCFE and obesity. Although the precise pathogenesis remains unknown, it is hypothesized that increased mechanical forces during a time of rapid pubertal growth lead to weakness at the physis. Among children who develop SCFE outside of the usual age distribution, endocrinopathies such as hypothyroidism, hypogonadism, and panhypopituitarism are often discovered.14
As with Perthes disease, SCFE may present as a chronic, subacute, or acute limp with referred pain to the groin, thigh, or knee. The patient with SCFE often has severe pain with internal rotation of the affected hip and will hold the hip in obligatory external rotation if it is flexed. Patients unable to bear weight on the affected side have unstable SCFE.
All patients with SCFE should be made nonweight bearing on the affected hip to prevent further slippage of the epiphysis. Definitive treatment involves in-situ fixation of the femoral neck with the proximal femoral epiphysis. Close orthopedic follow up is essential as roughly 50% of children with unilateral SCFE will go on to develop SCFE in the contralateral hip. Long-term complications of SCFE include osteonecrosis, joint space narrowing, and osteoarthritis.14,15
Osgood-Schlatter Disease
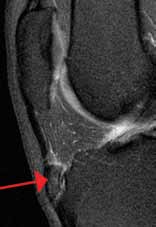
Osgood-Schlatter disease is a relatively benign cause of limp that is thought to occur due to repetitive trauma to the secondary ossification center tibial tubercle. Over time, the strong pull of the quadriceps muscle group on the patellar tendon causes a chronic avulsion at the site of the patellar tendon insertion on the tibial tuberosity. It most commonly develops in early puberty from ages 9 to 14 years. Affected children develop tenderness over the tibial tuberosity that is made worse with activity. On examination, pain can be elicited by having the child extend the knee against resistance or kneel. Lateral radiographs of the knee may be normal or demonstrate swelling, irregularity, or elevation of the tibial tubercle. Treatment consists of NSAIDs, ice, and physical therapy; activity restriction is generally unnecessary. Most cases resolve over 6 to 18 months as the growth plate at the secondary ossification center ossifies.16
Case Conclusion
Initial examination confirmed the presence of a limp; the differential in this age group includes toxic synovitis, septic arthritis, and osteomyelitis. Although Kailey appeared relatively well on examination, her history of recent fever and the worsening symptoms over the past week were concerning. The workup began with plain films of the pelvis and the right knee because of the possibility of referred pain and the lack of localizing signs on examination. In addition, laboratory evaluation was performed, including CBC, CRP, and ESR. Due to her young age, a rapid streptococcal assay, throat culture, or an ASO titer was not necessary.
Plain films of the pelvis and the right knee were normal. The patient’s WBC was unremarkable, but her ESR was 50 mm/hour and CRP was 25 mg/L. Given these elevated inflammatory markers, ultrasound of the right hip was ordered, which revealed a small effusion. An orthopedic specialist was consulted, who performed a sedated joint aspiration. Cell counts from the joint aspirate were sent for evaluation, as well as culture, gram stain, and a PCR for Kingella.
After joint aspiration, Kailey was admitted to the hospital overnight and was started on empiric treatment with IV clindamycin and ceftriaxone. The synovial fluid gram stain was negative, but the WBC was 65,000 cells/mm3. Over the next several days, her inflammatory markers trended downward, she remained afebrile, and her gait slowly improved. The synovial fluid culture remained negative, but the PCR was positive for Kingella. Kailey was discharged on hospital day 3 with a 21-day course of oral cephalexin.
Dr Kane is a fellow in the department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee. Dr McMorrow is an assistant professor of emergency medicine and assistant professor of pediatrics, department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee.
The child with limp represents a common scenario in the pediatric ED. Evaluation of such patients may be difficult due to vague clinical histories and nonspecific physical examination findings. The differential diagnosis is broad and includes mild self-limited processes (eg, toxic synovitis), as well as potentially limb and life-threatening etiologies (eg, osteomyelitis, malignancy). Careful attention to historical clues and a focused physical examination are often suggestive of a diagnosis, but laboratory and radiographic studies are necessary in most cases.
While trauma is the most common source of limp in children, infectious, inflammatory, and anatomic causes are also frequently encountered. This review focuses on several of the most important etiologies of limp in children: toxic synovitis, septic arthritis, osteomyelitis, Perthes disease, slipped capital femoral epiphysis (SCFE), and Osgood-Schlatter disease.
Case Presentation
Kailey, a 28-month-old girl, was brought to the ED by her parents, who stated that their child “isn’t walking right.” They noted that their daughter’s right leg had been bothering her for about a week, but that the limp had become more noticeable. Aside from a cold a week before presentation, both parents stated that the child has been healthy; they also denied any trauma or systemic symptoms such as rash, weight loss, vomiting, or diarrhea. The mother believed Kailey may have had a low-grade fever earlier in the week. There were no sick contacts at home, but the child did attend daycare.
On examination, Kailey was well-appearing on her mother’s lap. Her vital signs were unremarkable and she was afebrile. She was able to bear weight on the right leg but walked with a slightly antalgic gait, which became more apparent when she was asked to run across the room to her mother. Her right leg was normal appearing on examination with normal reflexes; however, the child appeared to wince when passively ranging her right hip and right knee joint. The emergency physician (EP) was not able to determine any particular areas of point tenderness. The remainder of the examination, including neurological and musculoskeletal examinations, was normal.
Regarding further history, the child’s parents informed the EP that they had taken their daughter to an urgent care clinic earlier in the week. X-rays taken of the girl’s right knee and hip at this visit were both normal; however, the girl’s limp had been getting worse.
Overview
Limp is a common presenting complaint in the pediatric ED, and its evaluation is often challenging as the clinical course of limp in a child varies from benign and self-limited to serious and limb-threatening. However, with careful attention to the history and physical examination, appropriate laboratory evaluation, and focused imaging studies, a diagnosis can be made in most cases and treatment initiated. Of paramount importance for clinicians is to remember that limp in a child nearly always represents an organic disease.1,2
While trauma is the most common cause of limp in children, infectious, inflammatory, and anatomic processes are other potential etiologies. A clue to the diagnosis may also be inferred from the patient’s age, as certain conditions such as fracture, Perthes disease, and transient synovitis are seen in younger children, while SCFE and Osgood-Schlatter are more common in children older than 10 years of age. Other serious conditions such as septic hip or osteomyelitis may be encountered at any age.
Initial Management
A thorough history and physical examination provide the basis for subsequent laboratory and radiographic testing of children presenting with a limp. The duration and localization of symptoms should be elicited; traumatic or infectious causes are more common among those presenting with acute (<2 weeks) complaints. The presence of systemic symptoms (eg, fever, weight loss, chills, rashes, recurrent arthralgias) increases the likelihood of underlying oncologic or rheumatologic process. Examination of the child begins with a full physical examination to uncover other possible etiologic clues such as other involved joints (juvenile idiopathic arthritis), signs of old bruising (nonaccidental trauma), firm lymph nodes (malignancy), abdominal pain (eg, appendicitis, psoas abscess, constipation), or limb-length discrepancy (developmental dysplasia of the hip).
Focused assessment of the limp itself involves watching the child walk or run; different variations of limp may also offer a clue to the diagnosis. An antalgic gait simply refers to one in which the affected leg spends less time in the weight-bearing stage, and it is most commonly seen with infection and trauma. Trendelenburg gait, frequently seen with SCFE and Perthes disease, is characterized by a downward tilt of the pelvis away from the affected side while the affected leg is bearing weight.
In many cases, it may be difficult to accurately characterize a limp due to a patient’s pain or lack of compliance. Evaluation of any limp should also focus on the joint above and below the child’s apparent main source of pain. This is particularly true of knee complaints as referred pain from the hip may often present as isolated thigh or knee pain. Areas of point tenderness, erythema, joint effusion, and warmth strongly point to an infectious source but are frequently absent early in disease presentation. While swelling and severe pain with passive movement of a joint indicate septic arthritis, limitation of joint movement at the hip can be seen with SCFE and Perthes disease.
Laboratory Studies
In most children presenting with limp, extensive laboratory testing is not needed for the diagnosis but is helpful when infectious, oncologic, and rheumatologic causes are considered. Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in most acute infectious processes. A complete blood count (CBC) should also be obtained in cases of suspected infection to evaluate the white blood cell count (WBC). Due to the high prevalence of joint infections and postinfectious processes caused by group A streptococcus (GAS), an antistreptolysin titer (ASO), throat culture, and rapid streptococcal antigen swab should be considered. If infection is suspected and a joint effusion is present, cell counts, gram stain, and culture from the synovial fluid should be sent to assist with diagnosis and antibiotic management. If a child appears septic, a blood culture should be obtained as well. With suspected osteomyelitis, cultures of the bone should be taken in the operating room in addition to a peripheral blood culture. Ideally, antibiotics should be withheld until cultures are sent unless the child appears acutely ill.
Diagnostic Imaging
Toxic Synovitis and Septic Arthritis
Clinicians may have difficulty differentiating between toxic synovitis (also known as transient synovitis) and septic arthritis of the hip in pediatric patients. In both toxic synovitis and septic arthritis, the child is unable to bear weight on the affected side, and ultrasound may demonstrate effusion.3,4
Toxic synovitis is thought to be a viral or postviral phenomenon, though the exact agent responsible for initiating the inflammatory cascade is not known. It has a relatively benign course and generally responds over 3 to 10 days to rest and nonsteroidal anti-inflammatory drugs (NSAIDs).5,6
Septic arthritis, however, is a serious infection with the capacity to cause permanent joint damage as well as spread into the blood or bone. Staphylococcal and streptococcal bacteria, including GAS and methicillin-resistant staphylococcus aureus (MRSA) are the most common causes of septic arthritis in the pediatric population.7 However, Kingella kingae, a gram-negative organism, is an increasingly recognized cause of septic arthritis in children younger than 3 years of age. Commonly found in the posterior pharynx, Kingella is very difficult to culture but may be detected by polymerase chain reaction (PCR) assays.8 The spectrum of GAS-related joint infections ranges from a postinfectious type that may be indistinguishable from toxic synovitis (but with evidence of recent GAS pharyngitis) to typical bacterial septic arthritis.9
Multiple clinical prediction rules and algorithms have been developed to assist with the management of these cases. The Kocher criteria, which include history of fever >101.3˚F, nonweight-bearing status, ESR >40 mm/hour, and WBC >12,000 cells/mm3 were found to be independent clinical predictors for the differentiation between transient arthritis and septic arthritis. Children with none of the risk factors had a 0.2% chance of septic arthritis while those with two risk factors had a 40% chance of septic arthritis. Subsequent validation studies among pediatric populations with a lower prevalence of septic arthritis found the positive predictive values to be lower; nevertheless, the Kocher criteria remain useful in guiding management.3,4
If a diagnosis of septic arthritis remains a consideration after the history, physical examination, and plain radiography, laboratory studies should be obtained, including a CBC, ESR, CRP, blood culture, rapid streptococcal assay, throat culture, and ASO titer. An ultrasound should also be performed to assess for the presence of a hip effusion.
Elevated inflammatory markers (ESR >40 mm/hour, CRP >20 mg/L, WBC >12,000 cells/mm3) and an effusion should prompt orthopedic consultation and arthrocentesis for synovial fluid-cell counts, gram stain, and culture. Synovial fluid WBC counts >50,000 cells/mm3 or a positive gram stain or culture is diagnostic of septic arthritis and requires treatment with intravenous (IV) antibiotics and likely operative intervention. The absence of an effusion on ultrasound, synovial fluid WBC counts <50,000 cells/mm3, and inflammatory markers that are not significantly elevated indicate an alternative diagnosis such as toxic synovitis. However, there may be cases in which the diagnosis is not clear due to ambiguous laboratory values from the blood or synovial fluid; in these cases the decision to proceed with IV antibiotics must be made in consultation with an orthopedist.10
Empiric antibiotic therapy in patients with septic arthritis should take into account the prevalence of MRSA in the community. Clindamycin is a popular choice due to its high oral bioavailability, which facilitates transition to a home regimen. Vancomycin is another option and can be reserved for more severely ill children. Empiric coverage for suspected septic arthritis in children younger than 3 years of age should also include an anti-gram negative agent, such as a cephalosporin, to treat Kingella.7
Osteomyelitis
Acute osteomyelitis is a common cause of limp among children and is usually caused by the hematogenous spread of bacteria. Staphylococcus aureus, including MRSA, is responsible for up to 90% of cases of osteomyelitis, though recent studies have identified Kingella as an etiologic agent among children younger age 3 years.7 The highly vascular metaphysis of the tibia and femur in children can become infected during times of otherwise asymptomatic bacteremia in healthy patients.8 Among younger children with less well-developed anatomic separation between the bone and joint space, it is possible for infection to spread into the joint space. Younger children with osteomyelitis may present with poorly localized pain with or without systemic symptoms; older children and those with more advanced disease may describe point tenderness on the bone. In many cases it may be difficult clinically to distinguish osteomyelitis from septic arthritis; excessive pain with passive range of motion of the joint is more indicative of septic arthritis.
Ideally, antibiotic therapy should be deferred until cultures from the site of infection are obtained operatively or via aspiration. The local staphylococcus aureus resistance pattern should guide empiric antibiotic therapy. With the rise of MRSA, clindamycin has become first-line therapy with vancomycin as an alternative for severely-ill or clindamycin-allergic patients. For children younger than 3 years of age with a more subacute presentation, the possibility of Kingella infection should be considered and treated with a cephalosporin. Some children with osteomyelitis will require operative debridement, though
many can be treated with antibiotics alone. The standard duration of antibiotic treatment is 4 to 6 weeks, with transition to an oral regimen once the patient is afebrile with downtrending inflammatory markers.7,8,11
Perthes Disease
Perthes disease (also called Legg-Calve-Perthes disease) is an idiopathic process that involves avascular necrosis and revascularization of the blood supply of the femoral head. The condition is most common in children ages 3 to 12 years and has a 4:1 male to female predominance. The remodeling takes place over the course of 2 to 4 years. During this process, the epiphysis of the femoral head is weakened and undergoes irreversible deformation that, if uncorrected, will persist throughout life. Severe degenerative arthritis may result and ultimately require hip replacement.12
Perthes disease typically presents as a subacute limp, sometimes with referred pain to the groin, thigh, or knee of the affected side. Range of motion of the hip may be limited, particularly in abduction and internal rotation. Plain films demonstrate necrotic avascular areas of the distal femoral head during active disease; after the remodeling process is complete, the femoral head often shows residual deformities. Magnetic resonance imaging is useful in cases in which plain film findings are subtle, particularly early in the course of the disease.12,13
Management of Perthes disease depends on the age of the child and clinical factors such as radiographic progression and range of motion of the hip. The goal of therapy is to limit damage to the femoral head during the revascularization process. In children younger than 5 years of age, nonsurgical management with an abduction splint to keep the femoral head contained and protected within the acetabulum may be used. Surgical osteotomies are used in older and more severely affected children to artificially contain the femoral head during the healing process.13
Slipped Capital Femoral Epiphysis
A common cause of limp among older children, the average age of presentation for SCFE is 13.5 years for boys and 12 years for girls. This condition occurs when the proximal femoral epiphysis slides posteriorly and inferiorly relative to the metaphysis. Epidemiological studies have established a connection between SCFE and obesity. Although the precise pathogenesis remains unknown, it is hypothesized that increased mechanical forces during a time of rapid pubertal growth lead to weakness at the physis. Among children who develop SCFE outside of the usual age distribution, endocrinopathies such as hypothyroidism, hypogonadism, and panhypopituitarism are often discovered.14
As with Perthes disease, SCFE may present as a chronic, subacute, or acute limp with referred pain to the groin, thigh, or knee. The patient with SCFE often has severe pain with internal rotation of the affected hip and will hold the hip in obligatory external rotation if it is flexed. Patients unable to bear weight on the affected side have unstable SCFE.
All patients with SCFE should be made nonweight bearing on the affected hip to prevent further slippage of the epiphysis. Definitive treatment involves in-situ fixation of the femoral neck with the proximal femoral epiphysis. Close orthopedic follow up is essential as roughly 50% of children with unilateral SCFE will go on to develop SCFE in the contralateral hip. Long-term complications of SCFE include osteonecrosis, joint space narrowing, and osteoarthritis.14,15
Osgood-Schlatter Disease
Osgood-Schlatter disease is a relatively benign cause of limp that is thought to occur due to repetitive trauma to the secondary ossification center tibial tubercle. Over time, the strong pull of the quadriceps muscle group on the patellar tendon causes a chronic avulsion at the site of the patellar tendon insertion on the tibial tuberosity. It most commonly develops in early puberty from ages 9 to 14 years. Affected children develop tenderness over the tibial tuberosity that is made worse with activity. On examination, pain can be elicited by having the child extend the knee against resistance or kneel. Lateral radiographs of the knee may be normal or demonstrate swelling, irregularity, or elevation of the tibial tubercle. Treatment consists of NSAIDs, ice, and physical therapy; activity restriction is generally unnecessary. Most cases resolve over 6 to 18 months as the growth plate at the secondary ossification center ossifies.16
Case Conclusion
Initial examination confirmed the presence of a limp; the differential in this age group includes toxic synovitis, septic arthritis, and osteomyelitis. Although Kailey appeared relatively well on examination, her history of recent fever and the worsening symptoms over the past week were concerning. The workup began with plain films of the pelvis and the right knee because of the possibility of referred pain and the lack of localizing signs on examination. In addition, laboratory evaluation was performed, including CBC, CRP, and ESR. Due to her young age, a rapid streptococcal assay, throat culture, or an ASO titer was not necessary.
Plain films of the pelvis and the right knee were normal. The patient’s WBC was unremarkable, but her ESR was 50 mm/hour and CRP was 25 mg/L. Given these elevated inflammatory markers, ultrasound of the right hip was ordered, which revealed a small effusion. An orthopedic specialist was consulted, who performed a sedated joint aspiration. Cell counts from the joint aspirate were sent for evaluation, as well as culture, gram stain, and a PCR for Kingella.
After joint aspiration, Kailey was admitted to the hospital overnight and was started on empiric treatment with IV clindamycin and ceftriaxone. The synovial fluid gram stain was negative, but the WBC was 65,000 cells/mm3. Over the next several days, her inflammatory markers trended downward, she remained afebrile, and her gait slowly improved. The synovial fluid culture remained negative, but the PCR was positive for Kingella. Kailey was discharged on hospital day 3 with a 21-day course of oral cephalexin.
Dr Kane is a fellow in the department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee. Dr McMorrow is an assistant professor of emergency medicine and assistant professor of pediatrics, department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee.
- Smith E, Anderson M, Foster H. The child with a limp: a symptom and not a diagnosis. Arch Dis Child Educ Pract Ed. 2012;97(5):185-193.
- Leung AK, Lemay JF. The limping child. J Pediatr Health Care. 2004;18(5):219-223.
- Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94(2):167,168.
- Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956-962.
- Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12(1):48-51.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23(1):32-36.
- Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13(5):451-460.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013;25(1):58-63.
- Mignemi ME, Martus JE, Bracikowski AC, Lovejoy SA, Mencio GA, Schoenecker JG. The spectrum of group A streptococcal joint pathology in the acute care setting. Pediatr Emerg Care. 2012;28(11):
1185-1189. - Rutz E, Spoerri M. Septic arthritis of the paediatric hip - A review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg. 2013;79(2):123-134.
- Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175-181.
- Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45(1):87-97.
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: current principles of diagnosis
and treatment. Dtsch Arztebl Int. 2009;106(31-32):517-523. - Novais EN, Millis MB. Slipped capital femoral epiphysis: prevalence, pathogenesis, and natural history. Clin Orthop Relat Res. 2012;470(12):3432-3438.
- Peck D. Slipped capital femoral epiphysis: diagnosis and management. Am Fam Physician. 2010;82(3):258-262.
- Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44-50.
- Smith E, Anderson M, Foster H. The child with a limp: a symptom and not a diagnosis. Arch Dis Child Educ Pract Ed. 2012;97(5):185-193.
- Leung AK, Lemay JF. The limping child. J Pediatr Health Care. 2004;18(5):219-223.
- Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94(2):167,168.
- Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956-962.
- Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12(1):48-51.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23(1):32-36.
- Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13(5):451-460.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013;25(1):58-63.
