User login
Toluene-2,5-Diamine Sulfate: The 2025 American Contact Dermatitis Society Allergen of the Year
Toluene-2,5-Diamine Sulfate: The 2025 American Contact Dermatitis Society Allergen of the Year
The American Contact Dermatitis Society selected toluene-2,5-diamine sulfate (PTDS) as the 2025 Allergen of the Year.1 Widely used as an alternative to para-phenylenediamine (PPD) in oxidative and permanent/semipermanent hair dyes, PTDS has emerged as a potent contact allergen with substantial cross-reactivity to PPD. In this article, we discuss PTDS as both a PPD alternative and a contact allergen as well as the clinical features of allergic contact dermatitis (ACD) to PTDS and practical recommendations for management in at-risk populations.
Background
Toluene-2,5-diamine sulfate is a compound formed by combining 2,5-diaminotoluene (PTD) with sulfuric acid, making it more water soluble and potentially less irritating than PTD alone.2 In this article, the terms PTDS and PTD will be used interchangeably due to their structural similarity.
Toluene-2,5-diamine sulfate commonly is used in oxidative and permanent/semipermanent hair dyes as an alternative to PPD, the most common hair dye contact allergen.3 Toluene-2,5-diamine sulfate also is a component used in color photography development and in dyes used for textiles, furs, leathers, and biologic stains.4 The prevalence of PTDS contact allergy likely is underreported due to its absence in routine patch test series such as the Thin-Layer Rapid Use Epicutaneous (T.R.U.E.) test (Smart Practice) and the American Contact Dermatitis Society Core 90 Series.
Cross-Reactivity Between PTDS and PPD
There is substantial cross-reactivity between PTDS and PPD, necessitating careful avoidance and alternative dye selection. The rate of cross-reactivity between these compounds is high, with some estimated to be more than 80% among patch tested individuals.5-9 In some cases, patients with a contact allergy to PPD are able to tolerate dyes containing PTDS. Studies conducted in Canada and Europe showed that 31.3% to 76.3% of patients with a contact allergy to PPD also had an allergy to PTDS or PTD.7,8,10 Stronger reactions to PPD also seem to be associated with an increased risk for cross-reaction.11
Clinical Manifestation of ACD to PTDS
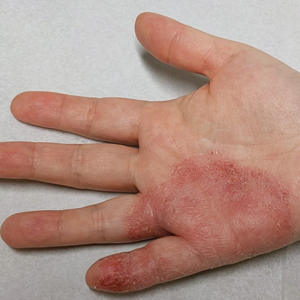
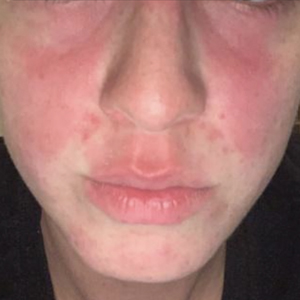
In the literature, case reports of ACD caused by PTDS are rare. The clinical manifestations of PTDS-ACD will closely mirror those described in PPD-ACD or PTD-ACD, reflecting the cross-reactivity between these aromatic amines. Generally, ACD to components in hair dyes manifests as a pruritic, erythematous, edematous, eczematous rash that can affect the margins of the scalp, ears, face, and/or neck. Severe cases can extend beyond the initial area of contact, potentially resulting in widespread involvement and systemic symptoms.12 Notably, the scalp often is spared, which may be attributable to protection provided by sebum or the hair itself covering the scalp.13
Two case reports described ACD of the eyebrows after application of PTD-containing hair dye.14,15 One patient developed severe bullous ACD involving the eyebrows and eyelashes with concurrent conjunctivitis,14 and the other experienced erythema, edema, burning, itching, and exudation at and around the eyebrows.15 The latter patient had prior exposure to PPD from a black henna tattoo, which may have led to an initial sensitization and subsequent cross-reactivity to PTD in the hair dye.
Another case report described a patient with erythema, edema, and scaling of the face, neck, and arms within 1 week of exposure to a new hair dye at a salon.16 Patch testing revealed a positive reaction to PPD on day 3, despite it not being a component of the hair dye. On day 7, the patient showed a delayed reaction to PTD, which was confirmed to be present in the dye.16 The implications of these findings are twofold. First, delayed patch test readings beyond day 5 could provide more sensitive interpretation. Second, this case highlights the cross-reactivity between these related compounds.
Hairdressers and users of hair care products are most commonly affected by PTDS contact allergy. Though hairdressers generally are at a higher risk, prevalence for PTD sensitization in a European patch tested population showed rates of 20% in hairdressers and 30.8% in consumers.17 The North American Contact Dermatitis Group reported PTDS sensitization in fewer than 2% of 4121 patients patch tested across 13 North American centers over a period of 1 year.18 This suggests potential underutilization of the more specific panels that include PTDS.
Hairdressers are at an increased risk of contact allergy to PTDS due to occupational exposure and are at higher risk for hand dermatitis due to frequent exposure to water. In a review of epidemiologic studies published between 2000 and 2021, the pooled lifetime prevalence of hand eczema in hairdressers was 38.2% compared to an estimated lifetime prevalence of 14.5% in the general population.19 Higher risk for hand eczema can increase the risk for sensitization to contact allergens including PPD and PTDS due to impaired barrier function, allowing allergen penetration through disrupted skin.20
Strategies for Management and Avoidance
Patients with suspected contact allergy to PTDS should avoid this compound and related dye chemicals such as PPD due to the high risk for ACD and frequent cross-reactivity. While PTDS-allergic patients should avoid products containing PPD, some patients allergic to PPD may be able to tolerate exposure to PTD or PTDS.7,8,10 Regardless, any suspected contact allergy should be supported by patch testing with PTDS and PPD to confirm sensitization. Patch test readings for PTDS/PTD could be delayed beyond day 5 if clinical suspicion is high and early patch test reading is noncontributory; however, more studies are needed to establish that later readings are more reliable for PTDS.
Occupational risk reduction in hairdressers is essential. Hairdressers as well as at-home users of hair dyes should be properly informed by their dermatologist or other trained health care professional about PTDS and PTD as potent allergens and should be provided with information on potential alternatives. They also should be counseled on proper skin protection, including single-use gloves and careful hand care through gentle cleansing and use of barrier creams to protect skin integrity and prevent contact dermatitis. Nitrile rubber gloves offer the best protection when handling hair dyes. Polyvinyl chloride or natural latex rubber gloves also may be sufficient; however, polyethylene gloves should be avoided, as they have been shown to have the fastest time to penetration.21 Gloves should be properly sized, and reuse should be avoided.
Because PTDS and PTD frequently are used in semipermanent and permanent hair dyes, temporary hair dyes (eg, henna-based dyes) may be safer alternatives, as they infrequently contain these allergens. Food, Drug, and Cosmetics (FD&C) and Drug and Cosmetics (D&C) dyes also are used in some semipermanent hair dyes and seem to have low cross-reactivity to PPD; therefore, these may be used in patients allergic to PTDS or PTD.22 However, these dyes require frequent reapplication, which may be unfavorable to some patients. Gallic acid–based hair dyes have been shown to be safe alternatives in patients with contact allergy to PTDS or PTD, though pretesting is recommended with a repeat open application test.23 The PPD derivative 2-methoxymethyl-para-phenylenediamine (ME-PPD) has reduced sensitization potential. In simulated hair dye use conditions, cross-reactivity to ME-PPD in patients with PPD contact allergy was 30% compared with 84% for PPD.24 However, in an open-use test in 25 PPD-allergic individuals, ME-PPD was reactive in 84% (21/25) and ME-PPD 2% patch testing was positive in 48% (12/25), suggesting that ME-PPD could be a potential alternative but is not universally tolerated.25
It is important to note that products purporting to be natural or botanical are not inherently safe and may themselves be allergenic.25 Patients should attempt a repeat open application test or patch testing prior to use of an alternative dye.
Given the prevalence of PTDS allergy, the fact that some PPD-allergic individuals may be able to tolerate hair dyes containing PTDS (assuming it tests negative), and the substantial quality of life and socioeconomic impacts of hair dye allergy, PTDS should be considered as an addition to standard patch test screening series.1
Final Thoughts
While initially popularized as an alternative to PPD in semipermanent and permanent hair dyes, PTDS now is emerging as a contact allergen with well-documented cross-reactivity to PPD. Dermatologists should consider patch testing for PTDS (and PPD) in individuals who regularly encounter this compound. This will guide further counseling and recommendations.
- Atwater AR, Botto N. Toluene-2,5-diamine sulfate: allergen of the year 2025. Dermatitis. 2025;36:3-11. doi:10.1089/derm.2024.0384
- National Center for Biotechnology Information. PubChem Compound Summary for 2,5-diamintoluene sulfate (CID 22856). Accessed Oct. 2, 2025. https://pubchem.ncbi.nlm.nih.gov/compound/2_5-Diaminotoluene-sulfate
- Søsted H, Rustemeyer T, Gonçalo M, et al. Contact allergy to common ingredients in hair dyes. Contact Dermatitis. 2013;69:32-39. doi:10.1111/cod.12077
- Burnett CL, Bergfeld WF, Belsito DV, et al. Final amended report of the safety assessment of toluene-2,5-diamine, toluene-2,5-diamine sulfate, and toluene-3,4-diamine as used in cosmetics. Int J Toxicol. 2010;29(3 suppl):61S-83S.
- Schmidt JD, Johansen JD, Nielsen MM, et al. Immune responses to hair dyes containing toluene-2,5-diamine. Br J Dermatol. 2014;170:352-359. doi:10.1111/bjd.12676
- Yazar K, Boman A, Lidén C. Potent skin sensitizers in oxidative hair dye products on the Swedish market. Contact Dermatitis. 2009;61:269-275. doi:10.1111/j.1600-0536.2009.01612.x
- Fautz R, Fuchs A, van der Walle H, et al. Hair dye-sensitized hairdressers: the cross-reaction pattern with new generation hair dyes. Contact Dermatitis. 2002;46:319-324. doi:10.1034/j.1600-0536.2002.460601.x
- Vogel TA, Heijnen RW, Coenraads PJ, et al. Two decades of p-phenyl-enediamine and toluene-2,5-diamine patch testing—focus on co-sensitizations in the European baseline series and cross-reactions with chemically related substances. Contact Dermatitis. 2017;76:81-88. doi:10.1111/cod.12619
- Skazik C, Grannemann S, Wilbers L, et al. Reactivity of in vitro activated human T lymphocytes to p-phenylenediamine and related substances. Contact Dermatitis. 2008;59:203-211. doi:10.1111/j.1600-0536.2008.01416.x
- LaBerge L, Pratt M, Fong B, et al. A 10-year review of p-phenylenediamine allergy and related para-amino compounds at the Ottawa Patch Test Clinic. Dermatitis. 2011;22:332. doi:10.2310/6620.2011.11044
- Thomas BR, White IR, McFadden JP, et al. Positive relationship—intensity of response to p-phenylenediamine on patch testing and cross-reactions with related allergens. Contact Dermatitis. 2014;71:98-101. doi:10.1111/cod.12255
- Helaskoski E, Suojalehto H, Virtanen H, et al. Occupational asthma, rhinitis, and contact urticaria caused by oxidative hair dyes in hairdressers. Ann Allergy Asthma Immunol. 2014;112:46-52. doi:10.1016/j.anai.2013.10.002
- Mukkanna KS, Stone NM, Ingram JR. Para-phenylenediamine allergy: current perspectives on diagnosis and management. J Asthma Allergy. 2017;10:9-15. doi:10.2147/JAA.S90265
- Søsted H, Rastogi SC, Thomsen JS. Allergic contact dermatitis from toluene-2,5-diamine in a cream dye for eyelashes and eyebrows—quantitative exposure assessment. Contact Dermatitis. 2007;57:195-196. doi:10.1111/j.1600-0536.2007.01105.x
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m‐aminophenol and toluene‐2,5‐diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52. doi:10.1111/cod.12987
- Bregnhøj A, Menne T. Primary sensitization to toluene-2,5-diamine giving rise to early positive patch reaction to p-phenylenediamine and late to toluene-2,5-diamine. Contact Dermatitis. 2008;59:189-190. doi:10.1111/j.1600-0536.2008.01407.x
- Uter W, Hallmann S, Gefeller O, et al. Contact allergy to ingredients of hair cosmetics in female hairdressers and female consumers—an update based on IVDK data 2013-2020. Contact Dermatitis. 2023;89:161-170. doi:10.1111/cod.14363
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- Havmose MS, Kezic S, Uter W, et al. Prevalence and incidence of hand eczema in hairdressers—a systematic review and meta‐analysis of the published literature from 2000–2021. Contact Dermatitis. 2022;86:254-265. doi:10.1111/cod.14048
- CDC. About skin exposures and effects. Published December 10, 2024. Accessed October 13, 2025. https://www.cdc.gov/niosh/skin-exposure/about/index.html
- Havmose M, Thyssen JP, Zachariae C, et al. Use of protective gloves by hairdressers: a review of efficacy and potential adverse effects. Contact Dermatitis. 2020;83:75-82. doi:10.1111/cod.13561
- Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter–update 2015. J Allergy Clin Immunol Pract. 2015;3(3 suppl):S1-S39. doi:10.1016/j.jaip.2015.02.009
- Choi Y, Lee JH, Kwon HB, et al. Skin testing of gallic acid-based hair dye in paraphenylenediamine/paratoluenediamine-reactive patients.J Dermatol. 2016;43:795-798. doi:10.1111/1346-8138.13226
- Blömeke B, Pot LM, Coenraads PJ, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine under hair dye use conditions in p-phenylenediamine-allergic individuals. Br J Dermatol. 2015;172:976-980. doi:10.1111/bjd.13412
- Schuttelaar ML, Dittmar D, Burgerhof JGM, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine in p-phenylenediamine-allergic individuals: results from open use testing and diagnostic patch testing. Contact Dermatitis. 2018;79:288-294. doi:10.1111/cod.13078
- Tran JM, Comstock JR, Reeder MJ. Natural is not always better: the prevalence of allergenic ingredients in "clean" beauty products. Dermatitis. 2022;33:215-219. doi:10.1097/DER.0000000000000863
The American Contact Dermatitis Society selected toluene-2,5-diamine sulfate (PTDS) as the 2025 Allergen of the Year.1 Widely used as an alternative to para-phenylenediamine (PPD) in oxidative and permanent/semipermanent hair dyes, PTDS has emerged as a potent contact allergen with substantial cross-reactivity to PPD. In this article, we discuss PTDS as both a PPD alternative and a contact allergen as well as the clinical features of allergic contact dermatitis (ACD) to PTDS and practical recommendations for management in at-risk populations.
Background
Toluene-2,5-diamine sulfate is a compound formed by combining 2,5-diaminotoluene (PTD) with sulfuric acid, making it more water soluble and potentially less irritating than PTD alone.2 In this article, the terms PTDS and PTD will be used interchangeably due to their structural similarity.
Toluene-2,5-diamine sulfate commonly is used in oxidative and permanent/semipermanent hair dyes as an alternative to PPD, the most common hair dye contact allergen.3 Toluene-2,5-diamine sulfate also is a component used in color photography development and in dyes used for textiles, furs, leathers, and biologic stains.4 The prevalence of PTDS contact allergy likely is underreported due to its absence in routine patch test series such as the Thin-Layer Rapid Use Epicutaneous (T.R.U.E.) test (Smart Practice) and the American Contact Dermatitis Society Core 90 Series.
Cross-Reactivity Between PTDS and PPD
There is substantial cross-reactivity between PTDS and PPD, necessitating careful avoidance and alternative dye selection. The rate of cross-reactivity between these compounds is high, with some estimated to be more than 80% among patch tested individuals.5-9 In some cases, patients with a contact allergy to PPD are able to tolerate dyes containing PTDS. Studies conducted in Canada and Europe showed that 31.3% to 76.3% of patients with a contact allergy to PPD also had an allergy to PTDS or PTD.7,8,10 Stronger reactions to PPD also seem to be associated with an increased risk for cross-reaction.11
Clinical Manifestation of ACD to PTDS
In the literature, case reports of ACD caused by PTDS are rare. The clinical manifestations of PTDS-ACD will closely mirror those described in PPD-ACD or PTD-ACD, reflecting the cross-reactivity between these aromatic amines. Generally, ACD to components in hair dyes manifests as a pruritic, erythematous, edematous, eczematous rash that can affect the margins of the scalp, ears, face, and/or neck. Severe cases can extend beyond the initial area of contact, potentially resulting in widespread involvement and systemic symptoms.12 Notably, the scalp often is spared, which may be attributable to protection provided by sebum or the hair itself covering the scalp.13
Two case reports described ACD of the eyebrows after application of PTD-containing hair dye.14,15 One patient developed severe bullous ACD involving the eyebrows and eyelashes with concurrent conjunctivitis,14 and the other experienced erythema, edema, burning, itching, and exudation at and around the eyebrows.15 The latter patient had prior exposure to PPD from a black henna tattoo, which may have led to an initial sensitization and subsequent cross-reactivity to PTD in the hair dye.
Another case report described a patient with erythema, edema, and scaling of the face, neck, and arms within 1 week of exposure to a new hair dye at a salon.16 Patch testing revealed a positive reaction to PPD on day 3, despite it not being a component of the hair dye. On day 7, the patient showed a delayed reaction to PTD, which was confirmed to be present in the dye.16 The implications of these findings are twofold. First, delayed patch test readings beyond day 5 could provide more sensitive interpretation. Second, this case highlights the cross-reactivity between these related compounds.
Hairdressers and users of hair care products are most commonly affected by PTDS contact allergy. Though hairdressers generally are at a higher risk, prevalence for PTD sensitization in a European patch tested population showed rates of 20% in hairdressers and 30.8% in consumers.17 The North American Contact Dermatitis Group reported PTDS sensitization in fewer than 2% of 4121 patients patch tested across 13 North American centers over a period of 1 year.18 This suggests potential underutilization of the more specific panels that include PTDS.
Hairdressers are at an increased risk of contact allergy to PTDS due to occupational exposure and are at higher risk for hand dermatitis due to frequent exposure to water. In a review of epidemiologic studies published between 2000 and 2021, the pooled lifetime prevalence of hand eczema in hairdressers was 38.2% compared to an estimated lifetime prevalence of 14.5% in the general population.19 Higher risk for hand eczema can increase the risk for sensitization to contact allergens including PPD and PTDS due to impaired barrier function, allowing allergen penetration through disrupted skin.20
Strategies for Management and Avoidance
Patients with suspected contact allergy to PTDS should avoid this compound and related dye chemicals such as PPD due to the high risk for ACD and frequent cross-reactivity. While PTDS-allergic patients should avoid products containing PPD, some patients allergic to PPD may be able to tolerate exposure to PTD or PTDS.7,8,10 Regardless, any suspected contact allergy should be supported by patch testing with PTDS and PPD to confirm sensitization. Patch test readings for PTDS/PTD could be delayed beyond day 5 if clinical suspicion is high and early patch test reading is noncontributory; however, more studies are needed to establish that later readings are more reliable for PTDS.
Occupational risk reduction in hairdressers is essential. Hairdressers as well as at-home users of hair dyes should be properly informed by their dermatologist or other trained health care professional about PTDS and PTD as potent allergens and should be provided with information on potential alternatives. They also should be counseled on proper skin protection, including single-use gloves and careful hand care through gentle cleansing and use of barrier creams to protect skin integrity and prevent contact dermatitis. Nitrile rubber gloves offer the best protection when handling hair dyes. Polyvinyl chloride or natural latex rubber gloves also may be sufficient; however, polyethylene gloves should be avoided, as they have been shown to have the fastest time to penetration.21 Gloves should be properly sized, and reuse should be avoided.
Because PTDS and PTD frequently are used in semipermanent and permanent hair dyes, temporary hair dyes (eg, henna-based dyes) may be safer alternatives, as they infrequently contain these allergens. Food, Drug, and Cosmetics (FD&C) and Drug and Cosmetics (D&C) dyes also are used in some semipermanent hair dyes and seem to have low cross-reactivity to PPD; therefore, these may be used in patients allergic to PTDS or PTD.22 However, these dyes require frequent reapplication, which may be unfavorable to some patients. Gallic acid–based hair dyes have been shown to be safe alternatives in patients with contact allergy to PTDS or PTD, though pretesting is recommended with a repeat open application test.23 The PPD derivative 2-methoxymethyl-para-phenylenediamine (ME-PPD) has reduced sensitization potential. In simulated hair dye use conditions, cross-reactivity to ME-PPD in patients with PPD contact allergy was 30% compared with 84% for PPD.24 However, in an open-use test in 25 PPD-allergic individuals, ME-PPD was reactive in 84% (21/25) and ME-PPD 2% patch testing was positive in 48% (12/25), suggesting that ME-PPD could be a potential alternative but is not universally tolerated.25
It is important to note that products purporting to be natural or botanical are not inherently safe and may themselves be allergenic.25 Patients should attempt a repeat open application test or patch testing prior to use of an alternative dye.
Given the prevalence of PTDS allergy, the fact that some PPD-allergic individuals may be able to tolerate hair dyes containing PTDS (assuming it tests negative), and the substantial quality of life and socioeconomic impacts of hair dye allergy, PTDS should be considered as an addition to standard patch test screening series.1
Final Thoughts
While initially popularized as an alternative to PPD in semipermanent and permanent hair dyes, PTDS now is emerging as a contact allergen with well-documented cross-reactivity to PPD. Dermatologists should consider patch testing for PTDS (and PPD) in individuals who regularly encounter this compound. This will guide further counseling and recommendations.
The American Contact Dermatitis Society selected toluene-2,5-diamine sulfate (PTDS) as the 2025 Allergen of the Year.1 Widely used as an alternative to para-phenylenediamine (PPD) in oxidative and permanent/semipermanent hair dyes, PTDS has emerged as a potent contact allergen with substantial cross-reactivity to PPD. In this article, we discuss PTDS as both a PPD alternative and a contact allergen as well as the clinical features of allergic contact dermatitis (ACD) to PTDS and practical recommendations for management in at-risk populations.
Background
Toluene-2,5-diamine sulfate is a compound formed by combining 2,5-diaminotoluene (PTD) with sulfuric acid, making it more water soluble and potentially less irritating than PTD alone.2 In this article, the terms PTDS and PTD will be used interchangeably due to their structural similarity.
Toluene-2,5-diamine sulfate commonly is used in oxidative and permanent/semipermanent hair dyes as an alternative to PPD, the most common hair dye contact allergen.3 Toluene-2,5-diamine sulfate also is a component used in color photography development and in dyes used for textiles, furs, leathers, and biologic stains.4 The prevalence of PTDS contact allergy likely is underreported due to its absence in routine patch test series such as the Thin-Layer Rapid Use Epicutaneous (T.R.U.E.) test (Smart Practice) and the American Contact Dermatitis Society Core 90 Series.
Cross-Reactivity Between PTDS and PPD
There is substantial cross-reactivity between PTDS and PPD, necessitating careful avoidance and alternative dye selection. The rate of cross-reactivity between these compounds is high, with some estimated to be more than 80% among patch tested individuals.5-9 In some cases, patients with a contact allergy to PPD are able to tolerate dyes containing PTDS. Studies conducted in Canada and Europe showed that 31.3% to 76.3% of patients with a contact allergy to PPD also had an allergy to PTDS or PTD.7,8,10 Stronger reactions to PPD also seem to be associated with an increased risk for cross-reaction.11
Clinical Manifestation of ACD to PTDS
In the literature, case reports of ACD caused by PTDS are rare. The clinical manifestations of PTDS-ACD will closely mirror those described in PPD-ACD or PTD-ACD, reflecting the cross-reactivity between these aromatic amines. Generally, ACD to components in hair dyes manifests as a pruritic, erythematous, edematous, eczematous rash that can affect the margins of the scalp, ears, face, and/or neck. Severe cases can extend beyond the initial area of contact, potentially resulting in widespread involvement and systemic symptoms.12 Notably, the scalp often is spared, which may be attributable to protection provided by sebum or the hair itself covering the scalp.13
Two case reports described ACD of the eyebrows after application of PTD-containing hair dye.14,15 One patient developed severe bullous ACD involving the eyebrows and eyelashes with concurrent conjunctivitis,14 and the other experienced erythema, edema, burning, itching, and exudation at and around the eyebrows.15 The latter patient had prior exposure to PPD from a black henna tattoo, which may have led to an initial sensitization and subsequent cross-reactivity to PTD in the hair dye.
Another case report described a patient with erythema, edema, and scaling of the face, neck, and arms within 1 week of exposure to a new hair dye at a salon.16 Patch testing revealed a positive reaction to PPD on day 3, despite it not being a component of the hair dye. On day 7, the patient showed a delayed reaction to PTD, which was confirmed to be present in the dye.16 The implications of these findings are twofold. First, delayed patch test readings beyond day 5 could provide more sensitive interpretation. Second, this case highlights the cross-reactivity between these related compounds.
Hairdressers and users of hair care products are most commonly affected by PTDS contact allergy. Though hairdressers generally are at a higher risk, prevalence for PTD sensitization in a European patch tested population showed rates of 20% in hairdressers and 30.8% in consumers.17 The North American Contact Dermatitis Group reported PTDS sensitization in fewer than 2% of 4121 patients patch tested across 13 North American centers over a period of 1 year.18 This suggests potential underutilization of the more specific panels that include PTDS.
Hairdressers are at an increased risk of contact allergy to PTDS due to occupational exposure and are at higher risk for hand dermatitis due to frequent exposure to water. In a review of epidemiologic studies published between 2000 and 2021, the pooled lifetime prevalence of hand eczema in hairdressers was 38.2% compared to an estimated lifetime prevalence of 14.5% in the general population.19 Higher risk for hand eczema can increase the risk for sensitization to contact allergens including PPD and PTDS due to impaired barrier function, allowing allergen penetration through disrupted skin.20
Strategies for Management and Avoidance
Patients with suspected contact allergy to PTDS should avoid this compound and related dye chemicals such as PPD due to the high risk for ACD and frequent cross-reactivity. While PTDS-allergic patients should avoid products containing PPD, some patients allergic to PPD may be able to tolerate exposure to PTD or PTDS.7,8,10 Regardless, any suspected contact allergy should be supported by patch testing with PTDS and PPD to confirm sensitization. Patch test readings for PTDS/PTD could be delayed beyond day 5 if clinical suspicion is high and early patch test reading is noncontributory; however, more studies are needed to establish that later readings are more reliable for PTDS.
Occupational risk reduction in hairdressers is essential. Hairdressers as well as at-home users of hair dyes should be properly informed by their dermatologist or other trained health care professional about PTDS and PTD as potent allergens and should be provided with information on potential alternatives. They also should be counseled on proper skin protection, including single-use gloves and careful hand care through gentle cleansing and use of barrier creams to protect skin integrity and prevent contact dermatitis. Nitrile rubber gloves offer the best protection when handling hair dyes. Polyvinyl chloride or natural latex rubber gloves also may be sufficient; however, polyethylene gloves should be avoided, as they have been shown to have the fastest time to penetration.21 Gloves should be properly sized, and reuse should be avoided.
Because PTDS and PTD frequently are used in semipermanent and permanent hair dyes, temporary hair dyes (eg, henna-based dyes) may be safer alternatives, as they infrequently contain these allergens. Food, Drug, and Cosmetics (FD&C) and Drug and Cosmetics (D&C) dyes also are used in some semipermanent hair dyes and seem to have low cross-reactivity to PPD; therefore, these may be used in patients allergic to PTDS or PTD.22 However, these dyes require frequent reapplication, which may be unfavorable to some patients. Gallic acid–based hair dyes have been shown to be safe alternatives in patients with contact allergy to PTDS or PTD, though pretesting is recommended with a repeat open application test.23 The PPD derivative 2-methoxymethyl-para-phenylenediamine (ME-PPD) has reduced sensitization potential. In simulated hair dye use conditions, cross-reactivity to ME-PPD in patients with PPD contact allergy was 30% compared with 84% for PPD.24 However, in an open-use test in 25 PPD-allergic individuals, ME-PPD was reactive in 84% (21/25) and ME-PPD 2% patch testing was positive in 48% (12/25), suggesting that ME-PPD could be a potential alternative but is not universally tolerated.25
It is important to note that products purporting to be natural or botanical are not inherently safe and may themselves be allergenic.25 Patients should attempt a repeat open application test or patch testing prior to use of an alternative dye.
Given the prevalence of PTDS allergy, the fact that some PPD-allergic individuals may be able to tolerate hair dyes containing PTDS (assuming it tests negative), and the substantial quality of life and socioeconomic impacts of hair dye allergy, PTDS should be considered as an addition to standard patch test screening series.1
Final Thoughts
While initially popularized as an alternative to PPD in semipermanent and permanent hair dyes, PTDS now is emerging as a contact allergen with well-documented cross-reactivity to PPD. Dermatologists should consider patch testing for PTDS (and PPD) in individuals who regularly encounter this compound. This will guide further counseling and recommendations.
- Atwater AR, Botto N. Toluene-2,5-diamine sulfate: allergen of the year 2025. Dermatitis. 2025;36:3-11. doi:10.1089/derm.2024.0384
- National Center for Biotechnology Information. PubChem Compound Summary for 2,5-diamintoluene sulfate (CID 22856). Accessed Oct. 2, 2025. https://pubchem.ncbi.nlm.nih.gov/compound/2_5-Diaminotoluene-sulfate
- Søsted H, Rustemeyer T, Gonçalo M, et al. Contact allergy to common ingredients in hair dyes. Contact Dermatitis. 2013;69:32-39. doi:10.1111/cod.12077
- Burnett CL, Bergfeld WF, Belsito DV, et al. Final amended report of the safety assessment of toluene-2,5-diamine, toluene-2,5-diamine sulfate, and toluene-3,4-diamine as used in cosmetics. Int J Toxicol. 2010;29(3 suppl):61S-83S.
- Schmidt JD, Johansen JD, Nielsen MM, et al. Immune responses to hair dyes containing toluene-2,5-diamine. Br J Dermatol. 2014;170:352-359. doi:10.1111/bjd.12676
- Yazar K, Boman A, Lidén C. Potent skin sensitizers in oxidative hair dye products on the Swedish market. Contact Dermatitis. 2009;61:269-275. doi:10.1111/j.1600-0536.2009.01612.x
- Fautz R, Fuchs A, van der Walle H, et al. Hair dye-sensitized hairdressers: the cross-reaction pattern with new generation hair dyes. Contact Dermatitis. 2002;46:319-324. doi:10.1034/j.1600-0536.2002.460601.x
- Vogel TA, Heijnen RW, Coenraads PJ, et al. Two decades of p-phenyl-enediamine and toluene-2,5-diamine patch testing—focus on co-sensitizations in the European baseline series and cross-reactions with chemically related substances. Contact Dermatitis. 2017;76:81-88. doi:10.1111/cod.12619
- Skazik C, Grannemann S, Wilbers L, et al. Reactivity of in vitro activated human T lymphocytes to p-phenylenediamine and related substances. Contact Dermatitis. 2008;59:203-211. doi:10.1111/j.1600-0536.2008.01416.x
- LaBerge L, Pratt M, Fong B, et al. A 10-year review of p-phenylenediamine allergy and related para-amino compounds at the Ottawa Patch Test Clinic. Dermatitis. 2011;22:332. doi:10.2310/6620.2011.11044
- Thomas BR, White IR, McFadden JP, et al. Positive relationship—intensity of response to p-phenylenediamine on patch testing and cross-reactions with related allergens. Contact Dermatitis. 2014;71:98-101. doi:10.1111/cod.12255
- Helaskoski E, Suojalehto H, Virtanen H, et al. Occupational asthma, rhinitis, and contact urticaria caused by oxidative hair dyes in hairdressers. Ann Allergy Asthma Immunol. 2014;112:46-52. doi:10.1016/j.anai.2013.10.002
- Mukkanna KS, Stone NM, Ingram JR. Para-phenylenediamine allergy: current perspectives on diagnosis and management. J Asthma Allergy. 2017;10:9-15. doi:10.2147/JAA.S90265
- Søsted H, Rastogi SC, Thomsen JS. Allergic contact dermatitis from toluene-2,5-diamine in a cream dye for eyelashes and eyebrows—quantitative exposure assessment. Contact Dermatitis. 2007;57:195-196. doi:10.1111/j.1600-0536.2007.01105.x
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m‐aminophenol and toluene‐2,5‐diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52. doi:10.1111/cod.12987
- Bregnhøj A, Menne T. Primary sensitization to toluene-2,5-diamine giving rise to early positive patch reaction to p-phenylenediamine and late to toluene-2,5-diamine. Contact Dermatitis. 2008;59:189-190. doi:10.1111/j.1600-0536.2008.01407.x
- Uter W, Hallmann S, Gefeller O, et al. Contact allergy to ingredients of hair cosmetics in female hairdressers and female consumers—an update based on IVDK data 2013-2020. Contact Dermatitis. 2023;89:161-170. doi:10.1111/cod.14363
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- Havmose MS, Kezic S, Uter W, et al. Prevalence and incidence of hand eczema in hairdressers—a systematic review and meta‐analysis of the published literature from 2000–2021. Contact Dermatitis. 2022;86:254-265. doi:10.1111/cod.14048
- CDC. About skin exposures and effects. Published December 10, 2024. Accessed October 13, 2025. https://www.cdc.gov/niosh/skin-exposure/about/index.html
- Havmose M, Thyssen JP, Zachariae C, et al. Use of protective gloves by hairdressers: a review of efficacy and potential adverse effects. Contact Dermatitis. 2020;83:75-82. doi:10.1111/cod.13561
- Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter–update 2015. J Allergy Clin Immunol Pract. 2015;3(3 suppl):S1-S39. doi:10.1016/j.jaip.2015.02.009
- Choi Y, Lee JH, Kwon HB, et al. Skin testing of gallic acid-based hair dye in paraphenylenediamine/paratoluenediamine-reactive patients.J Dermatol. 2016;43:795-798. doi:10.1111/1346-8138.13226
- Blömeke B, Pot LM, Coenraads PJ, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine under hair dye use conditions in p-phenylenediamine-allergic individuals. Br J Dermatol. 2015;172:976-980. doi:10.1111/bjd.13412
- Schuttelaar ML, Dittmar D, Burgerhof JGM, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine in p-phenylenediamine-allergic individuals: results from open use testing and diagnostic patch testing. Contact Dermatitis. 2018;79:288-294. doi:10.1111/cod.13078
- Tran JM, Comstock JR, Reeder MJ. Natural is not always better: the prevalence of allergenic ingredients in "clean" beauty products. Dermatitis. 2022;33:215-219. doi:10.1097/DER.0000000000000863
- Atwater AR, Botto N. Toluene-2,5-diamine sulfate: allergen of the year 2025. Dermatitis. 2025;36:3-11. doi:10.1089/derm.2024.0384
- National Center for Biotechnology Information. PubChem Compound Summary for 2,5-diamintoluene sulfate (CID 22856). Accessed Oct. 2, 2025. https://pubchem.ncbi.nlm.nih.gov/compound/2_5-Diaminotoluene-sulfate
- Søsted H, Rustemeyer T, Gonçalo M, et al. Contact allergy to common ingredients in hair dyes. Contact Dermatitis. 2013;69:32-39. doi:10.1111/cod.12077
- Burnett CL, Bergfeld WF, Belsito DV, et al. Final amended report of the safety assessment of toluene-2,5-diamine, toluene-2,5-diamine sulfate, and toluene-3,4-diamine as used in cosmetics. Int J Toxicol. 2010;29(3 suppl):61S-83S.
- Schmidt JD, Johansen JD, Nielsen MM, et al. Immune responses to hair dyes containing toluene-2,5-diamine. Br J Dermatol. 2014;170:352-359. doi:10.1111/bjd.12676
- Yazar K, Boman A, Lidén C. Potent skin sensitizers in oxidative hair dye products on the Swedish market. Contact Dermatitis. 2009;61:269-275. doi:10.1111/j.1600-0536.2009.01612.x
- Fautz R, Fuchs A, van der Walle H, et al. Hair dye-sensitized hairdressers: the cross-reaction pattern with new generation hair dyes. Contact Dermatitis. 2002;46:319-324. doi:10.1034/j.1600-0536.2002.460601.x
- Vogel TA, Heijnen RW, Coenraads PJ, et al. Two decades of p-phenyl-enediamine and toluene-2,5-diamine patch testing—focus on co-sensitizations in the European baseline series and cross-reactions with chemically related substances. Contact Dermatitis. 2017;76:81-88. doi:10.1111/cod.12619
- Skazik C, Grannemann S, Wilbers L, et al. Reactivity of in vitro activated human T lymphocytes to p-phenylenediamine and related substances. Contact Dermatitis. 2008;59:203-211. doi:10.1111/j.1600-0536.2008.01416.x
- LaBerge L, Pratt M, Fong B, et al. A 10-year review of p-phenylenediamine allergy and related para-amino compounds at the Ottawa Patch Test Clinic. Dermatitis. 2011;22:332. doi:10.2310/6620.2011.11044
- Thomas BR, White IR, McFadden JP, et al. Positive relationship—intensity of response to p-phenylenediamine on patch testing and cross-reactions with related allergens. Contact Dermatitis. 2014;71:98-101. doi:10.1111/cod.12255
- Helaskoski E, Suojalehto H, Virtanen H, et al. Occupational asthma, rhinitis, and contact urticaria caused by oxidative hair dyes in hairdressers. Ann Allergy Asthma Immunol. 2014;112:46-52. doi:10.1016/j.anai.2013.10.002
- Mukkanna KS, Stone NM, Ingram JR. Para-phenylenediamine allergy: current perspectives on diagnosis and management. J Asthma Allergy. 2017;10:9-15. doi:10.2147/JAA.S90265
- Søsted H, Rastogi SC, Thomsen JS. Allergic contact dermatitis from toluene-2,5-diamine in a cream dye for eyelashes and eyebrows—quantitative exposure assessment. Contact Dermatitis. 2007;57:195-196. doi:10.1111/j.1600-0536.2007.01105.x
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m‐aminophenol and toluene‐2,5‐diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52. doi:10.1111/cod.12987
- Bregnhøj A, Menne T. Primary sensitization to toluene-2,5-diamine giving rise to early positive patch reaction to p-phenylenediamine and late to toluene-2,5-diamine. Contact Dermatitis. 2008;59:189-190. doi:10.1111/j.1600-0536.2008.01407.x
- Uter W, Hallmann S, Gefeller O, et al. Contact allergy to ingredients of hair cosmetics in female hairdressers and female consumers—an update based on IVDK data 2013-2020. Contact Dermatitis. 2023;89:161-170. doi:10.1111/cod.14363
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- Havmose MS, Kezic S, Uter W, et al. Prevalence and incidence of hand eczema in hairdressers—a systematic review and meta‐analysis of the published literature from 2000–2021. Contact Dermatitis. 2022;86:254-265. doi:10.1111/cod.14048
- CDC. About skin exposures and effects. Published December 10, 2024. Accessed October 13, 2025. https://www.cdc.gov/niosh/skin-exposure/about/index.html
- Havmose M, Thyssen JP, Zachariae C, et al. Use of protective gloves by hairdressers: a review of efficacy and potential adverse effects. Contact Dermatitis. 2020;83:75-82. doi:10.1111/cod.13561
- Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter–update 2015. J Allergy Clin Immunol Pract. 2015;3(3 suppl):S1-S39. doi:10.1016/j.jaip.2015.02.009
- Choi Y, Lee JH, Kwon HB, et al. Skin testing of gallic acid-based hair dye in paraphenylenediamine/paratoluenediamine-reactive patients.J Dermatol. 2016;43:795-798. doi:10.1111/1346-8138.13226
- Blömeke B, Pot LM, Coenraads PJ, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine under hair dye use conditions in p-phenylenediamine-allergic individuals. Br J Dermatol. 2015;172:976-980. doi:10.1111/bjd.13412
- Schuttelaar ML, Dittmar D, Burgerhof JGM, et al. Cross-elicitation responses to 2-methoxymethyl-p-phenylenediamine in p-phenylenediamine-allergic individuals: results from open use testing and diagnostic patch testing. Contact Dermatitis. 2018;79:288-294. doi:10.1111/cod.13078
- Tran JM, Comstock JR, Reeder MJ. Natural is not always better: the prevalence of allergenic ingredients in "clean" beauty products. Dermatitis. 2022;33:215-219. doi:10.1097/DER.0000000000000863
Toluene-2,5-Diamine Sulfate: The 2025 American Contact Dermatitis Society Allergen of the Year
Toluene-2,5-Diamine Sulfate: The 2025 American Contact Dermatitis Society Allergen of the Year
Practice Points
- Toluene-2,5-diamine sulfate (PTDS) is a widely used alternative to para-phenylenediamine (PPD) that is itself a potent and likely underreported allergen.
- As high cross-reactivity has been reported, consider testing for both PTDS and PPD and possible delayed patch test reading.
- Allergic contact dermatitis to PTDS may manifest with erythema, edema, and/or pruritus, similar to PPD.
- Prevention entails avoidance of PTDS/PPD if sensitized, use of proper hand protection, and recommendation of alternative products.
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
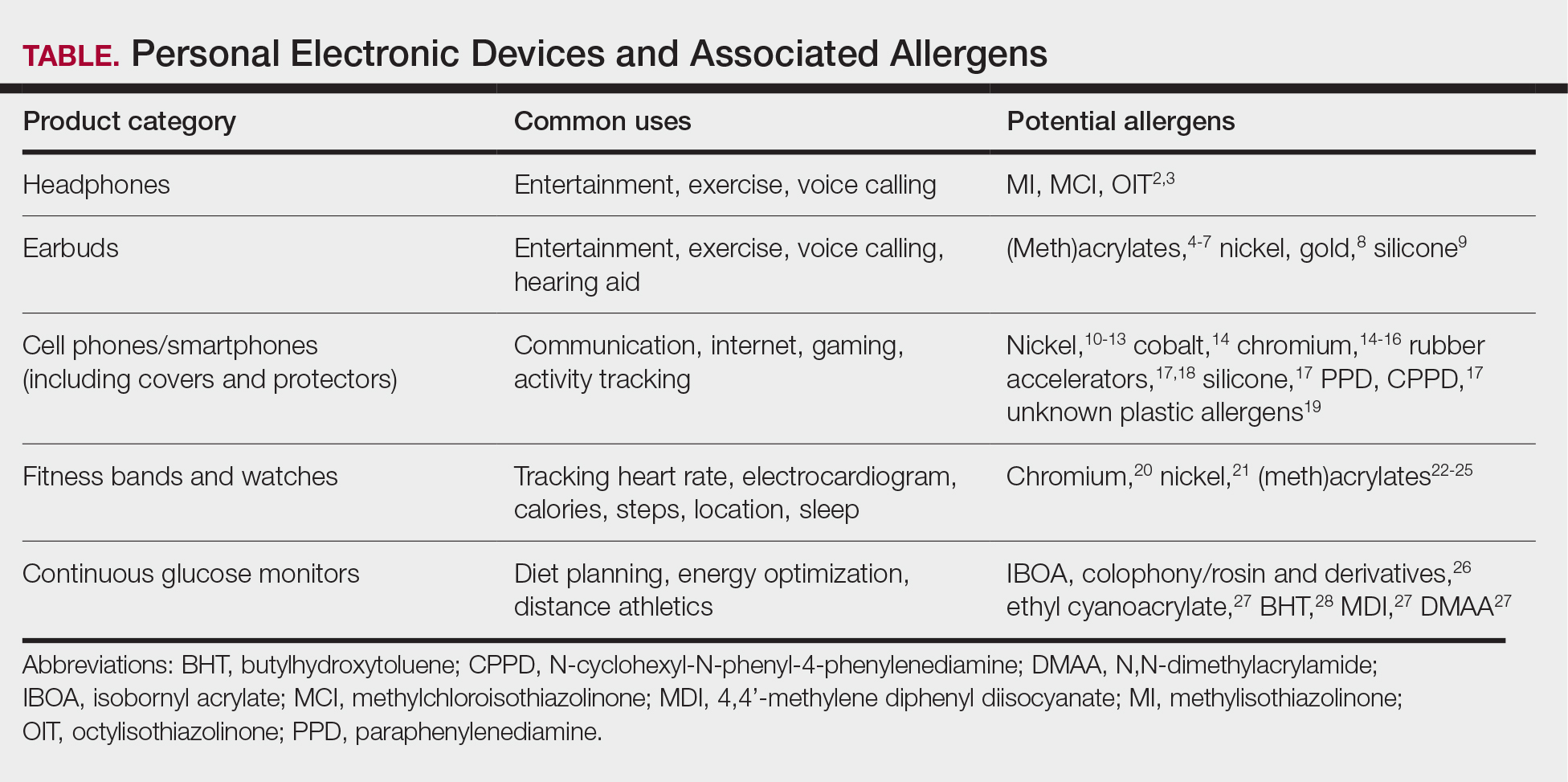
Personal electronic devices have become more common as consumer-driven health and entertainment practices continue to increase in popularity. A wide variety of devices including smartphones, headphones and earbuds, fitness watches, and continuous glucose monitors (CGMs) allow consumers to collect data and personalize their daily activities and health practices. The global market for fitness tracking devices alone was valued at $62.03 billion in 2024 and is projected to grow to $290.85 billion by 2032.1 Accordingly, the growing demand for continuous data tracking has led to new and prolonged skin contact with these devices, which have become emerging sources of allergic contact dermatitis (ACD). In this article, we provide a summary of the potential allergenicity of personal electronic devices with a focus on wearable devices, including clinical manifestations, reported allergens, and patch testing and management considerations (Table2-28).
Earbuds and Headphones
Wireless earbuds and headphones are used for listening to media and may contain microphones for voice calls. Earbuds are inserted into the ears while headphones are worn over the ears with a connecting band across the scalp. These devices frequently are worn during physical activity and thus in the setting of moist sweaty environments and mechanical friction on the skin. Depending on the style of the earbuds or headphones, associated ACD may manifest as acute or chronic pruritic eczema involving the inner and/or outer ears and potentially the periauricular areas or scalp.2 In a reported case of earbud ACD, the patient first presented to an otolaryngologist before being referred to a dermatologist for further evaluation and patch testing.9 Clinicians may be unfamiliar with these devices as a source of ACD or may potentially overlook inner ear canal manifestations, which may delay diagnosis.
Allergens reported in earbuds include (meth)acrylates,4-6 nickel, gold,8 and silicone.9 Apple AirPods and Samsung Galaxy Buds disclose the presence of acrylates and nickel.5,6 Cases also have been reported of ACD to gold earbud microphones8 and unknown allergens within silicone tips.4,9 Acrylates, named the 2012 Allergen of the Year by the American Contact Dermatitis Society,29 are used in a wide variety of consumer products as adhesives and coatings and are among the most frequently suspected headphone allergens.4 While fully polymerized acrylates theoretically are nonallergenic, residual acrylic monomers are potent allergens that may be found in in these products due to incomplete curing or polymer breakdown.29 It remains unclear whether earbud allergen concentrations are sufficient to induce sensitization or merely elicit ACD in previously sensitized users.29 Among patients with earbud ACD, the finding of inconsistent patch test reactions/cross-reactions led to the hypothesis that these headphones may contain an unidentified proprietary (meth)acrylate.4
Headphones, often utilized by runners and gymgoers for their comfort and fit, also have gained recent attention for their unique allergen profiles. In 2024, a case series described primary sensitization to octylisothiazolinone causing severe headphone-related ACD.3 This preservative, which is in the same family as methylchloroisothiazolinone/methylisothiazolinone, is used as a biocide in the leather or faux leather that encases the foam padding of headphones.3 Another case report highlighted ACD caused by methylisothiazolinone, methylchloroisothiazolinone, and octylisothiazolinone present in various components of a pair of headphones.2 These cases are notable, as European legislation limiting the use of methylchloroisothiazolinone/methylisothiazolinone in personal care products does not apply to inclusion of isothiazolinones in other product categories, such as detergents, paints, glues, and personal electronic devices.
Mobile Phones
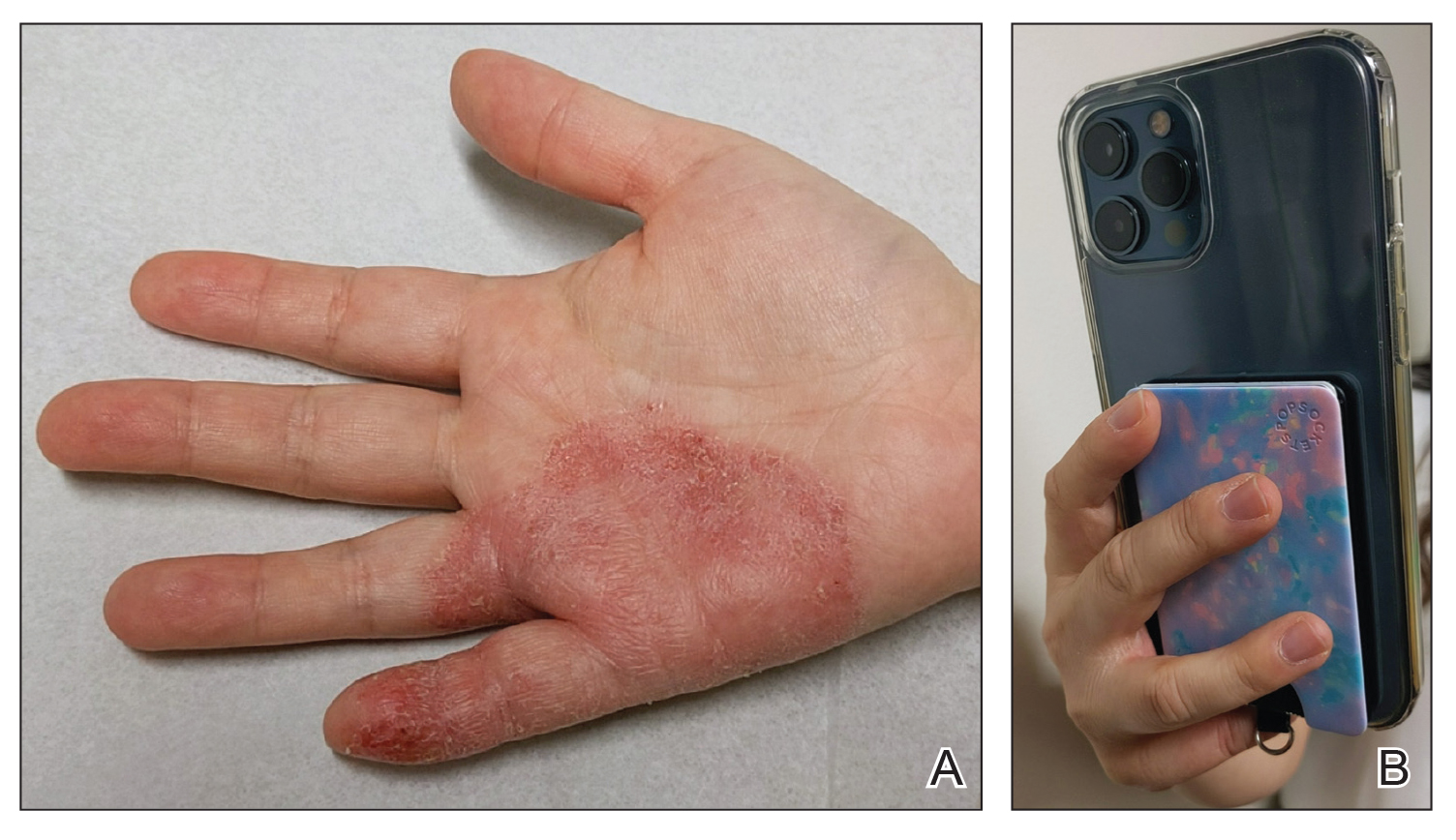
Mobile phones are a staple in modern society, used for a multitude of tasks including communication, internet browsing, entertainment, and activity tracking. In the early 2000s, mobile phone ACD primarily manifested on the lateral face, ears, and periauricular regions,12 as well as the thighs from carriage in pants pockets. Early cases of mobile phone ACD were attributed to metals including chromium16 and nickel.14 At that time, lengthy and frequent phone calls with the device against the ear were thought to increase exposure to metal allergens.30 More recently, as the utility of these devices has evolved, ACD has been reported to manifest on the fingers and hands associated with contact with cell phone cases, accessories, and screen protectors (Figure). In one report, a 17-year-old boy with chronic eczema of the palms was diagnosed with ACD to the rubber-related chemicals paraphenylenediamine and N-cyclohexyl-N-phenyl-4-phenylenediamine, confirmed via chemical analysis to be present in a phone case the patient used during daily gaming.17 Similarly, another case of palmar ACD resulted from thiuram rubber accelerators in a phone case.18 Most recently, a Japanese patient with a history of skin reactions to costume jewelry developed ACD involving the proximal middle finger due to exposure to nickel in a ring-grip phone case.11 While the European Union has enacted regulations regarding maximum nickel leaching in products that come into direct and prolonged contact with the skin, such regulations have not been implemented in Japan or the United States.11 International e-commerce makes these grips widely available, even in regions where strict metal regulations are in place. As screen time increases, it is important to consider all phone-related exposures including components of the case, screen protector, and main device body.
Watches
Smart watches and fitness bands are widely available to consumers and serve a variety of health and lifestyle functions. Features include fitness tracking, notification management, mobile payment, electrocardiography, navigation, and sleep and oxygen sensors. Multiple companies have produced hand- and wrist-based sensors for detailed wellness tracking within these categories. Allergic contact dermatitis to smart watches and wristbands manifests as eczematous lesions on the wrist (dorsal,21,22 volar,20 or circumferential involvement23,24).
(Meth)acrylates used to adhere screen protectors, house lithium ion batteries, and bind metal to plastic have been reported to cause ACD in smart watch users.22,25 In addition, there are at least 2 published reports of ACD to nickel in Apple Watches.21,31 Apple, having sold more than 229 million watches worldwide, has acknowledged the presence of trace acrylates and nickel in their watches (the latter falling below European Registration, Evaluation, Authorization, and Restriction of Chemicals limits).32 Hosoki et al20 identified ACD resulting from chromium exposure in the clasp of an Apple Watch band, which remains unreported by the manufacturer as a potential allergen.
Continuous Glucose Monitors
Continuous glucose monitoring systems provide users with dynamic information on their glycemic status and are associated with lower glycated hemoglobin and reduced episodes of hypoglycemia in patients with diabetes.33 Recently, growing interest in personalized health monitoring and performance optimization has expanded CGM use to individuals without diabetes; there are 2 over-the-counter CGM options currently available in the United States.34
Allergic contact dermatitis to CGMs in patients with diabetes is well characterized, manifesting as pruritic acute or chronic dermatitis at the sensor site.27 To date, we are unaware of published cases of ACD associated with use of CGM in individuals without diabetes; however, wearing a CGM during athletic activities and sweating could potentially increase adhesive degradation and/or penetration of allergens in the skin.6
Isobornyl acrylate, named the 2020 Allergen of the Year,35 is the most well-known contact allergen in glucose sensors.36,33 Initially suspected as a component of the CGM skin adhesive, isobornyl acrylate was found to leach from the device body onto the skin in users of one CGM device.36 Other reported allergens in CGM devices include colophony and related rosin derivatives, ethyl cyanoacrylate, and several chemicals that are not available as commercial patch test substances.27 Understanding these potential allergens is important for patch testing considerations as CGM use increases in individuals without diabetes.
Final Thoughts
Allergic contact dermatitis to personal electronic devices including wearables, sensors, and fitness trackers is an emerging problem that should be considered in cases of dermatitis of the wrists, hands, face, ears, or in any area that comes into contact with such devices. Although in-depth studies are lacking, certain wearable devices appear to introduce continuous, low-level allergen exposure that may be below the sensitization threshold but still is capable of eliciting ACD in previously sensitized users.21,26 Furthermore, increased allergen exposure is facilitated by prolonged skin contact, mechanical friction, and sweat.
Comprehensive patch testing often is necessary to diagnose cases of ACD to personal electronic devices.33 The thin-layer rapid use epicutaneous (T.R.U.E.) test does not include (meth)acrylates, which repeatedly have come up as culprit allergens.37 Isobornyl acrylate, a key allergen related to CGMs, is absent from standard patch test series.26 Nickel remains a common culprit in these devices despite adherence to European regulations.21 Since there is no obligation for manufacturers to declare all possible ingredients, chemical analysis can be useful in identifying potential allergens and directing the patch test strategy, but this is not feasible in general clinical practice outside the research setting.2
Following patch testing, patient education is essential to managing personal electronic device—induced ACD. Informed patients should switch to products that do not contain their triggers—although this may be more easily said than done, since incomplete ingredient disclosure from manufacturers may necessitate a frustrating and expensive trial-and-error approach. As wearable technology proliferates, device composition and potential contact allergen transparency must be prioritized by manufacturers and regulatory bodies. Until then, clinicians should stay on their toes regarding new and emerging clinical presentations and contact allergens in hopes of improving patient outcomes.
- Fitness tracker market size, share & industry analysis, by device type (smart watches, fitness bands, smart glasses, smart clothing, and others), by application (heart rate tracking, sleep measurement, glucose measurement, sports, running, and cycling tracking), by distribution channel (online, retail, and others), and regional forecast, 2025-2032. Fortune Business Insights. Updated June 9, 2025. Accessed June 25, 2025. https://www.fortunebusinessinsights.com/fitness-trackermarket-103358
- Caroppo ES, Stingeni L, Goracci L, et al. Wireless over-ear headphones: a new source of allergic contact dermatitis to isothiazolinones. Contact Dermatitis. 2024;90:621-625. doi:10.1111/cod.14528
- Menanteau M, Fenech G, Adam B, et al. Severe allergic contact dermatitis from octylisothiazolinone in over-ear headphones: a case series. Contact Dermatitis. 2025;92:291-298. doi:10.1111/cod.14733
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461. doi:10.1111/ijd.15954
- Fontane Hoyos CN, Goldminz AM. I’m all ears: common allergens in wireless in-ear headphones. Dermatitis. 2024;35:513-514. doi:10.1089/derm.2023.0251
- Lee LJ, Koh WL, Lim SPR. Allergic contact dermatitis to Apple AirPods Pro. Contact Dermatitis. 2022;86:127-129. doi:10.1111/cod.13987
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112. doi:10.1097/der.0000000000000735
- Hayakawa M, Suzuki C, Zhu Y, et al. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermatitis. 2022;86:328-330. doi:10.1111/cod.14036
- Hua W, Jin Y, Yao X, et al. Allergic contact dermatitis to in-ear headphones occurring in the external ear. Contact Dermatitis. 2024;91:83-85. doi:10.1111/cod.14556
- Guarneri F, Guarneri C, Cannavò SP. An unusual case of cell phone dermatitis. Contact Dermatitis. 2010;62:117. doi:10.1111 /j.1600-0536.2009.01674.x
- Ueda S, Akashi K, Washio K. A case of contact dermatitis caused by a cell phone grip ring. Contact Dermatitis. 2025;92:155-156. doi:10.1111/cod.14719
- Roberts H, Tate B. Nickel allergy presenting as mobile phone contact dermatitis. Australas J Dermatol. 2010;51:23-25. doi:10.1111 /j.1440-0960.2009.00580.x
- Livideanu C, Giordano-Labadie F, Paul C. Cellular phone addiction and allergic contact dermatitis to nickel. Contact Dermatitis. 2007;57:130- 131. doi:10.1111/j.1600-0536.2007.01090.x
- Rajpara A, Feldman SR. Cell phone allergic contact dermatitis: case report and review. Dermatol Online J. 2010;16:9.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Seishima M, Oyama Z, Yamamura M. Cellular phone dermatitis. Arch Dermatol. 2002;2:272-273.
- Corazza M, Schettini N, Catani M, et al. Pediatric allergic contact dermatitis due to rubber additives in a cellphone case. Dermatitis. 2021;32:E140-E141. doi:10.1097/der.0000000000000797
- Hamann D, Sköld MB, Hamann CR, et al. Thiuram allergic contact dermatitis on the hands after skin contact with a rubber cellphone case. Contact Dermatitis. 2019;80:130-131. doi:10.1111/cod.13140
- Williams PJ, King C, Arslanian V. Allergic contact dermatitis caused by a cell phone cover. Australas J Dermatol. 2012;53:76-77. doi:10.1111 /j.1440-0960.2011.00801.x
- Hosoki M, Tajima T, Miyagi M, et al. This report details a case of allergic contact dermatitis resulting from exposure to chromium in the clasp of an Apple Watch band. Dermatitis. Published online December 23, 2024. doi:10.1089/derm.2024.0171
- Levian B, Chan GC, Adler BL. Out of REACH: allergic contact dermatitis to nickel in an Apple Watch. Contact Dermatitis. 2024;90:99-101. doi:10.1111 /cod.14444
- Davies A, Stone N. Watch out! potential allergic contact dermatitis to acrylates in a smart watch. Contact Dermatitis. Published online December 26, 2024. doi:10.1111/cod.14749
- Gatica-Ortega ME, Mowitz M, Navarro-Triviño FJ, et al. Nonoccupational allergic contact dermatitis to 4-acryloylmorpholine in smartwatch screen protectors glue. Dermatitis. 2022;33:429-434. doi:10.1097 /der.0000000000000888
- Otero-Alonso A, Rodríguez-Vázquez V, López-Pesado I, et al. Smartwatch protective cover´s glue: a new non-occupational acrylate allergy. Contact Dermatitis. 2020;83:159-161. doi:10.1111/cod.13586
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Mowitz M, Hosseini S, Siemund I, et al. New device, ‘old’ allergens. allergic contact dermatitis caused by the Dexcom G7 glucose sensor. Contact Dermatitis. 2024;90:495-500. doi:10.1111/cod.14514
- de Groot A, van Oers EM, Ipenburg NA, et al. Allergic contact dermatitis caused by glucose sensors and insulin pumps: a full review: part 1: sensors and pumps, adverse cutaneous reactions, allergens, and diabetes devices causing allergic contact dermatitis. Contact Dermatitis. 2025;92:87-112. doi:10.1111/cod.14698
- Oppel E, Kamann S, Heinemann L, et al. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis. 2020;83:429-431. doi:10.1111/cod.13638
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- Tan S, Nixon R. Allergic contact dermatitis caused by chromium in a mobile phone. Contact Dermatitis. 2011;65:246-247. doi:10.1111 /j.1600-0536.2011.01955.x
- Ko WC, Yu J. Nickel allergy elicited by an Apple Watch. Dermatitis. 2022;33:E11-E12. doi:10.1097/der.0000000000000848
- Apple Support. Wearing your Apple Watch: for people who are sensitive to certain materials. Accessed June 27, 2025. https://support.apple.com/en-us/118234
- Seibold A. Minimizing adverse skin reactions to wearable continuous glucose monitoring sensors in patients with diabetes. J Diabetes Sci Technol. 2021;15:713-714. doi:10.1177/1932296820984763
- Klonoff DC, Nguyen KT, Xu NY, et al. Use of continuous glucose monitors by people without diabetes: an idea whose time has come? J Diabetes Sci Technol. 2023;17:1686-1697. doi:10.1177/19322968221110830
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12. doi:10.1097/der.0000000000000549
- Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. 2020;31:283-286. doi:10.1097/der.0000000000000575
- SmartPractice. Contact dermatitis products. SmartPractice. Accessed April 24, 2025. https://www.smartpractice.com/shop/category?id=581719&m=SPA
Personal electronic devices have become more common as consumer-driven health and entertainment practices continue to increase in popularity. A wide variety of devices including smartphones, headphones and earbuds, fitness watches, and continuous glucose monitors (CGMs) allow consumers to collect data and personalize their daily activities and health practices. The global market for fitness tracking devices alone was valued at $62.03 billion in 2024 and is projected to grow to $290.85 billion by 2032.1 Accordingly, the growing demand for continuous data tracking has led to new and prolonged skin contact with these devices, which have become emerging sources of allergic contact dermatitis (ACD). In this article, we provide a summary of the potential allergenicity of personal electronic devices with a focus on wearable devices, including clinical manifestations, reported allergens, and patch testing and management considerations (Table2-28).