- Mignemi ME, Martus JE, Bracikowski AC, Lovejoy SA, Mencio GA, Schoenecker JG. The spectrum of group A streptococcal joint pathology in the acute care setting. Pediatr Emerg Care. 2012;28(11):
1185-1189. - Rutz E, Spoerri M. Septic arthritis of the paediatric hip - A review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg. 2013;79(2):123-134.
- Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175-181.
- Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45(1):87-97.
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: current principles of diagnosis
and treatment. Dtsch Arztebl Int. 2009;106(31-32):517-523. - Novais EN, Millis MB. Slipped capital femoral epiphysis: prevalence, pathogenesis, and natural history. Clin Orthop Relat Res. 2012;470(12):3432-3438.
- Peck D. Slipped capital femoral epiphysis: diagnosis and management. Am Fam Physician. 2010;82(3):258-262.
- Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44-50.
Four Fracture Patterns Unique to Pediatric Patients
Case 1
A 2-year-old girl presented to the ED with arm pain. Her mother stated that her daughter was playing with a 5-year-old sibling when she heard the child cry- out in pain and noticed she was holding her right arm by her side, not wanting to move it. Neither child gave a reliable story of the injury.
Nursemaid’s Elbow
Nursemaid’s elbow, also known as pulled elbow, subluxation of the radial head, and most recently annular ligament displacement, is a common injury in children younger than age 6 years. One study estimates that the condition represented about 1% of injury-related ED visits in 2005.1
Patients with nursemaid’s elbow typically present holding the injured arm at their side, slightly flexed and pronated. These patients appear relatively comfortable until moved actively or passively. The classic history of nursemaid’s elbow includes a traction mechanism, with the child being pulled up by one arm or being grabbed by the arm suddenly to keep him or her out of harm’s way.2 Due to the laxity of connective tissues in children of this age, the head of the radius slips out of the annular ligament causing acute pain and decreased function.
Nursemaid’s elbow is usually diagnosed by history and examination alone, with special consideration to the mechanism of injury. There is rarely swelling or bruising.3 Passive flexion and extension at the elbow may be normal, but rotational maneuvers can be painful or fully resisted.
Reduction Techniques
In 2012, Cochrane updated its earlier review on nursemaid’s elbow and in 2013 followed up with an article in Pediatrics in Review.3,4 Each covered research on reduction techniques, summarizing studies comparing supination-flexion (SF) versus hyperpronation (HP) as the initial reduction maneuver. Given that these maneuvers are difficult to camouflage, studies tend to be pseudorandomized with assessment by a nonblinded healthcare provider, decreasing the strength of the studies. In the Cochrane review, four different trials that included 379 children under age 7 years were selected for the review. In all four studies, pronation was found to have the least chance of failed first attempt, the chosen outcome for this meta-analysis. The risk ratio of failure of reduction for pronation was 0.45 (95% confidence interval [CI], 0.28-0.73).
There is some data supporting hyperpronation to be less painful as well; however, the Cochrane reviewers felt there may have been reporting bias.4 Since the time of each of these reviews, another study comprised of 150 children was conducted and also favored similar practice styles, as the hyperpronation maneuver had 95% success rate on first attempt versus 68% first-time success with supination and flexion.5
Complications and Recurrence
In a small study aimed at identifying recurrence rates for nursemaid’s elbow, Teach and Schultzman6 studied 93 children for 1 year after probable or definite diagnosis of nursemaid’s elbow. Of these children, 23.7% had recurrent radial head subluxation. Children younger than age 2 years were found to have a relative risk of 2.6 (95% CI, 1.04-6.30) for one or more recurrences when compared to children older than age 2 years.
While the great majority of children with nursemaid’s elbow do not need referral to an orthopedist, those with two or more occurrences should be considered for referral to a specialist.
Case 2
A 6-year-old boy was presented to the ED by his father, who had placed the boy’s arm in a home-made sling. The child tearfully told the provider that he fell trying to catch himself after tripping over the house pet.
FOOSH Injury
The above case depicts a very common presentation in the ED—the so-called “FOOSH” (fall onto an outstretched hand) injury. This type of injury occurs with such frequency in both adults and children that it is one of the only injury patterns with a commonly used acronym. The bony injuries seen with FOOSH in children, however, have a different pattern than those in adults.
Pediatric fractures are unique due to the difference in the structure of the bones themselves. A child’s bones are more elastic than an adult’s bones, allowing them to bow and bend before they fracture.7 Despite this malleability, pediatric bones have been noted to have a thicker periosteum. For this reason, compression or impact may interrupt the periosteal sleeve, minimally yielding an incomplete interruption of the cortex unilaterally.
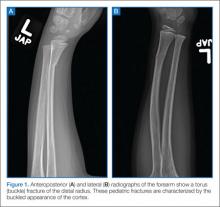
One fracture pattern commonly seen in children is the torus fracture. This type of fracture is also referred to as a buckle fracture as the bone cortex on radiographic imaging appears “buckled” as a result of the compressive forces on that side of the bone (Figure 1). Since the bone itself is minimally affected, these fractures are quite stable and not at risk for complications.
In comparison, a greenstick fracture, also unique to the pediatric population, is one in which the cortex shows plastic deformity on the side of the force or impact but is interrupted on the opposite side due to the tension of the impact itself. Greenstick fractures are frequently angulated and may require reduction for anatomic alignment, but long-term complications are typically minimal. These fracture patterns are distinguished from complete fractures (as seen in adults), which are quite unstable and generally require surgical intervention.
Of note, the location of pediatric forearm fractures varies with age as well. Diaphyseal fractures are more common in prepubescent children, whereas the highest incidence of physeal injuries occurs during large growth spurts, particularly throughout adolescence.7
Management
The remodeling potential of pediatric bones also makes management unique. Pediatric orthopedic literature has well-studied acceptable angles and degrees of appropriate displacement based largely on the age of the patient and proximity to a growth plate. Knowledge of these is imperative for definitive care of such fractures but is beyond the scope of this review.
Traditional treatment of pediatric forearm fractures includes immobilization of various types and duration to minimize pain and deformity while producing the best possible outcome. Several recent studies have aimed to determine best practice for the different fracture types with the goal of producing best alignment and return to function while decreasing cost, discomfort, and number of physician visits. Another concern among healthcare providers is the risk of refracture, which in buckle fractures is estimated at approximately 2% with a median time of 8 to 16 weeks after the initial injury.7
A 2010 review by Kennedy et al8 sought to determine if the refracture rate was affected by the technique used to immobilize torus fractures. The five studies used in this review had no reports of refracture in the 443 patients included in analysis, though only one of the studies (Plint et al) followed patients for more than 6 weeks.8,9 In this study, 75 patients were randomized to either a plaster removable splint or full below-elbow cast for 3 weeks; thereafter, they were followed for 6 months, during which time none experienced refracture.9
Another outcome from the same study assessed the ability of the patient to use the affected arm in the recovery period. While those in removable splints scored better during and immediately after cast removal, no differences were present after 1 week. Not surprisingly, families preferred the soft bandages or a removable splint for treatment.
Case 3
A 13-year-old boy presented to the ED with right ankle pain and difficulty bearing weight. He stated that he was playing basketball when he “rolled” his right ankle coming down from a rebound.
Ankle Fractures
Ankle fractures are among the most common acute injuries of the lower extremity in children, accounting for approximately 5% of pediatric fractures and 15% of physeal injuries.10 Ankle fractures also account for up to 40% of all injuries to the skeletally immature athlete.10,11 More specifically, distal fibular physeal fractures are the most common types of pediatric ankle fracture; however, they are associated with a relatively low risk for long-term complications. In contrast, distal tibial physeal fractures are associated with a higher risk for long-term complications.12,13
Presentation and Evaluation
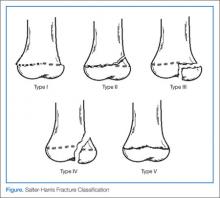
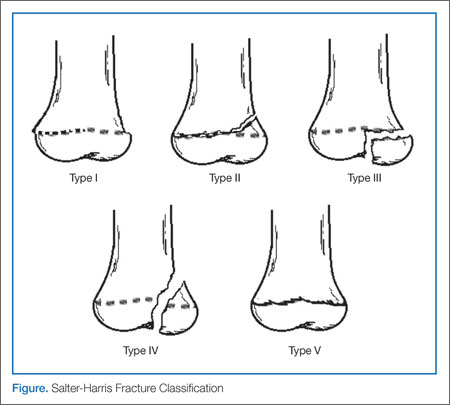
Typically, patients presenting with ankle fractures are too sore to bear weight, and swelling and ecchymosis can be identified anterior to the ankle. In addition, there may be diffuse tenderness throughout the ankle and point tenderness may be induced on the anterolateral aspect of the distal tibia.14 A complete evaluation of the entire lower extremity should be conducted before assuming that the injury is confined to the ankle, especially in children younger than age 5 years and/or who are nonverbal.10 When evaluating an ankle fracture, in general, orthopedic consultation should be obtained for children with neurovascular compromise, open fractures, and/or Salter-Harris III, IV, and V fractures.
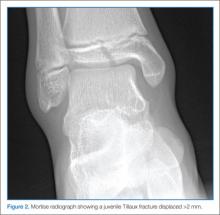
The juvenile Tillaux fracture represents a Salter-Harris III physeal injury that involves the anterolateral portion of the tibia. It usually occurs in children between ages 12 and 14 years as they approach skeletal maturity and who have a partially fused tibial physis. The common mechanism of injury is inversion of the ankle with the foot pointed away from the midline (supination with external rotation). This leads to avulsion of the lateral tibial epiphysis that is attached to the anterior inferior tibiofibular ligament. The uninvolved medial portion of the epiphysis is closed.10
Radiographic Imaging
Three radiographic views should be obtained in the evaluation of pediatric ankle injuries as Tillaux fractures or other subtle injuries could be easily missed if only two views are obtained. Interpretation of the radiographs must be correlated with the physical examination.10 The fracture line is usually best seen on a mortise view (Figure 2). Computed tomography (CT) is warranted in cases in which displacement greater than 2 mm is suspected because it better defines fracture displacement and can aid in surgical planning.14 Because of its sensitivity in detecting fractures displaced more than 2 mm, CT is now the preferred imaging modality in the assessment of juvenile Tillaux fractures.15
Definitive Management
There are two important goals when treating children with ankle fractures—achieving a satisfactory reduction and avoiding physeal arrest so as to minimize the risks of angular deformity, early arthrosis, leg-length inequality, and joint stiffness.11 Juvenile Tillaux fractures with greater than 2 mm of displacement require orthopedic consultation for closed or open reduction. Closed reduction is attempted by internally rotating the foot and applying direct pressure over the anterolateral tibia. If necessary, percutaneous pins can be used for stabilization of the reduction. If closed reduction is unsuccessful, open reduction is required. Care must be taken to assure no displacement occurs after casting; this requires weekly X-ray evaluation for the first 2 weeks.12
Patients with nondisplaced Salter-Harris III fractures are treated with long-leg casting for 4 weeks with conversion to a short-leg cast or boot for an additional 4 weeks. Patients should anticipate 8 weeks of nonweight-bearing. The patient is allowed to remove the boot for range-of-motion exercises but must remain nonweight-bearing for the first 2 weeks.14
Case 4
A 3-year-old previously healthy girl presented to the ED with a limp and difficulty bearing weight. Her mother reported that the child was playing in the yard when she caught her foot on a tree root, stumbled, and fell down. Since the incident, the child has been tearful, limping, and refusing to walk.
Tibial Fractures
Tibial fractures are among the most frequent types of orthopedic injuries in young children, with only femur and forearm fractures having a higher incidence of occurrence. Tibial fractures account for up to 15% of long bone fractures in children and adolescents.16,17 The mechanism of injury varies depending on the patient’s age. In young children, the most common cause of injury is from a seemingly minor twisting around a fixed foot or from a minor fall. In older children and adults, high-energy motor vehicle accidents and sports-related injuries are more common causes.
Fractures of the tibial shaft are typically short oblique or transverse fractures of the middle or distal third of the shaft. Thirty percent of tibial shaft fractures are associated with fractures of the fibula.16
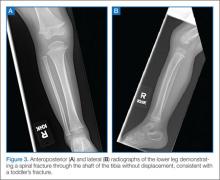
Toddler’s Fracture
The term toddler’s fracture refers to a nondisplaced oblique fracture of the tibial shaft without concomitant fibular fracture. It usually results from an indirect rotational or twisting force applied to the foot and lower leg.16-18 More specifically, the term describes a specialized case of spiral fracture of the distal tibia in patients aged 9 months to 3 years, when weight-bearing is just beginning.19,20 Such injuries commonly occur when a toddler stumbles and falls, or attempts to extricate the foot from between the bars of a crib. Often, however, the mechanism is minimal or unknown.18 Of those injuries that are witnessed, most caregivers report a minor twisting mechanism. Most children with toddler’s fracture are younger than age 6 years. Sixty-three of 76 such fractures reported by Dunbar et al17,19 occurred in children younger than 2.5 years of age. Toddler’s fractures occur more often in boys than girls, and in the right leg more often than the left. Most children will give a history of tripping or twisting their ankle.17
Evaluating the Toddler
Toddlers can be challenging patients as they can not relate history and are often uncooperative on examination. A child may present with a limp, diminished movement of the affected limb, or refuse to bear weight without a distinct history of injury. The onset of limping or refusal to bear weight after minor trauma, or without an obvious injury in a young ambulatory child, warrants a detailed examination looking for tenderness over the tibia, along with radiographic evaluation to rule out a toddler’s fracture.
The examination of the patient is rarely impressive as there is little swelling and bruising with most toddlers’ fractures. A complete clinical history is needed, including a detailed description of any observed traumatic event to exclude the existence of other injuries.
When no traumatic event is observed or an inconsistent history is provided, the physician should obtain a detailed social history, including a list of the child’s most recent caregivers and contacts.16 Because of mild clinical symptoms and frequent lack of a history of injury in this patient population, presentation for evaluation may be delayed. In such cases, by the time the extremity is examined, the fracture has begun to heal. This healing phase may be accompanied by periosteal new bone and, in the absence of a history, may erroneously suggest other, more ominous conditions such as osteomylelitis or tumor.17,18
Consideration of Abuse
Although tibial shaft fractures are rarely found in abused children, diagnosis of child abuse must be considered in cases where a tibial fracture is discovered in the nonambulatory child; his or her clinical history is inconsistent with the injury; and/or there are other physical findings suggestive of abuse. Investigation for suspected nonaccidental trauma includes a thorough physical examination, skeletal survey, and evaluation by social services personnel.16
Radiographic Imaging
Quality anteroposterior (AP) or lateral radiographs of the affected leg may show a hairline fracture, but these can easily be missed on initial plain films in almost a third of patients.21 An internal oblique view can aid in identifying nondisplaced toddler fractures.17 The AP view is the best view for observing the nondisplaced spiral fracture along the distal tibia (Figure 3).6 Occasionally, a fracture line is not identified on initial plain films and the first evidence of fracture becomes apparent on X-ray when new periosteal bone forms 7 to 10 days after the initial injury.
Definitive Treatment
Children with a classic history for a toddler’s fracture and an inability to bear weight should be immobilized with a long-leg splint or cast—even when X-rays are negative—until a definitive diagnosis can be made. Such fractures usually become visible on X-ray 7 to 10 days after injury as a result of new bone growth.22
When definitive diagnosis of a toddler’s fracture is made on plain radiographs, the child should either be immobilized in a long-leg splint with referral to an orthopedist within 5 to 7 days, or immediately casted.16
Conclusion
Fractures in both children and adults are among the most common injury-related presentations to the ED. Based on the structure and increased elasticity of bone in the pediatric patient, there are several fracture patterns unique to this population. Appropriate evaluation, diagnosis, and management in the ED helps to maximize and ensure long-term function and healing while minimizing trauma to the patient.
Dr McBride is an associate professor of pediatrics and pediatric emergency medicine, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
Dr Sutton is a pediatric resident, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
- Brown D. Emergency department visits for nursemaid’s elbow in the United States, 2005-2006. Orthop Nurs. 2009;28(4):161,162.
- Hardy RH. Pulled elbow. J R Coll Gen Pract. 1978;28(189):224-226.
- Browner EA. Nursemaid’s elbow (annular ligament displacement). Pediatr Rev. 2013;34(8):366,367.
- Krul M, van der Wouden JC,van Suijlekom-Smit LW, Koes BM. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database of Syst Rev. 2012;1:CD007759.
- Gunaydin YK, Katirci Y, Duymaz H, et al. Comparison of success and pain levels of supination-flexion and hyperpronation maneuvers in childhood nursemaid’s elbow cases. Am J Emerg Med. 2013;31(7):1078-1081.
- Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150(2):164-166.
- Dolan M and Waters PM. Fractures and dislocations of the forearm, wrist, and hand. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadephia, PA: Saunders Elsevier; 2009:159-206.
- Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19(1):77-81.
- Plint AC, Perry JJ, Correll R, Gaboury I, Lawtown L. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117(3):691-697.
- Kay RM, Matthys GA. Pediatric ankle fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(4):268-278.
- Marsh JS, Daigneault JP. Ankle injuries in the pediatric population. Curr Opin Pediatr. 2000;12(1):52-60
- Cummings RJ. Distal tibial and fibular fractures. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1096-1104.
- Boutis K, Willan AR, Babyn P, Narayanan UG, Alman B, Schuh S. A randomized, controlled trial of a removable brace versus casting in children with low-risk ankle fractures. Pediatrics. 2007;119(6):1256-1263.
- Wuerz TH, Gurd DP. Pediatric physeal ankle fracture. J Am Acad Orthop Surg. 2013;21(4):234-244.
- Horn BD, Crisci K, Krug M, Pizzutillo PD, MacEwen GD. Radiologic evaluation of juvenile Tillaux fractures of the distal tibia. J Pediatr Orthop. 2001;21(2):162-164.
- Mashru RP, Herman MJ, Pizzutillo PD. Tibial shaft fractures in children and adolescents. J Am Acad Orthop Surg. 2005;139(5):345-352.
- Heinrich SD, Mooney JF. Fractures of the shaft of the tibia and fibula. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1063,1064.
- John SD, Moorthy CS, Swischuk LE. Expanding the concept of the toddler’s fracture. Radiographics. 1997;17(2):367-376.
- Dunbar JS, Owen HF, Nogrady MB, McLeese R. Obscure tibial fracture of infants—the toddlers’ fracture. J Can Assoc Radiol 1964;15:136-144.
- Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8(3):208-211.
- Shravat BP, Harrop SN, Kane TP. Toddler’s fracture. J Accid Emerg Med. 1996;13(1):59-61.
- Halsey MF, Finzel KC, Carrion WV, Haralabatos SS, Gruber MA, Meinhard BP. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21(2):152-156.
Case 1
A 2-year-old girl presented to the ED with arm pain. Her mother stated that her daughter was playing with a 5-year-old sibling when she heard the child cry- out in pain and noticed she was holding her right arm by her side, not wanting to move it. Neither child gave a reliable story of the injury.
Nursemaid’s Elbow
Nursemaid’s elbow, also known as pulled elbow, subluxation of the radial head, and most recently annular ligament displacement, is a common injury in children younger than age 6 years. One study estimates that the condition represented about 1% of injury-related ED visits in 2005.1
Patients with nursemaid’s elbow typically present holding the injured arm at their side, slightly flexed and pronated. These patients appear relatively comfortable until moved actively or passively. The classic history of nursemaid’s elbow includes a traction mechanism, with the child being pulled up by one arm or being grabbed by the arm suddenly to keep him or her out of harm’s way.2 Due to the laxity of connective tissues in children of this age, the head of the radius slips out of the annular ligament causing acute pain and decreased function.
Nursemaid’s elbow is usually diagnosed by history and examination alone, with special consideration to the mechanism of injury. There is rarely swelling or bruising.3 Passive flexion and extension at the elbow may be normal, but rotational maneuvers can be painful or fully resisted.
Reduction Techniques
In 2012, Cochrane updated its earlier review on nursemaid’s elbow and in 2013 followed up with an article in Pediatrics in Review.3,4 Each covered research on reduction techniques, summarizing studies comparing supination-flexion (SF) versus hyperpronation (HP) as the initial reduction maneuver. Given that these maneuvers are difficult to camouflage, studies tend to be pseudorandomized with assessment by a nonblinded healthcare provider, decreasing the strength of the studies. In the Cochrane review, four different trials that included 379 children under age 7 years were selected for the review. In all four studies, pronation was found to have the least chance of failed first attempt, the chosen outcome for this meta-analysis. The risk ratio of failure of reduction for pronation was 0.45 (95% confidence interval [CI], 0.28-0.73).
There is some data supporting hyperpronation to be less painful as well; however, the Cochrane reviewers felt there may have been reporting bias.4 Since the time of each of these reviews, another study comprised of 150 children was conducted and also favored similar practice styles, as the hyperpronation maneuver had 95% success rate on first attempt versus 68% first-time success with supination and flexion.5
Complications and Recurrence
In a small study aimed at identifying recurrence rates for nursemaid’s elbow, Teach and Schultzman6 studied 93 children for 1 year after probable or definite diagnosis of nursemaid’s elbow. Of these children, 23.7% had recurrent radial head subluxation. Children younger than age 2 years were found to have a relative risk of 2.6 (95% CI, 1.04-6.30) for one or more recurrences when compared to children older than age 2 years.
While the great majority of children with nursemaid’s elbow do not need referral to an orthopedist, those with two or more occurrences should be considered for referral to a specialist.
Case 2
A 6-year-old boy was presented to the ED by his father, who had placed the boy’s arm in a home-made sling. The child tearfully told the provider that he fell trying to catch himself after tripping over the house pet.
FOOSH Injury
The above case depicts a very common presentation in the ED—the so-called “FOOSH” (fall onto an outstretched hand) injury. This type of injury occurs with such frequency in both adults and children that it is one of the only injury patterns with a commonly used acronym. The bony injuries seen with FOOSH in children, however, have a different pattern than those in adults.
Pediatric fractures are unique due to the difference in the structure of the bones themselves. A child’s bones are more elastic than an adult’s bones, allowing them to bow and bend before they fracture.7 Despite this malleability, pediatric bones have been noted to have a thicker periosteum. For this reason, compression or impact may interrupt the periosteal sleeve, minimally yielding an incomplete interruption of the cortex unilaterally.
One fracture pattern commonly seen in children is the torus fracture. This type of fracture is also referred to as a buckle fracture as the bone cortex on radiographic imaging appears “buckled” as a result of the compressive forces on that side of the bone (Figure 1). Since the bone itself is minimally affected, these fractures are quite stable and not at risk for complications.
In comparison, a greenstick fracture, also unique to the pediatric population, is one in which the cortex shows plastic deformity on the side of the force or impact but is interrupted on the opposite side due to the tension of the impact itself. Greenstick fractures are frequently angulated and may require reduction for anatomic alignment, but long-term complications are typically minimal. These fracture patterns are distinguished from complete fractures (as seen in adults), which are quite unstable and generally require surgical intervention.
Of note, the location of pediatric forearm fractures varies with age as well. Diaphyseal fractures are more common in prepubescent children, whereas the highest incidence of physeal injuries occurs during large growth spurts, particularly throughout adolescence.7
Management
The remodeling potential of pediatric bones also makes management unique. Pediatric orthopedic literature has well-studied acceptable angles and degrees of appropriate displacement based largely on the age of the patient and proximity to a growth plate. Knowledge of these is imperative for definitive care of such fractures but is beyond the scope of this review.
Traditional treatment of pediatric forearm fractures includes immobilization of various types and duration to minimize pain and deformity while producing the best possible outcome. Several recent studies have aimed to determine best practice for the different fracture types with the goal of producing best alignment and return to function while decreasing cost, discomfort, and number of physician visits. Another concern among healthcare providers is the risk of refracture, which in buckle fractures is estimated at approximately 2% with a median time of 8 to 16 weeks after the initial injury.7
A 2010 review by Kennedy et al8 sought to determine if the refracture rate was affected by the technique used to immobilize torus fractures. The five studies used in this review had no reports of refracture in the 443 patients included in analysis, though only one of the studies (Plint et al) followed patients for more than 6 weeks.8,9 In this study, 75 patients were randomized to either a plaster removable splint or full below-elbow cast for 3 weeks; thereafter, they were followed for 6 months, during which time none experienced refracture.9
Another outcome from the same study assessed the ability of the patient to use the affected arm in the recovery period. While those in removable splints scored better during and immediately after cast removal, no differences were present after 1 week. Not surprisingly, families preferred the soft bandages or a removable splint for treatment.
Case 3
A 13-year-old boy presented to the ED with right ankle pain and difficulty bearing weight. He stated that he was playing basketball when he “rolled” his right ankle coming down from a rebound.
Ankle Fractures
Ankle fractures are among the most common acute injuries of the lower extremity in children, accounting for approximately 5% of pediatric fractures and 15% of physeal injuries.10 Ankle fractures also account for up to 40% of all injuries to the skeletally immature athlete.10,11 More specifically, distal fibular physeal fractures are the most common types of pediatric ankle fracture; however, they are associated with a relatively low risk for long-term complications. In contrast, distal tibial physeal fractures are associated with a higher risk for long-term complications.12,13
Presentation and Evaluation
Typically, patients presenting with ankle fractures are too sore to bear weight, and swelling and ecchymosis can be identified anterior to the ankle. In addition, there may be diffuse tenderness throughout the ankle and point tenderness may be induced on the anterolateral aspect of the distal tibia.14 A complete evaluation of the entire lower extremity should be conducted before assuming that the injury is confined to the ankle, especially in children younger than age 5 years and/or who are nonverbal.10 When evaluating an ankle fracture, in general, orthopedic consultation should be obtained for children with neurovascular compromise, open fractures, and/or Salter-Harris III, IV, and V fractures.
The juvenile Tillaux fracture represents a Salter-Harris III physeal injury that involves the anterolateral portion of the tibia. It usually occurs in children between ages 12 and 14 years as they approach skeletal maturity and who have a partially fused tibial physis. The common mechanism of injury is inversion of the ankle with the foot pointed away from the midline (supination with external rotation). This leads to avulsion of the lateral tibial epiphysis that is attached to the anterior inferior tibiofibular ligament. The uninvolved medial portion of the epiphysis is closed.10
Radiographic Imaging
Three radiographic views should be obtained in the evaluation of pediatric ankle injuries as Tillaux fractures or other subtle injuries could be easily missed if only two views are obtained. Interpretation of the radiographs must be correlated with the physical examination.10 The fracture line is usually best seen on a mortise view (Figure 2). Computed tomography (CT) is warranted in cases in which displacement greater than 2 mm is suspected because it better defines fracture displacement and can aid in surgical planning.14 Because of its sensitivity in detecting fractures displaced more than 2 mm, CT is now the preferred imaging modality in the assessment of juvenile Tillaux fractures.15
Definitive Management
There are two important goals when treating children with ankle fractures—achieving a satisfactory reduction and avoiding physeal arrest so as to minimize the risks of angular deformity, early arthrosis, leg-length inequality, and joint stiffness.11 Juvenile Tillaux fractures with greater than 2 mm of displacement require orthopedic consultation for closed or open reduction. Closed reduction is attempted by internally rotating the foot and applying direct pressure over the anterolateral tibia. If necessary, percutaneous pins can be used for stabilization of the reduction. If closed reduction is unsuccessful, open reduction is required. Care must be taken to assure no displacement occurs after casting; this requires weekly X-ray evaluation for the first 2 weeks.12
Patients with nondisplaced Salter-Harris III fractures are treated with long-leg casting for 4 weeks with conversion to a short-leg cast or boot for an additional 4 weeks. Patients should anticipate 8 weeks of nonweight-bearing. The patient is allowed to remove the boot for range-of-motion exercises but must remain nonweight-bearing for the first 2 weeks.14
Case 4
A 3-year-old previously healthy girl presented to the ED with a limp and difficulty bearing weight. Her mother reported that the child was playing in the yard when she caught her foot on a tree root, stumbled, and fell down. Since the incident, the child has been tearful, limping, and refusing to walk.
Tibial Fractures
Tibial fractures are among the most frequent types of orthopedic injuries in young children, with only femur and forearm fractures having a higher incidence of occurrence. Tibial fractures account for up to 15% of long bone fractures in children and adolescents.16,17 The mechanism of injury varies depending on the patient’s age. In young children, the most common cause of injury is from a seemingly minor twisting around a fixed foot or from a minor fall. In older children and adults, high-energy motor vehicle accidents and sports-related injuries are more common causes.
Fractures of the tibial shaft are typically short oblique or transverse fractures of the middle or distal third of the shaft. Thirty percent of tibial shaft fractures are associated with fractures of the fibula.16
Toddler’s Fracture
The term toddler’s fracture refers to a nondisplaced oblique fracture of the tibial shaft without concomitant fibular fracture. It usually results from an indirect rotational or twisting force applied to the foot and lower leg.16-18 More specifically, the term describes a specialized case of spiral fracture of the distal tibia in patients aged 9 months to 3 years, when weight-bearing is just beginning.19,20 Such injuries commonly occur when a toddler stumbles and falls, or attempts to extricate the foot from between the bars of a crib. Often, however, the mechanism is minimal or unknown.18 Of those injuries that are witnessed, most caregivers report a minor twisting mechanism. Most children with toddler’s fracture are younger than age 6 years. Sixty-three of 76 such fractures reported by Dunbar et al17,19 occurred in children younger than 2.5 years of age. Toddler’s fractures occur more often in boys than girls, and in the right leg more often than the left. Most children will give a history of tripping or twisting their ankle.17
Evaluating the Toddler
Toddlers can be challenging patients as they can not relate history and are often uncooperative on examination. A child may present with a limp, diminished movement of the affected limb, or refuse to bear weight without a distinct history of injury. The onset of limping or refusal to bear weight after minor trauma, or without an obvious injury in a young ambulatory child, warrants a detailed examination looking for tenderness over the tibia, along with radiographic evaluation to rule out a toddler’s fracture.
The examination of the patient is rarely impressive as there is little swelling and bruising with most toddlers’ fractures. A complete clinical history is needed, including a detailed description of any observed traumatic event to exclude the existence of other injuries.
When no traumatic event is observed or an inconsistent history is provided, the physician should obtain a detailed social history, including a list of the child’s most recent caregivers and contacts.16 Because of mild clinical symptoms and frequent lack of a history of injury in this patient population, presentation for evaluation may be delayed. In such cases, by the time the extremity is examined, the fracture has begun to heal. This healing phase may be accompanied by periosteal new bone and, in the absence of a history, may erroneously suggest other, more ominous conditions such as osteomylelitis or tumor.17,18
Consideration of Abuse
Although tibial shaft fractures are rarely found in abused children, diagnosis of child abuse must be considered in cases where a tibial fracture is discovered in the nonambulatory child; his or her clinical history is inconsistent with the injury; and/or there are other physical findings suggestive of abuse. Investigation for suspected nonaccidental trauma includes a thorough physical examination, skeletal survey, and evaluation by social services personnel.16
Radiographic Imaging
Quality anteroposterior (AP) or lateral radiographs of the affected leg may show a hairline fracture, but these can easily be missed on initial plain films in almost a third of patients.21 An internal oblique view can aid in identifying nondisplaced toddler fractures.17 The AP view is the best view for observing the nondisplaced spiral fracture along the distal tibia (Figure 3).6 Occasionally, a fracture line is not identified on initial plain films and the first evidence of fracture becomes apparent on X-ray when new periosteal bone forms 7 to 10 days after the initial injury.
Definitive Treatment
Children with a classic history for a toddler’s fracture and an inability to bear weight should be immobilized with a long-leg splint or cast—even when X-rays are negative—until a definitive diagnosis can be made. Such fractures usually become visible on X-ray 7 to 10 days after injury as a result of new bone growth.22
When definitive diagnosis of a toddler’s fracture is made on plain radiographs, the child should either be immobilized in a long-leg splint with referral to an orthopedist within 5 to 7 days, or immediately casted.16
Conclusion
Fractures in both children and adults are among the most common injury-related presentations to the ED. Based on the structure and increased elasticity of bone in the pediatric patient, there are several fracture patterns unique to this population. Appropriate evaluation, diagnosis, and management in the ED helps to maximize and ensure long-term function and healing while minimizing trauma to the patient.
Dr McBride is an associate professor of pediatrics and pediatric emergency medicine, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
Dr Sutton is a pediatric resident, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
Case 1
A 2-year-old girl presented to the ED with arm pain. Her mother stated that her daughter was playing with a 5-year-old sibling when she heard the child cry- out in pain and noticed she was holding her right arm by her side, not wanting to move it. Neither child gave a reliable story of the injury.
Nursemaid’s Elbow
Nursemaid’s elbow, also known as pulled elbow, subluxation of the radial head, and most recently annular ligament displacement, is a common injury in children younger than age 6 years. One study estimates that the condition represented about 1% of injury-related ED visits in 2005.1
Patients with nursemaid’s elbow typically present holding the injured arm at their side, slightly flexed and pronated. These patients appear relatively comfortable until moved actively or passively. The classic history of nursemaid’s elbow includes a traction mechanism, with the child being pulled up by one arm or being grabbed by the arm suddenly to keep him or her out of harm’s way.2 Due to the laxity of connective tissues in children of this age, the head of the radius slips out of the annular ligament causing acute pain and decreased function.
Nursemaid’s elbow is usually diagnosed by history and examination alone, with special consideration to the mechanism of injury. There is rarely swelling or bruising.3 Passive flexion and extension at the elbow may be normal, but rotational maneuvers can be painful or fully resisted.
Reduction Techniques
In 2012, Cochrane updated its earlier review on nursemaid’s elbow and in 2013 followed up with an article in Pediatrics in Review.3,4 Each covered research on reduction techniques, summarizing studies comparing supination-flexion (SF) versus hyperpronation (HP) as the initial reduction maneuver. Given that these maneuvers are difficult to camouflage, studies tend to be pseudorandomized with assessment by a nonblinded healthcare provider, decreasing the strength of the studies. In the Cochrane review, four different trials that included 379 children under age 7 years were selected for the review. In all four studies, pronation was found to have the least chance of failed first attempt, the chosen outcome for this meta-analysis. The risk ratio of failure of reduction for pronation was 0.45 (95% confidence interval [CI], 0.28-0.73).
There is some data supporting hyperpronation to be less painful as well; however, the Cochrane reviewers felt there may have been reporting bias.4 Since the time of each of these reviews, another study comprised of 150 children was conducted and also favored similar practice styles, as the hyperpronation maneuver had 95% success rate on first attempt versus 68% first-time success with supination and flexion.5
Complications and Recurrence
In a small study aimed at identifying recurrence rates for nursemaid’s elbow, Teach and Schultzman6 studied 93 children for 1 year after probable or definite diagnosis of nursemaid’s elbow. Of these children, 23.7% had recurrent radial head subluxation. Children younger than age 2 years were found to have a relative risk of 2.6 (95% CI, 1.04-6.30) for one or more recurrences when compared to children older than age 2 years.
While the great majority of children with nursemaid’s elbow do not need referral to an orthopedist, those with two or more occurrences should be considered for referral to a specialist.
Case 2
A 6-year-old boy was presented to the ED by his father, who had placed the boy’s arm in a home-made sling. The child tearfully told the provider that he fell trying to catch himself after tripping over the house pet.
FOOSH Injury
The above case depicts a very common presentation in the ED—the so-called “FOOSH” (fall onto an outstretched hand) injury. This type of injury occurs with such frequency in both adults and children that it is one of the only injury patterns with a commonly used acronym. The bony injuries seen with FOOSH in children, however, have a different pattern than those in adults.
Pediatric fractures are unique due to the difference in the structure of the bones themselves. A child’s bones are more elastic than an adult’s bones, allowing them to bow and bend before they fracture.7 Despite this malleability, pediatric bones have been noted to have a thicker periosteum. For this reason, compression or impact may interrupt the periosteal sleeve, minimally yielding an incomplete interruption of the cortex unilaterally.
One fracture pattern commonly seen in children is the torus fracture. This type of fracture is also referred to as a buckle fracture as the bone cortex on radiographic imaging appears “buckled” as a result of the compressive forces on that side of the bone (Figure 1). Since the bone itself is minimally affected, these fractures are quite stable and not at risk for complications.
In comparison, a greenstick fracture, also unique to the pediatric population, is one in which the cortex shows plastic deformity on the side of the force or impact but is interrupted on the opposite side due to the tension of the impact itself. Greenstick fractures are frequently angulated and may require reduction for anatomic alignment, but long-term complications are typically minimal. These fracture patterns are distinguished from complete fractures (as seen in adults), which are quite unstable and generally require surgical intervention.
Of note, the location of pediatric forearm fractures varies with age as well. Diaphyseal fractures are more common in prepubescent children, whereas the highest incidence of physeal injuries occurs during large growth spurts, particularly throughout adolescence.7
Management
The remodeling potential of pediatric bones also makes management unique. Pediatric orthopedic literature has well-studied acceptable angles and degrees of appropriate displacement based largely on the age of the patient and proximity to a growth plate. Knowledge of these is imperative for definitive care of such fractures but is beyond the scope of this review.
Traditional treatment of pediatric forearm fractures includes immobilization of various types and duration to minimize pain and deformity while producing the best possible outcome. Several recent studies have aimed to determine best practice for the different fracture types with the goal of producing best alignment and return to function while decreasing cost, discomfort, and number of physician visits. Another concern among healthcare providers is the risk of refracture, which in buckle fractures is estimated at approximately 2% with a median time of 8 to 16 weeks after the initial injury.7
A 2010 review by Kennedy et al8 sought to determine if the refracture rate was affected by the technique used to immobilize torus fractures. The five studies used in this review had no reports of refracture in the 443 patients included in analysis, though only one of the studies (Plint et al) followed patients for more than 6 weeks.8,9 In this study, 75 patients were randomized to either a plaster removable splint or full below-elbow cast for 3 weeks; thereafter, they were followed for 6 months, during which time none experienced refracture.9
Another outcome from the same study assessed the ability of the patient to use the affected arm in the recovery period. While those in removable splints scored better during and immediately after cast removal, no differences were present after 1 week. Not surprisingly, families preferred the soft bandages or a removable splint for treatment.
Case 3
A 13-year-old boy presented to the ED with right ankle pain and difficulty bearing weight. He stated that he was playing basketball when he “rolled” his right ankle coming down from a rebound.
Ankle Fractures
Ankle fractures are among the most common acute injuries of the lower extremity in children, accounting for approximately 5% of pediatric fractures and 15% of physeal injuries.10 Ankle fractures also account for up to 40% of all injuries to the skeletally immature athlete.10,11 More specifically, distal fibular physeal fractures are the most common types of pediatric ankle fracture; however, they are associated with a relatively low risk for long-term complications. In contrast, distal tibial physeal fractures are associated with a higher risk for long-term complications.12,13
Presentation and Evaluation
Typically, patients presenting with ankle fractures are too sore to bear weight, and swelling and ecchymosis can be identified anterior to the ankle. In addition, there may be diffuse tenderness throughout the ankle and point tenderness may be induced on the anterolateral aspect of the distal tibia.14 A complete evaluation of the entire lower extremity should be conducted before assuming that the injury is confined to the ankle, especially in children younger than age 5 years and/or who are nonverbal.10 When evaluating an ankle fracture, in general, orthopedic consultation should be obtained for children with neurovascular compromise, open fractures, and/or Salter-Harris III, IV, and V fractures.
The juvenile Tillaux fracture represents a Salter-Harris III physeal injury that involves the anterolateral portion of the tibia. It usually occurs in children between ages 12 and 14 years as they approach skeletal maturity and who have a partially fused tibial physis. The common mechanism of injury is inversion of the ankle with the foot pointed away from the midline (supination with external rotation). This leads to avulsion of the lateral tibial epiphysis that is attached to the anterior inferior tibiofibular ligament. The uninvolved medial portion of the epiphysis is closed.10
Radiographic Imaging
Three radiographic views should be obtained in the evaluation of pediatric ankle injuries as Tillaux fractures or other subtle injuries could be easily missed if only two views are obtained. Interpretation of the radiographs must be correlated with the physical examination.10 The fracture line is usually best seen on a mortise view (Figure 2). Computed tomography (CT) is warranted in cases in which displacement greater than 2 mm is suspected because it better defines fracture displacement and can aid in surgical planning.14 Because of its sensitivity in detecting fractures displaced more than 2 mm, CT is now the preferred imaging modality in the assessment of juvenile Tillaux fractures.15
Definitive Management
There are two important goals when treating children with ankle fractures—achieving a satisfactory reduction and avoiding physeal arrest so as to minimize the risks of angular deformity, early arthrosis, leg-length inequality, and joint stiffness.11 Juvenile Tillaux fractures with greater than 2 mm of displacement require orthopedic consultation for closed or open reduction. Closed reduction is attempted by internally rotating the foot and applying direct pressure over the anterolateral tibia. If necessary, percutaneous pins can be used for stabilization of the reduction. If closed reduction is unsuccessful, open reduction is required. Care must be taken to assure no displacement occurs after casting; this requires weekly X-ray evaluation for the first 2 weeks.12
Patients with nondisplaced Salter-Harris III fractures are treated with long-leg casting for 4 weeks with conversion to a short-leg cast or boot for an additional 4 weeks. Patients should anticipate 8 weeks of nonweight-bearing. The patient is allowed to remove the boot for range-of-motion exercises but must remain nonweight-bearing for the first 2 weeks.14
Case 4
A 3-year-old previously healthy girl presented to the ED with a limp and difficulty bearing weight. Her mother reported that the child was playing in the yard when she caught her foot on a tree root, stumbled, and fell down. Since the incident, the child has been tearful, limping, and refusing to walk.