Earbuds and Headphones
Wireless earbuds and headphones are used for listening to media and may contain microphones for voice calls. Earbuds are inserted into the ears while headphones are worn over the ears with a connecting band across the scalp. These devices frequently are worn during physical activity and thus in the setting of moist sweaty environments and mechanical friction on the skin. Depending on the style of the earbuds or headphones, associated ACD may manifest as acute or chronic pruritic eczema involving the inner and/or outer ears and potentially the periauricular areas or scalp.2 In a reported case of earbud ACD, the patient first presented to an otolaryngologist before being referred to a dermatologist for further evaluation and patch testing.9 Clinicians may be unfamiliar with these devices as a source of ACD or may potentially overlook inner ear canal manifestations, which may delay diagnosis.
Allergens reported in earbuds include (meth)acrylates,4-6 nickel, gold,8 and silicone.9 Apple AirPods and Samsung Galaxy Buds disclose the presence of acrylates and nickel.5,6 Cases also have been reported of ACD to gold earbud microphones8 and unknown allergens within silicone tips.4,9 Acrylates, named the 2012 Allergen of the Year by the American Contact Dermatitis Society,29 are used in a wide variety of consumer products as adhesives and coatings and are among the most frequently suspected headphone allergens.4 While fully polymerized acrylates theoretically are nonallergenic, residual acrylic monomers are potent allergens that may be found in in these products due to incomplete curing or polymer breakdown.29 It remains unclear whether earbud allergen concentrations are sufficient to induce sensitization or merely elicit ACD in previously sensitized users.29 Among patients with earbud ACD, the finding of inconsistent patch test reactions/cross-reactions led to the hypothesis that these headphones may contain an unidentified proprietary (meth)acrylate.4
Headphones, often utilized by runners and gymgoers for their comfort and fit, also have gained recent attention for their unique allergen profiles. In 2024, a case series described primary sensitization to octylisothiazolinone causing severe headphone-related ACD.3 This preservative, which is in the same family as methylchloroisothiazolinone/methylisothiazolinone, is used as a biocide in the leather or faux leather that encases the foam padding of headphones.3 Another case report highlighted ACD caused by methylisothiazolinone, methylchloroisothiazolinone, and octylisothiazolinone present in various components of a pair of headphones.2 These cases are notable, as European legislation limiting the use of methylchloroisothiazolinone/methylisothiazolinone in personal care products does not apply to inclusion of isothiazolinones in other product categories, such as detergents, paints, glues, and personal electronic devices.
Mobile Phones
Mobile phones are a staple in modern society, used for a multitude of tasks including communication, internet browsing, entertainment, and activity tracking. In the early 2000s, mobile phone ACD primarily manifested on the lateral face, ears, and periauricular regions,12 as well as the thighs from carriage in pants pockets. Early cases of mobile phone ACD were attributed to metals including chromium16 and nickel.14 At that time, lengthy and frequent phone calls with the device against the ear were thought to increase exposure to metal allergens.30 More recently, as the utility of these devices has evolved, ACD has been reported to manifest on the fingers and hands associated with contact with cell phone cases, accessories, and screen protectors (Figure). In one report, a 17-year-old boy with chronic eczema of the palms was diagnosed with ACD to the rubber-related chemicals paraphenylenediamine and N-cyclohexyl-N-phenyl-4-phenylenediamine, confirmed via chemical analysis to be present in a phone case the patient used during daily gaming.17 Similarly, another case of palmar ACD resulted from thiuram rubber accelerators in a phone case.18 Most recently, a Japanese patient with a history of skin reactions to costume jewelry developed ACD involving the proximal middle finger due to exposure to nickel in a ring-grip phone case.11 While the European Union has enacted regulations regarding maximum nickel leaching in products that come into direct and prolonged contact with the skin, such regulations have not been implemented in Japan or the United States.11 International e-commerce makes these grips widely available, even in regions where strict metal regulations are in place. As screen time increases, it is important to consider all phone-related exposures including components of the case, screen protector, and main device body.

Watches
Smart watches and fitness bands are widely available to consumers and serve a variety of health and lifestyle functions. Features include fitness tracking, notification management, mobile payment, electrocardiography, navigation, and sleep and oxygen sensors. Multiple companies have produced hand- and wrist-based sensors for detailed wellness tracking within these categories. Allergic contact dermatitis to smart watches and wristbands manifests as eczematous lesions on the wrist (dorsal,21,22 volar,20 or circumferential involvement23,24).
(Meth)acrylates used to adhere screen protectors, house lithium ion batteries, and bind metal to plastic have been reported to cause ACD in smart watch users.22,25 In addition, there are at least 2 published reports of ACD to nickel in Apple Watches.21,31 Apple, having sold more than 229 million watches worldwide, has acknowledged the presence of trace acrylates and nickel in their watches (the latter falling below European Registration, Evaluation, Authorization, and Restriction of Chemicals limits).32 Hosoki et al20 identified ACD resulting from chromium exposure in the clasp of an Apple Watch band, which remains unreported by the manufacturer as a potential allergen.
Continuous Glucose Monitors
Continuous glucose monitoring systems provide users with dynamic information on their glycemic status and are associated with lower glycated hemoglobin and reduced episodes of hypoglycemia in patients with diabetes.33 Recently, growing interest in personalized health monitoring and performance optimization has expanded CGM use to individuals without diabetes; there are 2 over-the-counter CGM options currently available in the United States.34
Allergic contact dermatitis to CGMs in patients with diabetes is well characterized, manifesting as pruritic acute or chronic dermatitis at the sensor site.27 To date, we are unaware of published cases of ACD associated with use of CGM in individuals without diabetes; however, wearing a CGM during athletic activities and sweating could potentially increase adhesive degradation and/or penetration of allergens in the skin.6
Isobornyl acrylate, named the 2020 Allergen of the Year,35 is the most well-known contact allergen in glucose sensors.36,33 Initially suspected as a component of the CGM skin adhesive, isobornyl acrylate was found to leach from the device body onto the skin in users of one CGM device.36 Other reported allergens in CGM devices include colophony and related rosin derivatives, ethyl cyanoacrylate, and several chemicals that are not available as commercial patch test substances.27 Understanding these potential allergens is important for patch testing considerations as CGM use increases in individuals without diabetes.
Final Thoughts
Allergic contact dermatitis to personal electronic devices including wearables, sensors, and fitness trackers is an emerging problem that should be considered in cases of dermatitis of the wrists, hands, face, ears, or in any area that comes into contact with such devices. Although in-depth studies are lacking, certain wearable devices appear to introduce continuous, low-level allergen exposure that may be below the sensitization threshold but still is capable of eliciting ACD in previously sensitized users.21,26 Furthermore, increased allergen exposure is facilitated by prolonged skin contact, mechanical friction, and sweat.
Comprehensive patch testing often is necessary to diagnose cases of ACD to personal electronic devices.33 The thin-layer rapid use epicutaneous (T.R.U.E.) test does not include (meth)acrylates, which repeatedly have come up as culprit allergens.37 Isobornyl acrylate, a key allergen related to CGMs, is absent from standard patch test series.26 Nickel remains a common culprit in these devices despite adherence to European regulations.21 Since there is no obligation for manufacturers to declare all possible ingredients, chemical analysis can be useful in identifying potential allergens and directing the patch test strategy, but this is not feasible in general clinical practice outside the research setting.2
Following patch testing, patient education is essential to managing personal electronic device—induced ACD. Informed patients should switch to products that do not contain their triggers—although this may be more easily said than done, since incomplete ingredient disclosure from manufacturers may necessitate a frustrating and expensive trial-and-error approach. As wearable technology proliferates, device composition and potential contact allergen transparency must be prioritized by manufacturers and regulatory bodies. Until then, clinicians should stay on their toes regarding new and emerging clinical presentations and contact allergens in hopes of improving patient outcomes.
Personal electronic devices have become more common as consumer-driven health and entertainment practices continue to increase in popularity. A wide variety of devices including smartphones, headphones and earbuds, fitness watches, and continuous glucose monitors (CGMs) allow consumers to collect data and personalize their daily activities and health practices. The global market for fitness tracking devices alone was valued at $62.03 billion in 2024 and is projected to grow to $290.85 billion by 2032.1 Accordingly, the growing demand for continuous data tracking has led to new and prolonged skin contact with these devices, which have become emerging sources of allergic contact dermatitis (ACD). In this article, we provide a summary of the potential allergenicity of personal electronic devices with a focus on wearable devices, including clinical manifestations, reported allergens, and patch testing and management considerations (Table2-28).

Earbuds and Headphones
Wireless earbuds and headphones are used for listening to media and may contain microphones for voice calls. Earbuds are inserted into the ears while headphones are worn over the ears with a connecting band across the scalp. These devices frequently are worn during physical activity and thus in the setting of moist sweaty environments and mechanical friction on the skin. Depending on the style of the earbuds or headphones, associated ACD may manifest as acute or chronic pruritic eczema involving the inner and/or outer ears and potentially the periauricular areas or scalp.2 In a reported case of earbud ACD, the patient first presented to an otolaryngologist before being referred to a dermatologist for further evaluation and patch testing.9 Clinicians may be unfamiliar with these devices as a source of ACD or may potentially overlook inner ear canal manifestations, which may delay diagnosis.
Allergens reported in earbuds include (meth)acrylates,4-6 nickel, gold,8 and silicone.9 Apple AirPods and Samsung Galaxy Buds disclose the presence of acrylates and nickel.5,6 Cases also have been reported of ACD to gold earbud microphones8 and unknown allergens within silicone tips.4,9 Acrylates, named the 2012 Allergen of the Year by the American Contact Dermatitis Society,29 are used in a wide variety of consumer products as adhesives and coatings and are among the most frequently suspected headphone allergens.4 While fully polymerized acrylates theoretically are nonallergenic, residual acrylic monomers are potent allergens that may be found in in these products due to incomplete curing or polymer breakdown.29 It remains unclear whether earbud allergen concentrations are sufficient to induce sensitization or merely elicit ACD in previously sensitized users.29 Among patients with earbud ACD, the finding of inconsistent patch test reactions/cross-reactions led to the hypothesis that these headphones may contain an unidentified proprietary (meth)acrylate.4
Headphones, often utilized by runners and gymgoers for their comfort and fit, also have gained recent attention for their unique allergen profiles. In 2024, a case series described primary sensitization to octylisothiazolinone causing severe headphone-related ACD.3 This preservative, which is in the same family as methylchloroisothiazolinone/methylisothiazolinone, is used as a biocide in the leather or faux leather that encases the foam padding of headphones.3 Another case report highlighted ACD caused by methylisothiazolinone, methylchloroisothiazolinone, and octylisothiazolinone present in various components of a pair of headphones.2 These cases are notable, as European legislation limiting the use of methylchloroisothiazolinone/methylisothiazolinone in personal care products does not apply to inclusion of isothiazolinones in other product categories, such as detergents, paints, glues, and personal electronic devices.
Mobile Phones
Mobile phones are a staple in modern society, used for a multitude of tasks including communication, internet browsing, entertainment, and activity tracking. In the early 2000s, mobile phone ACD primarily manifested on the lateral face, ears, and periauricular regions,12 as well as the thighs from carriage in pants pockets. Early cases of mobile phone ACD were attributed to metals including chromium16 and nickel.14 At that time, lengthy and frequent phone calls with the device against the ear were thought to increase exposure to metal allergens.30 More recently, as the utility of these devices has evolved, ACD has been reported to manifest on the fingers and hands associated with contact with cell phone cases, accessories, and screen protectors (Figure). In one report, a 17-year-old boy with chronic eczema of the palms was diagnosed with ACD to the rubber-related chemicals paraphenylenediamine and N-cyclohexyl-N-phenyl-4-phenylenediamine, confirmed via chemical analysis to be present in a phone case the patient used during daily gaming.17 Similarly, another case of palmar ACD resulted from thiuram rubber accelerators in a phone case.18 Most recently, a Japanese patient with a history of skin reactions to costume jewelry developed ACD involving the proximal middle finger due to exposure to nickel in a ring-grip phone case.11 While the European Union has enacted regulations regarding maximum nickel leaching in products that come into direct and prolonged contact with the skin, such regulations have not been implemented in Japan or the United States.11 International e-commerce makes these grips widely available, even in regions where strict metal regulations are in place. As screen time increases, it is important to consider all phone-related exposures including components of the case, screen protector, and main device body.