Tibial Fractures
Tibial fractures are among the most frequent types of orthopedic injuries in young children, with only femur and forearm fractures having a higher incidence of occurrence. Tibial fractures account for up to 15% of long bone fractures in children and adolescents.16,17 The mechanism of injury varies depending on the patient’s age. In young children, the most common cause of injury is from a seemingly minor twisting around a fixed foot or from a minor fall. In older children and adults, high-energy motor vehicle accidents and sports-related injuries are more common causes.
Fractures of the tibial shaft are typically short oblique or transverse fractures of the middle or distal third of the shaft. Thirty percent of tibial shaft fractures are associated with fractures of the fibula.16
Toddler’s Fracture
The term toddler’s fracture refers to a nondisplaced oblique fracture of the tibial shaft without concomitant fibular fracture. It usually results from an indirect rotational or twisting force applied to the foot and lower leg.16-18 More specifically, the term describes a specialized case of spiral fracture of the distal tibia in patients aged 9 months to 3 years, when weight-bearing is just beginning.19,20 Such injuries commonly occur when a toddler stumbles and falls, or attempts to extricate the foot from between the bars of a crib. Often, however, the mechanism is minimal or unknown.18 Of those injuries that are witnessed, most caregivers report a minor twisting mechanism. Most children with toddler’s fracture are younger than age 6 years. Sixty-three of 76 such fractures reported by Dunbar et al17,19 occurred in children younger than 2.5 years of age. Toddler’s fractures occur more often in boys than girls, and in the right leg more often than the left. Most children will give a history of tripping or twisting their ankle.17
Evaluating the Toddler
Toddlers can be challenging patients as they can not relate history and are often uncooperative on examination. A child may present with a limp, diminished movement of the affected limb, or refuse to bear weight without a distinct history of injury. The onset of limping or refusal to bear weight after minor trauma, or without an obvious injury in a young ambulatory child, warrants a detailed examination looking for tenderness over the tibia, along with radiographic evaluation to rule out a toddler’s fracture.
The examination of the patient is rarely impressive as there is little swelling and bruising with most toddlers’ fractures. A complete clinical history is needed, including a detailed description of any observed traumatic event to exclude the existence of other injuries.
When no traumatic event is observed or an inconsistent history is provided, the physician should obtain a detailed social history, including a list of the child’s most recent caregivers and contacts.16 Because of mild clinical symptoms and frequent lack of a history of injury in this patient population, presentation for evaluation may be delayed. In such cases, by the time the extremity is examined, the fracture has begun to heal. This healing phase may be accompanied by periosteal new bone and, in the absence of a history, may erroneously suggest other, more ominous conditions such as osteomylelitis or tumor.17,18
Consideration of Abuse
Although tibial shaft fractures are rarely found in abused children, diagnosis of child abuse must be considered in cases where a tibial fracture is discovered in the nonambulatory child; his or her clinical history is inconsistent with the injury; and/or there are other physical findings suggestive of abuse. Investigation for suspected nonaccidental trauma includes a thorough physical examination, skeletal survey, and evaluation by social services personnel.16
Radiographic Imaging
Quality anteroposterior (AP) or lateral radiographs of the affected leg may show a hairline fracture, but these can easily be missed on initial plain films in almost a third of patients.21 An internal oblique view can aid in identifying nondisplaced toddler fractures.17 The AP view is the best view for observing the nondisplaced spiral fracture along the distal tibia (Figure 3).6 Occasionally, a fracture line is not identified on initial plain films and the first evidence of fracture becomes apparent on X-ray when new periosteal bone forms 7 to 10 days after the initial injury.
Definitive Treatment
Children with a classic history for a toddler’s fracture and an inability to bear weight should be immobilized with a long-leg splint or cast—even when X-rays are negative—until a definitive diagnosis can be made. Such fractures usually become visible on X-ray 7 to 10 days after injury as a result of new bone growth.22
When definitive diagnosis of a toddler’s fracture is made on plain radiographs, the child should either be immobilized in a long-leg splint with referral to an orthopedist within 5 to 7 days, or immediately casted.16
Conclusion
Fractures in both children and adults are among the most common injury-related presentations to the ED. Based on the structure and increased elasticity of bone in the pediatric patient, there are several fracture patterns unique to this population. Appropriate evaluation, diagnosis, and management in the ED helps to maximize and ensure long-term function and healing while minimizing trauma to the patient.
Dr McBride is an associate professor of pediatrics and pediatric emergency medicine, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
Dr Sutton is a pediatric resident, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
- Brown D. Emergency department visits for nursemaid’s elbow in the United States, 2005-2006. Orthop Nurs. 2009;28(4):161,162.
- Hardy RH. Pulled elbow. J R Coll Gen Pract. 1978;28(189):224-226.
- Browner EA. Nursemaid’s elbow (annular ligament displacement). Pediatr Rev. 2013;34(8):366,367.
- Krul M, van der Wouden JC,van Suijlekom-Smit LW, Koes BM. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database of Syst Rev. 2012;1:CD007759.
- Gunaydin YK, Katirci Y, Duymaz H, et al. Comparison of success and pain levels of supination-flexion and hyperpronation maneuvers in childhood nursemaid’s elbow cases. Am J Emerg Med. 2013;31(7):1078-1081.
- Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150(2):164-166.
- Dolan M and Waters PM. Fractures and dislocations of the forearm, wrist, and hand. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadephia, PA: Saunders Elsevier; 2009:159-206.
- Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19(1):77-81.
- Plint AC, Perry JJ, Correll R, Gaboury I, Lawtown L. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117(3):691-697.
- Kay RM, Matthys GA. Pediatric ankle fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(4):268-278.
- Marsh JS, Daigneault JP. Ankle injuries in the pediatric population. Curr Opin Pediatr. 2000;12(1):52-60
- Cummings RJ. Distal tibial and fibular fractures. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1096-1104.
- Boutis K, Willan AR, Babyn P, Narayanan UG, Alman B, Schuh S. A randomized, controlled trial of a removable brace versus casting in children with low-risk ankle fractures. Pediatrics. 2007;119(6):1256-1263.
- Wuerz TH, Gurd DP. Pediatric physeal ankle fracture. J Am Acad Orthop Surg. 2013;21(4):234-244.
- Horn BD, Crisci K, Krug M, Pizzutillo PD, MacEwen GD. Radiologic evaluation of juvenile Tillaux fractures of the distal tibia. J Pediatr Orthop. 2001;21(2):162-164.
- Mashru RP, Herman MJ, Pizzutillo PD. Tibial shaft fractures in children and adolescents. J Am Acad Orthop Surg. 2005;139(5):345-352.
- Heinrich SD, Mooney JF. Fractures of the shaft of the tibia and fibula. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1063,1064.
- John SD, Moorthy CS, Swischuk LE. Expanding the concept of the toddler’s fracture. Radiographics. 1997;17(2):367-376.
- Dunbar JS, Owen HF, Nogrady MB, McLeese R. Obscure tibial fracture of infants—the toddlers’ fracture. J Can Assoc Radiol 1964;15:136-144.
- Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8(3):208-211.
- Shravat BP, Harrop SN, Kane TP. Toddler’s fracture. J Accid Emerg Med. 1996;13(1):59-61.
- Halsey MF, Finzel KC, Carrion WV, Haralabatos SS, Gruber MA, Meinhard BP. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21(2):152-156.
- Brown D. Emergency department visits for nursemaid’s elbow in the United States, 2005-2006. Orthop Nurs. 2009;28(4):161,162.
- Hardy RH. Pulled elbow. J R Coll Gen Pract. 1978;28(189):224-226.
- Browner EA. Nursemaid’s elbow (annular ligament displacement). Pediatr Rev. 2013;34(8):366,367.
- Krul M, van der Wouden JC,van Suijlekom-Smit LW, Koes BM. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database of Syst Rev. 2012;1:CD007759.
- Gunaydin YK, Katirci Y, Duymaz H, et al. Comparison of success and pain levels of supination-flexion and hyperpronation maneuvers in childhood nursemaid’s elbow cases. Am J Emerg Med. 2013;31(7):1078-1081.
- Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150(2):164-166.
- Dolan M and Waters PM. Fractures and dislocations of the forearm, wrist, and hand. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadephia, PA: Saunders Elsevier; 2009:159-206.
- Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19(1):77-81.
- Plint AC, Perry JJ, Correll R, Gaboury I, Lawtown L. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117(3):691-697.
- Kay RM, Matthys GA. Pediatric ankle fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(4):268-278.
- Marsh JS, Daigneault JP. Ankle injuries in the pediatric population. Curr Opin Pediatr. 2000;12(1):52-60
- Cummings RJ. Distal tibial and fibular fractures. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1096-1104.
- Boutis K, Willan AR, Babyn P, Narayanan UG, Alman B, Schuh S. A randomized, controlled trial of a removable brace versus casting in children with low-risk ankle fractures. Pediatrics. 2007;119(6):1256-1263.
- Wuerz TH, Gurd DP. Pediatric physeal ankle fracture. J Am Acad Orthop Surg. 2013;21(4):234-244.
- Horn BD, Crisci K, Krug M, Pizzutillo PD, MacEwen GD. Radiologic evaluation of juvenile Tillaux fractures of the distal tibia. J Pediatr Orthop. 2001;21(2):162-164.
- Mashru RP, Herman MJ, Pizzutillo PD. Tibial shaft fractures in children and adolescents. J Am Acad Orthop Surg. 2005;139(5):345-352.
- Heinrich SD, Mooney JF. Fractures of the shaft of the tibia and fibula. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1063,1064.
- John SD, Moorthy CS, Swischuk LE. Expanding the concept of the toddler’s fracture. Radiographics. 1997;17(2):367-376.
- Dunbar JS, Owen HF, Nogrady MB, McLeese R. Obscure tibial fracture of infants—the toddlers’ fracture. J Can Assoc Radiol 1964;15:136-144.
- Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8(3):208-211.
- Shravat BP, Harrop SN, Kane TP. Toddler’s fracture. J Accid Emerg Med. 1996;13(1):59-61.
- Halsey MF, Finzel KC, Carrion WV, Haralabatos SS, Gruber MA, Meinhard BP. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21(2):152-156.
Pediatric Orthopedic Basics
Case 1
A mother presented to the ED with her 8-year-old daughter after she witnessed the child fall off her bicycle onto the sidewalk. When she fell, the girl landed onto her outstretched arms and sustained minor abrasions to her palms and knees, but did not hit her head or lose consciousness. Upon falling, the child immediately cried that her left arm hurt and kept holding it guarded near her body.
She was seated on her mother’s lap in the examination room, appearing anxious but in no acute distress. The treating EP observed the superficial abrasions from across the room and obtained a detailed history.
The patient was afebrile, and her vital signs were stable with the exception of mild tachycardia. After a couple of minutes, the EP slowly approached the child and was able to perform a basic examination. There was no obvious deformity to her left upper extremity, and only mild swelling over the wrist. She was able to move her fingers well and had excellent capillary refill distally. The child remained calm during manual palpation of the anatomic snuff box. However, she immediately pulled away and began to cry upon palpation more proximally over the distal forearm. The EP discussed his concerns with the child’s mother and explained that further evaluation was necessary.
Case 2
A mother and father presented to the ED carrying their 6-year-old boy, stating that the child had been limping since they picked him up from a neighbor’s house an hour earlier and was now refusing to walk. The father noted that a group of children had been jumping on a trampoline unsupervised, but he did not witness any injury to his son. Both parents said that the boy had been well up until that day.
At presentation, the child was afebrile and his vital signs were stable. The EP asked the parents to coax the child to walk across the room. During the walk, the boy was reluctant to bear weight on his right foot. Careful inspection of his lower limb revealed no external signs of trauma, and it appeared neurovascularly intact. Careful palpation elicited tenderness directly over the physis at the distal fibula and near the lateral malleolus. While considering the broad differential for a limping child, the physician was primarily concerned with point tenderness on examination and informed the parents that radiographic imaging was warranted.
Overview
Pediatric musculoskeletal (MSK) injury and orthopedic trauma now comprise more than 10% of visits to the ED.1 Fractures in particular are becoming more commonplace with the increasing number of children actively involved in athletic sports and high-risk activities.
The general approach to acute-care management of these children has evolved, trending more toward splinting the fractured extremity and away from traditional casting. There are many benefits to splinting. The most important is arguably the reduced risk of developing compartment syndrome due to a splint’s ability to expand with accompanied swelling.2 This article reviews the unique characteristics of pediatric bone development and initial management of pediatric fractures, as well as basic splinting techniques and unique indications that require further orthopedic consultation.
Physiological Differences in Children
The MSK system of a child differs greatly from that of an adult. The bones themselves are much more porous and malleable during childhood, making them more susceptible to traumatic injury. The growing periosteum and the developing physes are particularly vulnerable, accounting for up to 20% of pediatric fractures (see the Figure illustrating the Salter-Harris classification in the next section).3 This is particularly true at a young age, when ligamentous adherence out-performs the bony integrity itself, making fractures more common than sprains and tears. The opposite is true in adults, who are much more likely to experience sprains before succumbing to fracture. Furthermore, since the periosteum is still very active in children, the fractured bone is much more likely to remodel, lending to less deformity and overall better outcomes in most cases.3 Nonunion is extremely rare in children.
Initial Management
Approaching the Pediatric Patient
Special consideration should be given when initially approaching an injured child, so as not to cause additional undue fear or anxiety to the patient. It is helpful to take an extra moment upon entering the room to simply observe the child’s positioning, posture, or reluctance to move a particular limb. Obtaining a careful, detailed history from a distance is recommended before too quickly approaching the patient. In addition, asking the caregiver to serve as proxy during the initial physical examination may also prove helpful in localizing the pain. In the obscure case, such as the child refusing to bear weight, it is good to keep a broad differential and inspect for non-MSK injury (eg, painful hernia, testicular torsion, foreign body lodged in the bottom of the foot). Utilizing a “log-rolling” technique with the palms of one’s hands on the patient’s thigh may reveal hip pathology. Simply observing the preoccupied child walk around the unit while watching from behind may also aid in the evaluation.
However, when the injury such as an open fracture or severe displacement is obvious, immediate stabilization is critical so as not to permit any additional harm. An arm board is typically used to accomplish this. In addition, pain control should never be overlooked, either with intravenous opioids or more appropriate oral or intranasal analgesia.4,5 In cases of significant trauma, always remember ABC assessment (airway, breathing/oxygenation, and circulation), despite the eagerness to give attention to what may be an obvious fracture.
Workup
Although the use of ultrasound and other modalities is becoming more popular in some settings, it is still commonplace to begin the evaluation of a potential fracture or dislocation with plain film X-rays. Before sending a patient to radiology, always stabilize any unstable fracture to avoid further injury or potentiate neurovascular compromise. In most cases, three views, including anteroposterior, true lateral, and oblique, are obtained. If a fracture is unclear, it may be helpful to image the opposite extremity for comparison. The location of a fracture and its characteristics greatly influence acute-care management, as well as patient disposition, the need for consultation with orthopedics, and follow-up expectations and instructions.
Salter-Harris Fracture Classification
The physes of bones in growing children are particularly vulnerable sites of fracture since they have not yet fused. The five generally accepted types of fracture according to risk of growth disturbance are illustrated in the above Figure and are differentiated as follows:
Type I. This type of fracture is exclusive involvement of the physis itself, separating the metaphysis from the epiphysis. Since plain films may not reveal any visible fracture, the clinician should have a high index of suspicion if the physical examination elicits point tenderness over the growth plate. When in doubt if a fracture is present, always splint. Type I fractures of the physis tend to heal well, without significant consequence.
Type II. As with type I fractures, type II involve the physis, but also have a fragment of displaced metaphysis—the most common of all physeal fractures. Without significant displacement, these fractures also tend to have good outcomes.
Type III. Rather than involving the physis and metaphysis, type III fractures involve the epiphysis and therefore the joint itself. It is because of the epiphyseal displacement that these fractures tend to have a worse prognosis with joint disability and growth arrest. Thus, establishing alignment is imperative. The distal tibial Tillaux fracture is an example and requires internal fixation for optimal healing.
Type IV. Similar to a type III fracture, with the fracture extending proximally through a segment of the metaphysis, type IV fractures are treated similar to type III fractures. Due to joint involvement, an orthopedic consultation is warranted.
Type V. This type represents compression fractures of the physis. As the visibility of these fractures is poor on plain films, diagnosis can be challenging. However, history of axial compression injury may help lead the clinician to an accurate diagnosis. Since there is a high incidence of growth disturbance in type V fractures, compression affecting other areas such as the spine should also be considered.
Certainly not all pediatric fractures will involve a physis. A detailed description and management of other unique types of pediatric fractures is discussed in other articles in this feature.
Splinting Basics
Once the decision is made to apply a splint to a fracture, certain basic precautions should first be taken. Initially, any significant lacerations or abrasions should be thoroughly irrigated, cleansed, and dressed appropriately. Next, the physician should reevaluate and document both neurological status and perfusion of the area, particularly distal to the fracture site.
One commonly overlooked step in management of any fracture is pain control. It is advisable to consider administering medication prior to splinting on a case-by-case basis and for all fractures requiring reduction.
Materials and Methods
Prepackaged fiberglass splints have become a popular, efficient, and less-messy material of choice in pediatric splinting. Alternatively, plaster of Paris—although a bit more cumbersome—has some advantages, including low cost and a tendency to mold more easily to the extremity being splinted.7 When using plaster, strips should be cut a little longer than the anticipated length needed since they may shrink during curing. The unaffected limb should be used to gauge the measurement needed.
Regardless of the material chosen, all splinting should begin with the application of a stockinette tube dressing over the skin, leaving a distal opening over fingers or toes. This should be followed by a padding material (eg, Webril), beginning distally and rolling proximally, being sure to have approximately 50% overlap of each roll. Extra padding should be rolled over any bony prominence (eg, ulnar stylus) to avoid discomfort or pressure sores once the splint is applied.2
Between 8 and 10 layers of plaster (additional layers for lower extremity splints) should be wetted with room-temperature water. Hot water should never be used as this will intensify the exothermic reaction that occurs when curing and could cause burns.2 The limb should be kept in the anatomic position while the plaster is being molded to the shape of the extremity, allowing 15 to 20 minutes to dry.1 Once dry, an elastic bandage such as an Ace wrap may be placed over the entire cast to hold it secure in place. If fiberglass is used, it is helpful to squeeze out extra water before molding to the extremity. Again, an additional padding roll should be employed to avoid any discomfort or pressure beneath the splint.
In both fiberglass and plaster splinting, the edges of either type of material should not be abrasive to the skin; this can be avoided by rolling over excess padding and stockinette to create a round soft edge on either end.7 Finally, the patient should be fitted with a shoulder sling or crutches (if age appropriate) to further immobilize the injured extremity and avoid any movement or weight bearing.
Types of Splints
The type of splint depends of the location and characteristics of the fracture being immobilized. The following are a few examples of the more popular splinting techniques indicated for common pediatric fractures.
Long-Arm Posterior Splint. This splint is useful for most forearm and elbow fractures. The splint length should extend from midlength of the humerus to the palmar crease, and the width should be semicircular. In addition, an anatomic position of 90˚ flexion of the elbow should be maintained, with the hand in a neutral position and slight dorsiflexion. It is generally accepted to slightly pronate the forearm when splinting a supracondylar fracture. Orthopedics should always be consulted if the fracture involves the elbow.