Watches
Smart watches and fitness bands are widely available to consumers and serve a variety of health and lifestyle functions. Features include fitness tracking, notification management, mobile payment, electrocardiography, navigation, and sleep and oxygen sensors. Multiple companies have produced hand- and wrist-based sensors for detailed wellness tracking within these categories. Allergic contact dermatitis to smart watches and wristbands manifests as eczematous lesions on the wrist (dorsal,21,22 volar,20 or circumferential involvement23,24).
(Meth)acrylates used to adhere screen protectors, house lithium ion batteries, and bind metal to plastic have been reported to cause ACD in smart watch users.22,25 In addition, there are at least 2 published reports of ACD to nickel in Apple Watches.21,31 Apple, having sold more than 229 million watches worldwide, has acknowledged the presence of trace acrylates and nickel in their watches (the latter falling below European Registration, Evaluation, Authorization, and Restriction of Chemicals limits).32 Hosoki et al20 identified ACD resulting from chromium exposure in the clasp of an Apple Watch band, which remains unreported by the manufacturer as a potential allergen.
Continuous Glucose Monitors
Continuous glucose monitoring systems provide users with dynamic information on their glycemic status and are associated with lower glycated hemoglobin and reduced episodes of hypoglycemia in patients with diabetes.33 Recently, growing interest in personalized health monitoring and performance optimization has expanded CGM use to individuals without diabetes; there are 2 over-the-counter CGM options currently available in the United States.34
Allergic contact dermatitis to CGMs in patients with diabetes is well characterized, manifesting as pruritic acute or chronic dermatitis at the sensor site.27 To date, we are unaware of published cases of ACD associated with use of CGM in individuals without diabetes; however, wearing a CGM during athletic activities and sweating could potentially increase adhesive degradation and/or penetration of allergens in the skin.6
Isobornyl acrylate, named the 2020 Allergen of the Year,35 is the most well-known contact allergen in glucose sensors.36,33 Initially suspected as a component of the CGM skin adhesive, isobornyl acrylate was found to leach from the device body onto the skin in users of one CGM device.36 Other reported allergens in CGM devices include colophony and related rosin derivatives, ethyl cyanoacrylate, and several chemicals that are not available as commercial patch test substances.27 Understanding these potential allergens is important for patch testing considerations as CGM use increases in individuals without diabetes.
Final Thoughts
Allergic contact dermatitis to personal electronic devices including wearables, sensors, and fitness trackers is an emerging problem that should be considered in cases of dermatitis of the wrists, hands, face, ears, or in any area that comes into contact with such devices. Although in-depth studies are lacking, certain wearable devices appear to introduce continuous, low-level allergen exposure that may be below the sensitization threshold but still is capable of eliciting ACD in previously sensitized users.21,26 Furthermore, increased allergen exposure is facilitated by prolonged skin contact, mechanical friction, and sweat.
Comprehensive patch testing often is necessary to diagnose cases of ACD to personal electronic devices.33 The thin-layer rapid use epicutaneous (T.R.U.E.) test does not include (meth)acrylates, which repeatedly have come up as culprit allergens.37 Isobornyl acrylate, a key allergen related to CGMs, is absent from standard patch test series.26 Nickel remains a common culprit in these devices despite adherence to European regulations.21 Since there is no obligation for manufacturers to declare all possible ingredients, chemical analysis can be useful in identifying potential allergens and directing the patch test strategy, but this is not feasible in general clinical practice outside the research setting.2
Following patch testing, patient education is essential to managing personal electronic device—induced ACD. Informed patients should switch to products that do not contain their triggers—although this may be more easily said than done, since incomplete ingredient disclosure from manufacturers may necessitate a frustrating and expensive trial-and-error approach. As wearable technology proliferates, device composition and potential contact allergen transparency must be prioritized by manufacturers and regulatory bodies. Until then, clinicians should stay on their toes regarding new and emerging clinical presentations and contact allergens in hopes of improving patient outcomes.
- Fitness tracker market size, share & industry analysis, by device type (smart watches, fitness bands, smart glasses, smart clothing, and others), by application (heart rate tracking, sleep measurement, glucose measurement, sports, running, and cycling tracking), by distribution channel (online, retail, and others), and regional forecast, 2025-2032. Fortune Business Insights. Updated June 9, 2025. Accessed June 25, 2025. https://www.fortunebusinessinsights.com/fitness-trackermarket-103358
- Caroppo ES, Stingeni L, Goracci L, et al. Wireless over-ear headphones: a new source of allergic contact dermatitis to isothiazolinones. Contact Dermatitis. 2024;90:621-625. doi:10.1111/cod.14528
- Menanteau M, Fenech G, Adam B, et al. Severe allergic contact dermatitis from octylisothiazolinone in over-ear headphones: a case series. Contact Dermatitis. 2025;92:291-298. doi:10.1111/cod.14733
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461. doi:10.1111/ijd.15954
- Fontane Hoyos CN, Goldminz AM. I’m all ears: common allergens in wireless in-ear headphones. Dermatitis. 2024;35:513-514. doi:10.1089/derm.2023.0251
- Lee LJ, Koh WL, Lim SPR. Allergic contact dermatitis to Apple AirPods Pro. Contact Dermatitis. 2022;86:127-129. doi:10.1111/cod.13987
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112. doi:10.1097/der.0000000000000735
- Hayakawa M, Suzuki C, Zhu Y, et al. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermatitis. 2022;86:328-330. doi:10.1111/cod.14036
- Hua W, Jin Y, Yao X, et al. Allergic contact dermatitis to in-ear headphones occurring in the external ear. Contact Dermatitis. 2024;91:83-85. doi:10.1111/cod.14556
- Guarneri F, Guarneri C, Cannavò SP. An unusual case of cell phone dermatitis. Contact Dermatitis. 2010;62:117. doi:10.1111 /j.1600-0536.2009.01674.x
- Ueda S, Akashi K, Washio K. A case of contact dermatitis caused by a cell phone grip ring. Contact Dermatitis. 2025;92:155-156. doi:10.1111/cod.14719
- Roberts H, Tate B. Nickel allergy presenting as mobile phone contact dermatitis. Australas J Dermatol. 2010;51:23-25. doi:10.1111 /j.1440-0960.2009.00580.x
- Livideanu C, Giordano-Labadie F, Paul C. Cellular phone addiction and allergic contact dermatitis to nickel. Contact Dermatitis. 2007;57:130- 131. doi:10.1111/j.1600-0536.2007.01090.x
- Rajpara A, Feldman SR. Cell phone allergic contact dermatitis: case report and review. Dermatol Online J. 2010;16:9.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Seishima M, Oyama Z, Yamamura M. Cellular phone dermatitis. Arch Dermatol. 2002;2:272-273.
- Corazza M, Schettini N, Catani M, et al. Pediatric allergic contact dermatitis due to rubber additives in a cellphone case. Dermatitis. 2021;32:E140-E141. doi:10.1097/der.0000000000000797
- Hamann D, Sköld MB, Hamann CR, et al. Thiuram allergic contact dermatitis on the hands after skin contact with a rubber cellphone case. Contact Dermatitis. 2019;80:130-131. doi:10.1111/cod.13140
- Williams PJ, King C, Arslanian V. Allergic contact dermatitis caused by a cell phone cover. Australas J Dermatol. 2012;53:76-77. doi:10.1111 /j.1440-0960.2011.00801.x
- Hosoki M, Tajima T, Miyagi M, et al. This report details a case of allergic contact dermatitis resulting from exposure to chromium in the clasp of an Apple Watch band. Dermatitis. Published online December 23, 2024. doi:10.1089/derm.2024.0171
- Levian B, Chan GC, Adler BL. Out of REACH: allergic contact dermatitis to nickel in an Apple Watch. Contact Dermatitis. 2024;90:99-101. doi:10.1111 /cod.14444
- Davies A, Stone N. Watch out! potential allergic contact dermatitis to acrylates in a smart watch. Contact Dermatitis. Published online December 26, 2024. doi:10.1111/cod.14749
- Gatica-Ortega ME, Mowitz M, Navarro-Triviño FJ, et al. Nonoccupational allergic contact dermatitis to 4-acryloylmorpholine in smartwatch screen protectors glue. Dermatitis. 2022;33:429-434. doi:10.1097 /der.0000000000000888
- Otero-Alonso A, Rodríguez-Vázquez V, López-Pesado I, et al. Smartwatch protective cover´s glue: a new non-occupational acrylate allergy. Contact Dermatitis. 2020;83:159-161. doi:10.1111/cod.13586
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Mowitz M, Hosseini S, Siemund I, et al. New device, ‘old’ allergens. allergic contact dermatitis caused by the Dexcom G7 glucose sensor. Contact Dermatitis. 2024;90:495-500. doi:10.1111/cod.14514
- de Groot A, van Oers EM, Ipenburg NA, et al. Allergic contact dermatitis caused by glucose sensors and insulin pumps: a full review: part 1: sensors and pumps, adverse cutaneous reactions, allergens, and diabetes devices causing allergic contact dermatitis. Contact Dermatitis. 2025;92:87-112. doi:10.1111/cod.14698
- Oppel E, Kamann S, Heinemann L, et al. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis. 2020;83:429-431. doi:10.1111/cod.13638
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- Tan S, Nixon R. Allergic contact dermatitis caused by chromium in a mobile phone. Contact Dermatitis. 2011;65:246-247. doi:10.1111 /j.1600-0536.2011.01955.x
- Ko WC, Yu J. Nickel allergy elicited by an Apple Watch. Dermatitis. 2022;33:E11-E12. doi:10.1097/der.0000000000000848
- Apple Support. Wearing your Apple Watch: for people who are sensitive to certain materials. Accessed June 27, 2025. https://support.apple.com/en-us/118234
- Seibold A. Minimizing adverse skin reactions to wearable continuous glucose monitoring sensors in patients with diabetes. J Diabetes Sci Technol. 2021;15:713-714. doi:10.1177/1932296820984763
- Klonoff DC, Nguyen KT, Xu NY, et al. Use of continuous glucose monitors by people without diabetes: an idea whose time has come? J Diabetes Sci Technol. 2023;17:1686-1697. doi:10.1177/19322968221110830
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12. doi:10.1097/der.0000000000000549
- Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. 2020;31:283-286. doi:10.1097/der.0000000000000575
- SmartPractice. Contact dermatitis products. SmartPractice. Accessed April 24, 2025. https://www.smartpractice.com/shop/category?id=581719&m=SPA
- Fitness tracker market size, share & industry analysis, by device type (smart watches, fitness bands, smart glasses, smart clothing, and others), by application (heart rate tracking, sleep measurement, glucose measurement, sports, running, and cycling tracking), by distribution channel (online, retail, and others), and regional forecast, 2025-2032. Fortune Business Insights. Updated June 9, 2025. Accessed June 25, 2025. https://www.fortunebusinessinsights.com/fitness-trackermarket-103358
- Caroppo ES, Stingeni L, Goracci L, et al. Wireless over-ear headphones: a new source of allergic contact dermatitis to isothiazolinones. Contact Dermatitis. 2024;90:621-625. doi:10.1111/cod.14528
- Menanteau M, Fenech G, Adam B, et al. Severe allergic contact dermatitis from octylisothiazolinone in over-ear headphones: a case series. Contact Dermatitis. 2025;92:291-298. doi:10.1111/cod.14733
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461. doi:10.1111/ijd.15954
- Fontane Hoyos CN, Goldminz AM. I’m all ears: common allergens in wireless in-ear headphones. Dermatitis. 2024;35:513-514. doi:10.1089/derm.2023.0251
- Lee LJ, Koh WL, Lim SPR. Allergic contact dermatitis to Apple AirPods Pro. Contact Dermatitis. 2022;86:127-129. doi:10.1111/cod.13987
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112. doi:10.1097/der.0000000000000735
- Hayakawa M, Suzuki C, Zhu Y, et al. Allergic contact dermatitis to gold in the parts of in-ear headphones. Contact Dermatitis. 2022;86:328-330. doi:10.1111/cod.14036
- Hua W, Jin Y, Yao X, et al. Allergic contact dermatitis to in-ear headphones occurring in the external ear. Contact Dermatitis. 2024;91:83-85. doi:10.1111/cod.14556
- Guarneri F, Guarneri C, Cannavò SP. An unusual case of cell phone dermatitis. Contact Dermatitis. 2010;62:117. doi:10.1111 /j.1600-0536.2009.01674.x
- Ueda S, Akashi K, Washio K. A case of contact dermatitis caused by a cell phone grip ring. Contact Dermatitis. 2025;92:155-156. doi:10.1111/cod.14719
- Roberts H, Tate B. Nickel allergy presenting as mobile phone contact dermatitis. Australas J Dermatol. 2010;51:23-25. doi:10.1111 /j.1440-0960.2009.00580.x
- Livideanu C, Giordano-Labadie F, Paul C. Cellular phone addiction and allergic contact dermatitis to nickel. Contact Dermatitis. 2007;57:130- 131. doi:10.1111/j.1600-0536.2007.01090.x
- Rajpara A, Feldman SR. Cell phone allergic contact dermatitis: case report and review. Dermatol Online J. 2010;16:9.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Seishima M, Oyama Z, Yamamura M. Cellular phone dermatitis. Arch Dermatol. 2002;2:272-273.
- Corazza M, Schettini N, Catani M, et al. Pediatric allergic contact dermatitis due to rubber additives in a cellphone case. Dermatitis. 2021;32:E140-E141. doi:10.1097/der.0000000000000797
- Hamann D, Sköld MB, Hamann CR, et al. Thiuram allergic contact dermatitis on the hands after skin contact with a rubber cellphone case. Contact Dermatitis. 2019;80:130-131. doi:10.1111/cod.13140
- Williams PJ, King C, Arslanian V. Allergic contact dermatitis caused by a cell phone cover. Australas J Dermatol. 2012;53:76-77. doi:10.1111 /j.1440-0960.2011.00801.x
- Hosoki M, Tajima T, Miyagi M, et al. This report details a case of allergic contact dermatitis resulting from exposure to chromium in the clasp of an Apple Watch band. Dermatitis. Published online December 23, 2024. doi:10.1089/derm.2024.0171
- Levian B, Chan GC, Adler BL. Out of REACH: allergic contact dermatitis to nickel in an Apple Watch. Contact Dermatitis. 2024;90:99-101. doi:10.1111 /cod.14444
- Davies A, Stone N. Watch out! potential allergic contact dermatitis to acrylates in a smart watch. Contact Dermatitis. Published online December 26, 2024. doi:10.1111/cod.14749
- Gatica-Ortega ME, Mowitz M, Navarro-Triviño FJ, et al. Nonoccupational allergic contact dermatitis to 4-acryloylmorpholine in smartwatch screen protectors glue. Dermatitis. 2022;33:429-434. doi:10.1097 /der.0000000000000888
- Otero-Alonso A, Rodríguez-Vázquez V, López-Pesado I, et al. Smartwatch protective cover´s glue: a new non-occupational acrylate allergy. Contact Dermatitis. 2020;83:159-161. doi:10.1111/cod.13586
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Mowitz M, Hosseini S, Siemund I, et al. New device, ‘old’ allergens. allergic contact dermatitis caused by the Dexcom G7 glucose sensor. Contact Dermatitis. 2024;90:495-500. doi:10.1111/cod.14514
- de Groot A, van Oers EM, Ipenburg NA, et al. Allergic contact dermatitis caused by glucose sensors and insulin pumps: a full review: part 1: sensors and pumps, adverse cutaneous reactions, allergens, and diabetes devices causing allergic contact dermatitis. Contact Dermatitis. 2025;92:87-112. doi:10.1111/cod.14698
- Oppel E, Kamann S, Heinemann L, et al. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis. 2020;83:429-431. doi:10.1111/cod.13638
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- Tan S, Nixon R. Allergic contact dermatitis caused by chromium in a mobile phone. Contact Dermatitis. 2011;65:246-247. doi:10.1111 /j.1600-0536.2011.01955.x
- Ko WC, Yu J. Nickel allergy elicited by an Apple Watch. Dermatitis. 2022;33:E11-E12. doi:10.1097/der.0000000000000848
- Apple Support. Wearing your Apple Watch: for people who are sensitive to certain materials. Accessed June 27, 2025. https://support.apple.com/en-us/118234
- Seibold A. Minimizing adverse skin reactions to wearable continuous glucose monitoring sensors in patients with diabetes. J Diabetes Sci Technol. 2021;15:713-714. doi:10.1177/1932296820984763
- Klonoff DC, Nguyen KT, Xu NY, et al. Use of continuous glucose monitors by people without diabetes: an idea whose time has come? J Diabetes Sci Technol. 2023;17:1686-1697. doi:10.1177/19322968221110830
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12. doi:10.1097/der.0000000000000549
- Khatsenko K, Khin Y, Maibach H. Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis. 2020;31:283-286. doi:10.1097/der.0000000000000575
- SmartPractice. Contact dermatitis products. SmartPractice. Accessed April 24, 2025. https://www.smartpractice.com/shop/category?id=581719&m=SPA
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
Wear and Flare: Allergic Contact Dermatitis to Personal Electronic Devices
PRACTICE POINTS
- Personal electronic devices including smart phones, headphones, watches, and continuous glucose monitors represent an emerging source of allergic contact dermatitis.
- Reactions often are localized to areas of skin contact including the face, ears, wrists, and hands.
- Reported allergens in personal electronic devices include (meth)acrylates, metals, and rubber compounds.
- Patch testing is key in detecting and avoiding culprit allergens, but a major challenge is lack of transparency regarding device composition and ingredients.
Continuous Testing Method for Contact Allergy to Topical Therapies in the Management of Chronic and Postoperative Wounds
Patients who undergo cutaneous surgery and chronic wound care often are exposed to various topical
Practice Gap
Contact allergies are common in patients with postoperative or chronic wounds. When patch tested, approximately 80% of patients with chronic venous ulcers demonstrated at least 1 positive allergic reaction based on a Canadian study.3 Similarly, postoperative ACD in dermatologic surgery occurs in more than 1.6% of cases in North America and Europe, a rate that is similar to or higher than the rate of postoperative infection, approximately 1% to 2%.4 Postoperative patients and those with chronic wounds have multiple risk factors for ACD. Firstly, applying topical therapies to inflamed or compromised skin increases the risk for contact sensitization.5 Additionally, multiple topical therapies containing known allergenic components may be recommended for wound care, including impregnated or organic dressings, antibiotic ointments, adhesives, antiseptic washes, and topical therapies containing inactive ingredients such as lanolin derivatives.6 Contact with numerous compounds at the same time increases the risk for a contact allergy as well as co-sensitization.7 Similarly, the longer topical agents are applied, the greater the risk for a contact allergy, with sensitization liable to occur at any point during treatment.
Preventive topical antibiotics have garnered a negative reputation among dermatologists, often due to varying data on their efficacy and the overuse of highly allergenic over-the-counter topical antibiotics such as neomycin.8 However, data also have suggested that topical antibiotics can reduce postoperative infections in higher risk surgical cases, specifically certain head and neck surgeries.9 Likewise, topical antibiotics are useful for wound colonization with Pseudomonas, which can remain superficial and slow down healing without progressing to a systemic infection.10 Such cases can be successfully treated or prevented with topical therapies, thereby bypassing the more concerning adverse effects of systemic antibiotics. In particular, systemic fluoroquinolones often are used to treat Pseudomonas and can have many serious adverse effects, including tendon rupture, drug interactions, and arrhythmias.11 Therefore, it is worth implementing topical treatments for wounds colonized with Pseudomonas to spare patients these potential complications.
When a postoperative patient develops a rash at the surgical site, it is critical to differentiate between wound infection and contact allergy, as the treatments for these two conditions may be mutually exclusive and treating the wrong condition may exacerbate the other, such as mistakenly using topical corticosteroids for a wound infection.7 Prompt treatment is necessary for wound infections, as time is limited for patch testing when a rash is already present and the diagnosis is questionable. Allergic contact dermatitis typically erupts 48 to 96 hours following exposure to a contact allergen, often manifesting as intensely pruritic erythematous patches or vesicles.6 Wound infections are characterized by pain and warmth, with erythema and edema present in both conditions. Postoperative infections manifest usually 4 to 7 days following surgery.12 Despite these differences, pruritus and pain are common in the wound healing process; thus, differentiating an infection from ACD on a clinical basis alone is not always possible. Furthermore, presentation of a contact allergy may be delayed beyond the typical 96-hour timeframe if a patient is newly sensitized to an allergen, causing the timeline of rash development to appear similar to that of a wound infection. In such cases, systemic antibiotics often are prescribed empirically; hence, clearer and timelier differentiation between contact allergy and wound infection reduces unnecessary antibiotic prescriptions, thereby avoiding systemic adverse effects and promoting responsible antibiotic stewardship.12
The Technique
Since potentially allergenic topical therapies often are indicated in wound management, we propose that patients serve as internal controls to test continuously for contact allergy sensitization. We recommend that patients apply a small amount of the topical agent, product, or dressing to the inner forearm each time they apply it to the wound. If the patient is sensitized to the product initially or becomes sensitized during treatment, evidence of ACD will be visible not only at the site of the wound but also in the area of secondary application. The inner forearm is recommended for convenience and reproducibility, but a patient may choose a different site as long as it remains consistent. Although certain contact allergens rarely may react solely at a site of inflamed skin, our team has quickly identified ACD and avoided misdiagnosis of chronic or postsurgical wound infection using this approach.13 Subsequent patch testing is indicated when a contact allergy is detected.
Practice Implications
Topical therapies including ointments, washes, and dressing components have the potential to cause sensitization and contact allergy. Despite the concern for development of ACD, topical antibiotics play a useful role in cutaneous surgery.7 Synchronous testing for contact allergy when managing wounds with topical therapies could improve diagnostic accuracy when an allergic reaction occurs. This technique provides a means of harnessing the benefits of topical agents while monitoring the risk for ACD in postoperative and chronic wound care settings.
Butler L, Mowad C. Allergic contact dermatitis in dermatologic surgery: review of common allergens. Dermatitis. 2013;24:215-221. doi:10.1097/DER.0b013e3182a0d3a9
So SP, Yoon JY, Kim JW. Postoperative contact dermatitis caused by skin adhesives used in orthopedic surgery: incidence, characteristics, and difference from surgical site infection. Medicine (Baltimore). 2021;100:e26053. doi:10.1097/md.0000000000026053
Alavi A, Sibbald RG, Ladizinski B, et al. Wound-related allergic/irritant contact dermatitis. Adv Skin Wound Care. 2016;29:278-286. doi:10.1097/01.ASW.0000482834.94375.1e
Sheth VM, Weitzul S. Postoperative topical antimicrobial use. Dermatitis. 2008;19:181-189.
Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317.e1. doi:10.1016/j.jaad.2016.03.010
Cook KA, Kelso JM. Surgery-related contact dermatitis: a review of potential irritants and allergens. J Allergy Clin Immunol Pract. 2017;5:1234-1240. doi:10.1016/j.jaip.2017.03.001
Kreft B, Wohlrab J. Contact allergies to topical antibiotic applications. Allergol Select. 2022;6:18-26. doi:10.5414/alx02253e
Scherrer MAR, Abreu ÉP, Rocha VB. Neomycin: sources of contact and sensitization evaluation in 1162 patients treated at a tertiary service. An Bras Dermatol. 2023;98:487-492. doi:10.1016/j.abd.2022.07.008
Ashraf DC, Idowu OO, Wang Q, et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology. 2020;127:1747-1754. doi:10.1016/j.ophtha.2020.07.032
Zielin´ska M, Pawłowska A, Orzeł A, et al. Wound microbiota and its impact on wound healing. Int J Mol Sci. 2023;24:17318. doi:10.3390/ijms242417318
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161-164. doi:10.18773/austprescr.2021.035
Ken KM, Johnson MM, Leitenberger JJ, et al. Postoperative infections in dermatologic surgery: the role of wound cultures. Dermatol Surg. 2020;46:1294-1299. doi:10.1097/dss.0000000000002317
Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
Patients who undergo cutaneous surgery and chronic wound care often are exposed to various topical
Practice Gap
Contact allergies are common in patients with postoperative or chronic wounds. When patch tested, approximately 80% of patients with chronic venous ulcers demonstrated at least 1 positive allergic reaction based on a Canadian study.3 Similarly, postoperative ACD in dermatologic surgery occurs in more than 1.6% of cases in North America and Europe, a rate that is similar to or higher than the rate of postoperative infection, approximately 1% to 2%.4 Postoperative patients and those with chronic wounds have multiple risk factors for ACD. Firstly, applying topical therapies to inflamed or compromised skin increases the risk for contact sensitization.5 Additionally, multiple topical therapies containing known allergenic components may be recommended for wound care, including impregnated or organic dressings, antibiotic ointments, adhesives, antiseptic washes, and topical therapies containing inactive ingredients such as lanolin derivatives.6 Contact with numerous compounds at the same time increases the risk for a contact allergy as well as co-sensitization.7 Similarly, the longer topical agents are applied, the greater the risk for a contact allergy, with sensitization liable to occur at any point during treatment.
Preventive topical antibiotics have garnered a negative reputation among dermatologists, often due to varying data on their efficacy and the overuse of highly allergenic over-the-counter topical antibiotics such as neomycin.8 However, data also have suggested that topical antibiotics can reduce postoperative infections in higher risk surgical cases, specifically certain head and neck surgeries.9 Likewise, topical antibiotics are useful for wound colonization with Pseudomonas, which can remain superficial and slow down healing without progressing to a systemic infection.10 Such cases can be successfully treated or prevented with topical therapies, thereby bypassing the more concerning adverse effects of systemic antibiotics. In particular, systemic fluoroquinolones often are used to treat Pseudomonas and can have many serious adverse effects, including tendon rupture, drug interactions, and arrhythmias.11 Therefore, it is worth implementing topical treatments for wounds colonized with Pseudomonas to spare patients these potential complications.
When a postoperative patient develops a rash at the surgical site, it is critical to differentiate between wound infection and contact allergy, as the treatments for these two conditions may be mutually exclusive and treating the wrong condition may exacerbate the other, such as mistakenly using topical corticosteroids for a wound infection.7 Prompt treatment is necessary for wound infections, as time is limited for patch testing when a rash is already present and the diagnosis is questionable. Allergic contact dermatitis typically erupts 48 to 96 hours following exposure to a contact allergen, often manifesting as intensely pruritic erythematous patches or vesicles.6 Wound infections are characterized by pain and warmth, with erythema and edema present in both conditions. Postoperative infections manifest usually 4 to 7 days following surgery.12 Despite these differences, pruritus and pain are common in the wound healing process; thus, differentiating an infection from ACD on a clinical basis alone is not always possible. Furthermore, presentation of a contact allergy may be delayed beyond the typical 96-hour timeframe if a patient is newly sensitized to an allergen, causing the timeline of rash development to appear similar to that of a wound infection. In such cases, systemic antibiotics often are prescribed empirically; hence, clearer and timelier differentiation between contact allergy and wound infection reduces unnecessary antibiotic prescriptions, thereby avoiding systemic adverse effects and promoting responsible antibiotic stewardship.12
The Technique
Since potentially allergenic topical therapies often are indicated in wound management, we propose that patients serve as internal controls to test continuously for contact allergy sensitization. We recommend that patients apply a small amount of the topical agent, product, or dressing to the inner forearm each time they apply it to the wound. If the patient is sensitized to the product initially or becomes sensitized during treatment, evidence of ACD will be visible not only at the site of the wound but also in the area of secondary application. The inner forearm is recommended for convenience and reproducibility, but a patient may choose a different site as long as it remains consistent. Although certain contact allergens rarely may react solely at a site of inflamed skin, our team has quickly identified ACD and avoided misdiagnosis of chronic or postsurgical wound infection using this approach.13 Subsequent patch testing is indicated when a contact allergy is detected.
Practice Implications
Topical therapies including ointments, washes, and dressing components have the potential to cause sensitization and contact allergy. Despite the concern for development of ACD, topical antibiotics play a useful role in cutaneous surgery.7 Synchronous testing for contact allergy when managing wounds with topical therapies could improve diagnostic accuracy when an allergic reaction occurs. This technique provides a means of harnessing the benefits of topical agents while monitoring the risk for ACD in postoperative and chronic wound care settings.
Patients who undergo cutaneous surgery and chronic wound care often are exposed to various topical
Practice Gap
Contact allergies are common in patients with postoperative or chronic wounds. When patch tested, approximately 80% of patients with chronic venous ulcers demonstrated at least 1 positive allergic reaction based on a Canadian study.3 Similarly, postoperative ACD in dermatologic surgery occurs in more than 1.6% of cases in North America and Europe, a rate that is similar to or higher than the rate of postoperative infection, approximately 1% to 2%.4 Postoperative patients and those with chronic wounds have multiple risk factors for ACD. Firstly, applying topical therapies to inflamed or compromised skin increases the risk for contact sensitization.5 Additionally, multiple topical therapies containing known allergenic components may be recommended for wound care, including impregnated or organic dressings, antibiotic ointments, adhesives, antiseptic washes, and topical therapies containing inactive ingredients such as lanolin derivatives.6 Contact with numerous compounds at the same time increases the risk for a contact allergy as well as co-sensitization.7 Similarly, the longer topical agents are applied, the greater the risk for a contact allergy, with sensitization liable to occur at any point during treatment.
Preventive topical antibiotics have garnered a negative reputation among dermatologists, often due to varying data on their efficacy and the overuse of highly allergenic over-the-counter topical antibiotics such as neomycin.8 However, data also have suggested that topical antibiotics can reduce postoperative infections in higher risk surgical cases, specifically certain head and neck surgeries.9 Likewise, topical antibiotics are useful for wound colonization with Pseudomonas, which can remain superficial and slow down healing without progressing to a systemic infection.10 Such cases can be successfully treated or prevented with topical therapies, thereby bypassing the more concerning adverse effects of systemic antibiotics. In particular, systemic fluoroquinolones often are used to treat Pseudomonas and can have many serious adverse effects, including tendon rupture, drug interactions, and arrhythmias.11 Therefore, it is worth implementing topical treatments for wounds colonized with Pseudomonas to spare patients these potential complications.
When a postoperative patient develops a rash at the surgical site, it is critical to differentiate between wound infection and contact allergy, as the treatments for these two conditions may be mutually exclusive and treating the wrong condition may exacerbate the other, such as mistakenly using topical corticosteroids for a wound infection.7 Prompt treatment is necessary for wound infections, as time is limited for patch testing when a rash is already present and the diagnosis is questionable. Allergic contact dermatitis typically erupts 48 to 96 hours following exposure to a contact allergen, often manifesting as intensely pruritic erythematous patches or vesicles.6 Wound infections are characterized by pain and warmth, with erythema and edema present in both conditions. Postoperative infections manifest usually 4 to 7 days following surgery.12 Despite these differences, pruritus and pain are common in the wound healing process; thus, differentiating an infection from ACD on a clinical basis alone is not always possible. Furthermore, presentation of a contact allergy may be delayed beyond the typical 96-hour timeframe if a patient is newly sensitized to an allergen, causing the timeline of rash development to appear similar to that of a wound infection. In such cases, systemic antibiotics often are prescribed empirically; hence, clearer and timelier differentiation between contact allergy and wound infection reduces unnecessary antibiotic prescriptions, thereby avoiding systemic adverse effects and promoting responsible antibiotic stewardship.12
The Technique
Since potentially allergenic topical therapies often are indicated in wound management, we propose that patients serve as internal controls to test continuously for contact allergy sensitization. We recommend that patients apply a small amount of the topical agent, product, or dressing to the inner forearm each time they apply it to the wound. If the patient is sensitized to the product initially or becomes sensitized during treatment, evidence of ACD will be visible not only at the site of the wound but also in the area of secondary application. The inner forearm is recommended for convenience and reproducibility, but a patient may choose a different site as long as it remains consistent. Although certain contact allergens rarely may react solely at a site of inflamed skin, our team has quickly identified ACD and avoided misdiagnosis of chronic or postsurgical wound infection using this approach.13 Subsequent patch testing is indicated when a contact allergy is detected.
Practice Implications
Topical therapies including ointments, washes, and dressing components have the potential to cause sensitization and contact allergy. Despite the concern for development of ACD, topical antibiotics play a useful role in cutaneous surgery.7 Synchronous testing for contact allergy when managing wounds with topical therapies could improve diagnostic accuracy when an allergic reaction occurs. This technique provides a means of harnessing the benefits of topical agents while monitoring the risk for ACD in postoperative and chronic wound care settings.
Butler L, Mowad C. Allergic contact dermatitis in dermatologic surgery: review of common allergens. Dermatitis. 2013;24:215-221. doi:10.1097/DER.0b013e3182a0d3a9
So SP, Yoon JY, Kim JW. Postoperative contact dermatitis caused by skin adhesives used in orthopedic surgery: incidence, characteristics, and difference from surgical site infection. Medicine (Baltimore). 2021;100:e26053. doi:10.1097/md.0000000000026053
Alavi A, Sibbald RG, Ladizinski B, et al. Wound-related allergic/irritant contact dermatitis. Adv Skin Wound Care. 2016;29:278-286. doi:10.1097/01.ASW.0000482834.94375.1e
Sheth VM, Weitzul S. Postoperative topical antimicrobial use. Dermatitis. 2008;19:181-189.
Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317.e1. doi:10.1016/j.jaad.2016.03.010
Cook KA, Kelso JM. Surgery-related contact dermatitis: a review of potential irritants and allergens. J Allergy Clin Immunol Pract. 2017;5:1234-1240. doi:10.1016/j.jaip.2017.03.001
Kreft B, Wohlrab J. Contact allergies to topical antibiotic applications. Allergol Select. 2022;6:18-26. doi:10.5414/alx02253e
Scherrer MAR, Abreu ÉP, Rocha VB. Neomycin: sources of contact and sensitization evaluation in 1162 patients treated at a tertiary service. An Bras Dermatol. 2023;98:487-492. doi:10.1016/j.abd.2022.07.008
Ashraf DC, Idowu OO, Wang Q, et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology. 2020;127:1747-1754. doi:10.1016/j.ophtha.2020.07.032
Zielin´ska M, Pawłowska A, Orzeł A, et al. Wound microbiota and its impact on wound healing. Int J Mol Sci. 2023;24:17318. doi:10.3390/ijms242417318
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161-164. doi:10.18773/austprescr.2021.035
Ken KM, Johnson MM, Leitenberger JJ, et al. Postoperative infections in dermatologic surgery: the role of wound cultures. Dermatol Surg. 2020;46:1294-1299. doi:10.1097/dss.0000000000002317
Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
Butler L, Mowad C. Allergic contact dermatitis in dermatologic surgery: review of common allergens. Dermatitis. 2013;24:215-221. doi:10.1097/DER.0b013e3182a0d3a9
So SP, Yoon JY, Kim JW. Postoperative contact dermatitis caused by skin adhesives used in orthopedic surgery: incidence, characteristics, and difference from surgical site infection. Medicine (Baltimore). 2021;100:e26053. doi:10.1097/md.0000000000026053
Alavi A, Sibbald RG, Ladizinski B, et al. Wound-related allergic/irritant contact dermatitis. Adv Skin Wound Care. 2016;29:278-286. doi:10.1097/01.ASW.0000482834.94375.1e
Sheth VM, Weitzul S. Postoperative topical antimicrobial use. Dermatitis. 2008;19:181-189.
Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317.e1. doi:10.1016/j.jaad.2016.03.010
Cook KA, Kelso JM. Surgery-related contact dermatitis: a review of potential irritants and allergens. J Allergy Clin Immunol Pract. 2017;5:1234-1240. doi:10.1016/j.jaip.2017.03.001
Kreft B, Wohlrab J. Contact allergies to topical antibiotic applications. Allergol Select. 2022;6:18-26. doi:10.5414/alx02253e
Scherrer MAR, Abreu ÉP, Rocha VB. Neomycin: sources of contact and sensitization evaluation in 1162 patients treated at a tertiary service. An Bras Dermatol. 2023;98:487-492. doi:10.1016/j.abd.2022.07.008
Ashraf DC, Idowu OO, Wang Q, et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology. 2020;127:1747-1754. doi:10.1016/j.ophtha.2020.07.032
Zielin´ska M, Pawłowska A, Orzeł A, et al. Wound microbiota and its impact on wound healing. Int J Mol Sci. 2023;24:17318. doi:10.3390/ijms242417318
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161-164. doi:10.18773/austprescr.2021.035
Ken KM, Johnson MM, Leitenberger JJ, et al. Postoperative infections in dermatologic surgery: the role of wound cultures. Dermatol Surg. 2020;46:1294-1299. doi:10.1097/dss.0000000000002317
Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
Erythematous Rash on the Face and Neck
The Diagnosis: Allergic Contact Dermatitis
In our patient, the erythematous pruritic rash on the face and neck, the lack of systemic symptoms, and her history of atopic dermatitis suggested a diagnosis of allergic contact dermatitis (ACD). She underwent patch testing with standard, fragrance, and cosmetic panels in addition to 6 of her personal care products. Her first patch test, which was read on day 2, showed a positive reaction to isopropyl myristate (IPM), a penetration enhancer used in cosmetics, topical medications (eg, tretinoin), and cosmeceuticals. The reading on day 5 showed a 2+ reaction to IPM, which was found in several of her personal care products, including her shampoo, leave-in conditioner, and eczema-calming cream. Isopropyl myristate is used in these products because of its ability to enhance their penetration into the skin and also can be found in commercially used products such as hand sanitizers. The patient was given information on this allergen and how to identify and avoid triggers. At follow-up, the ACD had resolved with avoidance of IPM.
Contact dermatitis is an inflammatory skin condition that is triggered by contact with a specific causative agent. There are 2 types of contact dermatitis: irritant and allergic; the irritant type is more common (approximately 80% of cases worldwide).1 Allergic contact dermatitis is a type IV (delayed-type) hypersensitivity reaction; common causative agents include shampoos, moisturizers, makeup, certain metals (eg, nickel), fragrances, latex, and certain plants (eg, poison ivy).2 In cases of ACD, a new reaction can develop from exposure to a product that the patient has used for years. It manifests clinically as erythema, pruritus, scaling, and vesicle formation.1 Certain populations, such as those with atopic dermatitis, are more prone to developing ACD due to a breakdown of the skin barrier, frequent use of topical products, and immune dysregulation.1,2 Patch testing performed by dermatologists and allergists is the gold standard for diagnosing ACD.1,3
Annually, allergists, dermatologists, and primary care physicians see thousands of cases of contact dermatitis.1 Early recognition and appropriate treatment can help reduce the severity and duration of symptoms and improve patient outcomes. The main treatment for ACD is identification of the causative agent followed by patient education on how to identify and avoid triggers.2 Once patch testing has been completed, patients can be given access to the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) to help them identify and avoid products that contain triggering allergens.
Topical corticosteroids are the first-line pharmacologic treatments for atopic dermatitis.4 When our patient presented with the facial rash, her atopic dermatitis had been well controlled with both dupilumab and topical triamcinolone. The lack of response to previously successful therapies in a new area of involvement made a flare of atopic dermatitis less likely. For flares of ACD after exposure, topical corticosteroids and topical calcineurin inhibitors can help. If needed due to severity, oral corticosteroids also can be used.1
Dermatomyositis is an inflammatory myopathy that has several skin manifestations, including a heliotrope rash and poikiloderma.5 While our patient’s rash covered the periorbital area, she did not have other classic skin findings of dermatomyositis, such as nail-fold capillary changes or poikiloderma in a shawl or holster distribution.6 She also lacked signs of systemic involvement including myositis and elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and creatine kinase levels.5
Erythematotelangiectatic rosacea is characterized by telangiectasias and transient flushing and erythema on the central face.5 Rosacea typically is triggered by temperature changes, alcohol consumption, sun exposure, spicy foods, and stress5 and would be expected to involve the nose, which was not observed in our patient. The fixed nature of our patient’s patches and the absence of telangiectasias also argued against this diagnosis.
The classic cutaneous finding of systemic lupus erythematosus is a malar rash, which appears as erythematous patches or thin plaques across the bridge of the nose and over the cheeks, sparing the nasolabial folds.5 Systemic lupus erythematosus is associated with laboratory abnormalities, such as positive antinuclear antibodies and elevated CRP and ESR levels.5 Our patient had notable sparing of the nose, negative antinuclear antibodies, and normal CRP and ESR levels, making systemic lupus erythematosus unlikely. Systemic lupus erythematosus also can manifest with photosensitivity,7 and involvement of the submental skin in our patient argued against a photosensitive eruption.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76. doi:10.1016/j.mcna.2019.08.012
- Fonacier LS, Sher JM. Allergic contact dermatitis. Ann Allergy Asthma Immunol. 2014;113:9-12. doi:10.1016/j.anai.2014.03.018
- Uyesugi BA, Sheehan MP. Patch testing pearls. Clin Rev Allergy Immunol. 2019;56:110-118. doi:10.1007/s12016-018-8715-y
- Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2018;14(suppl 2):52. doi:10.1186/s13223-018-0281-6
- Naji S. Malar rash. StatPearls. Updated September 4, 2023. Accessed June 30, 2025. https://www.statpearls.com/point-of-care/24661
- Muro Y, Sugiura K, Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features—a comprehensive review. Clin Rev Allergy Immunol. 2016;51:293-302. doi:10.1007 /s12016-015-8496-5
- Hannon CW, McCourt C, Lima HC, et al. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst Rev. 2021;3(3):CD007478. doi:10.1002/14651858.CD007478.pub2
The Diagnosis: Allergic Contact Dermatitis
In our patient, the erythematous pruritic rash on the face and neck, the lack of systemic symptoms, and her history of atopic dermatitis suggested a diagnosis of allergic contact dermatitis (ACD). She underwent patch testing with standard, fragrance, and cosmetic panels in addition to 6 of her personal care products. Her first patch test, which was read on day 2, showed a positive reaction to isopropyl myristate (IPM), a penetration enhancer used in cosmetics, topical medications (eg, tretinoin), and cosmeceuticals. The reading on day 5 showed a 2+ reaction to IPM, which was found in several of her personal care products, including her shampoo, leave-in conditioner, and eczema-calming cream. Isopropyl myristate is used in these products because of its ability to enhance their penetration into the skin and also can be found in commercially used products such as hand sanitizers. The patient was given information on this allergen and how to identify and avoid triggers. At follow-up, the ACD had resolved with avoidance of IPM.
Contact dermatitis is an inflammatory skin condition that is triggered by contact with a specific causative agent. There are 2 types of contact dermatitis: irritant and allergic; the irritant type is more common (approximately 80% of cases worldwide).1 Allergic contact dermatitis is a type IV (delayed-type) hypersensitivity reaction; common causative agents include shampoos, moisturizers, makeup, certain metals (eg, nickel), fragrances, latex, and certain plants (eg, poison ivy).2 In cases of ACD, a new reaction can develop from exposure to a product that the patient has used for years. It manifests clinically as erythema, pruritus, scaling, and vesicle formation.1 Certain populations, such as those with atopic dermatitis, are more prone to developing ACD due to a breakdown of the skin barrier, frequent use of topical products, and immune dysregulation.1,2 Patch testing performed by dermatologists and allergists is the gold standard for diagnosing ACD.1,3
Annually, allergists, dermatologists, and primary care physicians see thousands of cases of contact dermatitis.1 Early recognition and appropriate treatment can help reduce the severity and duration of symptoms and improve patient outcomes. The main treatment for ACD is identification of the causative agent followed by patient education on how to identify and avoid triggers.2 Once patch testing has been completed, patients can be given access to the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) to help them identify and avoid products that contain triggering allergens.
Topical corticosteroids are the first-line pharmacologic treatments for atopic dermatitis.4 When our patient presented with the facial rash, her atopic dermatitis had been well controlled with both dupilumab and topical triamcinolone. The lack of response to previously successful therapies in a new area of involvement made a flare of atopic dermatitis less likely. For flares of ACD after exposure, topical corticosteroids and topical calcineurin inhibitors can help. If needed due to severity, oral corticosteroids also can be used.1
Dermatomyositis is an inflammatory myopathy that has several skin manifestations, including a heliotrope rash and poikiloderma.5 While our patient’s rash covered the periorbital area, she did not have other classic skin findings of dermatomyositis, such as nail-fold capillary changes or poikiloderma in a shawl or holster distribution.6 She also lacked signs of systemic involvement including myositis and elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and creatine kinase levels.5
Erythematotelangiectatic rosacea is characterized by telangiectasias and transient flushing and erythema on the central face.5 Rosacea typically is triggered by temperature changes, alcohol consumption, sun exposure, spicy foods, and stress5 and would be expected to involve the nose, which was not observed in our patient. The fixed nature of our patient’s patches and the absence of telangiectasias also argued against this diagnosis.
The classic cutaneous finding of systemic lupus erythematosus is a malar rash, which appears as erythematous patches or thin plaques across the bridge of the nose and over the cheeks, sparing the nasolabial folds.5 Systemic lupus erythematosus is associated with laboratory abnormalities, such as positive antinuclear antibodies and elevated CRP and ESR levels.5 Our patient had notable sparing of the nose, negative antinuclear antibodies, and normal CRP and ESR levels, making systemic lupus erythematosus unlikely. Systemic lupus erythematosus also can manifest with photosensitivity,7 and involvement of the submental skin in our patient argued against a photosensitive eruption.
The Diagnosis: Allergic Contact Dermatitis
In our patient, the erythematous pruritic rash on the face and neck, the lack of systemic symptoms, and her history of atopic dermatitis suggested a diagnosis of allergic contact dermatitis (ACD). She underwent patch testing with standard, fragrance, and cosmetic panels in addition to 6 of her personal care products. Her first patch test, which was read on day 2, showed a positive reaction to isopropyl myristate (IPM), a penetration enhancer used in cosmetics, topical medications (eg, tretinoin), and cosmeceuticals. The reading on day 5 showed a 2+ reaction to IPM, which was found in several of her personal care products, including her shampoo, leave-in conditioner, and eczema-calming cream. Isopropyl myristate is used in these products because of its ability to enhance their penetration into the skin and also can be found in commercially used products such as hand sanitizers. The patient was given information on this allergen and how to identify and avoid triggers. At follow-up, the ACD had resolved with avoidance of IPM.
Contact dermatitis is an inflammatory skin condition that is triggered by contact with a specific causative agent. There are 2 types of contact dermatitis: irritant and allergic; the irritant type is more common (approximately 80% of cases worldwide).1 Allergic contact dermatitis is a type IV (delayed-type) hypersensitivity reaction; common causative agents include shampoos, moisturizers, makeup, certain metals (eg, nickel), fragrances, latex, and certain plants (eg, poison ivy).2 In cases of ACD, a new reaction can develop from exposure to a product that the patient has used for years. It manifests clinically as erythema, pruritus, scaling, and vesicle formation.1 Certain populations, such as those with atopic dermatitis, are more prone to developing ACD due to a breakdown of the skin barrier, frequent use of topical products, and immune dysregulation.1,2 Patch testing performed by dermatologists and allergists is the gold standard for diagnosing ACD.1,3
Annually, allergists, dermatologists, and primary care physicians see thousands of cases of contact dermatitis.1 Early recognition and appropriate treatment can help reduce the severity and duration of symptoms and improve patient outcomes. The main treatment for ACD is identification of the causative agent followed by patient education on how to identify and avoid triggers.2 Once patch testing has been completed, patients can be given access to the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) to help them identify and avoid products that contain triggering allergens.
Topical corticosteroids are the first-line pharmacologic treatments for atopic dermatitis.4 When our patient presented with the facial rash, her atopic dermatitis had been well controlled with both dupilumab and topical triamcinolone. The lack of response to previously successful therapies in a new area of involvement made a flare of atopic dermatitis less likely. For flares of ACD after exposure, topical corticosteroids and topical calcineurin inhibitors can help. If needed due to severity, oral corticosteroids also can be used.1
Dermatomyositis is an inflammatory myopathy that has several skin manifestations, including a heliotrope rash and poikiloderma.5 While our patient’s rash covered the periorbital area, she did not have other classic skin findings of dermatomyositis, such as nail-fold capillary changes or poikiloderma in a shawl or holster distribution.6 She also lacked signs of systemic involvement including myositis and elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and creatine kinase levels.5
Erythematotelangiectatic rosacea is characterized by telangiectasias and transient flushing and erythema on the central face.5 Rosacea typically is triggered by temperature changes, alcohol consumption, sun exposure, spicy foods, and stress5 and would be expected to involve the nose, which was not observed in our patient. The fixed nature of our patient’s patches and the absence of telangiectasias also argued against this diagnosis.
The classic cutaneous finding of systemic lupus erythematosus is a malar rash, which appears as erythematous patches or thin plaques across the bridge of the nose and over the cheeks, sparing the nasolabial folds.5 Systemic lupus erythematosus is associated with laboratory abnormalities, such as positive antinuclear antibodies and elevated CRP and ESR levels.5 Our patient had notable sparing of the nose, negative antinuclear antibodies, and normal CRP and ESR levels, making systemic lupus erythematosus unlikely. Systemic lupus erythematosus also can manifest with photosensitivity,7 and involvement of the submental skin in our patient argued against a photosensitive eruption.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76. doi:10.1016/j.mcna.2019.08.012
- Fonacier LS, Sher JM. Allergic contact dermatitis. Ann Allergy Asthma Immunol. 2014;113:9-12. doi:10.1016/j.anai.2014.03.018
- Uyesugi BA, Sheehan MP. Patch testing pearls. Clin Rev Allergy Immunol. 2019;56:110-118. doi:10.1007/s12016-018-8715-y
- Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2018;14(suppl 2):52. doi:10.1186/s13223-018-0281-6
- Naji S. Malar rash. StatPearls. Updated September 4, 2023. Accessed June 30, 2025. https://www.statpearls.com/point-of-care/24661
- Muro Y, Sugiura K, Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features—a comprehensive review. Clin Rev Allergy Immunol. 2016;51:293-302. doi:10.1007 /s12016-015-8496-5
- Hannon CW, McCourt C, Lima HC, et al. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst Rev. 2021;3(3):CD007478. doi:10.1002/14651858.CD007478.pub2
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76. doi:10.1016/j.mcna.2019.08.012
- Fonacier LS, Sher JM. Allergic contact dermatitis. Ann Allergy Asthma Immunol. 2014;113:9-12. doi:10.1016/j.anai.2014.03.018
- Uyesugi BA, Sheehan MP. Patch testing pearls. Clin Rev Allergy Immunol. 2019;56:110-118. doi:10.1007/s12016-018-8715-y
- Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2018;14(suppl 2):52. doi:10.1186/s13223-018-0281-6
- Naji S. Malar rash. StatPearls. Updated September 4, 2023. Accessed June 30, 2025. https://www.statpearls.com/point-of-care/24661
- Muro Y, Sugiura K, Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features—a comprehensive review. Clin Rev Allergy Immunol. 2016;51:293-302. doi:10.1007 /s12016-015-8496-5
- Hannon CW, McCourt C, Lima HC, et al. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst Rev. 2021;3(3):CD007478. doi:10.1002/14651858.CD007478.pub2
A 23-year-old woman with atopic dermatitis and seasonal allergic rhinitis presented to the dermatology department with an erythematous pruritic rash of 1 year’s duration involving the forehead, periorbital and submental skin, and neck. The patient’s atopic dermatitis was stable and had been well controlled with dupilumab and topical triamcinolone as needed for flares. The patient denied any other symptoms including fever, fatigue, and muscle weakness. Physical examination of the hands and nails revealed no abnormalities. She was treated with topical triamcinolone acetonide 0.1% without improvement. Short-term prednisone tapers fully resolved the rash, but it recurred within 5 days after discontinuation of prednisone. Results of testing for rheumatoid factor, antinuclear antibodies, complete blood count, comprehensive metabolic panel, C-reactive protein, erythrocyte sedimentation rate, and antistreptolysin O antibodies were unremarkable.
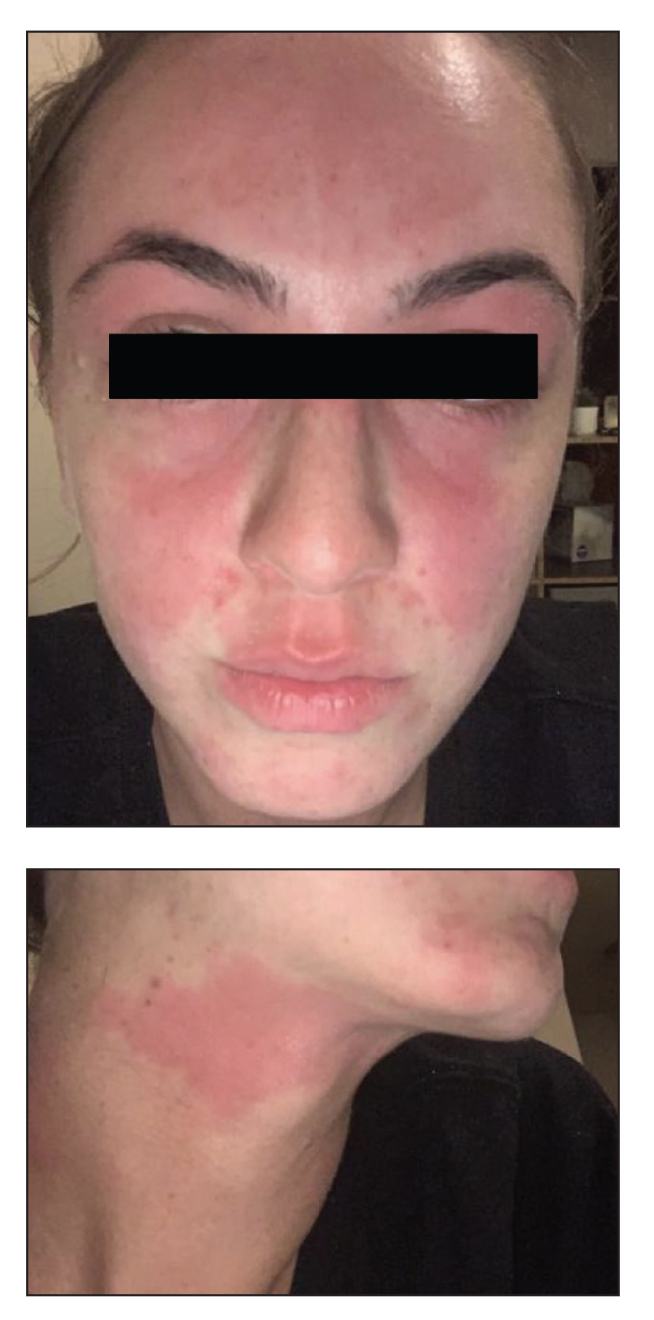
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
The yellow fly (Diachlorus ferrugatus) is a flying biting insect belonging to the order Diptera, family Tabanidae, which also includes deer flies (genus Chrysops) and horse flies (genus Tabanus).1 They are different from stinging insects of the order Hymenoptera (bees, wasps, yellow jackets, and hornets). As the name suggests, the yellow fly has a distinct yellow appearance, and adult yellow flies have a body length of approximately 1 cm.1,2 Distinguishing features of the yellow fly include prominently dark forelegs (the remaining legs are yellow), dark purple to black eyes with 2 fluorescent green lines, and a yellow abdomen with black hairs along the lateral regions and a broad central yellow stripe.1-3 Their wings have longitudinal black veins with clear spaces in between and a conspicuous brown patch at the apex (Figure 1A). In comparison, horse flies are darker and larger (Figure 1B), and deer flies are similar in shape but have stripes on the abdomen and thorax and mottled wings with dark patches near the apex (Figure 1C).1

The Tabanidae family comprises 4455 species belonging to 137 genera and is notorious for bites that result in localized pain, swelling, itching, and discomfort.4 While some Tabanidae species are mechanical or biologic vectors of pathogens (eg, Loa loa, equine infectious anemia virus, Trypanosoma species, cattle and sheep anthrax and tularemia), yellow flies do not appear to play a considerable role in disease transmission.4,5 Nonetheless, their bites can cause discomfort and create a nuisance for individuals residing within their distribution areas as well as for agricultural livestock, contributing to lower weight gain and milk production.1
Yellow flies are a commonly occurring species in the southeastern United States; their distribution spans several states, including New Jersey, Florida, and Texas.1,2 In Florida, specifically, yellow flies exhibit a seasonal pattern, with peak activity typically occurring from April through June.6-9 Activity levels are heightened around sunset as well as sunrise.1,9 Tabanids can be found in forests, parks, and gardens—particularly those that contain waterways such as freshwater lakes and streams—and typically stay near shaded woodlands that are prone to flooding.9
Tabanids go through the life cycle stages of egg, larva, pupa, and adult; the life cycle typically spans 1 year, with the adults living 30 to 60 days.1 Mating occurs soon after adults emerge from the pupal case in the soil.1,10 Females then are attracted to large dark moving objects and will feed on blood to develop eggs.2,10 Only female members of the Tabanidae family have modifications of the mouth parts that allow wounding of the skin (Figure 2). Their bites introduce saliva to the skin containing anticoagulants and other likely allergens. The tongue is used to lap between 20 to 600 microliters of blood.11 Males feed primarily on pollen and nectar.10 Most tabanid bites result in transient wheal-and-flare reactions, but some can result in more severe allergic reactions such as in our reported case.10 Rarely, anaphylactic reactions have been documented.10,12

Case Report
A 48-year-old man presented with swelling of the left hand following a yellow fly bite to the wrist 30 minutes prior while he worked outside at a ranch in central Florida (Figure 3). The patient was afebrile and reported no respiratory or gastrointestinal symptoms. The left hand and forearm were warm to the touch and appeared red and edematous (Figure 4). He was not tachycardic and did not appear to be in any distress. The patient reported that he had worked on the ranch for several years, and during that time had noted he was developing worsening localized reactions to yellow fly bites. He had visually identified the offending insect prior to the current presentation and had trapped some flies in previous incidents. Recently he had experienced rapid swelling at the bite sites but had never experienced respiratory difficulties or signs of systemic allergic reactions. He previously had used topical steroids when bites resulted in mild wheal-and-flare reactions, but he reported that these were no longer effective.
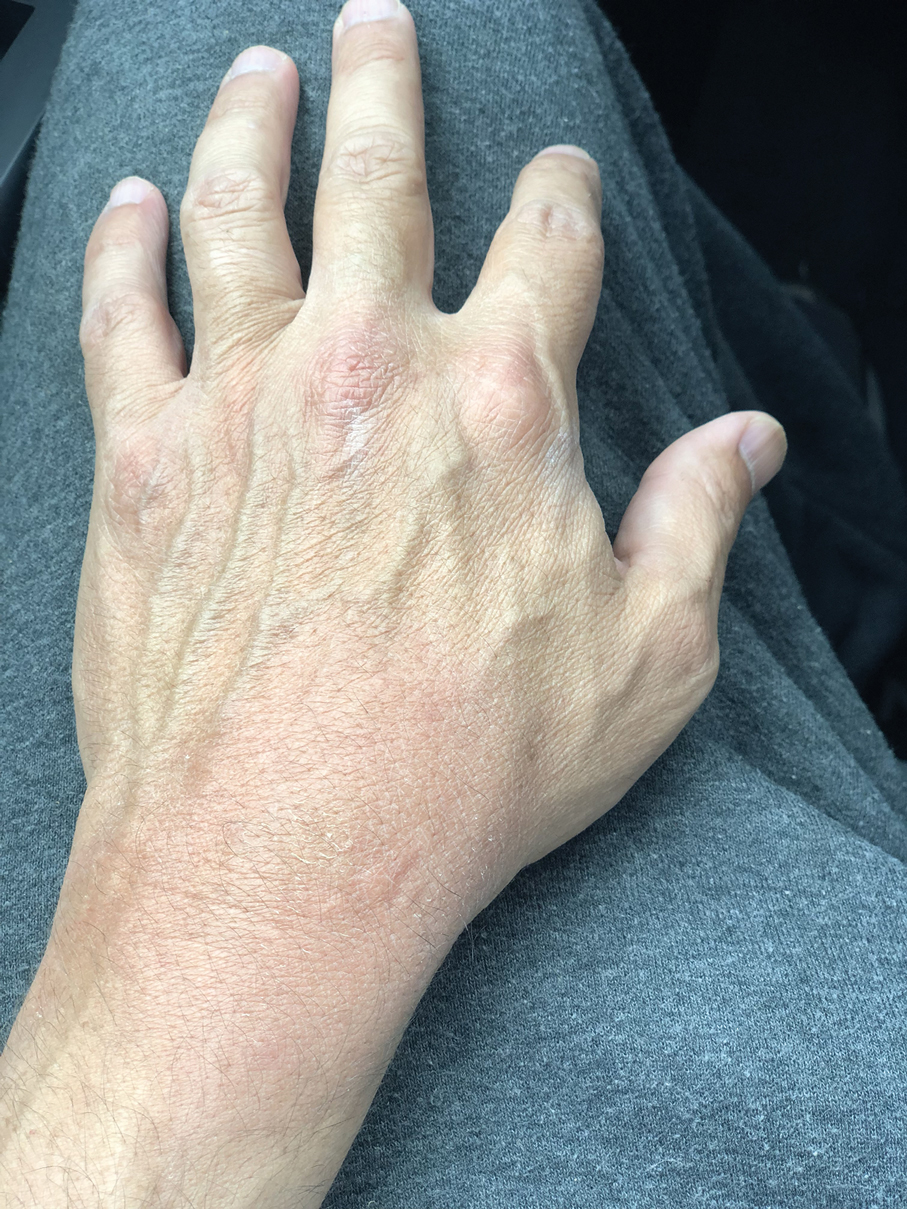

Management of the current bite reaction included oral prednisone tapered over 1 week from 40 mg to 10 mg daily as well as oral cetirizine 10 mg daily. Although bacterial cellulitis was considered in the differential diagnosis, no oral antibiotics were prescribed given the patient’s history of similar clinical presentations following yellow fly bites. His symptoms resolved within a few hours of his dose of prednisone. Incidentally, our patient has been able to control the progression of subsequent hypersensitivity reactions to yellow fly bites with a single 20-mg dose of prednisone administered at the onset of the bite.
Comment
In general, blood-feeding (hematophagous) insects rarely cause anaphylaxis and are more likely to cause cutaneous hypersensitivity reactions, possibly due to the small amount of antigen injected from a bite.13,14 The immediate wheal-and-flare reaction is an IgE-mediated type 1 immune reaction compared to a less common type 4 T-cell mediated delayed hypersensitivity reaction.14,15 There are many protein allergens in the saliva of biting insects that are not well characterized. Relevant allergens include a 69 kDa salivary gland protein as well as a Tab y 1 (anticoagulant), Tab y 2 (hyaluronidase), and Tab y 5 (antigen 5–related venom protein).11,15-17 Some of these proteins have structural homology between insects of different orders and can cause cross-reactivity in patients who also are allergic to Hymenoptera stings (wasp-horsefly syndrome).12,16
Our patient’s cutaneous reaction was localized and clinically manifested with rapidly progressive erythema and edema at the bite location. He did not exhibit signs of a systemic reaction such as angioedema, respiratory or gastrointestinal symptoms, tachycardia, or hypotension. Management of affected patients depends on the extent of the reaction and may include oral or parenteral antihistamines as well as oral steroids for more severe edema.11 Anaphylactic reactions generally respond to subcutaneous epinephrine.15 It would be prudent for patients with a relevant anaphylactic history to carry an autoinjectable epinephrine pen in case of difficulty breathing or general malaise following a bite. Besides avoidance of insect bites, personal protection methods include wearing long-sleeved shirts and pants and using insect repellents containing diethyl toluamide (DEET), citronella, or geraniol.1
At present, diagnosis of cutaneous reactions to yellow fly bites is best made based on the patient’s personal history.14 If the offending fly is trapped, it can be identified. As most patients cannot differentiate between insects, it may be helpful for dermatologists to know that a small amount of blood at the bite site is suggestive of a fly bite rather than a sting from a member of the order Hymenoptera. Currently, there are no consistently useful extracts for intradermal skin testing.11 Although there are several commercially available serum-specific IgE tests for suspected horse fly reactions, their usefulness is doubtful without further information on sensitivity and specificity as well as the allergen utilized.11,18,19 The use of allergen immunotherapy to induce hyposensitization in patients who experience cutaneous reactions is not standardized and poses some risks including severe allergic reactions requiring facilities for resuscitation, variability of response patterns, and supporting evidence is weak.11
Final Thoughts
Cutaneous reactions to yellow fly bites rarely are described in the dermatology literature. The salivary proteins implicated in inducing an allergic response and cross-reactivity of D ferrugatus with other biting and stinging insects as well as the natural course of immune reactions over time need to be further characterized.
- Squitier JM. Deer flies, yellow flies, and horse flies, Chrysops, Diachlorus and Tabanus spp. (Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN155
- Fairchild GB, Weems HB Jr, Fasulo TR. Yellow fly, Diachlorus ferrugatus (Fabricius)(Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN595
- Mullens BA. Horse flies and deer flies (Tabanidae). In: Mullen G, Durden L. Med Vet Entomol. Elsevier Science; 2009:327-344.
- Akhoundi M, Sereno D, Marteau A, et al. Who bites me? A tentative discriminative key to diagnose hematophagous ectoparasites biting using clinical manifestations. Diagnostics (Basel). 2020;10:308.
- Cheng TC. General Parasitology. 2nd ed. Elsevier Science; 2021:660.
- Wells K, Varnadoe C, Dorman D, et al. Survey of the distribution and seasonal activity of yellow flies (Diptera: Tabanidae) in Florida, USA. J Vector Ecol. 2019;44:235-242.
- Hribar LJ, Leppla NC, Beshear RJ, et al. Seasonal abundance of Diachlorus ferrugatus (Diptera: Tabanidae) in Monroe County, Florida. Florida Scientist. 2003;66:52-54.
- Fairchild GB, Weems HV. Diachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular. 1973;139.
- Cilek JE, Schreiber ET. Diel host-seeking activity of adult Diachlorus ferrugatus (F.) (Diptera: Tabanidae) in Northwestern Florida. J Entomol Sci. 1999;34:462-466.
- Sean S. Tabanids (horseflies). Dermatol Online J. 1999;5:6.
- Whyte AF, Popeseu FD, Carlson J. Tabanidae insect (horsefly and deerfly) allergy in humans: a review of the literature. Clin Exp Allergy. 2020;50:886-893.
- Buonomo A, Rizzi A, Aruanno A, et al. Anaphylaxis after horsefly sting: a strange case of wasp-horsefly syndrome. Postepi Dermatol Alergol. 2021;2:331-332.
- Freye HB, Litwin C. Coexistent anaphylaxis to Diptera and Hymenoptera. Ann Allergy Asthma Immunol. 1996 76:270-272.
- Hemmer W, Wantke F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol Select. 2020;4:97-104.
- Ewan PW. Allergy to insect stings: a review. J R Soc Med. 1985;78:234-239.
- Ma D, Li Y, Dong J, et al. Purification and characterization of two new allergens from the salivary glands of the horsefly Tabanus yao. Allergy. 2011;66:101-109.
- Hemmer W, Focke M, Vieluf D, et al. Anaphylaxis induced by horsefly bites: identification of a 69 kd IgE-binding protein from Chrysops spp. (Diptera: Tabanidae) by western blot analysis. J Allergy Clin Immunol. 1998;101:134-136.
- Mayo Clinic Laboratories. Test catalog: horse fly. Accessed March 11, 2025. https://www.mayocliniclabs.com/search?q=horse%20fly
- HealthLabs.com. Horsefly allergy test. Accessed March 11, 2025. https://www.healthlabs.com/horsefly-allergy-testing
The yellow fly (Diachlorus ferrugatus) is a flying biting insect belonging to the order Diptera, family Tabanidae, which also includes deer flies (genus Chrysops) and horse flies (genus Tabanus).1 They are different from stinging insects of the order Hymenoptera (bees, wasps, yellow jackets, and hornets). As the name suggests, the yellow fly has a distinct yellow appearance, and adult yellow flies have a body length of approximately 1 cm.1,2 Distinguishing features of the yellow fly include prominently dark forelegs (the remaining legs are yellow), dark purple to black eyes with 2 fluorescent green lines, and a yellow abdomen with black hairs along the lateral regions and a broad central yellow stripe.1-3 Their wings have longitudinal black veins with clear spaces in between and a conspicuous brown patch at the apex (Figure 1A). In comparison, horse flies are darker and larger (Figure 1B), and deer flies are similar in shape but have stripes on the abdomen and thorax and mottled wings with dark patches near the apex (Figure 1C).1

The Tabanidae family comprises 4455 species belonging to 137 genera and is notorious for bites that result in localized pain, swelling, itching, and discomfort.4 While some Tabanidae species are mechanical or biologic vectors of pathogens (eg, Loa loa, equine infectious anemia virus, Trypanosoma species, cattle and sheep anthrax and tularemia), yellow flies do not appear to play a considerable role in disease transmission.4,5 Nonetheless, their bites can cause discomfort and create a nuisance for individuals residing within their distribution areas as well as for agricultural livestock, contributing to lower weight gain and milk production.1
Yellow flies are a commonly occurring species in the southeastern United States; their distribution spans several states, including New Jersey, Florida, and Texas.1,2 In Florida, specifically, yellow flies exhibit a seasonal pattern, with peak activity typically occurring from April through June.6-9 Activity levels are heightened around sunset as well as sunrise.1,9 Tabanids can be found in forests, parks, and gardens—particularly those that contain waterways such as freshwater lakes and streams—and typically stay near shaded woodlands that are prone to flooding.9
Tabanids go through the life cycle stages of egg, larva, pupa, and adult; the life cycle typically spans 1 year, with the adults living 30 to 60 days.1 Mating occurs soon after adults emerge from the pupal case in the soil.1,10 Females then are attracted to large dark moving objects and will feed on blood to develop eggs.2,10 Only female members of the Tabanidae family have modifications of the mouth parts that allow wounding of the skin (Figure 2). Their bites introduce saliva to the skin containing anticoagulants and other likely allergens. The tongue is used to lap between 20 to 600 microliters of blood.11 Males feed primarily on pollen and nectar.10 Most tabanid bites result in transient wheal-and-flare reactions, but some can result in more severe allergic reactions such as in our reported case.10 Rarely, anaphylactic reactions have been documented.10,12

Case Report
A 48-year-old man presented with swelling of the left hand following a yellow fly bite to the wrist 30 minutes prior while he worked outside at a ranch in central Florida (Figure 3). The patient was afebrile and reported no respiratory or gastrointestinal symptoms. The left hand and forearm were warm to the touch and appeared red and edematous (Figure 4). He was not tachycardic and did not appear to be in any distress. The patient reported that he had worked on the ranch for several years, and during that time had noted he was developing worsening localized reactions to yellow fly bites. He had visually identified the offending insect prior to the current presentation and had trapped some flies in previous incidents. Recently he had experienced rapid swelling at the bite sites but had never experienced respiratory difficulties or signs of systemic allergic reactions. He previously had used topical steroids when bites resulted in mild wheal-and-flare reactions, but he reported that these were no longer effective.


Management of the current bite reaction included oral prednisone tapered over 1 week from 40 mg to 10 mg daily as well as oral cetirizine 10 mg daily. Although bacterial cellulitis was considered in the differential diagnosis, no oral antibiotics were prescribed given the patient’s history of similar clinical presentations following yellow fly bites. His symptoms resolved within a few hours of his dose of prednisone. Incidentally, our patient has been able to control the progression of subsequent hypersensitivity reactions to yellow fly bites with a single 20-mg dose of prednisone administered at the onset of the bite.
Comment
In general, blood-feeding (hematophagous) insects rarely cause anaphylaxis and are more likely to cause cutaneous hypersensitivity reactions, possibly due to the small amount of antigen injected from a bite.13,14 The immediate wheal-and-flare reaction is an IgE-mediated type 1 immune reaction compared to a less common type 4 T-cell mediated delayed hypersensitivity reaction.14,15 There are many protein allergens in the saliva of biting insects that are not well characterized. Relevant allergens include a 69 kDa salivary gland protein as well as a Tab y 1 (anticoagulant), Tab y 2 (hyaluronidase), and Tab y 5 (antigen 5–related venom protein).11,15-17 Some of these proteins have structural homology between insects of different orders and can cause cross-reactivity in patients who also are allergic to Hymenoptera stings (wasp-horsefly syndrome).12,16
Our patient’s cutaneous reaction was localized and clinically manifested with rapidly progressive erythema and edema at the bite location. He did not exhibit signs of a systemic reaction such as angioedema, respiratory or gastrointestinal symptoms, tachycardia, or hypotension. Management of affected patients depends on the extent of the reaction and may include oral or parenteral antihistamines as well as oral steroids for more severe edema.11 Anaphylactic reactions generally respond to subcutaneous epinephrine.15 It would be prudent for patients with a relevant anaphylactic history to carry an autoinjectable epinephrine pen in case of difficulty breathing or general malaise following a bite. Besides avoidance of insect bites, personal protection methods include wearing long-sleeved shirts and pants and using insect repellents containing diethyl toluamide (DEET), citronella, or geraniol.1
At present, diagnosis of cutaneous reactions to yellow fly bites is best made based on the patient’s personal history.14 If the offending fly is trapped, it can be identified. As most patients cannot differentiate between insects, it may be helpful for dermatologists to know that a small amount of blood at the bite site is suggestive of a fly bite rather than a sting from a member of the order Hymenoptera. Currently, there are no consistently useful extracts for intradermal skin testing.11 Although there are several commercially available serum-specific IgE tests for suspected horse fly reactions, their usefulness is doubtful without further information on sensitivity and specificity as well as the allergen utilized.11,18,19 The use of allergen immunotherapy to induce hyposensitization in patients who experience cutaneous reactions is not standardized and poses some risks including severe allergic reactions requiring facilities for resuscitation, variability of response patterns, and supporting evidence is weak.11
Final Thoughts
Cutaneous reactions to yellow fly bites rarely are described in the dermatology literature. The salivary proteins implicated in inducing an allergic response and cross-reactivity of D ferrugatus with other biting and stinging insects as well as the natural course of immune reactions over time need to be further characterized.
The yellow fly (Diachlorus ferrugatus) is a flying biting insect belonging to the order Diptera, family Tabanidae, which also includes deer flies (genus Chrysops) and horse flies (genus Tabanus).1 They are different from stinging insects of the order Hymenoptera (bees, wasps, yellow jackets, and hornets). As the name suggests, the yellow fly has a distinct yellow appearance, and adult yellow flies have a body length of approximately 1 cm.1,2 Distinguishing features of the yellow fly include prominently dark forelegs (the remaining legs are yellow), dark purple to black eyes with 2 fluorescent green lines, and a yellow abdomen with black hairs along the lateral regions and a broad central yellow stripe.1-3 Their wings have longitudinal black veins with clear spaces in between and a conspicuous brown patch at the apex (Figure 1A). In comparison, horse flies are darker and larger (Figure 1B), and deer flies are similar in shape but have stripes on the abdomen and thorax and mottled wings with dark patches near the apex (Figure 1C).1

The Tabanidae family comprises 4455 species belonging to 137 genera and is notorious for bites that result in localized pain, swelling, itching, and discomfort.4 While some Tabanidae species are mechanical or biologic vectors of pathogens (eg, Loa loa, equine infectious anemia virus, Trypanosoma species, cattle and sheep anthrax and tularemia), yellow flies do not appear to play a considerable role in disease transmission.4,5 Nonetheless, their bites can cause discomfort and create a nuisance for individuals residing within their distribution areas as well as for agricultural livestock, contributing to lower weight gain and milk production.1
Yellow flies are a commonly occurring species in the southeastern United States; their distribution spans several states, including New Jersey, Florida, and Texas.1,2 In Florida, specifically, yellow flies exhibit a seasonal pattern, with peak activity typically occurring from April through June.6-9 Activity levels are heightened around sunset as well as sunrise.1,9 Tabanids can be found in forests, parks, and gardens—particularly those that contain waterways such as freshwater lakes and streams—and typically stay near shaded woodlands that are prone to flooding.9
Tabanids go through the life cycle stages of egg, larva, pupa, and adult; the life cycle typically spans 1 year, with the adults living 30 to 60 days.1 Mating occurs soon after adults emerge from the pupal case in the soil.1,10 Females then are attracted to large dark moving objects and will feed on blood to develop eggs.2,10 Only female members of the Tabanidae family have modifications of the mouth parts that allow wounding of the skin (Figure 2). Their bites introduce saliva to the skin containing anticoagulants and other likely allergens. The tongue is used to lap between 20 to 600 microliters of blood.11 Males feed primarily on pollen and nectar.10 Most tabanid bites result in transient wheal-and-flare reactions, but some can result in more severe allergic reactions such as in our reported case.10 Rarely, anaphylactic reactions have been documented.10,12

Case Report
A 48-year-old man presented with swelling of the left hand following a yellow fly bite to the wrist 30 minutes prior while he worked outside at a ranch in central Florida (Figure 3). The patient was afebrile and reported no respiratory or gastrointestinal symptoms. The left hand and forearm were warm to the touch and appeared red and edematous (Figure 4). He was not tachycardic and did not appear to be in any distress. The patient reported that he had worked on the ranch for several years, and during that time had noted he was developing worsening localized reactions to yellow fly bites. He had visually identified the offending insect prior to the current presentation and had trapped some flies in previous incidents. Recently he had experienced rapid swelling at the bite sites but had never experienced respiratory difficulties or signs of systemic allergic reactions. He previously had used topical steroids when bites resulted in mild wheal-and-flare reactions, but he reported that these were no longer effective.