Ulnar “Gutter” Splint. Useful for nondisplaced, minimally-angulated metacarpal “boxer’s fracture” or fourth and fifth phalangeal fractures, the length of the ulnar splint should extend from the distal phalanx to proximal forearm. Splint width should enclose both the volar and dorsum surfaces of the fourth and fifth metacarpals. In addition, padding should be placed between the digits for comfort. The metacarpophalangeal joints should be positioned at 70˚, and the proximal phalangeal angle at approximately 20˚ flexion2; this will help minimize the risk of contractures.
Forearm “Sugar-Tong” Splint. These splints are indicated for immobilization of a distal radius fracture or wrist injury. Distal radial fractures are by far the most common fractures encountered in the pediatric population,8 and splinting for angulation less than 15˚ is preferred.9,10 For proper stabilization, a long U-shaped splint should originate at the palmar crease, wrap around the elbow, and end at the metacarpophalangeal joint dorsally. Again, the hand should be dorsiflexed, and a soft rolled edge should be kept on the palmar crease to allow full finger flexion to near 90˚.
Thumb Spica Splint. A thumb spica splint is useful to immobilize uncomplicated fracture of the first metacarpal or proximal phalanx or when scaphoid (navicular) bone fracture is suspected. A semicircumferential molding of the radial forearm should be formed, extending to the thumbnail bed, and wrapping around the thumb. The proper hand positioning is slightly dorsiflexed, with thumb abducted slightly, as if holding a glass of water.2 If there is any doubt of a navicular fracture (rare in prepubescent children), the clinician should never hesitate to splint!
Long-Leg Posterior Splint. This type of splint is appropriate for immobilization of midshaft tibia/fibula fractures or most knee injuries. Full length of the splint should start beneath the inferior gluteal fold and extend to the ball of the foot, leaving the toes free. The ankle should be at 90˚ flexion and the knee should remain just slightly flexed, never locked straight. Orthopedics should always be consulted in cases of proximal tibia/fibula fractures or knee joint involvement.
Posterior Ankle Splint. Essentially a shorter version of a long-leg splint extending proximally to just below the knee, the posterior ankle splint is useful to immobilize ankle fractures, foot fractures, and severe ankle sprains. The distal fibula and occasional tibia physes are another common site of pediatric fractures, particularly in obese or more active children.11,12 When using either a long- or short-leg posterior ankle splint, it is helpful to hold the foot at 90˚ flexion until the material hardens or the proper angle may be lost. A recall that displaced or Salter-Harris type III or IV physeal fractures justify orthopedics consult. Nonweight-bearing, use of crutches, ice, and elevation are all important points for recovery in 3 to 6 weeks.
Lower Extremity Stirrup “Sugar-Tong” Splint. This splint is indicated for additional ankle stabilization. It runs in a U-shape (not unlike a forearm sugar-tong splint) from just below the knee around the calcaneus, and it must be wide enough to encase the ankle but not so wide that the two sides overlap when molded. It is very important to add extra padding around both malleoli and beneath the calcaneus to reduce the likelihood of pressure sores. Crutches are essential to avoid weight-bearing in patients old enough to use them. Some pediatric orthopedists advise avoiding this type of splint in the smaller, noncompliant, active child.
Complications
Although splinting has many advantages over casting in the acute-care setting, several potential complications may develop. Although rare, thermal burns to the underlying skin may occur if excessively warm or hot water is used on plaster or fiberglass due to the exothermic reaction during the hardening process. Therefore, the use of room-temperature water is always recommended. Despite the noncircumferential nature of a splint, it is still possible to develop significant swelling following splint application, which can lead to neurovascular compromise, compartment syndrome, infection, or pressure ulcers.7 The patient and caregiver should be advised to return to the ED immediately for evaluation if serious signs and symptoms such as pain, numbness, tingling, dusky color of skin, or poor capillary refill develop.
Case 1 Conclusion
The EP in this case elected to obtain plain X-rays of the patient’s left forearm, including the wrist and elbow. The results demonstrated a disruption of the cortical integrity of the distal radius, consistent with a buckle fracture. The angulation was estimated at merely 10˚. The bones of the wrist and elbow appeared normal. The EP concluded that a consult with orthopedics was not required urgently, and immobilized the patient’s arm using a fiberglass sugar-tong splint, keeping her elbow at 90˚, the forearm in a neutral position, and hand slightly dorsiflexed. A nurse assisted in keeping the child still to ensure the splint was shaped around the arm and hardened in this position. The child was provided with a sling, and supportive-care measures, including analgesia with nonsteroidal anti-inflammatory drugs as needed, ice, rest, and the importance of keeping the splint dry, were reviewed with her parents. The EP also stressed the importance of surveying for any loss of sensation or perfusion to the patient’s hand and fingers, and recommended follow up with orthopedics 1 week from discharge.
Case 2 Conclusion
Multiple views of the patient’s ankle were obtained on X-ray and showed no apparent fracture or dislocation. Additional films of the opposite ankle were obtained for comparison, but both appeared quite similar except for mild soft-tissue swelling of the affected side. Since point tenderness was reproducible over the distal fibular physis, the EP elected to place a short-leg posterior splint, maintaining good anatomic position with extra padding around the malleoli. The parents were instructed on proper elevation, ice to reduce inflammation, and the use of pain medication if needed.
One week after discharge, the treating EP received a letter from the child’s orthopedist, informing him that at the follow-up appointment, a repeat ankle film revealed periosteal changes and a type I Salter-Harris distal fibula fracture. Immobilization for an additional 3 weeks and supportive care was indicated.
Dr Del Re is an instructor of pediatrics and an intermediate care pediatrician, Rady Children’s Hospital, San Diego, California. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Bachman D, Santora S. Musculoskeletal trauma. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:1335-1375.
- Klig JE. Splinting procedures. In: King C, Henretig FM, eds. Texbook of Pediatric Emergency Procedures. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:919-931.
- Wilkins KE. The incidence of fractures in children. In: Rockwood CA, Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia, PA: Lippincott-Raven; 1996:3-17.
- Mahar PJ, Rana JA, Kennedy CS, Christopher NC. A randomized clinical trial of oral transmucosal fentanyl citrate versus intravenous morphine sulfate for initial control of pain in children with extremity injuries. Pediatr Emerg Care. 2007;23(8):544-548.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155-1161.
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
- Boyd AS, Benjamin HJ, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-22.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33(6):503-505.
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. CMAJ. 2010;182(14):1507-1512.
- Firmin F, Crouch R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): a literature review. Int Emerg Nurs. 2009;17(3):173-178.
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14(4):423-430.
- Blackburn EW, Aronsson DD, Rubright JH, Lisle JW. Ankle fractures in children. J Bone Joint Surg Am. 2012; 94(13):1234-1244.
Case 1
A mother presented to the ED with her 8-year-old daughter after she witnessed the child fall off her bicycle onto the sidewalk. When she fell, the girl landed onto her outstretched arms and sustained minor abrasions to her palms and knees, but did not hit her head or lose consciousness. Upon falling, the child immediately cried that her left arm hurt and kept holding it guarded near her body.
She was seated on her mother’s lap in the examination room, appearing anxious but in no acute distress. The treating EP observed the superficial abrasions from across the room and obtained a detailed history.
The patient was afebrile, and her vital signs were stable with the exception of mild tachycardia. After a couple of minutes, the EP slowly approached the child and was able to perform a basic examination. There was no obvious deformity to her left upper extremity, and only mild swelling over the wrist. She was able to move her fingers well and had excellent capillary refill distally. The child remained calm during manual palpation of the anatomic snuff box. However, she immediately pulled away and began to cry upon palpation more proximally over the distal forearm. The EP discussed his concerns with the child’s mother and explained that further evaluation was necessary.
Case 2
A mother and father presented to the ED carrying their 6-year-old boy, stating that the child had been limping since they picked him up from a neighbor’s house an hour earlier and was now refusing to walk. The father noted that a group of children had been jumping on a trampoline unsupervised, but he did not witness any injury to his son. Both parents said that the boy had been well up until that day.
At presentation, the child was afebrile and his vital signs were stable. The EP asked the parents to coax the child to walk across the room. During the walk, the boy was reluctant to bear weight on his right foot. Careful inspection of his lower limb revealed no external signs of trauma, and it appeared neurovascularly intact. Careful palpation elicited tenderness directly over the physis at the distal fibula and near the lateral malleolus. While considering the broad differential for a limping child, the physician was primarily concerned with point tenderness on examination and informed the parents that radiographic imaging was warranted.
Overview
Pediatric musculoskeletal (MSK) injury and orthopedic trauma now comprise more than 10% of visits to the ED.1 Fractures in particular are becoming more commonplace with the increasing number of children actively involved in athletic sports and high-risk activities.
The general approach to acute-care management of these children has evolved, trending more toward splinting the fractured extremity and away from traditional casting. There are many benefits to splinting. The most important is arguably the reduced risk of developing compartment syndrome due to a splint’s ability to expand with accompanied swelling.2 This article reviews the unique characteristics of pediatric bone development and initial management of pediatric fractures, as well as basic splinting techniques and unique indications that require further orthopedic consultation.
Physiological Differences in Children
The MSK system of a child differs greatly from that of an adult. The bones themselves are much more porous and malleable during childhood, making them more susceptible to traumatic injury. The growing periosteum and the developing physes are particularly vulnerable, accounting for up to 20% of pediatric fractures (see the Figure illustrating the Salter-Harris classification in the next section).3 This is particularly true at a young age, when ligamentous adherence out-performs the bony integrity itself, making fractures more common than sprains and tears. The opposite is true in adults, who are much more likely to experience sprains before succumbing to fracture. Furthermore, since the periosteum is still very active in children, the fractured bone is much more likely to remodel, lending to less deformity and overall better outcomes in most cases.3 Nonunion is extremely rare in children.
Initial Management
Approaching the Pediatric Patient
Special consideration should be given when initially approaching an injured child, so as not to cause additional undue fear or anxiety to the patient. It is helpful to take an extra moment upon entering the room to simply observe the child’s positioning, posture, or reluctance to move a particular limb. Obtaining a careful, detailed history from a distance is recommended before too quickly approaching the patient. In addition, asking the caregiver to serve as proxy during the initial physical examination may also prove helpful in localizing the pain. In the obscure case, such as the child refusing to bear weight, it is good to keep a broad differential and inspect for non-MSK injury (eg, painful hernia, testicular torsion, foreign body lodged in the bottom of the foot). Utilizing a “log-rolling” technique with the palms of one’s hands on the patient’s thigh may reveal hip pathology. Simply observing the preoccupied child walk around the unit while watching from behind may also aid in the evaluation.
However, when the injury such as an open fracture or severe displacement is obvious, immediate stabilization is critical so as not to permit any additional harm. An arm board is typically used to accomplish this. In addition, pain control should never be overlooked, either with intravenous opioids or more appropriate oral or intranasal analgesia.4,5 In cases of significant trauma, always remember ABC assessment (airway, breathing/oxygenation, and circulation), despite the eagerness to give attention to what may be an obvious fracture.
Workup
Although the use of ultrasound and other modalities is becoming more popular in some settings, it is still commonplace to begin the evaluation of a potential fracture or dislocation with plain film X-rays. Before sending a patient to radiology, always stabilize any unstable fracture to avoid further injury or potentiate neurovascular compromise. In most cases, three views, including anteroposterior, true lateral, and oblique, are obtained. If a fracture is unclear, it may be helpful to image the opposite extremity for comparison. The location of a fracture and its characteristics greatly influence acute-care management, as well as patient disposition, the need for consultation with orthopedics, and follow-up expectations and instructions.
Salter-Harris Fracture Classification
The physes of bones in growing children are particularly vulnerable sites of fracture since they have not yet fused. The five generally accepted types of fracture according to risk of growth disturbance are illustrated in the above Figure and are differentiated as follows:
Type I. This type of fracture is exclusive involvement of the physis itself, separating the metaphysis from the epiphysis. Since plain films may not reveal any visible fracture, the clinician should have a high index of suspicion if the physical examination elicits point tenderness over the growth plate. When in doubt if a fracture is present, always splint. Type I fractures of the physis tend to heal well, without significant consequence.
Type II. As with type I fractures, type II involve the physis, but also have a fragment of displaced metaphysis—the most common of all physeal fractures. Without significant displacement, these fractures also tend to have good outcomes.
Type III. Rather than involving the physis and metaphysis, type III fractures involve the epiphysis and therefore the joint itself. It is because of the epiphyseal displacement that these fractures tend to have a worse prognosis with joint disability and growth arrest. Thus, establishing alignment is imperative. The distal tibial Tillaux fracture is an example and requires internal fixation for optimal healing.
Type IV. Similar to a type III fracture, with the fracture extending proximally through a segment of the metaphysis, type IV fractures are treated similar to type III fractures. Due to joint involvement, an orthopedic consultation is warranted.
Type V. This type represents compression fractures of the physis. As the visibility of these fractures is poor on plain films, diagnosis can be challenging. However, history of axial compression injury may help lead the clinician to an accurate diagnosis. Since there is a high incidence of growth disturbance in type V fractures, compression affecting other areas such as the spine should also be considered.
Certainly not all pediatric fractures will involve a physis. A detailed description and management of other unique types of pediatric fractures is discussed in other articles in this feature.
Splinting Basics
Once the decision is made to apply a splint to a fracture, certain basic precautions should first be taken. Initially, any significant lacerations or abrasions should be thoroughly irrigated, cleansed, and dressed appropriately. Next, the physician should reevaluate and document both neurological status and perfusion of the area, particularly distal to the fracture site.
One commonly overlooked step in management of any fracture is pain control. It is advisable to consider administering medication prior to splinting on a case-by-case basis and for all fractures requiring reduction.
Materials and Methods
Prepackaged fiberglass splints have become a popular, efficient, and less-messy material of choice in pediatric splinting. Alternatively, plaster of Paris—although a bit more cumbersome—has some advantages, including low cost and a tendency to mold more easily to the extremity being splinted.7 When using plaster, strips should be cut a little longer than the anticipated length needed since they may shrink during curing. The unaffected limb should be used to gauge the measurement needed.
Regardless of the material chosen, all splinting should begin with the application of a stockinette tube dressing over the skin, leaving a distal opening over fingers or toes. This should be followed by a padding material (eg, Webril), beginning distally and rolling proximally, being sure to have approximately 50% overlap of each roll. Extra padding should be rolled over any bony prominence (eg, ulnar stylus) to avoid discomfort or pressure sores once the splint is applied.2
Between 8 and 10 layers of plaster (additional layers for lower extremity splints) should be wetted with room-temperature water. Hot water should never be used as this will intensify the exothermic reaction that occurs when curing and could cause burns.2 The limb should be kept in the anatomic position while the plaster is being molded to the shape of the extremity, allowing 15 to 20 minutes to dry.1 Once dry, an elastic bandage such as an Ace wrap may be placed over the entire cast to hold it secure in place. If fiberglass is used, it is helpful to squeeze out extra water before molding to the extremity. Again, an additional padding roll should be employed to avoid any discomfort or pressure beneath the splint.
In both fiberglass and plaster splinting, the edges of either type of material should not be abrasive to the skin; this can be avoided by rolling over excess padding and stockinette to create a round soft edge on either end.7 Finally, the patient should be fitted with a shoulder sling or crutches (if age appropriate) to further immobilize the injured extremity and avoid any movement or weight bearing.
Types of Splints
The type of splint depends of the location and characteristics of the fracture being immobilized. The following are a few examples of the more popular splinting techniques indicated for common pediatric fractures.
Long-Arm Posterior Splint. This splint is useful for most forearm and elbow fractures. The splint length should extend from midlength of the humerus to the palmar crease, and the width should be semicircular. In addition, an anatomic position of 90˚ flexion of the elbow should be maintained, with the hand in a neutral position and slight dorsiflexion. It is generally accepted to slightly pronate the forearm when splinting a supracondylar fracture. Orthopedics should always be consulted if the fracture involves the elbow.
Ulnar “Gutter” Splint. Useful for nondisplaced, minimally-angulated metacarpal “boxer’s fracture” or fourth and fifth phalangeal fractures, the length of the ulnar splint should extend from the distal phalanx to proximal forearm. Splint width should enclose both the volar and dorsum surfaces of the fourth and fifth metacarpals. In addition, padding should be placed between the digits for comfort. The metacarpophalangeal joints should be positioned at 70˚, and the proximal phalangeal angle at approximately 20˚ flexion2; this will help minimize the risk of contractures.
Forearm “Sugar-Tong” Splint. These splints are indicated for immobilization of a distal radius fracture or wrist injury. Distal radial fractures are by far the most common fractures encountered in the pediatric population,8 and splinting for angulation less than 15˚ is preferred.9,10 For proper stabilization, a long U-shaped splint should originate at the palmar crease, wrap around the elbow, and end at the metacarpophalangeal joint dorsally. Again, the hand should be dorsiflexed, and a soft rolled edge should be kept on the palmar crease to allow full finger flexion to near 90˚.
Thumb Spica Splint. A thumb spica splint is useful to immobilize uncomplicated fracture of the first metacarpal or proximal phalanx or when scaphoid (navicular) bone fracture is suspected. A semicircumferential molding of the radial forearm should be formed, extending to the thumbnail bed, and wrapping around the thumb. The proper hand positioning is slightly dorsiflexed, with thumb abducted slightly, as if holding a glass of water.2 If there is any doubt of a navicular fracture (rare in prepubescent children), the clinician should never hesitate to splint!
Long-Leg Posterior Splint. This type of splint is appropriate for immobilization of midshaft tibia/fibula fractures or most knee injuries. Full length of the splint should start beneath the inferior gluteal fold and extend to the ball of the foot, leaving the toes free. The ankle should be at 90˚ flexion and the knee should remain just slightly flexed, never locked straight. Orthopedics should always be consulted in cases of proximal tibia/fibula fractures or knee joint involvement.
Posterior Ankle Splint. Essentially a shorter version of a long-leg splint extending proximally to just below the knee, the posterior ankle splint is useful to immobilize ankle fractures, foot fractures, and severe ankle sprains. The distal fibula and occasional tibia physes are another common site of pediatric fractures, particularly in obese or more active children.11,12 When using either a long- or short-leg posterior ankle splint, it is helpful to hold the foot at 90˚ flexion until the material hardens or the proper angle may be lost. A recall that displaced or Salter-Harris type III or IV physeal fractures justify orthopedics consult. Nonweight-bearing, use of crutches, ice, and elevation are all important points for recovery in 3 to 6 weeks.
Lower Extremity Stirrup “Sugar-Tong” Splint. This splint is indicated for additional ankle stabilization. It runs in a U-shape (not unlike a forearm sugar-tong splint) from just below the knee around the calcaneus, and it must be wide enough to encase the ankle but not so wide that the two sides overlap when molded. It is very important to add extra padding around both malleoli and beneath the calcaneus to reduce the likelihood of pressure sores. Crutches are essential to avoid weight-bearing in patients old enough to use them. Some pediatric orthopedists advise avoiding this type of splint in the smaller, noncompliant, active child.
Complications
Although splinting has many advantages over casting in the acute-care setting, several potential complications may develop. Although rare, thermal burns to the underlying skin may occur if excessively warm or hot water is used on plaster or fiberglass due to the exothermic reaction during the hardening process. Therefore, the use of room-temperature water is always recommended. Despite the noncircumferential nature of a splint, it is still possible to develop significant swelling following splint application, which can lead to neurovascular compromise, compartment syndrome, infection, or pressure ulcers.7 The patient and caregiver should be advised to return to the ED immediately for evaluation if serious signs and symptoms such as pain, numbness, tingling, dusky color of skin, or poor capillary refill develop.
Case 1 Conclusion
The EP in this case elected to obtain plain X-rays of the patient’s left forearm, including the wrist and elbow. The results demonstrated a disruption of the cortical integrity of the distal radius, consistent with a buckle fracture. The angulation was estimated at merely 10˚. The bones of the wrist and elbow appeared normal. The EP concluded that a consult with orthopedics was not required urgently, and immobilized the patient’s arm using a fiberglass sugar-tong splint, keeping her elbow at 90˚, the forearm in a neutral position, and hand slightly dorsiflexed. A nurse assisted in keeping the child still to ensure the splint was shaped around the arm and hardened in this position. The child was provided with a sling, and supportive-care measures, including analgesia with nonsteroidal anti-inflammatory drugs as needed, ice, rest, and the importance of keeping the splint dry, were reviewed with her parents. The EP also stressed the importance of surveying for any loss of sensation or perfusion to the patient’s hand and fingers, and recommended follow up with orthopedics 1 week from discharge.