Management of the current bite reaction included oral prednisone tapered over 1 week from 40 mg to 10 mg daily as well as oral cetirizine 10 mg daily. Although bacterial cellulitis was considered in the differential diagnosis, no oral antibiotics were prescribed given the patient’s history of similar clinical presentations following yellow fly bites. His symptoms resolved within a few hours of his dose of prednisone. Incidentally, our patient has been able to control the progression of subsequent hypersensitivity reactions to yellow fly bites with a single 20-mg dose of prednisone administered at the onset of the bite.
Comment
In general, blood-feeding (hematophagous) insects rarely cause anaphylaxis and are more likely to cause cutaneous hypersensitivity reactions, possibly due to the small amount of antigen injected from a bite.13,14 The immediate wheal-and-flare reaction is an IgE-mediated type 1 immune reaction compared to a less common type 4 T-cell mediated delayed hypersensitivity reaction.14,15 There are many protein allergens in the saliva of biting insects that are not well characterized. Relevant allergens include a 69 kDa salivary gland protein as well as a Tab y 1 (anticoagulant), Tab y 2 (hyaluronidase), and Tab y 5 (antigen 5–related venom protein).11,15-17 Some of these proteins have structural homology between insects of different orders and can cause cross-reactivity in patients who also are allergic to Hymenoptera stings (wasp-horsefly syndrome).12,16
Our patient’s cutaneous reaction was localized and clinically manifested with rapidly progressive erythema and edema at the bite location. He did not exhibit signs of a systemic reaction such as angioedema, respiratory or gastrointestinal symptoms, tachycardia, or hypotension. Management of affected patients depends on the extent of the reaction and may include oral or parenteral antihistamines as well as oral steroids for more severe edema.11 Anaphylactic reactions generally respond to subcutaneous epinephrine.15 It would be prudent for patients with a relevant anaphylactic history to carry an autoinjectable epinephrine pen in case of difficulty breathing or general malaise following a bite. Besides avoidance of insect bites, personal protection methods include wearing long-sleeved shirts and pants and using insect repellents containing diethyl toluamide (DEET), citronella, or geraniol.1
At present, diagnosis of cutaneous reactions to yellow fly bites is best made based on the patient’s personal history.14 If the offending fly is trapped, it can be identified. As most patients cannot differentiate between insects, it may be helpful for dermatologists to know that a small amount of blood at the bite site is suggestive of a fly bite rather than a sting from a member of the order Hymenoptera. Currently, there are no consistently useful extracts for intradermal skin testing.11 Although there are several commercially available serum-specific IgE tests for suspected horse fly reactions, their usefulness is doubtful without further information on sensitivity and specificity as well as the allergen utilized.11,18,19 The use of allergen immunotherapy to induce hyposensitization in patients who experience cutaneous reactions is not standardized and poses some risks including severe allergic reactions requiring facilities for resuscitation, variability of response patterns, and supporting evidence is weak.11
Final Thoughts
Cutaneous reactions to yellow fly bites rarely are described in the dermatology literature. The salivary proteins implicated in inducing an allergic response and cross-reactivity of D ferrugatus with other biting and stinging insects as well as the natural course of immune reactions over time need to be further characterized.
- Squitier JM. Deer flies, yellow flies, and horse flies, Chrysops, Diachlorus and Tabanus spp. (Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN155
- Fairchild GB, Weems HB Jr, Fasulo TR. Yellow fly, Diachlorus ferrugatus (Fabricius)(Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN595
- Mullens BA. Horse flies and deer flies (Tabanidae). In: Mullen G, Durden L. Med Vet Entomol. Elsevier Science; 2009:327-344.
- Akhoundi M, Sereno D, Marteau A, et al. Who bites me? A tentative discriminative key to diagnose hematophagous ectoparasites biting using clinical manifestations. Diagnostics (Basel). 2020;10:308.
- Cheng TC. General Parasitology. 2nd ed. Elsevier Science; 2021:660.
- Wells K, Varnadoe C, Dorman D, et al. Survey of the distribution and seasonal activity of yellow flies (Diptera: Tabanidae) in Florida, USA. J Vector Ecol. 2019;44:235-242.
- Hribar LJ, Leppla NC, Beshear RJ, et al. Seasonal abundance of Diachlorus ferrugatus (Diptera: Tabanidae) in Monroe County, Florida. Florida Scientist. 2003;66:52-54.
- Fairchild GB, Weems HV. Diachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular. 1973;139.
- Cilek JE, Schreiber ET. Diel host-seeking activity of adult Diachlorus ferrugatus (F.) (Diptera: Tabanidae) in Northwestern Florida. J Entomol Sci. 1999;34:462-466.
- Sean S. Tabanids (horseflies). Dermatol Online J. 1999;5:6.
- Whyte AF, Popeseu FD, Carlson J. Tabanidae insect (horsefly and deerfly) allergy in humans: a review of the literature. Clin Exp Allergy. 2020;50:886-893.
- Buonomo A, Rizzi A, Aruanno A, et al. Anaphylaxis after horsefly sting: a strange case of wasp-horsefly syndrome. Postepi Dermatol Alergol. 2021;2:331-332.
- Freye HB, Litwin C. Coexistent anaphylaxis to Diptera and Hymenoptera. Ann Allergy Asthma Immunol. 1996 76:270-272.
- Hemmer W, Wantke F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol Select. 2020;4:97-104.
- Ewan PW. Allergy to insect stings: a review. J R Soc Med. 1985;78:234-239.
- Ma D, Li Y, Dong J, et al. Purification and characterization of two new allergens from the salivary glands of the horsefly Tabanus yao. Allergy. 2011;66:101-109.
- Hemmer W, Focke M, Vieluf D, et al. Anaphylaxis induced by horsefly bites: identification of a 69 kd IgE-binding protein from Chrysops spp. (Diptera: Tabanidae) by western blot analysis. J Allergy Clin Immunol. 1998;101:134-136.
- Mayo Clinic Laboratories. Test catalog: horse fly. Accessed March 11, 2025. https://www.mayocliniclabs.com/search?q=horse%20fly
- HealthLabs.com. Horsefly allergy test. Accessed March 11, 2025. https://www.healthlabs.com/horsefly-allergy-testing
- Squitier JM. Deer flies, yellow flies, and horse flies, Chrysops, Diachlorus and Tabanus spp. (Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN155
- Fairchild GB, Weems HB Jr, Fasulo TR. Yellow fly, Diachlorus ferrugatus (Fabricius)(Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN595
- Mullens BA. Horse flies and deer flies (Tabanidae). In: Mullen G, Durden L. Med Vet Entomol. Elsevier Science; 2009:327-344.
- Akhoundi M, Sereno D, Marteau A, et al. Who bites me? A tentative discriminative key to diagnose hematophagous ectoparasites biting using clinical manifestations. Diagnostics (Basel). 2020;10:308.
- Cheng TC. General Parasitology. 2nd ed. Elsevier Science; 2021:660.
- Wells K, Varnadoe C, Dorman D, et al. Survey of the distribution and seasonal activity of yellow flies (Diptera: Tabanidae) in Florida, USA. J Vector Ecol. 2019;44:235-242.
- Hribar LJ, Leppla NC, Beshear RJ, et al. Seasonal abundance of Diachlorus ferrugatus (Diptera: Tabanidae) in Monroe County, Florida. Florida Scientist. 2003;66:52-54.
- Fairchild GB, Weems HV. Diachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular. 1973;139.
- Cilek JE, Schreiber ET. Diel host-seeking activity of adult Diachlorus ferrugatus (F.) (Diptera: Tabanidae) in Northwestern Florida. J Entomol Sci. 1999;34:462-466.
- Sean S. Tabanids (horseflies). Dermatol Online J. 1999;5:6.
- Whyte AF, Popeseu FD, Carlson J. Tabanidae insect (horsefly and deerfly) allergy in humans: a review of the literature. Clin Exp Allergy. 2020;50:886-893.
- Buonomo A, Rizzi A, Aruanno A, et al. Anaphylaxis after horsefly sting: a strange case of wasp-horsefly syndrome. Postepi Dermatol Alergol. 2021;2:331-332.
- Freye HB, Litwin C. Coexistent anaphylaxis to Diptera and Hymenoptera. Ann Allergy Asthma Immunol. 1996 76:270-272.
- Hemmer W, Wantke F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol Select. 2020;4:97-104.
- Ewan PW. Allergy to insect stings: a review. J R Soc Med. 1985;78:234-239.
- Ma D, Li Y, Dong J, et al. Purification and characterization of two new allergens from the salivary glands of the horsefly Tabanus yao. Allergy. 2011;66:101-109.
- Hemmer W, Focke M, Vieluf D, et al. Anaphylaxis induced by horsefly bites: identification of a 69 kd IgE-binding protein from Chrysops spp. (Diptera: Tabanidae) by western blot analysis. J Allergy Clin Immunol. 1998;101:134-136.
- Mayo Clinic Laboratories. Test catalog: horse fly. Accessed March 11, 2025. https://www.mayocliniclabs.com/search?q=horse%20fly
- HealthLabs.com. Horsefly allergy test. Accessed March 11, 2025. https://www.healthlabs.com/horsefly-allergy-testing
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
PRACTICE POINTS
- Diachlorus ferrugatus, commonly known as the yellow fly, belongs to the Tabanidae family of insects that also includes deer flies and horse flies.
- The female yellow fly can instill a painful bite in humans and can cause local and systemic allergic reactions.
- Medical management of yellow fly bites is dictated by the severity of the reaction.
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15
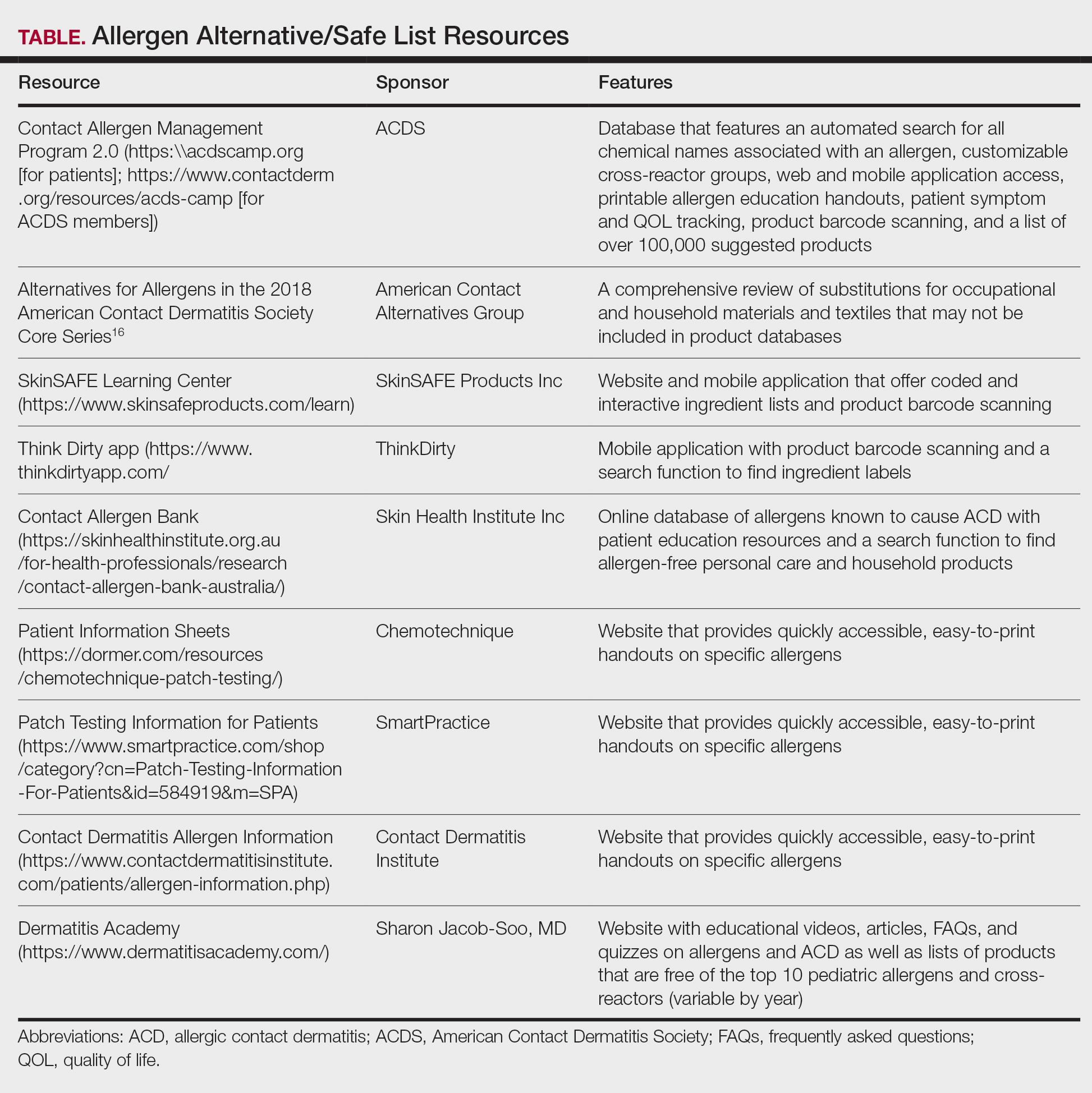
Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.
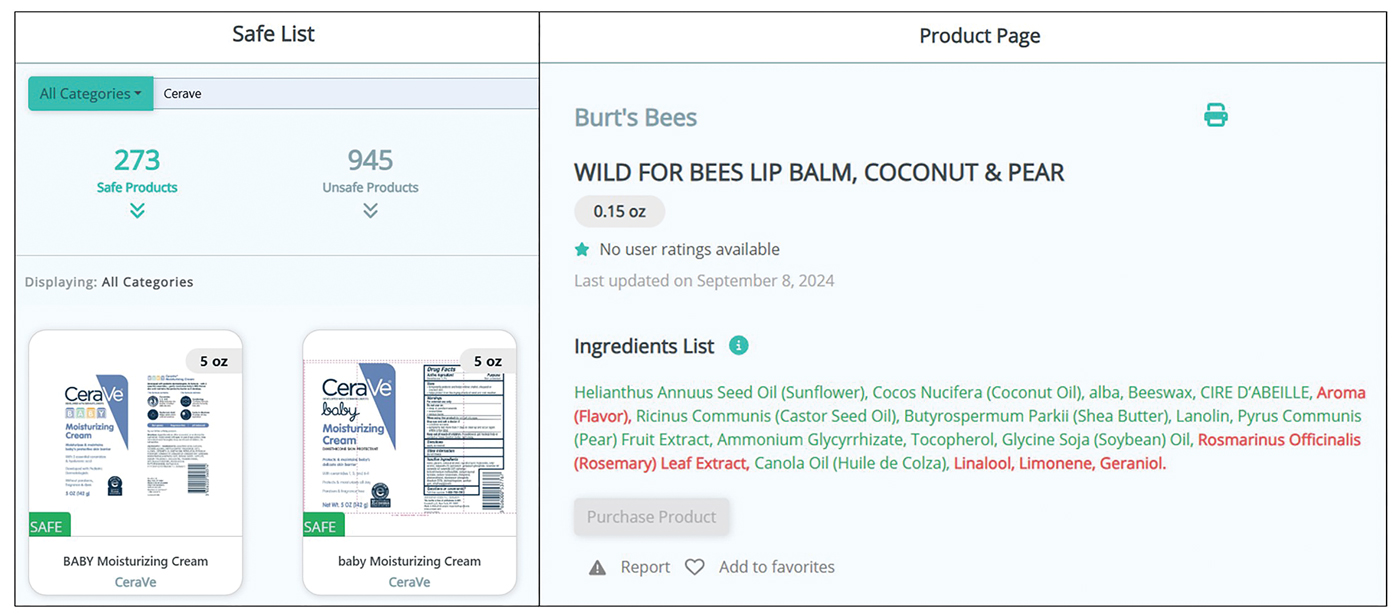
Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.
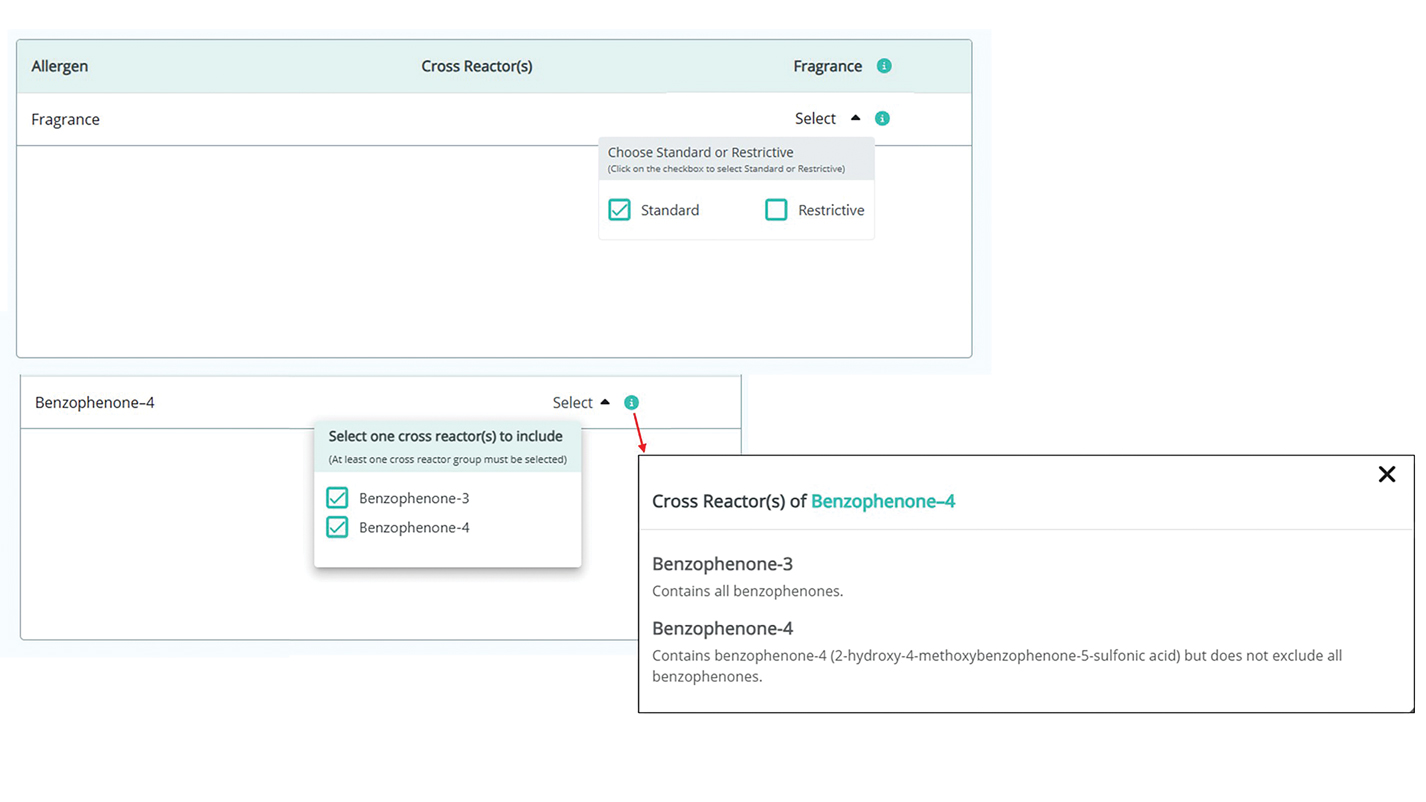
Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
PRACTICE POINTS
- Comprehensive patient education is critical for appropriate allergen avoidance after patch testing, and allergen databases and product safe lists are invaluable tools to complement clinical guidance.
- The updated Contact Allergen Management Program 2.0 offers an updated approach to patient guidance, including a database of more than 100,000 products and an easy-to-use platform to find safe, allergen-free products.
- Interactive learning resources, product pages, and quality-of-life tracking tools can help equip patients with information to encourage further autonomy in allergen avoidance.
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
To the Editor:
Uveitis associated with tattoos is common, yet the etiology and optimal treatment options for this phenomenon remain unclear. Possible causes include a delayed hypersensitivity reaction to tattoo ink antigen or systemic sarcoidosis localized to the skin.1 Long-term treatment options include topical, intralesional, and systemic corticosteroids or immunosuppressants.2 Short-term options often include direct surgical excision and laser treatment. However, laser removal of tattoo pigment typically involves multiple sessions over the course of years, and there is a risk for antigen dispersal that may lead to anaphylaxis. Determining the most effective and safe treatment for a patient with progressive and severe ocular symptoms can be challenging. We describe a patient with cutaneous blue ink tattoos who developed chronic bilateral glaucoma, iritis, uveitis, and ocular hypertension that was refractory to multiple systemic medications and ophthalmologic procedures but responded to CO2 laser ablation.
A 27-year-old man with an active smoking history presented to our laser surgery center with a rash of approximately 4 years’ duration in areas with blue tattoo ink on both forearms. He was referred by his ophthalmologist due to bilateral uveitis and iritis and subsequent ocular hypertension and glaucoma that developed approximately 5 years after tattoo placement on the bilateral forearms. When the rash first appeared, the skin in the areas of the blue tattoo ink had hyperpigmented pruritic plaques. The patient was treated by a dermatologist with topical steroids to help reduce the itching and inflammation. Around the same time, he also started having ocular symptoms—vitreous floaters, erythema, eye pain, and blurriness—and was diagnosed with iritis of unclear etiology by ophthalmology. Figure 1 documents the patient’s clinical course. Due to escalating intraocular pressure and symptoms, he was referred to a glaucoma specialist and a rheumatologist. Systemic and rheumatologic medical conditions were ruled out with negative results on a series of blood tests (eg, rheumatoid factor, HLA-B27, antinuclear antibody, lysozyme, interferon gamma release assay, erythrocyte sedimentation rate, C-reactive protein, hepatitis B/C virus, Treponema pallidum, HIV), and magnetic resonance imaging of the brain was negative, ruling out demyelinating disease. Laboratory workup for sarcoidosis also was performed. The angiotensin-converting enzyme level was 30 U/L (reference range, 9-67 U/L), and a chest radiograph and computed tomography with contrast indicated no evidence of cardiopulmonary involvement. Although sarcoidosis could not be definitively ruled out, no other cause could be determined, and the patient’s glaucoma specialist diagnosed him with tattoo-associated uveitis. The patient was started on brimonidine, latanoprost, prednisolone, and dorzolamidetimolol eye drops, as well as acetazolamide (500 mg twice daily) and oral prednisone (various doses). Over the next 3 years, the patient continued to have symptoms, and immunosuppressant medications—methotrexate 20-25 mg weekly and adalimumab 40 mg every 2 weeks—were added to his treatment regimen. The patient also underwent bilateral ophthalmologic procedures, including a Baerveldt glaucoma implant procedure in the left eye and circumferential trabeculectomy in the right eye.
Despite these medications and procedures, the patient’s symptoms and intraocular pressure had not improved. At the current visit, punch biopsy of the tattooed skin and histologic examination showed dermal lymphoplasmacytic inflammation with scattered foreign-body giant cells associated with blue tattoo ink and overlying hyperkeratosis and spongiosis, consistent with allergic contact dermatitis (Figure 2). Because both immunosuppressant medications and ophthalmologic procedures had failed to control the progression of the ocular symptoms and the patient was at risk for permanent blindness, surgical excision and laser tattoo removal were considered as potential treatment options. Due to the large surface area and circumferential nature of the tattoos, there was a notable risk for disfiguring scars at both recipient and donor sites with surgical excision followed by graft placement. Thus, CO2 laser ablation was the preferred treatment option. However, this procedure was not without risk for anaphylaxis if the tattoo pigment were to be released into systemic circulation. Thus, at the first visit, ablation was performed on 3 test spots and the patient was prescribed cetirizine, diphenhydramine, and prophylactic prednisone for a few days. The patient then received a total of 5 fully ablative CO2 laser sessions (pulse energy: 200 mJ [15 J/cm2]; computerized pattern generator: 2-8-9 [85.2 J/cm2]; rate: 200 Hz [20 W], 3 passes) over 13 months to remove all visible blue ink in stages (Figure 3). Even with a shortened time course (as more time between laser sessions typically is preferred), the treatments were well tolerated with only mild hypertrophic scarring that responded to intralesional steroids (triamcinolone 10 mg/mL). On repeat skin biopsy during the treatment course, the superficial dermis demonstrated mostly scar tissue and near-total pigment removal—a 90% to 95% reduction in blue ink from prior biopsy—and minimal inflammation (Figure 4). Scant fine to coarse pigment deposition was seen in the deep dermis next to subcutaneous fat, which was unchanged from the previous biopsy. The patient’s ophthalmologic symptoms were tracked via improvement in intraocular pressure and stabilization of his vision, indicating rapid and complete resolution of the glaucoma after the last laser treatment. With resolution of his ocular symptoms, the patient was tapered off all immunosuppressant medications. The patient was lost to follow-up approximately 2 years after the final laser treatment.
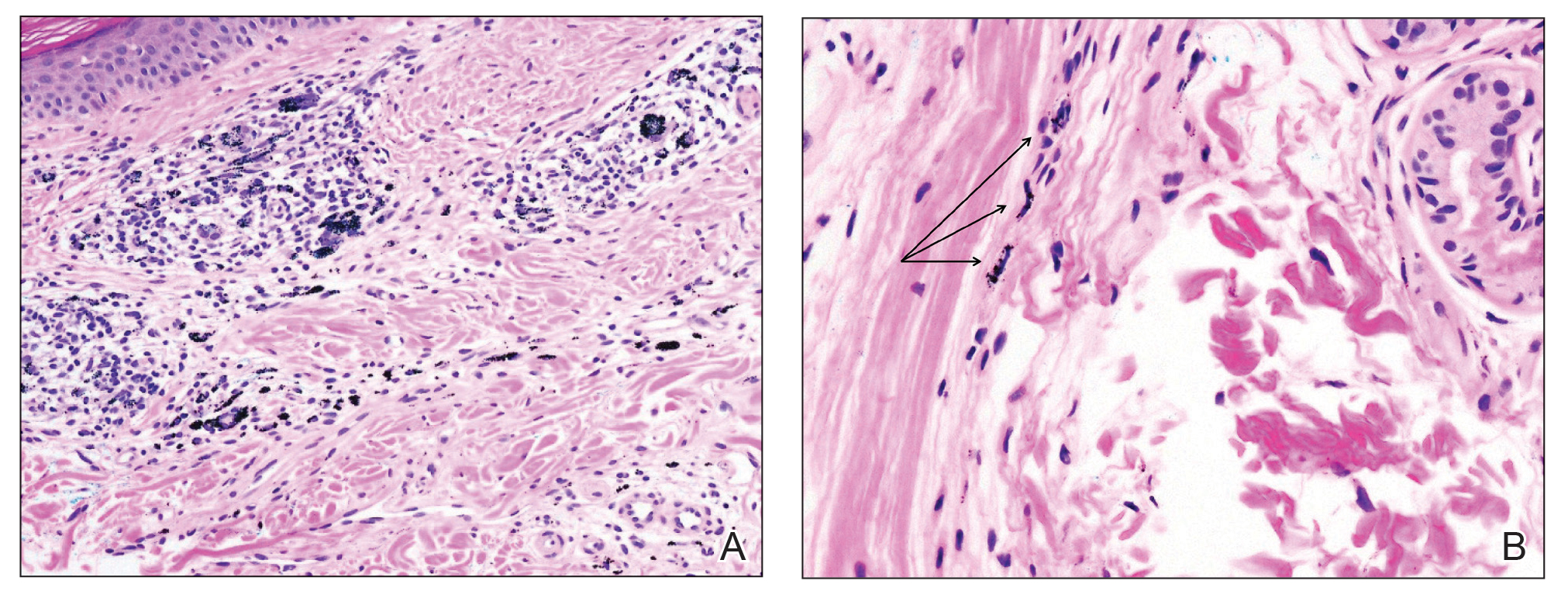

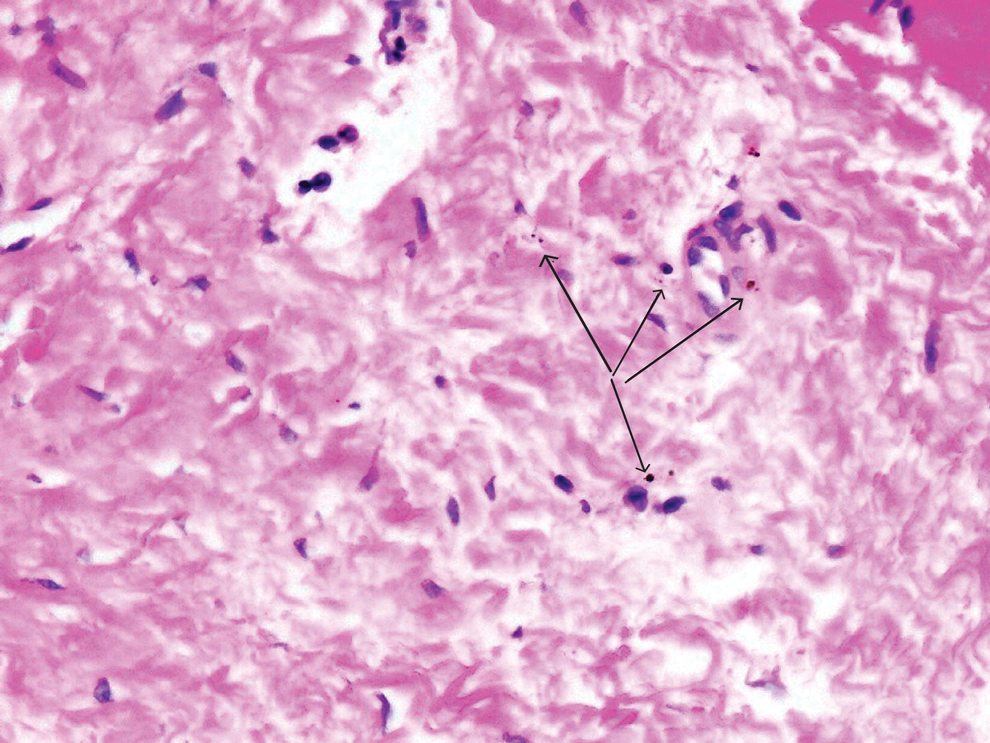
Tattoo-associated uveitis initially was described in 1969 in 3 patients with light blue tattoos who developed tattoo granulomas and simultaneous uveitis. These cases were successfully treated with excision.3 Multiple cases have been reported since, often with bilateral uveitis and tattoos demonstrating noncaseating granulomatous inflammation that were treated with steroids.4 In 2018, a diagnosis of exclusion was proposed for uveitis associated with granulomatous tattoo reaction without sarcoidosis: tattoo granulomas with uveitis (TAGU).1
In this case, sarcoidosis initially was high on the differential diagnosis. Sarcoidosis is an immune-mediated systemic disease of unknown etiology characterized by the presence of widespread noncaseating epithelioid cell granulomas, primarily seen in the pulmonary and lymphatic systems. However, it often initially manifests with cutaneous involvement with noncaseating “naked” granulomas in the dermis and subcutaneous tissue. Although TAGU cases have demonstrated noncaseating granulomas in association with dermal tattoo pigment on histopathology,1,4 dermal lymphoplasmacytic inflammation with scattered foreign body giant cells was noted in our patient, which was more consistent with allergic contact dermatitis. Thus, it is important to consider that TAGU can be seen with varying histologic patterns. In patients with tattoos, sarcoidosis can manifest grossly as a papulonodular cutaneous reaction.5 Active smoking is associated with a decreased risk for sarcoidosis, and those who smoke are statistically more likely to have tattoos than the general population,6,7 so our patient’s smoking history may be relevant. However, sarcoidosis was an unlikely diagnosis due to the serum angiotensin-converting enzyme level; results of a chest radiograph (bilateral adenopathy and coarse reticular opacities) and computed tomography (hilar and mediastinal adenopathy); and nonsarcoidal histopathology.
An allergic reaction to tattoo ink is caused by a delayed-type hypersensitivity reaction to a pigment hapten that can develop abruptly months to years after tattoo placement—1 year after tattoo placement in our patient. This reaction was seen in our patient’s blue pigment tattoos, although it is more commonly seen in red pigment tattoos.8 Although the etiology of TAGU is poorly understood, it also is hypothesized to be a delayed-type hypersensitivity response to tattoo ink particles, suggested by the pattern of lymphocytes infiltrating the tattoo and atypical T-cell infiltrate on vitreous biopsy.9,10 Further research is required to elucidate the relationship between tattoos and uveitis.
Q-switched lasers (eg, 532-nm or 1064-nm Nd:YAG, alexandrite, or ruby lasers) are the standard treatment options for uncomplicated tattoo removal and employ a high-intensity, ultrashort pulse duration.11 However Q-switched lasers require multiple sessions and target pigment-containing cells, releasing the tattoo particles into systemic circulation, which can potentially induce a severe allergic response.12 In contrast, CO2 lasers use a different mechanism, emitting energy at a wavelength of 10,600 nm, which is absorbed by intracellular water and allows for the ablation of the superficial epidermis along with the embedded ink with subsequent re-epithelialization, as well as heat-mediated thermal injury to allow for dermal collagen remodeling.13 In a 2021 retrospective study of ablative laser therapy for allergic tattoo reactions, patients were treated with the 10,600-nm ablative CO2 laser and noted improvements in itching and burning with minimal adverse events.12 Although using a CO2 laser may not be considered a firstline treatment option for TAGU, the refractory clinical course and notable morbidity of surgical excision necessitated the use of ablative laser in our case.
Tattoo granulomas with uveitis is a rare diagnosis with the potential for serious permanent sequelae including blindness. Existing treatments such as topical and oral corticosteroids, immunosuppressants, surgical excision, and Q-switched lasers all are possible options, but in a patient with progressive ocular symptoms with other potential rheumatologic conditions and sarcoidosis ruled out, fully ablative CO2 laser may be an effective treatment option. Our case demonstrated the successful treatment of TAGU with CO2 laser ablation. Given the unclear etiology of TAGU and the limited evidence on treatment options and efficacy, our case contributes to the body of literature that can inform clinical management of this unusual and serious reaction.
- Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852-1861. doi:10.1111/jdv.15070
- Tiew S. Tattoo-associated panuveitis: a 10-year follow-up. Eur J Ophthalmol. 2019;29(1 suppl):18-21. doi:10.1177/1120672119846341
- Rorsman H, Brehmer-Andersson E, Dahlquist I, et al. Tattoo granuloma and uveitis. Lancet. 1969;2:27-28. doi:10.1016/s0140-6736(69)92600-2
- Ostheimer TA, Burkholder BM, Leung TG, et al. Tattoo-associated uveitis. Am J Ophthalmol. 2014;158:637-643.e1. doi:10.1016/j.ajo.2014.05.019
- Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679-686. doi:10.1159/000453315
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383: 1155-1167. doi:10.1016/S0140-6736(13)60680-7
- Kluger N. Epidemiology of tattoos in industrialized countries. Curr Probl Dermatol. 2015;48:6-20. doi:10.1159/000369175
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82. doi:10.1111/cod.13423
- Mansour AM, Chan CC. Recurrent uveitis preceded by swelling of skin tattoos. Am J Ophthalmol. 1991;111:515-516. doi:10.1016/s0002-9394(14)72395-5
- Reddy AK, Shildkrot Y, Newman SA, et al. T-lymphocyte predominance and cellular atypia in tattoo-associated uveitis. JAMA Ophthalmol. 2015;133:1356-1357. doi:10.1001/jamaophthalmol.2015.3354
- Wenzel SM. Current concepts in laser tattoo removal. Skin Therapy Lett. 2010;15:3-5.
- van der Bent SAS, Huisman S, Rustemeyer T, et al. Ablative laser surgery for allergic tattoo reactions: a retrospective study. mLasers Med Sci. 2021;36:1241-1248. doi:10.1007/s10103-020-03164-2
- Yumeen S, Khan T. Laser carbon dioxide resurfacing. In: StatPearls. StatPearls Publishing; April 23, 2023. Accessed March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560544/
To the Editor:
Uveitis associated with tattoos is common, yet the etiology and optimal treatment options for this phenomenon remain unclear. Possible causes include a delayed hypersensitivity reaction to tattoo ink antigen or systemic sarcoidosis localized to the skin.1 Long-term treatment options include topical, intralesional, and systemic corticosteroids or immunosuppressants.2 Short-term options often include direct surgical excision and laser treatment. However, laser removal of tattoo pigment typically involves multiple sessions over the course of years, and there is a risk for antigen dispersal that may lead to anaphylaxis. Determining the most effective and safe treatment for a patient with progressive and severe ocular symptoms can be challenging. We describe a patient with cutaneous blue ink tattoos who developed chronic bilateral glaucoma, iritis, uveitis, and ocular hypertension that was refractory to multiple systemic medications and ophthalmologic procedures but responded to CO2 laser ablation.
A 27-year-old man with an active smoking history presented to our laser surgery center with a rash of approximately 4 years’ duration in areas with blue tattoo ink on both forearms. He was referred by his ophthalmologist due to bilateral uveitis and iritis and subsequent ocular hypertension and glaucoma that developed approximately 5 years after tattoo placement on the bilateral forearms. When the rash first appeared, the skin in the areas of the blue tattoo ink had hyperpigmented pruritic plaques. The patient was treated by a dermatologist with topical steroids to help reduce the itching and inflammation. Around the same time, he also started having ocular symptoms—vitreous floaters, erythema, eye pain, and blurriness—and was diagnosed with iritis of unclear etiology by ophthalmology. Figure 1 documents the patient’s clinical course. Due to escalating intraocular pressure and symptoms, he was referred to a glaucoma specialist and a rheumatologist. Systemic and rheumatologic medical conditions were ruled out with negative results on a series of blood tests (eg, rheumatoid factor, HLA-B27, antinuclear antibody, lysozyme, interferon gamma release assay, erythrocyte sedimentation rate, C-reactive protein, hepatitis B/C virus, Treponema pallidum, HIV), and magnetic resonance imaging of the brain was negative, ruling out demyelinating disease. Laboratory workup for sarcoidosis also was performed. The angiotensin-converting enzyme level was 30 U/L (reference range, 9-67 U/L), and a chest radiograph and computed tomography with contrast indicated no evidence of cardiopulmonary involvement. Although sarcoidosis could not be definitively ruled out, no other cause could be determined, and the patient’s glaucoma specialist diagnosed him with tattoo-associated uveitis. The patient was started on brimonidine, latanoprost, prednisolone, and dorzolamidetimolol eye drops, as well as acetazolamide (500 mg twice daily) and oral prednisone (various doses). Over the next 3 years, the patient continued to have symptoms, and immunosuppressant medications—methotrexate 20-25 mg weekly and adalimumab 40 mg every 2 weeks—were added to his treatment regimen. The patient also underwent bilateral ophthalmologic procedures, including a Baerveldt glaucoma implant procedure in the left eye and circumferential trabeculectomy in the right eye.

Despite these medications and procedures, the patient’s symptoms and intraocular pressure had not improved. At the current visit, punch biopsy of the tattooed skin and histologic examination showed dermal lymphoplasmacytic inflammation with scattered foreign-body giant cells associated with blue tattoo ink and overlying hyperkeratosis and spongiosis, consistent with allergic contact dermatitis (Figure 2). Because both immunosuppressant medications and ophthalmologic procedures had failed to control the progression of the ocular symptoms and the patient was at risk for permanent blindness, surgical excision and laser tattoo removal were considered as potential treatment options. Due to the large surface area and circumferential nature of the tattoos, there was a notable risk for disfiguring scars at both recipient and donor sites with surgical excision followed by graft placement. Thus, CO2 laser ablation was the preferred treatment option. However, this procedure was not without risk for anaphylaxis if the tattoo pigment were to be released into systemic circulation. Thus, at the first visit, ablation was performed on 3 test spots and the patient was prescribed cetirizine, diphenhydramine, and prophylactic prednisone for a few days. The patient then received a total of 5 fully ablative CO2 laser sessions (pulse energy: 200 mJ [15 J/cm2]; computerized pattern generator: 2-8-9 [85.2 J/cm2]; rate: 200 Hz [20 W], 3 passes) over 13 months to remove all visible blue ink in stages (Figure 3). Even with a shortened time course (as more time between laser sessions typically is preferred), the treatments were well tolerated with only mild hypertrophic scarring that responded to intralesional steroids (triamcinolone 10 mg/mL). On repeat skin biopsy during the treatment course, the superficial dermis demonstrated mostly scar tissue and near-total pigment removal—a 90% to 95% reduction in blue ink from prior biopsy—and minimal inflammation (Figure 4). Scant fine to coarse pigment deposition was seen in the deep dermis next to subcutaneous fat, which was unchanged from the previous biopsy. The patient’s ophthalmologic symptoms were tracked via improvement in intraocular pressure and stabilization of his vision, indicating rapid and complete resolution of the glaucoma after the last laser treatment. With resolution of his ocular symptoms, the patient was tapered off all immunosuppressant medications. The patient was lost to follow-up approximately 2 years after the final laser treatment.



Tattoo-associated uveitis initially was described in 1969 in 3 patients with light blue tattoos who developed tattoo granulomas and simultaneous uveitis. These cases were successfully treated with excision.3 Multiple cases have been reported since, often with bilateral uveitis and tattoos demonstrating noncaseating granulomatous inflammation that were treated with steroids.4 In 2018, a diagnosis of exclusion was proposed for uveitis associated with granulomatous tattoo reaction without sarcoidosis: tattoo granulomas with uveitis (TAGU).1
In this case, sarcoidosis initially was high on the differential diagnosis. Sarcoidosis is an immune-mediated systemic disease of unknown etiology characterized by the presence of widespread noncaseating epithelioid cell granulomas, primarily seen in the pulmonary and lymphatic systems. However, it often initially manifests with cutaneous involvement with noncaseating “naked” granulomas in the dermis and subcutaneous tissue. Although TAGU cases have demonstrated noncaseating granulomas in association with dermal tattoo pigment on histopathology,1,4 dermal lymphoplasmacytic inflammation with scattered foreign body giant cells was noted in our patient, which was more consistent with allergic contact dermatitis. Thus, it is important to consider that TAGU can be seen with varying histologic patterns. In patients with tattoos, sarcoidosis can manifest grossly as a papulonodular cutaneous reaction.5 Active smoking is associated with a decreased risk for sarcoidosis, and those who smoke are statistically more likely to have tattoos than the general population,6,7 so our patient’s smoking history may be relevant. However, sarcoidosis was an unlikely diagnosis due to the serum angiotensin-converting enzyme level; results of a chest radiograph (bilateral adenopathy and coarse reticular opacities) and computed tomography (hilar and mediastinal adenopathy); and nonsarcoidal histopathology.
An allergic reaction to tattoo ink is caused by a delayed-type hypersensitivity reaction to a pigment hapten that can develop abruptly months to years after tattoo placement—1 year after tattoo placement in our patient. This reaction was seen in our patient’s blue pigment tattoos, although it is more commonly seen in red pigment tattoos.8 Although the etiology of TAGU is poorly understood, it also is hypothesized to be a delayed-type hypersensitivity response to tattoo ink particles, suggested by the pattern of lymphocytes infiltrating the tattoo and atypical T-cell infiltrate on vitreous biopsy.9,10 Further research is required to elucidate the relationship between tattoos and uveitis.
Q-switched lasers (eg, 532-nm or 1064-nm Nd:YAG, alexandrite, or ruby lasers) are the standard treatment options for uncomplicated tattoo removal and employ a high-intensity, ultrashort pulse duration.11 However Q-switched lasers require multiple sessions and target pigment-containing cells, releasing the tattoo particles into systemic circulation, which can potentially induce a severe allergic response.12 In contrast, CO2 lasers use a different mechanism, emitting energy at a wavelength of 10,600 nm, which is absorbed by intracellular water and allows for the ablation of the superficial epidermis along with the embedded ink with subsequent re-epithelialization, as well as heat-mediated thermal injury to allow for dermal collagen remodeling.13 In a 2021 retrospective study of ablative laser therapy for allergic tattoo reactions, patients were treated with the 10,600-nm ablative CO2 laser and noted improvements in itching and burning with minimal adverse events.12 Although using a CO2 laser may not be considered a firstline treatment option for TAGU, the refractory clinical course and notable morbidity of surgical excision necessitated the use of ablative laser in our case.
Tattoo granulomas with uveitis is a rare diagnosis with the potential for serious permanent sequelae including blindness. Existing treatments such as topical and oral corticosteroids, immunosuppressants, surgical excision, and Q-switched lasers all are possible options, but in a patient with progressive ocular symptoms with other potential rheumatologic conditions and sarcoidosis ruled out, fully ablative CO2 laser may be an effective treatment option. Our case demonstrated the successful treatment of TAGU with CO2 laser ablation. Given the unclear etiology of TAGU and the limited evidence on treatment options and efficacy, our case contributes to the body of literature that can inform clinical management of this unusual and serious reaction.
To the Editor:
Uveitis associated with tattoos is common, yet the etiology and optimal treatment options for this phenomenon remain unclear. Possible causes include a delayed hypersensitivity reaction to tattoo ink antigen or systemic sarcoidosis localized to the skin.1 Long-term treatment options include topical, intralesional, and systemic corticosteroids or immunosuppressants.2 Short-term options often include direct surgical excision and laser treatment. However, laser removal of tattoo pigment typically involves multiple sessions over the course of years, and there is a risk for antigen dispersal that may lead to anaphylaxis. Determining the most effective and safe treatment for a patient with progressive and severe ocular symptoms can be challenging. We describe a patient with cutaneous blue ink tattoos who developed chronic bilateral glaucoma, iritis, uveitis, and ocular hypertension that was refractory to multiple systemic medications and ophthalmologic procedures but responded to CO2 laser ablation.
A 27-year-old man with an active smoking history presented to our laser surgery center with a rash of approximately 4 years’ duration in areas with blue tattoo ink on both forearms. He was referred by his ophthalmologist due to bilateral uveitis and iritis and subsequent ocular hypertension and glaucoma that developed approximately 5 years after tattoo placement on the bilateral forearms. When the rash first appeared, the skin in the areas of the blue tattoo ink had hyperpigmented pruritic plaques. The patient was treated by a dermatologist with topical steroids to help reduce the itching and inflammation. Around the same time, he also started having ocular symptoms—vitreous floaters, erythema, eye pain, and blurriness—and was diagnosed with iritis of unclear etiology by ophthalmology. Figure 1 documents the patient’s clinical course. Due to escalating intraocular pressure and symptoms, he was referred to a glaucoma specialist and a rheumatologist. Systemic and rheumatologic medical conditions were ruled out with negative results on a series of blood tests (eg, rheumatoid factor, HLA-B27, antinuclear antibody, lysozyme, interferon gamma release assay, erythrocyte sedimentation rate, C-reactive protein, hepatitis B/C virus, Treponema pallidum, HIV), and magnetic resonance imaging of the brain was negative, ruling out demyelinating disease. Laboratory workup for sarcoidosis also was performed. The angiotensin-converting enzyme level was 30 U/L (reference range, 9-67 U/L), and a chest radiograph and computed tomography with contrast indicated no evidence of cardiopulmonary involvement. Although sarcoidosis could not be definitively ruled out, no other cause could be determined, and the patient’s glaucoma specialist diagnosed him with tattoo-associated uveitis. The patient was started on brimonidine, latanoprost, prednisolone, and dorzolamidetimolol eye drops, as well as acetazolamide (500 mg twice daily) and oral prednisone (various doses). Over the next 3 years, the patient continued to have symptoms, and immunosuppressant medications—methotrexate 20-25 mg weekly and adalimumab 40 mg every 2 weeks—were added to his treatment regimen. The patient also underwent bilateral ophthalmologic procedures, including a Baerveldt glaucoma implant procedure in the left eye and circumferential trabeculectomy in the right eye.

Despite these medications and procedures, the patient’s symptoms and intraocular pressure had not improved. At the current visit, punch biopsy of the tattooed skin and histologic examination showed dermal lymphoplasmacytic inflammation with scattered foreign-body giant cells associated with blue tattoo ink and overlying hyperkeratosis and spongiosis, consistent with allergic contact dermatitis (Figure 2). Because both immunosuppressant medications and ophthalmologic procedures had failed to control the progression of the ocular symptoms and the patient was at risk for permanent blindness, surgical excision and laser tattoo removal were considered as potential treatment options. Due to the large surface area and circumferential nature of the tattoos, there was a notable risk for disfiguring scars at both recipient and donor sites with surgical excision followed by graft placement. Thus, CO2 laser ablation was the preferred treatment option. However, this procedure was not without risk for anaphylaxis if the tattoo pigment were to be released into systemic circulation. Thus, at the first visit, ablation was performed on 3 test spots and the patient was prescribed cetirizine, diphenhydramine, and prophylactic prednisone for a few days. The patient then received a total of 5 fully ablative CO2 laser sessions (pulse energy: 200 mJ [15 J/cm2]; computerized pattern generator: 2-8-9 [85.2 J/cm2]; rate: 200 Hz [20 W], 3 passes) over 13 months to remove all visible blue ink in stages (Figure 3). Even with a shortened time course (as more time between laser sessions typically is preferred), the treatments were well tolerated with only mild hypertrophic scarring that responded to intralesional steroids (triamcinolone 10 mg/mL). On repeat skin biopsy during the treatment course, the superficial dermis demonstrated mostly scar tissue and near-total pigment removal—a 90% to 95% reduction in blue ink from prior biopsy—and minimal inflammation (Figure 4). Scant fine to coarse pigment deposition was seen in the deep dermis next to subcutaneous fat, which was unchanged from the previous biopsy. The patient’s ophthalmologic symptoms were tracked via improvement in intraocular pressure and stabilization of his vision, indicating rapid and complete resolution of the glaucoma after the last laser treatment. With resolution of his ocular symptoms, the patient was tapered off all immunosuppressant medications. The patient was lost to follow-up approximately 2 years after the final laser treatment.



Tattoo-associated uveitis initially was described in 1969 in 3 patients with light blue tattoos who developed tattoo granulomas and simultaneous uveitis. These cases were successfully treated with excision.3 Multiple cases have been reported since, often with bilateral uveitis and tattoos demonstrating noncaseating granulomatous inflammation that were treated with steroids.4 In 2018, a diagnosis of exclusion was proposed for uveitis associated with granulomatous tattoo reaction without sarcoidosis: tattoo granulomas with uveitis (TAGU).1
In this case, sarcoidosis initially was high on the differential diagnosis. Sarcoidosis is an immune-mediated systemic disease of unknown etiology characterized by the presence of widespread noncaseating epithelioid cell granulomas, primarily seen in the pulmonary and lymphatic systems. However, it often initially manifests with cutaneous involvement with noncaseating “naked” granulomas in the dermis and subcutaneous tissue. Although TAGU cases have demonstrated noncaseating granulomas in association with dermal tattoo pigment on histopathology,1,4 dermal lymphoplasmacytic inflammation with scattered foreign body giant cells was noted in our patient, which was more consistent with allergic contact dermatitis. Thus, it is important to consider that TAGU can be seen with varying histologic patterns. In patients with tattoos, sarcoidosis can manifest grossly as a papulonodular cutaneous reaction.5 Active smoking is associated with a decreased risk for sarcoidosis, and those who smoke are statistically more likely to have tattoos than the general population,6,7 so our patient’s smoking history may be relevant. However, sarcoidosis was an unlikely diagnosis due to the serum angiotensin-converting enzyme level; results of a chest radiograph (bilateral adenopathy and coarse reticular opacities) and computed tomography (hilar and mediastinal adenopathy); and nonsarcoidal histopathology.
An allergic reaction to tattoo ink is caused by a delayed-type hypersensitivity reaction to a pigment hapten that can develop abruptly months to years after tattoo placement—1 year after tattoo placement in our patient. This reaction was seen in our patient’s blue pigment tattoos, although it is more commonly seen in red pigment tattoos.8 Although the etiology of TAGU is poorly understood, it also is hypothesized to be a delayed-type hypersensitivity response to tattoo ink particles, suggested by the pattern of lymphocytes infiltrating the tattoo and atypical T-cell infiltrate on vitreous biopsy.9,10 Further research is required to elucidate the relationship between tattoos and uveitis.
Q-switched lasers (eg, 532-nm or 1064-nm Nd:YAG, alexandrite, or ruby lasers) are the standard treatment options for uncomplicated tattoo removal and employ a high-intensity, ultrashort pulse duration.11 However Q-switched lasers require multiple sessions and target pigment-containing cells, releasing the tattoo particles into systemic circulation, which can potentially induce a severe allergic response.12 In contrast, CO2 lasers use a different mechanism, emitting energy at a wavelength of 10,600 nm, which is absorbed by intracellular water and allows for the ablation of the superficial epidermis along with the embedded ink with subsequent re-epithelialization, as well as heat-mediated thermal injury to allow for dermal collagen remodeling.13 In a 2021 retrospective study of ablative laser therapy for allergic tattoo reactions, patients were treated with the 10,600-nm ablative CO2 laser and noted improvements in itching and burning with minimal adverse events.12 Although using a CO2 laser may not be considered a firstline treatment option for TAGU, the refractory clinical course and notable morbidity of surgical excision necessitated the use of ablative laser in our case.
Tattoo granulomas with uveitis is a rare diagnosis with the potential for serious permanent sequelae including blindness. Existing treatments such as topical and oral corticosteroids, immunosuppressants, surgical excision, and Q-switched lasers all are possible options, but in a patient with progressive ocular symptoms with other potential rheumatologic conditions and sarcoidosis ruled out, fully ablative CO2 laser may be an effective treatment option. Our case demonstrated the successful treatment of TAGU with CO2 laser ablation. Given the unclear etiology of TAGU and the limited evidence on treatment options and efficacy, our case contributes to the body of literature that can inform clinical management of this unusual and serious reaction.
- Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852-1861. doi:10.1111/jdv.15070
- Tiew S. Tattoo-associated panuveitis: a 10-year follow-up. Eur J Ophthalmol. 2019;29(1 suppl):18-21. doi:10.1177/1120672119846341
- Rorsman H, Brehmer-Andersson E, Dahlquist I, et al. Tattoo granuloma and uveitis. Lancet. 1969;2:27-28. doi:10.1016/s0140-6736(69)92600-2
- Ostheimer TA, Burkholder BM, Leung TG, et al. Tattoo-associated uveitis. Am J Ophthalmol. 2014;158:637-643.e1. doi:10.1016/j.ajo.2014.05.019
- Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679-686. doi:10.1159/000453315
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383: 1155-1167. doi:10.1016/S0140-6736(13)60680-7
- Kluger N. Epidemiology of tattoos in industrialized countries. Curr Probl Dermatol. 2015;48:6-20. doi:10.1159/000369175
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82. doi:10.1111/cod.13423
- Mansour AM, Chan CC. Recurrent uveitis preceded by swelling of skin tattoos. Am J Ophthalmol. 1991;111:515-516. doi:10.1016/s0002-9394(14)72395-5
- Reddy AK, Shildkrot Y, Newman SA, et al. T-lymphocyte predominance and cellular atypia in tattoo-associated uveitis. JAMA Ophthalmol. 2015;133:1356-1357. doi:10.1001/jamaophthalmol.2015.3354
- Wenzel SM. Current concepts in laser tattoo removal. Skin Therapy Lett. 2010;15:3-5.
- van der Bent SAS, Huisman S, Rustemeyer T, et al. Ablative laser surgery for allergic tattoo reactions: a retrospective study. mLasers Med Sci. 2021;36:1241-1248. doi:10.1007/s10103-020-03164-2
- Yumeen S, Khan T. Laser carbon dioxide resurfacing. In: StatPearls. StatPearls Publishing; April 23, 2023. Accessed March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560544/
- Kluger N. Tattoo-associated uveitis with or without systemic sarcoidosis: a comparative review of the literature. J Eur Acad Dermatol Venereol. 2018;32:1852-1861. doi:10.1111/jdv.15070
- Tiew S. Tattoo-associated panuveitis: a 10-year follow-up. Eur J Ophthalmol. 2019;29(1 suppl):18-21. doi:10.1177/1120672119846341
- Rorsman H, Brehmer-Andersson E, Dahlquist I, et al. Tattoo granuloma and uveitis. Lancet. 1969;2:27-28. doi:10.1016/s0140-6736(69)92600-2
- Ostheimer TA, Burkholder BM, Leung TG, et al. Tattoo-associated uveitis. Am J Ophthalmol. 2014;158:637-643.e1. doi:10.1016/j.ajo.2014.05.019
- Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology. 2016;232:679-686. doi:10.1159/000453315
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383: 1155-1167. doi:10.1016/S0140-6736(13)60680-7
- Kluger N. Epidemiology of tattoos in industrialized countries. Curr Probl Dermatol. 2015;48:6-20. doi:10.1159/000369175
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82. doi:10.1111/cod.13423
- Mansour AM, Chan CC. Recurrent uveitis preceded by swelling of skin tattoos. Am J Ophthalmol. 1991;111:515-516. doi:10.1016/s0002-9394(14)72395-5
- Reddy AK, Shildkrot Y, Newman SA, et al. T-lymphocyte predominance and cellular atypia in tattoo-associated uveitis. JAMA Ophthalmol. 2015;133:1356-1357. doi:10.1001/jamaophthalmol.2015.3354
- Wenzel SM. Current concepts in laser tattoo removal. Skin Therapy Lett. 2010;15:3-5.
- van der Bent SAS, Huisman S, Rustemeyer T, et al. Ablative laser surgery for allergic tattoo reactions: a retrospective study. mLasers Med Sci. 2021;36:1241-1248. doi:10.1007/s10103-020-03164-2
- Yumeen S, Khan T. Laser carbon dioxide resurfacing. In: StatPearls. StatPearls Publishing; April 23, 2023. Accessed March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560544/
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
Tattoo Granulomas With Uveitis Successfully Treated With CO2 Laser Ablation
PRACTICE POINTS
- Dermatologists should be aware that uveitis can develop as a delayed hypersensitivity reaction to tattoo ink, particularly in patients with blue ink tattoos.
- It is important to rule out systemic conditions such as sarcoidosis in patients presenting with uveitis and a history of tattoos.
- In a patient with progressive ocular symptoms, carbon dioxide laser ablation may be an effective treatment option if other potential rheumatologic conditions and sarcoidosis have been ruled out and other therapies have not resulted in improvement of symptoms.
- Continuous monitoring of ocular symptoms and intraocular pressure is vital to prevent complications such as glaucoma and potential blindness.
Managing Contact Dermatitis Related to Amputee Care
Managing Contact Dermatitis Related to Amputee Care
Amputees who use prosthetic devices are particularly susceptible to contact dermatitis due to moisture, irritation, and prolonged contact with components of the device. Contact dermatitis accounts for approximately one-third of the dermatoses encountered by amputees who wear a prosthesis.1 Diagnosing allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) is challenging due to errors of omission from the differential and the substantial clinical overlap with other eczematous dermatoses. Diagnosis relies on patient history, clinical examination, exposure assessment, diagnostic testing, and a high index of suspicion. Conventionally, ACD comprises approximately 20% of all contact dermatitis cases, whereas ICD accounts for 80%.2 Symptoms vary between the 2 conditions, with pruritus more common in ACD and burning and soreness more common in ICD.3 Onset of dermatitis relative to exposure is crucial, with ICD often manifesting more quickly and ACD requiring an initial sensitization phase.4 Additionally, the complexity of ICD as a condition with variable features adds to the diagnostic difficulty, especially when allergens also have irritant effects.
Understanding these 2 primary types of contact dermatitis is crucial for effective management and prevention strategies in amputees who use prosthetics. In this article, we describe common causes of ACD and ICD related to amputee prosthetics and propose a tailored patch testing panel in order to better diagnose ACD in this patient population.
Allergic Contact Dermatitis
Allergic contact dermatitis occurs when the skin comes into contact with a substance to which the individual is sensitized. In amputees who use prosthetics, the socket and sock liner materials are frequent culprits for triggering allergic reactions. Components such as rubber, metals (eg, nickel), adhesives, and various plastic monomers can induce ACD in susceptible individuals. Additionally, chronic friction and sweat augment hapten penetration, increasing the risk of developing ACD.5
Contact allergens (typically small molecules under 500 Da) penetrate the skin, engage dendritic cells, activate T lymphocytes, and trigger the immune response and memory.6 The skin contains a substantial population of memory T cells, with CD8+ T cells in the epidermis and CD4+ T cells in the dermis, expressing markers that facilitate skin reactivity. The balance between effector and regulatory T cells, which can produce suppressive cytokines such as IL-10, promotes clinical tolerance to allergens such as nickel.
Textile-driven ACD presents with a distinct clinical pattern, often manifesting as patchy generalized dermatitis that coincides with sites where garments fit most snugly. This presentation can mimic other forms of dermatitis, such as nummular or asteatotic dermatitis. The skin beneath undergarments such as underwear or prosthetic socks may be spared, as these act as shields from contact allergens. Notably, the face and hands typically are spared unless the patient has a cross-reaction to formaldehyde-based preservatives found in personal care products.4
Allergy to Components of the Prosthetic Socket and Sock Liner
A prosthesis consists of several key components, including a socket, sleeve, liner, and stump shrinker (eFigure 1). The prosthetic socket, custom-made to fit the residual limb, is the upper part of the prosthesis, while the lower part consists of prosthetic components such as joints and terminal devices ordered to meet individual needs. Prosthetic sleeves provide suspension by securely holding the prosthetic limb in place, while liners offer cushioning and protection to the residual limb, enhancing comfort and reducing friction. Stump shrinkers aid in reducing swelling and shaping the residual limb, facilitating a better fit for the prosthetic socket. Together, these components work in harmony to optimize stability, comfort, and functionality for the user, enabling them to navigate daily activities with greater ease and confidence. Common allergens found in components of the socket and sock liner include rubbers and other elastomers, metals, plastics, adhesives, and textiles.
Rubbers and Other Elastomers—Consumables, including liners, knee sleeves, and socks, are tailored to each client and utilize materials such as silicone and natural and synthetic rubbers for comfort and secure fit. Allergic reactions to natural rubber latex, more commonly used in earlier prosthetics, are associated with both type I and type IV hypersensitivity reactions.4 Proteins inherent to natural rubber are overwhelmingly associated with an immediate urticarial eruption, whereas chemical additives used to produce latex are mostly linked to delayed hypersensitivity reactions, manifesting as allergic reactions ranging from mild itching to severe skin blistering.4
Vulcanization is the process of using heat and other accelerators to manufacture rubber. Common rubber accelerators include thiurams (the most common allergen associated with rubbers and other elastomers), carbamates/carba mix, 1,3-diphenylguanidine, and mercaptobenzothiazole.4 Thiourea is an implicated cause of ACD to neoprene rubber.7 These sensitizing chemicals are all included in the North American 80 Comprehensive Series; only thiuram mix, carba mix, and mercaptobenzothiazole are available in the T.R.U.E. TEST (SmartPractice). Sensitization often occurs due to repeated exposure, particularly in individuals who have undergone multiple prosthetic fittings. Many modern prospective liners utilize a medical-grade silicone as an elastomer for its high flexibility; silicone is considered biologically nonreactive and generally is considered a rare cause of ACD.8
Metals—Nickel, a ubiquitous allergen found in metal alloys used in prosthetic hardware, can cause localized itching, redness, and even blistering upon contact with the skin. Other metals, such as cobalt and chromium, also may trigger allergic reactions in susceptible individuals. Though many elastic fitting prosthetic socks contain silver fibers to reduce odors and friction-causing blisters, pure silver used in clothing or jewelry rarely causes dermatitis.4
Plastics and Adhesives—Leg prosthesis sockets typically are finished with the application of varnish, plastics, and/or resins—all potential allergens—to improve the appearance of the device and protect it from external agents.9 Polyester plastics themselves can cause ICD, only rarely leading to ACD.4 Incomplete curing during their manufacture may result in inadvertent exposure to epoxy resins or other phenol- formaldehyde resins such as 4-tert-butylcatechol and 4-tert-butylphenol formaldehyde, demonstrated causes of ACD in amputees.10 Adhesives used in sock liners or tapes to secure prosthetic devices can contain ingredients such as acrylates (a well-known cause of nail allergens) and other formaldehyde resins.4 Additionally, benzophenone commonly is added to paints and rubbers as a UV light absorber, reducing UV degradation and enhancing the material’s durability under light exposure.11
Textiles—Cotton, a common component in prosthetic sock liners, is almost 100% cellulose and typically does not cause ACD; however, synthetic fibers such as polypropylene and elastane (spandex) can elicit allergic reactions.4 Allergy to textiles often is driven by the chemicals used in the manufacturing process, particularly textile finishes, dyes, and formaldehyde resins, which are commonly used as fabric treatments. Disperse dyes are another common cause of allergic reactions. Para-phenylenediamine, a dye found in permanent hair dye and other darkly colored fabrics, is a potent sensitizer that may cross-react with other compounds that also contain similar amine groups, such as ester anesthetics, sunscreens containing para-aminobenzoic acid, other para dyes, and sulfonamides.12 Sweat can exacerbate these reactions by causing allergens to leach out of textiles, increasing skin exposure. Additionally, prosthetics containing leather may trigger allergies to potassium dichromate and other chromium compounds used in the leather-tanning process.12
Allergy to Personal Care Products
Skin protectants and prosthetic cleansers are crucial in dermatologic care for amputees, working together to safeguard the skin and maintain prosthetic hygiene. Skin protectants form a barrier against irritation, friction, and moisture, protecting the residual limb from damage and enhancing comfort and mobility. Meanwhile, prosthetic cleansers remove sweat, oils, and bacteria from the prosthetic socket, reducing the risk of infections and odors and ensuring the longevity and optimal function of the prosthetic device. Together, they support skin health, comfort, and overall quality of life for amputees.
The socket should be cleaned with warm water prior to use, but more importantly, immediately after removing the prosthesis. If cleaning products are used at night, residual haptens may remain on the device, increasing the risk of sensitization. Common contact irritants found in personal care products utilized in amputee care include sulfates, surfactants, preservatives, and fragrances (eTable 1).4 Additionally, common household cleaners and disinfectants can damage the prosthesis, leading to breakdown and the release of the monomers, precipitating ACD.
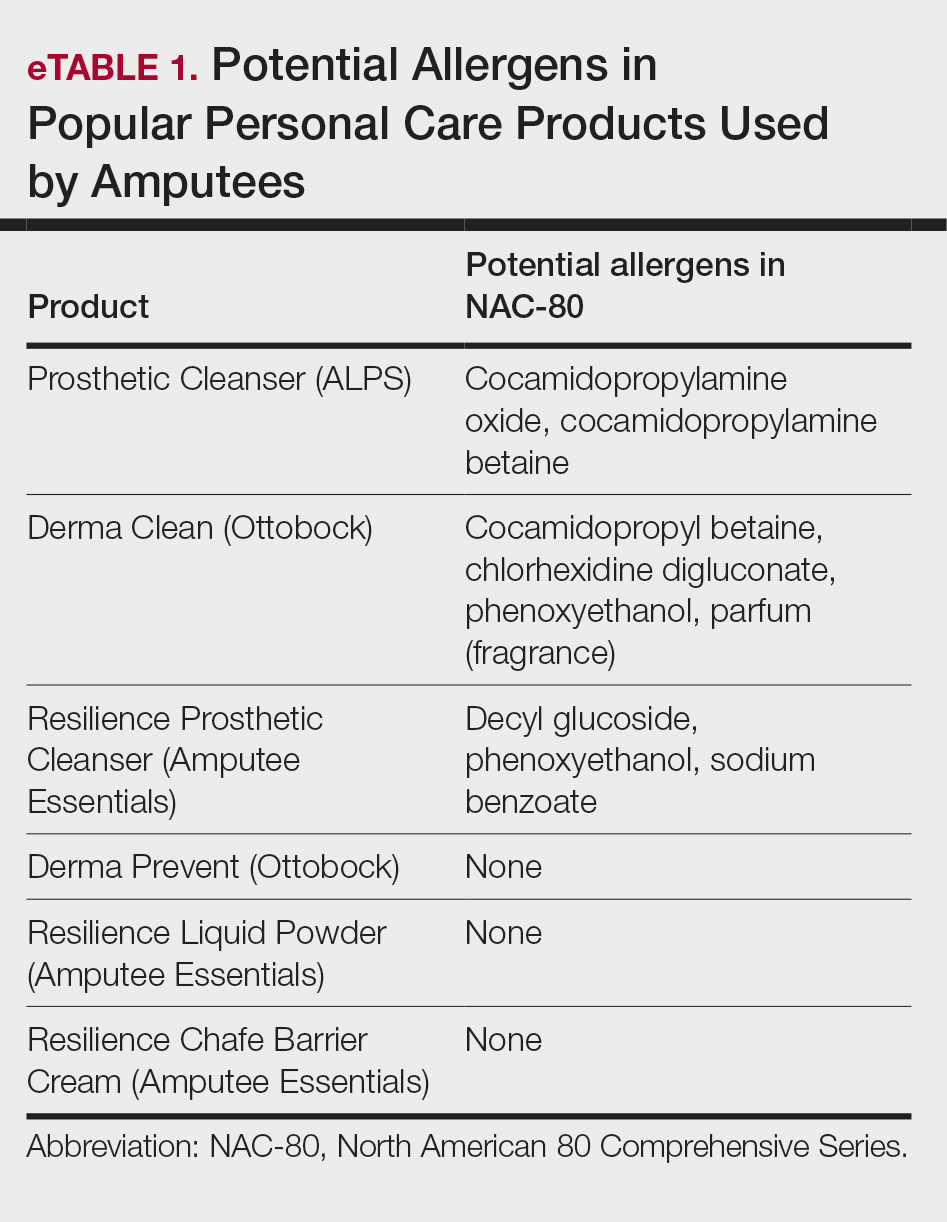
Patch Testing to Identify Causative Allergens
Patch testing is a valuable tool for identifying specific allergens responsible for ACD in amputees. This procedure involves applying small amounts of suspected allergens to the patient’s skin under occlusion and leaving the patches in place for 48 hours. After removal, the skin is assessed for reactions at 48 hours, with additional assessments conducted according to International Contact Dermatitis Research Group guidelines, typically at 72 and 96 hours, to identify delayed responses. This diagnostic approach helps pinpoint the substances to which the individual is allergic, enabling targeted avoidance strategies and treatment recommendations. Two widely used patch tests—the T.R.U.E. TEST, a preassembled patch test encompassing 35 allergens, and the North American 80 Comprehensive Series, which includes 80 allergens—demonstrate a sensitivity range between 70% and 80%.13,14 eTable 2 shows a recommended custom contact dermatitis panel to assess the most common causes of ACD related to amputee care.
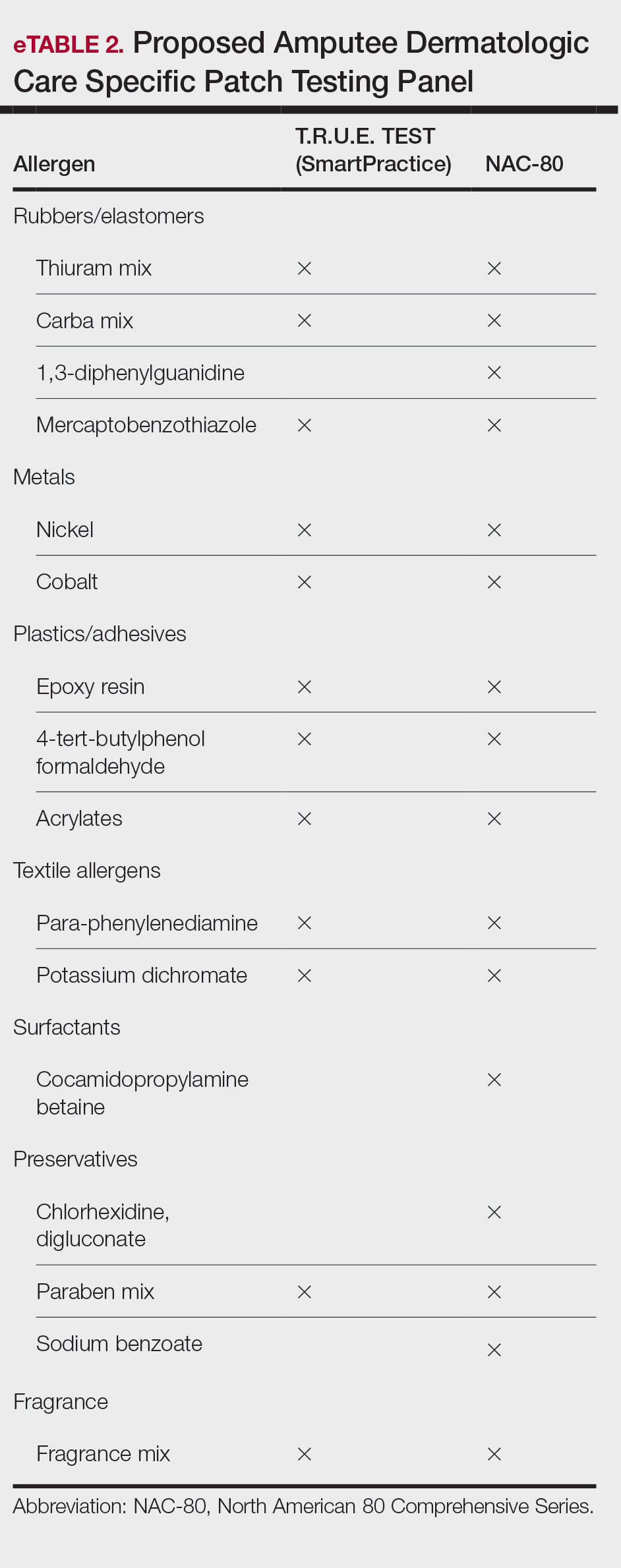
Irritant Contact Dermatitis
Irritant contact dermatitis occurs when the skin’s protective barrier is damaged by repeated exposure to a particular irritant. In amputees, perspiration, friction, and pressure from prosthetic devices can exacerbate irritant reactions, leading to skin maceration, breakdown, and increased transepidermal penetration. Sweat accumulation within the prosthetic socket creates a moist environment conducive to ICD. The combination of sweat and friction can strip the skin of its natural oils, leading to dryness, chafing, and maceration. Continuous exposure to moisture also can exacerbate existing dermatitis and compromise skin integrity.4 Additionally, chronic irritation may increase transepidermal penetration of haptens, potentiating the development of ACD.15
Management of ICD in amputees involves a combination of treatments aimed at reducing friction, reducing sweating, and restoring barrier protection. Strategies to minimize mechanical trauma to the skin include ensuring proper socket fit, managing moisture, and protecting the skin. Using moisture-wicking sock liners and breathable prosthetic materials can help keep the skin dry. Topical antiperspirants containing aluminum chloride or similar compounds that help to block sweat glands often are the first line of treatment. Oral anticholinergics may be prescribed to reduce overall sweating, though they can have systemic side effects. Iontophoresis, a procedure where the affected area is exposed to a mild electrical current, can also be effective, especially for sweating of the hands and feet, though its application in amputees might be more limited.14
Recently, 2 treatments have emerged as options for managing excessive sweating (hyperhidrosis) in amputees: botulinum toxin injections and laser hair removal. By inhibiting the release of acetylcholine from sweat glands, botulinum toxin effectively reduces sweat production, thereby alleviating perspiration-induced skin irritation. Approximately 2 to 3 units of botulinum toxin at a dilution of 100 units in 1 mL of bacteriostatic saline 0.9% are injected transdermally at 1-cm intervals in a circumferential pattern on the skin covered by the prosthesis socket (typically a total of 300-500 units are utilized in the procedure)(eFigure 2).16 Laser hair removal can assist amputees with hyperhidrosis by reducing hair in the residual limb area, which decreases sweat retention and the potential for skin irritation due to friction.
Final Thoughts
In amputee dermatologic care, individuals with limb loss are particularly prone to contact dermatitis due to moisture, friction, and prolonged contact with prosthetic components. Diagnosing ACD and ICD is challenging due to overlapping symptoms and the potential for simultaneous occurrence. Distinguishing between these conditions is crucial for effective management. Understanding their causes, particularly in relation to prosthetic use, is essential for developing targeted prevention and treatment strategies, including the use of tailored patch testing panels to better diagnose ACD in amputees.
- Lyon CC, Kulkarni J, Zimersonc E, et al. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501-507.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2018;56:99-109.
- Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis: A Practical Approach. Springer; 2021:57-92.
- Fisher AA, Rietschel RL, Fowler JF. Fisher’s Contact Dermatitis. BC Decker Inc; 2008.
- Johansen JD, Frosch PJ, Lepoittevin JP. Contact Dermatitis. Springer; 2010:43-90.
- Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens: the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473-487.
- Johnson R. Wrist dermatitis: contact allergy to neoprene in a keyboard wrist rest. Am J Contact Dermat. 1997;8:172-174.
- Adams RM. Occupational Skin Disease. WB Saunders; 1999:501-551.
- Requena L, Vázquez F, Requena C, et al. Epoxy dermatitis of an amputation stump. Contact Dermatitis. 1986;14:320.
- Freeman S. Contact dermatitis of a limb stump caused by p-tertiary butyl catechol in the artificial limb. Contact Dermatitis. 1986;14:68-69.
- Heurung AR, Raju SI, Warshaw EM. Benzophenones. Dermatitis. 2014;25:3-10.
- Manneschi V, Palmerio B, Pauluzzi P, et al. Contact dermatitis from myoelectric prostheses. Contact Dermatitis. 1989;21:116-117.
- Heinrich D, Altmeyer P, Brasch J. “New” techniques for more sensitive patch testing? J Dtsch Dermatol Ges. 2011;9:889-896.
- James WD. Contact dermatitis update. Presented at: Walter Reed National Military Medical Center; April 18, 2024.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486.
Amputees who use prosthetic devices are particularly susceptible to contact dermatitis due to moisture, irritation, and prolonged contact with components of the device. Contact dermatitis accounts for approximately one-third of the dermatoses encountered by amputees who wear a prosthesis.1 Diagnosing allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) is challenging due to errors of omission from the differential and the substantial clinical overlap with other eczematous dermatoses. Diagnosis relies on patient history, clinical examination, exposure assessment, diagnostic testing, and a high index of suspicion. Conventionally, ACD comprises approximately 20% of all contact dermatitis cases, whereas ICD accounts for 80%.2 Symptoms vary between the 2 conditions, with pruritus more common in ACD and burning and soreness more common in ICD.3 Onset of dermatitis relative to exposure is crucial, with ICD often manifesting more quickly and ACD requiring an initial sensitization phase.4 Additionally, the complexity of ICD as a condition with variable features adds to the diagnostic difficulty, especially when allergens also have irritant effects.
Understanding these 2 primary types of contact dermatitis is crucial for effective management and prevention strategies in amputees who use prosthetics. In this article, we describe common causes of ACD and ICD related to amputee prosthetics and propose a tailored patch testing panel in order to better diagnose ACD in this patient population.
Allergic Contact Dermatitis
Allergic contact dermatitis occurs when the skin comes into contact with a substance to which the individual is sensitized. In amputees who use prosthetics, the socket and sock liner materials are frequent culprits for triggering allergic reactions. Components such as rubber, metals (eg, nickel), adhesives, and various plastic monomers can induce ACD in susceptible individuals. Additionally, chronic friction and sweat augment hapten penetration, increasing the risk of developing ACD.5
Contact allergens (typically small molecules under 500 Da) penetrate the skin, engage dendritic cells, activate T lymphocytes, and trigger the immune response and memory.6 The skin contains a substantial population of memory T cells, with CD8+ T cells in the epidermis and CD4+ T cells in the dermis, expressing markers that facilitate skin reactivity. The balance between effector and regulatory T cells, which can produce suppressive cytokines such as IL-10, promotes clinical tolerance to allergens such as nickel.
Textile-driven ACD presents with a distinct clinical pattern, often manifesting as patchy generalized dermatitis that coincides with sites where garments fit most snugly. This presentation can mimic other forms of dermatitis, such as nummular or asteatotic dermatitis. The skin beneath undergarments such as underwear or prosthetic socks may be spared, as these act as shields from contact allergens. Notably, the face and hands typically are spared unless the patient has a cross-reaction to formaldehyde-based preservatives found in personal care products.4
Allergy to Components of the Prosthetic Socket and Sock Liner
A prosthesis consists of several key components, including a socket, sleeve, liner, and stump shrinker (eFigure 1). The prosthetic socket, custom-made to fit the residual limb, is the upper part of the prosthesis, while the lower part consists of prosthetic components such as joints and terminal devices ordered to meet individual needs. Prosthetic sleeves provide suspension by securely holding the prosthetic limb in place, while liners offer cushioning and protection to the residual limb, enhancing comfort and reducing friction. Stump shrinkers aid in reducing swelling and shaping the residual limb, facilitating a better fit for the prosthetic socket. Together, these components work in harmony to optimize stability, comfort, and functionality for the user, enabling them to navigate daily activities with greater ease and confidence. Common allergens found in components of the socket and sock liner include rubbers and other elastomers, metals, plastics, adhesives, and textiles.