Case 2 Conclusion
Multiple views of the patient’s ankle were obtained on X-ray and showed no apparent fracture or dislocation. Additional films of the opposite ankle were obtained for comparison, but both appeared quite similar except for mild soft-tissue swelling of the affected side. Since point tenderness was reproducible over the distal fibular physis, the EP elected to place a short-leg posterior splint, maintaining good anatomic position with extra padding around the malleoli. The parents were instructed on proper elevation, ice to reduce inflammation, and the use of pain medication if needed.
One week after discharge, the treating EP received a letter from the child’s orthopedist, informing him that at the follow-up appointment, a repeat ankle film revealed periosteal changes and a type I Salter-Harris distal fibula fracture. Immobilization for an additional 3 weeks and supportive care was indicated.
Dr Del Re is an instructor of pediatrics and an intermediate care pediatrician, Rady Children’s Hospital, San Diego, California. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
Case 1
A mother presented to the ED with her 8-year-old daughter after she witnessed the child fall off her bicycle onto the sidewalk. When she fell, the girl landed onto her outstretched arms and sustained minor abrasions to her palms and knees, but did not hit her head or lose consciousness. Upon falling, the child immediately cried that her left arm hurt and kept holding it guarded near her body.
She was seated on her mother’s lap in the examination room, appearing anxious but in no acute distress. The treating EP observed the superficial abrasions from across the room and obtained a detailed history.
The patient was afebrile, and her vital signs were stable with the exception of mild tachycardia. After a couple of minutes, the EP slowly approached the child and was able to perform a basic examination. There was no obvious deformity to her left upper extremity, and only mild swelling over the wrist. She was able to move her fingers well and had excellent capillary refill distally. The child remained calm during manual palpation of the anatomic snuff box. However, she immediately pulled away and began to cry upon palpation more proximally over the distal forearm. The EP discussed his concerns with the child’s mother and explained that further evaluation was necessary.
Case 2
A mother and father presented to the ED carrying their 6-year-old boy, stating that the child had been limping since they picked him up from a neighbor’s house an hour earlier and was now refusing to walk. The father noted that a group of children had been jumping on a trampoline unsupervised, but he did not witness any injury to his son. Both parents said that the boy had been well up until that day.
At presentation, the child was afebrile and his vital signs were stable. The EP asked the parents to coax the child to walk across the room. During the walk, the boy was reluctant to bear weight on his right foot. Careful inspection of his lower limb revealed no external signs of trauma, and it appeared neurovascularly intact. Careful palpation elicited tenderness directly over the physis at the distal fibula and near the lateral malleolus. While considering the broad differential for a limping child, the physician was primarily concerned with point tenderness on examination and informed the parents that radiographic imaging was warranted.
Overview
Pediatric musculoskeletal (MSK) injury and orthopedic trauma now comprise more than 10% of visits to the ED.1 Fractures in particular are becoming more commonplace with the increasing number of children actively involved in athletic sports and high-risk activities.
The general approach to acute-care management of these children has evolved, trending more toward splinting the fractured extremity and away from traditional casting. There are many benefits to splinting. The most important is arguably the reduced risk of developing compartment syndrome due to a splint’s ability to expand with accompanied swelling.2 This article reviews the unique characteristics of pediatric bone development and initial management of pediatric fractures, as well as basic splinting techniques and unique indications that require further orthopedic consultation.
Physiological Differences in Children
The MSK system of a child differs greatly from that of an adult. The bones themselves are much more porous and malleable during childhood, making them more susceptible to traumatic injury. The growing periosteum and the developing physes are particularly vulnerable, accounting for up to 20% of pediatric fractures (see the Figure illustrating the Salter-Harris classification in the next section).3 This is particularly true at a young age, when ligamentous adherence out-performs the bony integrity itself, making fractures more common than sprains and tears. The opposite is true in adults, who are much more likely to experience sprains before succumbing to fracture. Furthermore, since the periosteum is still very active in children, the fractured bone is much more likely to remodel, lending to less deformity and overall better outcomes in most cases.3 Nonunion is extremely rare in children.
Initial Management
Approaching the Pediatric Patient
Special consideration should be given when initially approaching an injured child, so as not to cause additional undue fear or anxiety to the patient. It is helpful to take an extra moment upon entering the room to simply observe the child’s positioning, posture, or reluctance to move a particular limb. Obtaining a careful, detailed history from a distance is recommended before too quickly approaching the patient. In addition, asking the caregiver to serve as proxy during the initial physical examination may also prove helpful in localizing the pain. In the obscure case, such as the child refusing to bear weight, it is good to keep a broad differential and inspect for non-MSK injury (eg, painful hernia, testicular torsion, foreign body lodged in the bottom of the foot). Utilizing a “log-rolling” technique with the palms of one’s hands on the patient’s thigh may reveal hip pathology. Simply observing the preoccupied child walk around the unit while watching from behind may also aid in the evaluation.
However, when the injury such as an open fracture or severe displacement is obvious, immediate stabilization is critical so as not to permit any additional harm. An arm board is typically used to accomplish this. In addition, pain control should never be overlooked, either with intravenous opioids or more appropriate oral or intranasal analgesia.4,5 In cases of significant trauma, always remember ABC assessment (airway, breathing/oxygenation, and circulation), despite the eagerness to give attention to what may be an obvious fracture.
Workup
Although the use of ultrasound and other modalities is becoming more popular in some settings, it is still commonplace to begin the evaluation of a potential fracture or dislocation with plain film X-rays. Before sending a patient to radiology, always stabilize any unstable fracture to avoid further injury or potentiate neurovascular compromise. In most cases, three views, including anteroposterior, true lateral, and oblique, are obtained. If a fracture is unclear, it may be helpful to image the opposite extremity for comparison. The location of a fracture and its characteristics greatly influence acute-care management, as well as patient disposition, the need for consultation with orthopedics, and follow-up expectations and instructions.
Salter-Harris Fracture Classification
The physes of bones in growing children are particularly vulnerable sites of fracture since they have not yet fused. The five generally accepted types of fracture according to risk of growth disturbance are illustrated in the above Figure and are differentiated as follows:
Type I. This type of fracture is exclusive involvement of the physis itself, separating the metaphysis from the epiphysis. Since plain films may not reveal any visible fracture, the clinician should have a high index of suspicion if the physical examination elicits point tenderness over the growth plate. When in doubt if a fracture is present, always splint. Type I fractures of the physis tend to heal well, without significant consequence.
Type II. As with type I fractures, type II involve the physis, but also have a fragment of displaced metaphysis—the most common of all physeal fractures. Without significant displacement, these fractures also tend to have good outcomes.
Type III. Rather than involving the physis and metaphysis, type III fractures involve the epiphysis and therefore the joint itself. It is because of the epiphyseal displacement that these fractures tend to have a worse prognosis with joint disability and growth arrest. Thus, establishing alignment is imperative. The distal tibial Tillaux fracture is an example and requires internal fixation for optimal healing.
Type IV. Similar to a type III fracture, with the fracture extending proximally through a segment of the metaphysis, type IV fractures are treated similar to type III fractures. Due to joint involvement, an orthopedic consultation is warranted.
Type V. This type represents compression fractures of the physis. As the visibility of these fractures is poor on plain films, diagnosis can be challenging. However, history of axial compression injury may help lead the clinician to an accurate diagnosis. Since there is a high incidence of growth disturbance in type V fractures, compression affecting other areas such as the spine should also be considered.
Certainly not all pediatric fractures will involve a physis. A detailed description and management of other unique types of pediatric fractures is discussed in other articles in this feature.
Splinting Basics
Once the decision is made to apply a splint to a fracture, certain basic precautions should first be taken. Initially, any significant lacerations or abrasions should be thoroughly irrigated, cleansed, and dressed appropriately. Next, the physician should reevaluate and document both neurological status and perfusion of the area, particularly distal to the fracture site.
One commonly overlooked step in management of any fracture is pain control. It is advisable to consider administering medication prior to splinting on a case-by-case basis and for all fractures requiring reduction.
Materials and Methods
Prepackaged fiberglass splints have become a popular, efficient, and less-messy material of choice in pediatric splinting. Alternatively, plaster of Paris—although a bit more cumbersome—has some advantages, including low cost and a tendency to mold more easily to the extremity being splinted.7 When using plaster, strips should be cut a little longer than the anticipated length needed since they may shrink during curing. The unaffected limb should be used to gauge the measurement needed.
Regardless of the material chosen, all splinting should begin with the application of a stockinette tube dressing over the skin, leaving a distal opening over fingers or toes. This should be followed by a padding material (eg, Webril), beginning distally and rolling proximally, being sure to have approximately 50% overlap of each roll. Extra padding should be rolled over any bony prominence (eg, ulnar stylus) to avoid discomfort or pressure sores once the splint is applied.2
Between 8 and 10 layers of plaster (additional layers for lower extremity splints) should be wetted with room-temperature water. Hot water should never be used as this will intensify the exothermic reaction that occurs when curing and could cause burns.2 The limb should be kept in the anatomic position while the plaster is being molded to the shape of the extremity, allowing 15 to 20 minutes to dry.1 Once dry, an elastic bandage such as an Ace wrap may be placed over the entire cast to hold it secure in place. If fiberglass is used, it is helpful to squeeze out extra water before molding to the extremity. Again, an additional padding roll should be employed to avoid any discomfort or pressure beneath the splint.
In both fiberglass and plaster splinting, the edges of either type of material should not be abrasive to the skin; this can be avoided by rolling over excess padding and stockinette to create a round soft edge on either end.7 Finally, the patient should be fitted with a shoulder sling or crutches (if age appropriate) to further immobilize the injured extremity and avoid any movement or weight bearing.
Types of Splints
The type of splint depends of the location and characteristics of the fracture being immobilized. The following are a few examples of the more popular splinting techniques indicated for common pediatric fractures.
Long-Arm Posterior Splint. This splint is useful for most forearm and elbow fractures. The splint length should extend from midlength of the humerus to the palmar crease, and the width should be semicircular. In addition, an anatomic position of 90˚ flexion of the elbow should be maintained, with the hand in a neutral position and slight dorsiflexion. It is generally accepted to slightly pronate the forearm when splinting a supracondylar fracture. Orthopedics should always be consulted if the fracture involves the elbow.
Ulnar “Gutter” Splint. Useful for nondisplaced, minimally-angulated metacarpal “boxer’s fracture” or fourth and fifth phalangeal fractures, the length of the ulnar splint should extend from the distal phalanx to proximal forearm. Splint width should enclose both the volar and dorsum surfaces of the fourth and fifth metacarpals. In addition, padding should be placed between the digits for comfort. The metacarpophalangeal joints should be positioned at 70˚, and the proximal phalangeal angle at approximately 20˚ flexion2; this will help minimize the risk of contractures.
Forearm “Sugar-Tong” Splint. These splints are indicated for immobilization of a distal radius fracture or wrist injury. Distal radial fractures are by far the most common fractures encountered in the pediatric population,8 and splinting for angulation less than 15˚ is preferred.9,10 For proper stabilization, a long U-shaped splint should originate at the palmar crease, wrap around the elbow, and end at the metacarpophalangeal joint dorsally. Again, the hand should be dorsiflexed, and a soft rolled edge should be kept on the palmar crease to allow full finger flexion to near 90˚.
Thumb Spica Splint. A thumb spica splint is useful to immobilize uncomplicated fracture of the first metacarpal or proximal phalanx or when scaphoid (navicular) bone fracture is suspected. A semicircumferential molding of the radial forearm should be formed, extending to the thumbnail bed, and wrapping around the thumb. The proper hand positioning is slightly dorsiflexed, with thumb abducted slightly, as if holding a glass of water.2 If there is any doubt of a navicular fracture (rare in prepubescent children), the clinician should never hesitate to splint!
Long-Leg Posterior Splint. This type of splint is appropriate for immobilization of midshaft tibia/fibula fractures or most knee injuries. Full length of the splint should start beneath the inferior gluteal fold and extend to the ball of the foot, leaving the toes free. The ankle should be at 90˚ flexion and the knee should remain just slightly flexed, never locked straight. Orthopedics should always be consulted in cases of proximal tibia/fibula fractures or knee joint involvement.
Posterior Ankle Splint. Essentially a shorter version of a long-leg splint extending proximally to just below the knee, the posterior ankle splint is useful to immobilize ankle fractures, foot fractures, and severe ankle sprains. The distal fibula and occasional tibia physes are another common site of pediatric fractures, particularly in obese or more active children.11,12 When using either a long- or short-leg posterior ankle splint, it is helpful to hold the foot at 90˚ flexion until the material hardens or the proper angle may be lost. A recall that displaced or Salter-Harris type III or IV physeal fractures justify orthopedics consult. Nonweight-bearing, use of crutches, ice, and elevation are all important points for recovery in 3 to 6 weeks.
Lower Extremity Stirrup “Sugar-Tong” Splint. This splint is indicated for additional ankle stabilization. It runs in a U-shape (not unlike a forearm sugar-tong splint) from just below the knee around the calcaneus, and it must be wide enough to encase the ankle but not so wide that the two sides overlap when molded. It is very important to add extra padding around both malleoli and beneath the calcaneus to reduce the likelihood of pressure sores. Crutches are essential to avoid weight-bearing in patients old enough to use them. Some pediatric orthopedists advise avoiding this type of splint in the smaller, noncompliant, active child.
Complications
Although splinting has many advantages over casting in the acute-care setting, several potential complications may develop. Although rare, thermal burns to the underlying skin may occur if excessively warm or hot water is used on plaster or fiberglass due to the exothermic reaction during the hardening process. Therefore, the use of room-temperature water is always recommended. Despite the noncircumferential nature of a splint, it is still possible to develop significant swelling following splint application, which can lead to neurovascular compromise, compartment syndrome, infection, or pressure ulcers.7 The patient and caregiver should be advised to return to the ED immediately for evaluation if serious signs and symptoms such as pain, numbness, tingling, dusky color of skin, or poor capillary refill develop.
Case 1 Conclusion
The EP in this case elected to obtain plain X-rays of the patient’s left forearm, including the wrist and elbow. The results demonstrated a disruption of the cortical integrity of the distal radius, consistent with a buckle fracture. The angulation was estimated at merely 10˚. The bones of the wrist and elbow appeared normal. The EP concluded that a consult with orthopedics was not required urgently, and immobilized the patient’s arm using a fiberglass sugar-tong splint, keeping her elbow at 90˚, the forearm in a neutral position, and hand slightly dorsiflexed. A nurse assisted in keeping the child still to ensure the splint was shaped around the arm and hardened in this position. The child was provided with a sling, and supportive-care measures, including analgesia with nonsteroidal anti-inflammatory drugs as needed, ice, rest, and the importance of keeping the splint dry, were reviewed with her parents. The EP also stressed the importance of surveying for any loss of sensation or perfusion to the patient’s hand and fingers, and recommended follow up with orthopedics 1 week from discharge.
Case 2 Conclusion
Multiple views of the patient’s ankle were obtained on X-ray and showed no apparent fracture or dislocation. Additional films of the opposite ankle were obtained for comparison, but both appeared quite similar except for mild soft-tissue swelling of the affected side. Since point tenderness was reproducible over the distal fibular physis, the EP elected to place a short-leg posterior splint, maintaining good anatomic position with extra padding around the malleoli. The parents were instructed on proper elevation, ice to reduce inflammation, and the use of pain medication if needed.
One week after discharge, the treating EP received a letter from the child’s orthopedist, informing him that at the follow-up appointment, a repeat ankle film revealed periosteal changes and a type I Salter-Harris distal fibula fracture. Immobilization for an additional 3 weeks and supportive care was indicated.
Dr Del Re is an instructor of pediatrics and an intermediate care pediatrician, Rady Children’s Hospital, San Diego, California. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Bachman D, Santora S. Musculoskeletal trauma. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:1335-1375.
- Klig JE. Splinting procedures. In: King C, Henretig FM, eds. Texbook of Pediatric Emergency Procedures. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:919-931.
- Wilkins KE. The incidence of fractures in children. In: Rockwood CA, Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia, PA: Lippincott-Raven; 1996:3-17.
- Mahar PJ, Rana JA, Kennedy CS, Christopher NC. A randomized clinical trial of oral transmucosal fentanyl citrate versus intravenous morphine sulfate for initial control of pain in children with extremity injuries. Pediatr Emerg Care. 2007;23(8):544-548.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155-1161.
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
- Boyd AS, Benjamin HJ, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-22.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33(6):503-505.
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. CMAJ. 2010;182(14):1507-1512.
- Firmin F, Crouch R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): a literature review. Int Emerg Nurs. 2009;17(3):173-178.
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14(4):423-430.
- Blackburn EW, Aronsson DD, Rubright JH, Lisle JW. Ankle fractures in children. J Bone Joint Surg Am. 2012; 94(13):1234-1244.
- Bachman D, Santora S. Musculoskeletal trauma. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:1335-1375.
- Klig JE. Splinting procedures. In: King C, Henretig FM, eds. Texbook of Pediatric Emergency Procedures. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:919-931.
- Wilkins KE. The incidence of fractures in children. In: Rockwood CA, Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia, PA: Lippincott-Raven; 1996:3-17.
- Mahar PJ, Rana JA, Kennedy CS, Christopher NC. A randomized clinical trial of oral transmucosal fentanyl citrate versus intravenous morphine sulfate for initial control of pain in children with extremity injuries. Pediatr Emerg Care. 2007;23(8):544-548.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155-1161.
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
- Boyd AS, Benjamin HJ, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-22.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33(6):503-505.
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. CMAJ. 2010;182(14):1507-1512.
- Firmin F, Crouch R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): a literature review. Int Emerg Nurs. 2009;17(3):173-178.
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14(4):423-430.
- Blackburn EW, Aronsson DD, Rubright JH, Lisle JW. Ankle fractures in children. J Bone Joint Surg Am. 2012; 94(13):1234-1244.
Pediatric Orthopedic Injuries
| Pediatric orthopedic injuries are a common presentation to the ED, representing 12% of pediatric visits.1 In this special feature, our authors focus on the challenge of evaluating and managing the pediatric orthopedic patient and spotlight conditions where the emergency physician (EP) might not have significant clinical experience. |
While many pediatric orthopedic injuries are the simple “bruises and bumps” of active childhood and need little more than pain control and parental education, there are some age-specific injuries that require truly emergent care in order to salvage an extremity or prevent loss of function. Differentiation between the urgent and emergent patient may not be obvious in the preverbal child or in a child whose radiographs show open growth plates and ossification centers obscuring interpretation.
This educational series begins by covering the physiological differences in pediatric musculoskeletal injuries and reviews both the general approach to examination and some pediatric splinting basics. With an enhanced awareness of the structural differences between growing and mature bones (along with their tendons and ligaments), subtle age-related injuries are less likely to be missed.
With the basics firmly in hand, we turn your attention to several common orthopedic injuries unique to children and review acute-care management. The third article deals with the frustrating diagnostic dilemma of “my child won’t walk” and explores some nontraumatic pediatric orthopedic presentations. Our series concludes with a review of some “high risk/can’t miss” pediatric injury patterns and presenting symptoms and also reminds us of injuries that might be suggestive of nonaccidental trauma as the underlying etiology.
While pediatric bones are indeed not simply little adult bones, EPs need not be intimidated in caring for these patients. A basic understanding of pediatric musculoskeletal physiology and an enhanced clinical awareness as to common injury patterns will equip most EPs with the knowledge necessary to ensure the best possible outcome for these children.
Reference
1. Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the pediatric risk of admission (PRISA) score using a multi-institutional sample. Ann Emerg Med. 2004;43(4):461-486.
| Pediatric orthopedic injuries are a common presentation to the ED, representing 12% of pediatric visits.1 In this special feature, our authors focus on the challenge of evaluating and managing the pediatric orthopedic patient and spotlight conditions where the emergency physician (EP) might not have significant clinical experience. |
While many pediatric orthopedic injuries are the simple “bruises and bumps” of active childhood and need little more than pain control and parental education, there are some age-specific injuries that require truly emergent care in order to salvage an extremity or prevent loss of function. Differentiation between the urgent and emergent patient may not be obvious in the preverbal child or in a child whose radiographs show open growth plates and ossification centers obscuring interpretation.