Rubbers and Other Elastomers—Consumables, including liners, knee sleeves, and socks, are tailored to each client and utilize materials such as silicone and natural and synthetic rubbers for comfort and secure fit. Allergic reactions to natural rubber latex, more commonly used in earlier prosthetics, are associated with both type I and type IV hypersensitivity reactions.4 Proteins inherent to natural rubber are overwhelmingly associated with an immediate urticarial eruption, whereas chemical additives used to produce latex are mostly linked to delayed hypersensitivity reactions, manifesting as allergic reactions ranging from mild itching to severe skin blistering.4
Vulcanization is the process of using heat and other accelerators to manufacture rubber. Common rubber accelerators include thiurams (the most common allergen associated with rubbers and other elastomers), carbamates/carba mix, 1,3-diphenylguanidine, and mercaptobenzothiazole.4 Thiourea is an implicated cause of ACD to neoprene rubber.7 These sensitizing chemicals are all included in the North American 80 Comprehensive Series; only thiuram mix, carba mix, and mercaptobenzothiazole are available in the T.R.U.E. TEST (SmartPractice). Sensitization often occurs due to repeated exposure, particularly in individuals who have undergone multiple prosthetic fittings. Many modern prospective liners utilize a medical-grade silicone as an elastomer for its high flexibility; silicone is considered biologically nonreactive and generally is considered a rare cause of ACD.8
Metals—Nickel, a ubiquitous allergen found in metal alloys used in prosthetic hardware, can cause localized itching, redness, and even blistering upon contact with the skin. Other metals, such as cobalt and chromium, also may trigger allergic reactions in susceptible individuals. Though many elastic fitting prosthetic socks contain silver fibers to reduce odors and friction-causing blisters, pure silver used in clothing or jewelry rarely causes dermatitis.4
Plastics and Adhesives—Leg prosthesis sockets typically are finished with the application of varnish, plastics, and/or resins—all potential allergens—to improve the appearance of the device and protect it from external agents.9 Polyester plastics themselves can cause ICD, only rarely leading to ACD.4 Incomplete curing during their manufacture may result in inadvertent exposure to epoxy resins or other phenol- formaldehyde resins such as 4-tert-butylcatechol and 4-tert-butylphenol formaldehyde, demonstrated causes of ACD in amputees.10 Adhesives used in sock liners or tapes to secure prosthetic devices can contain ingredients such as acrylates (a well-known cause of nail allergens) and other formaldehyde resins.4 Additionally, benzophenone commonly is added to paints and rubbers as a UV light absorber, reducing UV degradation and enhancing the material’s durability under light exposure.11
Textiles—Cotton, a common component in prosthetic sock liners, is almost 100% cellulose and typically does not cause ACD; however, synthetic fibers such as polypropylene and elastane (spandex) can elicit allergic reactions.4 Allergy to textiles often is driven by the chemicals used in the manufacturing process, particularly textile finishes, dyes, and formaldehyde resins, which are commonly used as fabric treatments. Disperse dyes are another common cause of allergic reactions. Para-phenylenediamine, a dye found in permanent hair dye and other darkly colored fabrics, is a potent sensitizer that may cross-react with other compounds that also contain similar amine groups, such as ester anesthetics, sunscreens containing para-aminobenzoic acid, other para dyes, and sulfonamides.12 Sweat can exacerbate these reactions by causing allergens to leach out of textiles, increasing skin exposure. Additionally, prosthetics containing leather may trigger allergies to potassium dichromate and other chromium compounds used in the leather-tanning process.12
Allergy to Personal Care Products
Skin protectants and prosthetic cleansers are crucial in dermatologic care for amputees, working together to safeguard the skin and maintain prosthetic hygiene. Skin protectants form a barrier against irritation, friction, and moisture, protecting the residual limb from damage and enhancing comfort and mobility. Meanwhile, prosthetic cleansers remove sweat, oils, and bacteria from the prosthetic socket, reducing the risk of infections and odors and ensuring the longevity and optimal function of the prosthetic device. Together, they support skin health, comfort, and overall quality of life for amputees.
The socket should be cleaned with warm water prior to use, but more importantly, immediately after removing the prosthesis. If cleaning products are used at night, residual haptens may remain on the device, increasing the risk of sensitization. Common contact irritants found in personal care products utilized in amputee care include sulfates, surfactants, preservatives, and fragrances (eTable 1).4 Additionally, common household cleaners and disinfectants can damage the prosthesis, leading to breakdown and the release of the monomers, precipitating ACD.

Patch Testing to Identify Causative Allergens
Patch testing is a valuable tool for identifying specific allergens responsible for ACD in amputees. This procedure involves applying small amounts of suspected allergens to the patient’s skin under occlusion and leaving the patches in place for 48 hours. After removal, the skin is assessed for reactions at 48 hours, with additional assessments conducted according to International Contact Dermatitis Research Group guidelines, typically at 72 and 96 hours, to identify delayed responses. This diagnostic approach helps pinpoint the substances to which the individual is allergic, enabling targeted avoidance strategies and treatment recommendations. Two widely used patch tests—the T.R.U.E. TEST, a preassembled patch test encompassing 35 allergens, and the North American 80 Comprehensive Series, which includes 80 allergens—demonstrate a sensitivity range between 70% and 80%.13,14 eTable 2 shows a recommended custom contact dermatitis panel to assess the most common causes of ACD related to amputee care.

Irritant Contact Dermatitis
Irritant contact dermatitis occurs when the skin’s protective barrier is damaged by repeated exposure to a particular irritant. In amputees, perspiration, friction, and pressure from prosthetic devices can exacerbate irritant reactions, leading to skin maceration, breakdown, and increased transepidermal penetration. Sweat accumulation within the prosthetic socket creates a moist environment conducive to ICD. The combination of sweat and friction can strip the skin of its natural oils, leading to dryness, chafing, and maceration. Continuous exposure to moisture also can exacerbate existing dermatitis and compromise skin integrity.4 Additionally, chronic irritation may increase transepidermal penetration of haptens, potentiating the development of ACD.15
Management of ICD in amputees involves a combination of treatments aimed at reducing friction, reducing sweating, and restoring barrier protection. Strategies to minimize mechanical trauma to the skin include ensuring proper socket fit, managing moisture, and protecting the skin. Using moisture-wicking sock liners and breathable prosthetic materials can help keep the skin dry. Topical antiperspirants containing aluminum chloride or similar compounds that help to block sweat glands often are the first line of treatment. Oral anticholinergics may be prescribed to reduce overall sweating, though they can have systemic side effects. Iontophoresis, a procedure where the affected area is exposed to a mild electrical current, can also be effective, especially for sweating of the hands and feet, though its application in amputees might be more limited.14
Recently, 2 treatments have emerged as options for managing excessive sweating (hyperhidrosis) in amputees: botulinum toxin injections and laser hair removal. By inhibiting the release of acetylcholine from sweat glands, botulinum toxin effectively reduces sweat production, thereby alleviating perspiration-induced skin irritation. Approximately 2 to 3 units of botulinum toxin at a dilution of 100 units in 1 mL of bacteriostatic saline 0.9% are injected transdermally at 1-cm intervals in a circumferential pattern on the skin covered by the prosthesis socket (typically a total of 300-500 units are utilized in the procedure)(eFigure 2).16 Laser hair removal can assist amputees with hyperhidrosis by reducing hair in the residual limb area, which decreases sweat retention and the potential for skin irritation due to friction.

Final Thoughts
In amputee dermatologic care, individuals with limb loss are particularly prone to contact dermatitis due to moisture, friction, and prolonged contact with prosthetic components. Diagnosing ACD and ICD is challenging due to overlapping symptoms and the potential for simultaneous occurrence. Distinguishing between these conditions is crucial for effective management. Understanding their causes, particularly in relation to prosthetic use, is essential for developing targeted prevention and treatment strategies, including the use of tailored patch testing panels to better diagnose ACD in amputees.
Amputees who use prosthetic devices are particularly susceptible to contact dermatitis due to moisture, irritation, and prolonged contact with components of the device. Contact dermatitis accounts for approximately one-third of the dermatoses encountered by amputees who wear a prosthesis.1 Diagnosing allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) is challenging due to errors of omission from the differential and the substantial clinical overlap with other eczematous dermatoses. Diagnosis relies on patient history, clinical examination, exposure assessment, diagnostic testing, and a high index of suspicion. Conventionally, ACD comprises approximately 20% of all contact dermatitis cases, whereas ICD accounts for 80%.2 Symptoms vary between the 2 conditions, with pruritus more common in ACD and burning and soreness more common in ICD.3 Onset of dermatitis relative to exposure is crucial, with ICD often manifesting more quickly and ACD requiring an initial sensitization phase.4 Additionally, the complexity of ICD as a condition with variable features adds to the diagnostic difficulty, especially when allergens also have irritant effects.
Understanding these 2 primary types of contact dermatitis is crucial for effective management and prevention strategies in amputees who use prosthetics. In this article, we describe common causes of ACD and ICD related to amputee prosthetics and propose a tailored patch testing panel in order to better diagnose ACD in this patient population.
Allergic Contact Dermatitis
Allergic contact dermatitis occurs when the skin comes into contact with a substance to which the individual is sensitized. In amputees who use prosthetics, the socket and sock liner materials are frequent culprits for triggering allergic reactions. Components such as rubber, metals (eg, nickel), adhesives, and various plastic monomers can induce ACD in susceptible individuals. Additionally, chronic friction and sweat augment hapten penetration, increasing the risk of developing ACD.5
Contact allergens (typically small molecules under 500 Da) penetrate the skin, engage dendritic cells, activate T lymphocytes, and trigger the immune response and memory.6 The skin contains a substantial population of memory T cells, with CD8+ T cells in the epidermis and CD4+ T cells in the dermis, expressing markers that facilitate skin reactivity. The balance between effector and regulatory T cells, which can produce suppressive cytokines such as IL-10, promotes clinical tolerance to allergens such as nickel.
Textile-driven ACD presents with a distinct clinical pattern, often manifesting as patchy generalized dermatitis that coincides with sites where garments fit most snugly. This presentation can mimic other forms of dermatitis, such as nummular or asteatotic dermatitis. The skin beneath undergarments such as underwear or prosthetic socks may be spared, as these act as shields from contact allergens. Notably, the face and hands typically are spared unless the patient has a cross-reaction to formaldehyde-based preservatives found in personal care products.4
Allergy to Components of the Prosthetic Socket and Sock Liner
A prosthesis consists of several key components, including a socket, sleeve, liner, and stump shrinker (eFigure 1). The prosthetic socket, custom-made to fit the residual limb, is the upper part of the prosthesis, while the lower part consists of prosthetic components such as joints and terminal devices ordered to meet individual needs. Prosthetic sleeves provide suspension by securely holding the prosthetic limb in place, while liners offer cushioning and protection to the residual limb, enhancing comfort and reducing friction. Stump shrinkers aid in reducing swelling and shaping the residual limb, facilitating a better fit for the prosthetic socket. Together, these components work in harmony to optimize stability, comfort, and functionality for the user, enabling them to navigate daily activities with greater ease and confidence. Common allergens found in components of the socket and sock liner include rubbers and other elastomers, metals, plastics, adhesives, and textiles.

Rubbers and Other Elastomers—Consumables, including liners, knee sleeves, and socks, are tailored to each client and utilize materials such as silicone and natural and synthetic rubbers for comfort and secure fit. Allergic reactions to natural rubber latex, more commonly used in earlier prosthetics, are associated with both type I and type IV hypersensitivity reactions.4 Proteins inherent to natural rubber are overwhelmingly associated with an immediate urticarial eruption, whereas chemical additives used to produce latex are mostly linked to delayed hypersensitivity reactions, manifesting as allergic reactions ranging from mild itching to severe skin blistering.4
Vulcanization is the process of using heat and other accelerators to manufacture rubber. Common rubber accelerators include thiurams (the most common allergen associated with rubbers and other elastomers), carbamates/carba mix, 1,3-diphenylguanidine, and mercaptobenzothiazole.4 Thiourea is an implicated cause of ACD to neoprene rubber.7 These sensitizing chemicals are all included in the North American 80 Comprehensive Series; only thiuram mix, carba mix, and mercaptobenzothiazole are available in the T.R.U.E. TEST (SmartPractice). Sensitization often occurs due to repeated exposure, particularly in individuals who have undergone multiple prosthetic fittings. Many modern prospective liners utilize a medical-grade silicone as an elastomer for its high flexibility; silicone is considered biologically nonreactive and generally is considered a rare cause of ACD.8
Metals—Nickel, a ubiquitous allergen found in metal alloys used in prosthetic hardware, can cause localized itching, redness, and even blistering upon contact with the skin. Other metals, such as cobalt and chromium, also may trigger allergic reactions in susceptible individuals. Though many elastic fitting prosthetic socks contain silver fibers to reduce odors and friction-causing blisters, pure silver used in clothing or jewelry rarely causes dermatitis.4
Plastics and Adhesives—Leg prosthesis sockets typically are finished with the application of varnish, plastics, and/or resins—all potential allergens—to improve the appearance of the device and protect it from external agents.9 Polyester plastics themselves can cause ICD, only rarely leading to ACD.4 Incomplete curing during their manufacture may result in inadvertent exposure to epoxy resins or other phenol- formaldehyde resins such as 4-tert-butylcatechol and 4-tert-butylphenol formaldehyde, demonstrated causes of ACD in amputees.10 Adhesives used in sock liners or tapes to secure prosthetic devices can contain ingredients such as acrylates (a well-known cause of nail allergens) and other formaldehyde resins.4 Additionally, benzophenone commonly is added to paints and rubbers as a UV light absorber, reducing UV degradation and enhancing the material’s durability under light exposure.11
Textiles—Cotton, a common component in prosthetic sock liners, is almost 100% cellulose and typically does not cause ACD; however, synthetic fibers such as polypropylene and elastane (spandex) can elicit allergic reactions.4 Allergy to textiles often is driven by the chemicals used in the manufacturing process, particularly textile finishes, dyes, and formaldehyde resins, which are commonly used as fabric treatments. Disperse dyes are another common cause of allergic reactions. Para-phenylenediamine, a dye found in permanent hair dye and other darkly colored fabrics, is a potent sensitizer that may cross-react with other compounds that also contain similar amine groups, such as ester anesthetics, sunscreens containing para-aminobenzoic acid, other para dyes, and sulfonamides.12 Sweat can exacerbate these reactions by causing allergens to leach out of textiles, increasing skin exposure. Additionally, prosthetics containing leather may trigger allergies to potassium dichromate and other chromium compounds used in the leather-tanning process.12
Allergy to Personal Care Products
Skin protectants and prosthetic cleansers are crucial in dermatologic care for amputees, working together to safeguard the skin and maintain prosthetic hygiene. Skin protectants form a barrier against irritation, friction, and moisture, protecting the residual limb from damage and enhancing comfort and mobility. Meanwhile, prosthetic cleansers remove sweat, oils, and bacteria from the prosthetic socket, reducing the risk of infections and odors and ensuring the longevity and optimal function of the prosthetic device. Together, they support skin health, comfort, and overall quality of life for amputees.
The socket should be cleaned with warm water prior to use, but more importantly, immediately after removing the prosthesis. If cleaning products are used at night, residual haptens may remain on the device, increasing the risk of sensitization. Common contact irritants found in personal care products utilized in amputee care include sulfates, surfactants, preservatives, and fragrances (eTable 1).4 Additionally, common household cleaners and disinfectants can damage the prosthesis, leading to breakdown and the release of the monomers, precipitating ACD.

Patch Testing to Identify Causative Allergens
Patch testing is a valuable tool for identifying specific allergens responsible for ACD in amputees. This procedure involves applying small amounts of suspected allergens to the patient’s skin under occlusion and leaving the patches in place for 48 hours. After removal, the skin is assessed for reactions at 48 hours, with additional assessments conducted according to International Contact Dermatitis Research Group guidelines, typically at 72 and 96 hours, to identify delayed responses. This diagnostic approach helps pinpoint the substances to which the individual is allergic, enabling targeted avoidance strategies and treatment recommendations. Two widely used patch tests—the T.R.U.E. TEST, a preassembled patch test encompassing 35 allergens, and the North American 80 Comprehensive Series, which includes 80 allergens—demonstrate a sensitivity range between 70% and 80%.13,14 eTable 2 shows a recommended custom contact dermatitis panel to assess the most common causes of ACD related to amputee care.

Irritant Contact Dermatitis
Irritant contact dermatitis occurs when the skin’s protective barrier is damaged by repeated exposure to a particular irritant. In amputees, perspiration, friction, and pressure from prosthetic devices can exacerbate irritant reactions, leading to skin maceration, breakdown, and increased transepidermal penetration. Sweat accumulation within the prosthetic socket creates a moist environment conducive to ICD. The combination of sweat and friction can strip the skin of its natural oils, leading to dryness, chafing, and maceration. Continuous exposure to moisture also can exacerbate existing dermatitis and compromise skin integrity.4 Additionally, chronic irritation may increase transepidermal penetration of haptens, potentiating the development of ACD.15
Management of ICD in amputees involves a combination of treatments aimed at reducing friction, reducing sweating, and restoring barrier protection. Strategies to minimize mechanical trauma to the skin include ensuring proper socket fit, managing moisture, and protecting the skin. Using moisture-wicking sock liners and breathable prosthetic materials can help keep the skin dry. Topical antiperspirants containing aluminum chloride or similar compounds that help to block sweat glands often are the first line of treatment. Oral anticholinergics may be prescribed to reduce overall sweating, though they can have systemic side effects. Iontophoresis, a procedure where the affected area is exposed to a mild electrical current, can also be effective, especially for sweating of the hands and feet, though its application in amputees might be more limited.14
Recently, 2 treatments have emerged as options for managing excessive sweating (hyperhidrosis) in amputees: botulinum toxin injections and laser hair removal. By inhibiting the release of acetylcholine from sweat glands, botulinum toxin effectively reduces sweat production, thereby alleviating perspiration-induced skin irritation. Approximately 2 to 3 units of botulinum toxin at a dilution of 100 units in 1 mL of bacteriostatic saline 0.9% are injected transdermally at 1-cm intervals in a circumferential pattern on the skin covered by the prosthesis socket (typically a total of 300-500 units are utilized in the procedure)(eFigure 2).16 Laser hair removal can assist amputees with hyperhidrosis by reducing hair in the residual limb area, which decreases sweat retention and the potential for skin irritation due to friction.

Final Thoughts
In amputee dermatologic care, individuals with limb loss are particularly prone to contact dermatitis due to moisture, friction, and prolonged contact with prosthetic components. Diagnosing ACD and ICD is challenging due to overlapping symptoms and the potential for simultaneous occurrence. Distinguishing between these conditions is crucial for effective management. Understanding their causes, particularly in relation to prosthetic use, is essential for developing targeted prevention and treatment strategies, including the use of tailored patch testing panels to better diagnose ACD in amputees.
- Lyon CC, Kulkarni J, Zimersonc E, et al. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501-507.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2018;56:99-109.
- Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis: A Practical Approach. Springer; 2021:57-92.
- Fisher AA, Rietschel RL, Fowler JF. Fisher’s Contact Dermatitis. BC Decker Inc; 2008.
- Johansen JD, Frosch PJ, Lepoittevin JP. Contact Dermatitis. Springer; 2010:43-90.
- Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens: the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473-487.
- Johnson R. Wrist dermatitis: contact allergy to neoprene in a keyboard wrist rest. Am J Contact Dermat. 1997;8:172-174.
- Adams RM. Occupational Skin Disease. WB Saunders; 1999:501-551.
- Requena L, Vázquez F, Requena C, et al. Epoxy dermatitis of an amputation stump. Contact Dermatitis. 1986;14:320.
- Freeman S. Contact dermatitis of a limb stump caused by p-tertiary butyl catechol in the artificial limb. Contact Dermatitis. 1986;14:68-69.
- Heurung AR, Raju SI, Warshaw EM. Benzophenones. Dermatitis. 2014;25:3-10.
- Manneschi V, Palmerio B, Pauluzzi P, et al. Contact dermatitis from myoelectric prostheses. Contact Dermatitis. 1989;21:116-117.
- Heinrich D, Altmeyer P, Brasch J. “New” techniques for more sensitive patch testing? J Dtsch Dermatol Ges. 2011;9:889-896.
- James WD. Contact dermatitis update. Presented at: Walter Reed National Military Medical Center; April 18, 2024.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486.
- Lyon CC, Kulkarni J, Zimersonc E, et al. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501-507.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2018;56:99-109.
- Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis: A Practical Approach. Springer; 2021:57-92.
- Fisher AA, Rietschel RL, Fowler JF. Fisher’s Contact Dermatitis. BC Decker Inc; 2008.
- Johansen JD, Frosch PJ, Lepoittevin JP. Contact Dermatitis. Springer; 2010:43-90.
- Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens: the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473-487.
- Johnson R. Wrist dermatitis: contact allergy to neoprene in a keyboard wrist rest. Am J Contact Dermat. 1997;8:172-174.
- Adams RM. Occupational Skin Disease. WB Saunders; 1999:501-551.
- Requena L, Vázquez F, Requena C, et al. Epoxy dermatitis of an amputation stump. Contact Dermatitis. 1986;14:320.
- Freeman S. Contact dermatitis of a limb stump caused by p-tertiary butyl catechol in the artificial limb. Contact Dermatitis. 1986;14:68-69.
- Heurung AR, Raju SI, Warshaw EM. Benzophenones. Dermatitis. 2014;25:3-10.
- Manneschi V, Palmerio B, Pauluzzi P, et al. Contact dermatitis from myoelectric prostheses. Contact Dermatitis. 1989;21:116-117.
- Heinrich D, Altmeyer P, Brasch J. “New” techniques for more sensitive patch testing? J Dtsch Dermatol Ges. 2011;9:889-896.
- James WD. Contact dermatitis update. Presented at: Walter Reed National Military Medical Center; April 18, 2024.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486.
Managing Contact Dermatitis Related to Amputee Care
Managing Contact Dermatitis Related to Amputee Care
PRACTICE POINTS
- Incorporating a tailored patch testing panel that includes common prosthetic-related allergens (eg, rubber, metals, adhesives) can greatly improve the diagnosis and treatment of allergic vs irritant contact dermatitis in amputees.
- Effective management of irritant contact dermatitis in amputees involves reducing moisture and friction in the prosthetic socket with moisture-wicking liners, ensuring proper fit, and utilizing treatments such as topical antiperspirants and botulinum toxin injections.
Pruritus: Diagnosing and Treating Older Adults
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Central Line Skin Reactions in Children: Survey Addresses Treatment Protocols in Use
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.