This educational series begins by covering the physiological differences in pediatric musculoskeletal injuries and reviews both the general approach to examination and some pediatric splinting basics. With an enhanced awareness of the structural differences between growing and mature bones (along with their tendons and ligaments), subtle age-related injuries are less likely to be missed.
With the basics firmly in hand, we turn your attention to several common orthopedic injuries unique to children and review acute-care management. The third article deals with the frustrating diagnostic dilemma of “my child won’t walk” and explores some nontraumatic pediatric orthopedic presentations. Our series concludes with a review of some “high risk/can’t miss” pediatric injury patterns and presenting symptoms and also reminds us of injuries that might be suggestive of nonaccidental trauma as the underlying etiology.
While pediatric bones are indeed not simply little adult bones, EPs need not be intimidated in caring for these patients. A basic understanding of pediatric musculoskeletal physiology and an enhanced clinical awareness as to common injury patterns will equip most EPs with the knowledge necessary to ensure the best possible outcome for these children.
| Pediatric orthopedic injuries are a common presentation to the ED, representing 12% of pediatric visits.1 In this special feature, our authors focus on the challenge of evaluating and managing the pediatric orthopedic patient and spotlight conditions where the emergency physician (EP) might not have significant clinical experience. |
While many pediatric orthopedic injuries are the simple “bruises and bumps” of active childhood and need little more than pain control and parental education, there are some age-specific injuries that require truly emergent care in order to salvage an extremity or prevent loss of function. Differentiation between the urgent and emergent patient may not be obvious in the preverbal child or in a child whose radiographs show open growth plates and ossification centers obscuring interpretation.
This educational series begins by covering the physiological differences in pediatric musculoskeletal injuries and reviews both the general approach to examination and some pediatric splinting basics. With an enhanced awareness of the structural differences between growing and mature bones (along with their tendons and ligaments), subtle age-related injuries are less likely to be missed.
With the basics firmly in hand, we turn your attention to several common orthopedic injuries unique to children and review acute-care management. The third article deals with the frustrating diagnostic dilemma of “my child won’t walk” and explores some nontraumatic pediatric orthopedic presentations. Our series concludes with a review of some “high risk/can’t miss” pediatric injury patterns and presenting symptoms and also reminds us of injuries that might be suggestive of nonaccidental trauma as the underlying etiology.
While pediatric bones are indeed not simply little adult bones, EPs need not be intimidated in caring for these patients. A basic understanding of pediatric musculoskeletal physiology and an enhanced clinical awareness as to common injury patterns will equip most EPs with the knowledge necessary to ensure the best possible outcome for these children.
Reference
1. Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the pediatric risk of admission (PRISA) score using a multi-institutional sample. Ann Emerg Med. 2004;43(4):461-486.
Reference
1. Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the pediatric risk of admission (PRISA) score using a multi-institutional sample. Ann Emerg Med. 2004;43(4):461-486.
DEA moves hydrocodone combination products to schedule II
The Drug Enforcement Administration is making it harder to prescribe hydrocodone combination products.
The move was expected, as the agency proposed in February to move hydrocodone combinations from schedule III to schedule II in response to requests from both the U.S. Department of Health & Human Services and the Food and Drug Administration.
Some physician groups have opposed the move, saying that it will lead to more administrative burdens, do nothing to curb abuse and diversion, and potentially decrease access to medications.
The DEA will publish the final rule on the rescheduling in the Federal Register on Aug. 22. Manufacturers, distributors, and prescribers will have to comply by Oct. 13.
The agency said it is time to rein in opioid prescribing and that rescheduling will help accomplish that goal.
"Almost 7 million Americans abuse controlled-substance prescription medications, including opioid painkillers, resulting in more deaths from prescription drug overdoses than auto accidents," DEA administrator Michele Leonhart said in a statement. "Today’s action recognizes that these products are some of the most addictive and potentially dangerous prescription medications available."
Hydrocodone combination products were placed on schedule III by Congress in 1970 when it created the Controlled Substances Act, in part because it was believed that adding acetaminophen or other non-narcotics might lessen the abuse potential. Hydrocodone itself was placed on schedule II.
Now, "the scientific, medical, and epidemiological data are robust and support rescheduling [of hydrocodone combination products] into schedule II," according to the final rule.
Data show that the products are widely diverted and abused at rates similar to that of oxycodone products, which are schedule II, said the agency, which added that abuse is associated with severe psychological or physical dependence, and many are being admitted to addiction treatment.
The hydrocodone combinations are also associated with large numbers of deaths, said the agency. More than 16,000 deaths in 2010 were due to abuse of opioids, including hydrocodone combinations, according to the DEA.
About 137 million prescriptions for hydrocodone combinations were dispensed in 2013, the agency said. The most frequently prescribed combination is hydrocodone/acetaminophen.
In comments to the proposed rule in April, the American Medical Association, along with a group of organizations and companies in the long-term care field, asked the agency to delay the final rule until an exception was made for nursing homes and other long-term care facilities. The AMA’s House of Delegates had also voted in 2013 to oppose rescheduling.
The American College of Emergency Physicians also urged against rescheduling, telling the DEA in April that it would not likely solve the abuse and diversion problems, but would lead to a greater administrative hassle for physicians.
The DEA said it received 573 comments after it proposed rescheduling, with 52% in favor, 41% opposed, and 7% taking no position. The agency received the most comments from the general public (44%; 250 comments) and pharmacists and pharmacy students (21%; 122 comments), physicians (13%; 73 comments), patients (6%; 35 comments), and midlevel practitioners (5%; 31 comments). Just over half of the physician comments supported, or supported with qualification, rescheduling.
Most of the commenters who were opposed to rescheduling were pharmacists, pharmacy students, and patients. Those opposed were concerned about how it would affect prescribing practices and patient access to medicine, and how it might impact long-term care facilities. Commenters also said that it would not prevent abuse or diversion.
The DEA said that although moving to schedule II does, for instance, prohibit refills, it would not necessarily block physicians from writing prescriptions for supplies of longer than 30 days, or from writing multiple prescriptions at once. State laws might have limits, however, and those will take precedence over the DEA rule.
The DEA rescheduling follows an FDA advisory committee recommendation in Jan. 2013 to do so, and the FDA’s backing of the proposal in Oct. 2013. HHS followed with its own recommendation to the DEA.
In comments on the proposed rule, manufacturers and pharmacies asked for more time to implement the rescheduling, but the DEA said no, citing high rates of abuse, overdose, and deaths relating to hydrocodone combination products.
The rescheduling goes into effect on Oct. 13, 45 days from the date of the rule’s publication in the Federal Register.
On Twitter @aliciaault
The Drug Enforcement Administration is making it harder to prescribe hydrocodone combination products.
The move was expected, as the agency proposed in February to move hydrocodone combinations from schedule III to schedule II in response to requests from both the U.S. Department of Health & Human Services and the Food and Drug Administration.
Some physician groups have opposed the move, saying that it will lead to more administrative burdens, do nothing to curb abuse and diversion, and potentially decrease access to medications.
The DEA will publish the final rule on the rescheduling in the Federal Register on Aug. 22. Manufacturers, distributors, and prescribers will have to comply by Oct. 13.
The agency said it is time to rein in opioid prescribing and that rescheduling will help accomplish that goal.
"Almost 7 million Americans abuse controlled-substance prescription medications, including opioid painkillers, resulting in more deaths from prescription drug overdoses than auto accidents," DEA administrator Michele Leonhart said in a statement. "Today’s action recognizes that these products are some of the most addictive and potentially dangerous prescription medications available."
Hydrocodone combination products were placed on schedule III by Congress in 1970 when it created the Controlled Substances Act, in part because it was believed that adding acetaminophen or other non-narcotics might lessen the abuse potential. Hydrocodone itself was placed on schedule II.
Now, "the scientific, medical, and epidemiological data are robust and support rescheduling [of hydrocodone combination products] into schedule II," according to the final rule.
Data show that the products are widely diverted and abused at rates similar to that of oxycodone products, which are schedule II, said the agency, which added that abuse is associated with severe psychological or physical dependence, and many are being admitted to addiction treatment.
The hydrocodone combinations are also associated with large numbers of deaths, said the agency. More than 16,000 deaths in 2010 were due to abuse of opioids, including hydrocodone combinations, according to the DEA.
About 137 million prescriptions for hydrocodone combinations were dispensed in 2013, the agency said. The most frequently prescribed combination is hydrocodone/acetaminophen.
In comments to the proposed rule in April, the American Medical Association, along with a group of organizations and companies in the long-term care field, asked the agency to delay the final rule until an exception was made for nursing homes and other long-term care facilities. The AMA’s House of Delegates had also voted in 2013 to oppose rescheduling.
The American College of Emergency Physicians also urged against rescheduling, telling the DEA in April that it would not likely solve the abuse and diversion problems, but would lead to a greater administrative hassle for physicians.
The DEA said it received 573 comments after it proposed rescheduling, with 52% in favor, 41% opposed, and 7% taking no position. The agency received the most comments from the general public (44%; 250 comments) and pharmacists and pharmacy students (21%; 122 comments), physicians (13%; 73 comments), patients (6%; 35 comments), and midlevel practitioners (5%; 31 comments). Just over half of the physician comments supported, or supported with qualification, rescheduling.
Most of the commenters who were opposed to rescheduling were pharmacists, pharmacy students, and patients. Those opposed were concerned about how it would affect prescribing practices and patient access to medicine, and how it might impact long-term care facilities. Commenters also said that it would not prevent abuse or diversion.
The DEA said that although moving to schedule II does, for instance, prohibit refills, it would not necessarily block physicians from writing prescriptions for supplies of longer than 30 days, or from writing multiple prescriptions at once. State laws might have limits, however, and those will take precedence over the DEA rule.
The DEA rescheduling follows an FDA advisory committee recommendation in Jan. 2013 to do so, and the FDA’s backing of the proposal in Oct. 2013. HHS followed with its own recommendation to the DEA.
In comments on the proposed rule, manufacturers and pharmacies asked for more time to implement the rescheduling, but the DEA said no, citing high rates of abuse, overdose, and deaths relating to hydrocodone combination products.
The rescheduling goes into effect on Oct. 13, 45 days from the date of the rule’s publication in the Federal Register.
On Twitter @aliciaault
The Drug Enforcement Administration is making it harder to prescribe hydrocodone combination products.
The move was expected, as the agency proposed in February to move hydrocodone combinations from schedule III to schedule II in response to requests from both the U.S. Department of Health & Human Services and the Food and Drug Administration.
Some physician groups have opposed the move, saying that it will lead to more administrative burdens, do nothing to curb abuse and diversion, and potentially decrease access to medications.
The DEA will publish the final rule on the rescheduling in the Federal Register on Aug. 22. Manufacturers, distributors, and prescribers will have to comply by Oct. 13.
The agency said it is time to rein in opioid prescribing and that rescheduling will help accomplish that goal.
"Almost 7 million Americans abuse controlled-substance prescription medications, including opioid painkillers, resulting in more deaths from prescription drug overdoses than auto accidents," DEA administrator Michele Leonhart said in a statement. "Today’s action recognizes that these products are some of the most addictive and potentially dangerous prescription medications available."
Hydrocodone combination products were placed on schedule III by Congress in 1970 when it created the Controlled Substances Act, in part because it was believed that adding acetaminophen or other non-narcotics might lessen the abuse potential. Hydrocodone itself was placed on schedule II.
Now, "the scientific, medical, and epidemiological data are robust and support rescheduling [of hydrocodone combination products] into schedule II," according to the final rule.
Data show that the products are widely diverted and abused at rates similar to that of oxycodone products, which are schedule II, said the agency, which added that abuse is associated with severe psychological or physical dependence, and many are being admitted to addiction treatment.
The hydrocodone combinations are also associated with large numbers of deaths, said the agency. More than 16,000 deaths in 2010 were due to abuse of opioids, including hydrocodone combinations, according to the DEA.
About 137 million prescriptions for hydrocodone combinations were dispensed in 2013, the agency said. The most frequently prescribed combination is hydrocodone/acetaminophen.
In comments to the proposed rule in April, the American Medical Association, along with a group of organizations and companies in the long-term care field, asked the agency to delay the final rule until an exception was made for nursing homes and other long-term care facilities. The AMA’s House of Delegates had also voted in 2013 to oppose rescheduling.
The American College of Emergency Physicians also urged against rescheduling, telling the DEA in April that it would not likely solve the abuse and diversion problems, but would lead to a greater administrative hassle for physicians.
The DEA said it received 573 comments after it proposed rescheduling, with 52% in favor, 41% opposed, and 7% taking no position. The agency received the most comments from the general public (44%; 250 comments) and pharmacists and pharmacy students (21%; 122 comments), physicians (13%; 73 comments), patients (6%; 35 comments), and midlevel practitioners (5%; 31 comments). Just over half of the physician comments supported, or supported with qualification, rescheduling.
Most of the commenters who were opposed to rescheduling were pharmacists, pharmacy students, and patients. Those opposed were concerned about how it would affect prescribing practices and patient access to medicine, and how it might impact long-term care facilities. Commenters also said that it would not prevent abuse or diversion.
The DEA said that although moving to schedule II does, for instance, prohibit refills, it would not necessarily block physicians from writing prescriptions for supplies of longer than 30 days, or from writing multiple prescriptions at once. State laws might have limits, however, and those will take precedence over the DEA rule.
The DEA rescheduling follows an FDA advisory committee recommendation in Jan. 2013 to do so, and the FDA’s backing of the proposal in Oct. 2013. HHS followed with its own recommendation to the DEA.
In comments on the proposed rule, manufacturers and pharmacies asked for more time to implement the rescheduling, but the DEA said no, citing high rates of abuse, overdose, and deaths relating to hydrocodone combination products.
The rescheduling goes into effect on Oct. 13, 45 days from the date of the rule’s publication in the Federal Register.
On Twitter @aliciaault
Study Outlines Risk Factors for ACL Re-Injury
SEATTLE—Identification and patient education regarding modifiable risk factors may minimize the chance of a future anterior cruciate ligament (ACL) tear, according to research presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine. “Our research suggests that a few risk factors such as, age, activity level and type of graft utilized may point to the possibility of re-injury,” said lead author, Christopher C. Kaeding, MD, of the Ohio State University in Columbus, and his research colleagues.
The researchers analyzed data from 2,695 patients through the MOON ACL database from 2002 to 2008. Subjects who had a primary ACL reconstruction with no history of contralateral knee surgery and had 2-year follow-up data were included. Subjects who had multi-ligament surgery were excluded.
Graft type, age, Marx score at time of index surgery, sport played post–ACL reconstruction, sex, smoking status, lateral meniscus tear at the time of ACL reconstruction, medial meniscus tear at the time of ACL reconstruction, body mass index (BMI), and MOON site were evaluated to determine their contribution to both ipsilateral retear and contralateral ACL tear.
The analysis was repeated using the 2002 to 2003 and 2007 to 2008 cohort and included age, graft, sex, and Marx. An analysis of variance with post-hoc analysis was performed to detect significant differences in age and Marx score by graft type over time.
Study findings also indicate:
• There were 165/2801 (5.89%) ipsilateral and 177/2801 (6.32%) contralateral ACL tears identified in the cohort at the two-year follow-up.
• The odds of ipsilateral retear are 1.68 times greater for hamstring autograft (P = 0.04) and 4.67 times greater for an allograft (P < 0.001) compared to auto-BTB.
• The odds of ipsilateral retear decrease by 8% for every yearly increase in age (P < 0.001) and increases by 6% for every increased point on the Marx score (P = 0.017).
• The odds of contralateral ACL tear increase by 7% for every increased point on the Marx score (P = 0.004) and decreases by 5% for every one-point increase in BMI (P = 0.03).
• In 2002 to 2003, there were 61 out of 815 (7.5%) retears compared to 37 out of 1056 (3.5%) in 2007 and 2008.
“The study highlights that younger age, higher activity levels at time of injury, and what type of graft used (allograft) may increase risk of same side ACL injury within two years. With individuals having higher activity levels and lower age, retears on the opposite leg were more prominent,” said Dr. Kaeding. “Physicians and physical therapists need to better educate their patients about continued neuromuscular training even after the immediate rehabilitation process has ended to help prevent future tears.”
SEATTLE—Identification and patient education regarding modifiable risk factors may minimize the chance of a future anterior cruciate ligament (ACL) tear, according to research presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine. “Our research suggests that a few risk factors such as, age, activity level and type of graft utilized may point to the possibility of re-injury,” said lead author, Christopher C. Kaeding, MD, of the Ohio State University in Columbus, and his research colleagues.
The researchers analyzed data from 2,695 patients through the MOON ACL database from 2002 to 2008. Subjects who had a primary ACL reconstruction with no history of contralateral knee surgery and had 2-year follow-up data were included. Subjects who had multi-ligament surgery were excluded.
Graft type, age, Marx score at time of index surgery, sport played post–ACL reconstruction, sex, smoking status, lateral meniscus tear at the time of ACL reconstruction, medial meniscus tear at the time of ACL reconstruction, body mass index (BMI), and MOON site were evaluated to determine their contribution to both ipsilateral retear and contralateral ACL tear.
The analysis was repeated using the 2002 to 2003 and 2007 to 2008 cohort and included age, graft, sex, and Marx. An analysis of variance with post-hoc analysis was performed to detect significant differences in age and Marx score by graft type over time.
Study findings also indicate:
• There were 165/2801 (5.89%) ipsilateral and 177/2801 (6.32%) contralateral ACL tears identified in the cohort at the two-year follow-up.
• The odds of ipsilateral retear are 1.68 times greater for hamstring autograft (P = 0.04) and 4.67 times greater for an allograft (P < 0.001) compared to auto-BTB.
• The odds of ipsilateral retear decrease by 8% for every yearly increase in age (P < 0.001) and increases by 6% for every increased point on the Marx score (P = 0.017).
• The odds of contralateral ACL tear increase by 7% for every increased point on the Marx score (P = 0.004) and decreases by 5% for every one-point increase in BMI (P = 0.03).
• In 2002 to 2003, there were 61 out of 815 (7.5%) retears compared to 37 out of 1056 (3.5%) in 2007 and 2008.
“The study highlights that younger age, higher activity levels at time of injury, and what type of graft used (allograft) may increase risk of same side ACL injury within two years. With individuals having higher activity levels and lower age, retears on the opposite leg were more prominent,” said Dr. Kaeding. “Physicians and physical therapists need to better educate their patients about continued neuromuscular training even after the immediate rehabilitation process has ended to help prevent future tears.”
SEATTLE—Identification and patient education regarding modifiable risk factors may minimize the chance of a future anterior cruciate ligament (ACL) tear, according to research presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine. “Our research suggests that a few risk factors such as, age, activity level and type of graft utilized may point to the possibility of re-injury,” said lead author, Christopher C. Kaeding, MD, of the Ohio State University in Columbus, and his research colleagues.
The researchers analyzed data from 2,695 patients through the MOON ACL database from 2002 to 2008. Subjects who had a primary ACL reconstruction with no history of contralateral knee surgery and had 2-year follow-up data were included. Subjects who had multi-ligament surgery were excluded.
Graft type, age, Marx score at time of index surgery, sport played post–ACL reconstruction, sex, smoking status, lateral meniscus tear at the time of ACL reconstruction, medial meniscus tear at the time of ACL reconstruction, body mass index (BMI), and MOON site were evaluated to determine their contribution to both ipsilateral retear and contralateral ACL tear.
The analysis was repeated using the 2002 to 2003 and 2007 to 2008 cohort and included age, graft, sex, and Marx. An analysis of variance with post-hoc analysis was performed to detect significant differences in age and Marx score by graft type over time.
Study findings also indicate:
• There were 165/2801 (5.89%) ipsilateral and 177/2801 (6.32%) contralateral ACL tears identified in the cohort at the two-year follow-up.
• The odds of ipsilateral retear are 1.68 times greater for hamstring autograft (P = 0.04) and 4.67 times greater for an allograft (P < 0.001) compared to auto-BTB.
• The odds of ipsilateral retear decrease by 8% for every yearly increase in age (P < 0.001) and increases by 6% for every increased point on the Marx score (P = 0.017).
• The odds of contralateral ACL tear increase by 7% for every increased point on the Marx score (P = 0.004) and decreases by 5% for every one-point increase in BMI (P = 0.03).
• In 2002 to 2003, there were 61 out of 815 (7.5%) retears compared to 37 out of 1056 (3.5%) in 2007 and 2008.
“The study highlights that younger age, higher activity levels at time of injury, and what type of graft used (allograft) may increase risk of same side ACL injury within two years. With individuals having higher activity levels and lower age, retears on the opposite leg were more prominent,” said Dr. Kaeding. “Physicians and physical therapists need to better educate their patients about continued neuromuscular training even after the immediate rehabilitation process has ended to help prevent future tears.”
Genetic Factors Linked to Slow Concussion Recovery in Athletes
SEATTLE—An investigation into the association of the (GT)n variable nucleotide tandem repeats (VNTR) within the GRIN2A gene and concussion recovery found that athletes carrying the long allele genotype are predisposed to prolonged recovery following a concussive injury. The findings were presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine.
“We identified that patients with a long allele in the (GT)n genotype were four times more likely to have a prolonged concussion recovery,” said lead author Jane McDevitt, PhD, from Temple University in Philadelphia, Pennsylvania, and her research colleagues.
Fifty-one athletes with a diagnosed concussion from a hospital concussion program completed a standardized initial evaluation. Concussion injury characteristics and acute signs and symptoms were assessed, followed by an objective screening that included vestibular ocular assessments, the BESS test, and an ImPACT exam.
Participants provided salivary samples for isolation of DNA. The number of (GT) VNTR within the promoter region of GRIN2A was genotyped. The long (L) allele was defined as an allele with 25 or more dinucleotide repeats in the GT tract. The short (S) allele was defined as an allele with < 25 dinucleotide repeats in the GT tract.
Based on the results of genetic analysis, participants were genotyped as LL homozygotes, SS homozygotes, or LS heterozygotes. Participants’ concussion recovery time was followed prospectively until the full return to play clearance date determined by the treating physician.
Participant’s recovery time was categorized as normal (≤ 20 days) or prolonged (> 20 days). The DNA region surrounding position (-975 to -776) in the promoter of GRIN2A was amplified by PCR, and was analyzed by capillary electrophoresis. Fragment length polymorphism analysis was performed by measuring the migration time of a PCR product, and extrapolation to the known fragments in the DNA standard ladder using computer software. The number of GT dinucleotide repeats was calculated using the following equation: n(GT)=(L -167)/2, where L is the length of the PCR fragment estimated in base pairs.
Results indicated there was a significant association between the GT VNTR (recessive model: LL versus SS + LS) and recovery, where the chance of prolonged recovery was 4.3 times greater for homozygous carriers of the long allele.
“Making the genetic connection in this data is an exciting step for concussion injury research,” said Dr. McDevitt. “Knowing this information could help improve monitoring and management of athletes who experience concussion, and may also aid in the development of genetic counseling in athletes exposed to concussive head impacts.”
SEATTLE—An investigation into the association of the (GT)n variable nucleotide tandem repeats (VNTR) within the GRIN2A gene and concussion recovery found that athletes carrying the long allele genotype are predisposed to prolonged recovery following a concussive injury. The findings were presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine.
“We identified that patients with a long allele in the (GT)n genotype were four times more likely to have a prolonged concussion recovery,” said lead author Jane McDevitt, PhD, from Temple University in Philadelphia, Pennsylvania, and her research colleagues.
Fifty-one athletes with a diagnosed concussion from a hospital concussion program completed a standardized initial evaluation. Concussion injury characteristics and acute signs and symptoms were assessed, followed by an objective screening that included vestibular ocular assessments, the BESS test, and an ImPACT exam.
Participants provided salivary samples for isolation of DNA. The number of (GT) VNTR within the promoter region of GRIN2A was genotyped. The long (L) allele was defined as an allele with 25 or more dinucleotide repeats in the GT tract. The short (S) allele was defined as an allele with < 25 dinucleotide repeats in the GT tract.
Based on the results of genetic analysis, participants were genotyped as LL homozygotes, SS homozygotes, or LS heterozygotes. Participants’ concussion recovery time was followed prospectively until the full return to play clearance date determined by the treating physician.
Participant’s recovery time was categorized as normal (≤ 20 days) or prolonged (> 20 days). The DNA region surrounding position (-975 to -776) in the promoter of GRIN2A was amplified by PCR, and was analyzed by capillary electrophoresis. Fragment length polymorphism analysis was performed by measuring the migration time of a PCR product, and extrapolation to the known fragments in the DNA standard ladder using computer software. The number of GT dinucleotide repeats was calculated using the following equation: n(GT)=(L -167)/2, where L is the length of the PCR fragment estimated in base pairs.
Results indicated there was a significant association between the GT VNTR (recessive model: LL versus SS + LS) and recovery, where the chance of prolonged recovery was 4.3 times greater for homozygous carriers of the long allele.
“Making the genetic connection in this data is an exciting step for concussion injury research,” said Dr. McDevitt. “Knowing this information could help improve monitoring and management of athletes who experience concussion, and may also aid in the development of genetic counseling in athletes exposed to concussive head impacts.”
SEATTLE—An investigation into the association of the (GT)n variable nucleotide tandem repeats (VNTR) within the GRIN2A gene and concussion recovery found that athletes carrying the long allele genotype are predisposed to prolonged recovery following a concussive injury. The findings were presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine.
“We identified that patients with a long allele in the (GT)n genotype were four times more likely to have a prolonged concussion recovery,” said lead author Jane McDevitt, PhD, from Temple University in Philadelphia, Pennsylvania, and her research colleagues.
Fifty-one athletes with a diagnosed concussion from a hospital concussion program completed a standardized initial evaluation. Concussion injury characteristics and acute signs and symptoms were assessed, followed by an objective screening that included vestibular ocular assessments, the BESS test, and an ImPACT exam.
Participants provided salivary samples for isolation of DNA. The number of (GT) VNTR within the promoter region of GRIN2A was genotyped. The long (L) allele was defined as an allele with 25 or more dinucleotide repeats in the GT tract. The short (S) allele was defined as an allele with < 25 dinucleotide repeats in the GT tract.
Based on the results of genetic analysis, participants were genotyped as LL homozygotes, SS homozygotes, or LS heterozygotes. Participants’ concussion recovery time was followed prospectively until the full return to play clearance date determined by the treating physician.
Participant’s recovery time was categorized as normal (≤ 20 days) or prolonged (> 20 days). The DNA region surrounding position (-975 to -776) in the promoter of GRIN2A was amplified by PCR, and was analyzed by capillary electrophoresis. Fragment length polymorphism analysis was performed by measuring the migration time of a PCR product, and extrapolation to the known fragments in the DNA standard ladder using computer software. The number of GT dinucleotide repeats was calculated using the following equation: n(GT)=(L -167)/2, where L is the length of the PCR fragment estimated in base pairs.
Results indicated there was a significant association between the GT VNTR (recessive model: LL versus SS + LS) and recovery, where the chance of prolonged recovery was 4.3 times greater for homozygous carriers of the long allele.
“Making the genetic connection in this data is an exciting step for concussion injury research,” said Dr. McDevitt. “Knowing this information could help improve monitoring and management of athletes who experience concussion, and may also aid in the development of genetic counseling in athletes exposed to concussive head impacts.”
Autografts May Extend Life of ACL Reconstructions
SEATTLE—The type of material used to create a new anterior cruciate ligament (ACL) may determine the length of time an athlete can stay in the game, according to a study presented at the 2014 Annual Meeting of the American Orthopaedic Society of Sports Medicine. “Our study results highlight that in a young athletic population, allografts fail more frequently than using autografts,” said Craig R. Bottoni, MD, lead author from Tripler Army Medical Center in Honolulu, Hawaii, and his team of researchers.
In their study, which lasted from June 2002 to August 2003, researchers randomized 99 patients with a symptomatic ACL-deficient knee (87 male; 95 active-duty military) to either hamstring autograft or tibialis posterior allograft for their ACL construction. All allografts were from a single tissue bank, aseptically processed and fresh frozen without terminal irradiation. Graft fixation was identical in all knees and all patients followed the same post-operative rehabilitation protocol, blinded to the therapists.
Preoperative and postoperative assessments were performed via examination and/or telephone and internet-based questionnaire to ascertain functional and subjective status using established knee metrics. The primary outcome measures were graft integrity, subjective knee stability, and functional status.
Overall, both groups were similar in demographics and preoperative activity level. The mean and median age of both groups was similar (29 and 26). Concomitant meniscal and chondral pathology, microfracture, and meniscal repairs performed at the time of reconstruction were similar in both groups.
At a minimum 10 years (range: 120-134 months) from surgery, 96 patients (97 knees) were contacted (2 patients were deceased and 1 was lost to follow-up). There were four (8.3%) autograft and 13 (26.5%) allograft failures, which required revision reconstruction. In the remaining patients whose graft was intact, there was no difference in the mean SANE, Tegner, or IKDC scores.
“After following the patients for 10 years, more than 80% of all grafts were intact and had maintained stability. However, those patients who had an allograft failed at a rate more than three times higher than those reconstructed with an autograft. This study was also of only one type of allograft—tibialis posterior. Therefore, we can make a strong statement about that type and not necessarily extrapolate to other types of allografts, most notably those with bone,” said Dr. Bottoni. “By better understanding why and how grafts fail in ACL reconstructions, we can increase the life span of these procedures and minimize future surgeries where feasible,” he said.
SEATTLE—The type of material used to create a new anterior cruciate ligament (ACL) may determine the length of time an athlete can stay in the game, according to a study presented at the 2014 Annual Meeting of the American Orthopaedic Society of Sports Medicine. “Our study results highlight that in a young athletic population, allografts fail more frequently than using autografts,” said Craig R. Bottoni, MD, lead author from Tripler Army Medical Center in Honolulu, Hawaii, and his team of researchers.
In their study, which lasted from June 2002 to August 2003, researchers randomized 99 patients with a symptomatic ACL-deficient knee (87 male; 95 active-duty military) to either hamstring autograft or tibialis posterior allograft for their ACL construction. All allografts were from a single tissue bank, aseptically processed and fresh frozen without terminal irradiation. Graft fixation was identical in all knees and all patients followed the same post-operative rehabilitation protocol, blinded to the therapists.
Preoperative and postoperative assessments were performed via examination and/or telephone and internet-based questionnaire to ascertain functional and subjective status using established knee metrics. The primary outcome measures were graft integrity, subjective knee stability, and functional status.
Overall, both groups were similar in demographics and preoperative activity level. The mean and median age of both groups was similar (29 and 26). Concomitant meniscal and chondral pathology, microfracture, and meniscal repairs performed at the time of reconstruction were similar in both groups.
At a minimum 10 years (range: 120-134 months) from surgery, 96 patients (97 knees) were contacted (2 patients were deceased and 1 was lost to follow-up). There were four (8.3%) autograft and 13 (26.5%) allograft failures, which required revision reconstruction. In the remaining patients whose graft was intact, there was no difference in the mean SANE, Tegner, or IKDC scores.
“After following the patients for 10 years, more than 80% of all grafts were intact and had maintained stability. However, those patients who had an allograft failed at a rate more than three times higher than those reconstructed with an autograft. This study was also of only one type of allograft—tibialis posterior. Therefore, we can make a strong statement about that type and not necessarily extrapolate to other types of allografts, most notably those with bone,” said Dr. Bottoni. “By better understanding why and how grafts fail in ACL reconstructions, we can increase the life span of these procedures and minimize future surgeries where feasible,” he said.
SEATTLE—The type of material used to create a new anterior cruciate ligament (ACL) may determine the length of time an athlete can stay in the game, according to a study presented at the 2014 Annual Meeting of the American Orthopaedic Society of Sports Medicine. “Our study results highlight that in a young athletic population, allografts fail more frequently than using autografts,” said Craig R. Bottoni, MD, lead author from Tripler Army Medical Center in Honolulu, Hawaii, and his team of researchers.
In their study, which lasted from June 2002 to August 2003, researchers randomized 99 patients with a symptomatic ACL-deficient knee (87 male; 95 active-duty military) to either hamstring autograft or tibialis posterior allograft for their ACL construction. All allografts were from a single tissue bank, aseptically processed and fresh frozen without terminal irradiation. Graft fixation was identical in all knees and all patients followed the same post-operative rehabilitation protocol, blinded to the therapists.
Preoperative and postoperative assessments were performed via examination and/or telephone and internet-based questionnaire to ascertain functional and subjective status using established knee metrics. The primary outcome measures were graft integrity, subjective knee stability, and functional status.
Overall, both groups were similar in demographics and preoperative activity level. The mean and median age of both groups was similar (29 and 26). Concomitant meniscal and chondral pathology, microfracture, and meniscal repairs performed at the time of reconstruction were similar in both groups.
At a minimum 10 years (range: 120-134 months) from surgery, 96 patients (97 knees) were contacted (2 patients were deceased and 1 was lost to follow-up). There were four (8.3%) autograft and 13 (26.5%) allograft failures, which required revision reconstruction. In the remaining patients whose graft was intact, there was no difference in the mean SANE, Tegner, or IKDC scores.
“After following the patients for 10 years, more than 80% of all grafts were intact and had maintained stability. However, those patients who had an allograft failed at a rate more than three times higher than those reconstructed with an autograft. This study was also of only one type of allograft—tibialis posterior. Therefore, we can make a strong statement about that type and not necessarily extrapolate to other types of allografts, most notably those with bone,” said Dr. Bottoni. “By better understanding why and how grafts fail in ACL reconstructions, we can increase the life span of these procedures and minimize future surgeries where feasible,” he said.
Young Athletes May Benefit From Early ACL Surgery
SEATTLE—It was previously believed that children and adolescents with anterior cruciate ligament (ACL) injuries should wait until skeletal maturity to have their knee injuries surgically corrected; however, this is no longer the case, according to a study presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine.
“In reviewing records of young patients who received ACL reconstructions, our data showed higher rates and severity of secondary meniscus injuries when surgery is delayed,” said study author Allen F. Anderson, MD, from the Tennessee Orthopaedic Alliance in Nashville, Tennessee.
Dr. Anderson’s findings suggest that early reconstruction is preferable to nonoperative treatment because increased time from injury to surgery may be associated with a higher rate of meniscal and articular cartilage injury.
In the study 135 consecutive patients, ages 8 to 16 years, (mean age, 13.8) with ACL tears were evaluated from 2000 and 2012. The time from surgery was divided into three groups based on timing—acute (< 6 weeks), subacute (6 weeks to 3 months), and chronic (> 3 months). The type and grade of lateral and medial meniscus tears was documented according to the ISAKOS Meniscal Documentation Criteria and chondral injury location and grade was documented according to ICRS Criteria.
A total of 112 meniscal tears (70 lateral, 42 medial) were found in the study cohort. Sixty two patients were treated acutely, 37 subacute, and 36 chronic. Eighty percent of the patients ages 8 to 12 years had a meniscal tear and 84% of patients ages 13 to 16 years had a meniscal tear. Multivariant logistic regression revealed the risk factors for lateral meniscus tears were younger age (P = .007) and increased time to surgery (P = .008).
Study findings also conclude:
• The odds ratio of lateral meniscus tears for patients who had a single episode of instability was 3.1.
• For time to surgery, the odds ratio was 1 for acute reconstruction, 2.6 for subacute, and 2.59 for reconstruction of chronic injuries.
• The odds ratio for increased grade of tear was 3.3 for a giving-way episode and 6.5 for increased time to surgery.
• For medial meniscus tears, the risk factors were: older age (P = .001), increasing time to surgery (P =.007), return to sports (P = .044), and instability episodes (P = .001).
• Risk factors for increasing grade of medial meniscus tears were: time to surgery, return to sports, and any instability episode (P = < .001 for all).
“These data provide evidence that initial nonoperative treatment of ACL tears in this age group carries a high risk of additional meniscal and chondral injury, which may result in long-term knee injury,” Dr. Anderson concluded.
SEATTLE—It was previously believed that children and adolescents with anterior cruciate ligament (ACL) injuries should wait until skeletal maturity to have their knee injuries surgically corrected; however, this is no longer the case, according to a study presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine.
“In reviewing records of young patients who received ACL reconstructions, our data showed higher rates and severity of secondary meniscus injuries when surgery is delayed,” said study author Allen F. Anderson, MD, from the Tennessee Orthopaedic Alliance in Nashville, Tennessee.
Dr. Anderson’s findings suggest that early reconstruction is preferable to nonoperative treatment because increased time from injury to surgery may be associated with a higher rate of meniscal and articular cartilage injury.
In the study 135 consecutive patients, ages 8 to 16 years, (mean age, 13.8) with ACL tears were evaluated from 2000 and 2012. The time from surgery was divided into three groups based on timing—acute (< 6 weeks), subacute (6 weeks to 3 months), and chronic (> 3 months). The type and grade of lateral and medial meniscus tears was documented according to the ISAKOS Meniscal Documentation Criteria and chondral injury location and grade was documented according to ICRS Criteria.
A total of 112 meniscal tears (70 lateral, 42 medial) were found in the study cohort. Sixty two patients were treated acutely, 37 subacute, and 36 chronic. Eighty percent of the patients ages 8 to 12 years had a meniscal tear and 84% of patients ages 13 to 16 years had a meniscal tear. Multivariant logistic regression revealed the risk factors for lateral meniscus tears were younger age (P = .007) and increased time to surgery (P = .008).
Study findings also conclude:
• The odds ratio of lateral meniscus tears for patients who had a single episode of instability was 3.1.
• For time to surgery, the odds ratio was 1 for acute reconstruction, 2.6 for subacute, and 2.59 for reconstruction of chronic injuries.
• The odds ratio for increased grade of tear was 3.3 for a giving-way episode and 6.5 for increased time to surgery.
• For medial meniscus tears, the risk factors were: older age (P = .001), increasing time to surgery (P =.007), return to sports (P = .044), and instability episodes (P = .001).
• Risk factors for increasing grade of medial meniscus tears were: time to surgery, return to sports, and any instability episode (P = < .001 for all).
“These data provide evidence that initial nonoperative treatment of ACL tears in this age group carries a high risk of additional meniscal and chondral injury, which may result in long-term knee injury,” Dr. Anderson concluded.
SEATTLE—It was previously believed that children and adolescents with anterior cruciate ligament (ACL) injuries should wait until skeletal maturity to have their knee injuries surgically corrected; however, this is no longer the case, according to a study presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine.
“In reviewing records of young patients who received ACL reconstructions, our data showed higher rates and severity of secondary meniscus injuries when surgery is delayed,” said study author Allen F. Anderson, MD, from the Tennessee Orthopaedic Alliance in Nashville, Tennessee.
Dr. Anderson’s findings suggest that early reconstruction is preferable to nonoperative treatment because increased time from injury to surgery may be associated with a higher rate of meniscal and articular cartilage injury.
In the study 135 consecutive patients, ages 8 to 16 years, (mean age, 13.8) with ACL tears were evaluated from 2000 and 2012. The time from surgery was divided into three groups based on timing—acute (< 6 weeks), subacute (6 weeks to 3 months), and chronic (> 3 months). The type and grade of lateral and medial meniscus tears was documented according to the ISAKOS Meniscal Documentation Criteria and chondral injury location and grade was documented according to ICRS Criteria.
A total of 112 meniscal tears (70 lateral, 42 medial) were found in the study cohort. Sixty two patients were treated acutely, 37 subacute, and 36 chronic. Eighty percent of the patients ages 8 to 12 years had a meniscal tear and 84% of patients ages 13 to 16 years had a meniscal tear. Multivariant logistic regression revealed the risk factors for lateral meniscus tears were younger age (P = .007) and increased time to surgery (P = .008).
Study findings also conclude:
• The odds ratio of lateral meniscus tears for patients who had a single episode of instability was 3.1.
• For time to surgery, the odds ratio was 1 for acute reconstruction, 2.6 for subacute, and 2.59 for reconstruction of chronic injuries.
• The odds ratio for increased grade of tear was 3.3 for a giving-way episode and 6.5 for increased time to surgery.
• For medial meniscus tears, the risk factors were: older age (P = .001), increasing time to surgery (P =.007), return to sports (P = .044), and instability episodes (P = .001).
• Risk factors for increasing grade of medial meniscus tears were: time to surgery, return to sports, and any instability episode (P = < .001 for all).
“These data provide evidence that initial nonoperative treatment of ACL tears in this age group carries a high risk of additional meniscal and chondral injury, which may result in long-term knee injury,” Dr. Anderson concluded.