User login
Optimal management of dysplastic nevi continues to evolve
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
San Diego – The way Benjamin Kelley, MD, sees it,
“There’s a confusion in the terminology, a term the late A. Bernard Ackerman, MD, called ‘patho-babel,’ ” Dr. Kelley, a Mohs micrographic surgeon and dermatopathologist in La Jolla, Calif., said at the annual Cutaneous Malignancy Update. “The idea of DN was originally used to describe a clinical melanoma syndrome. Now we use it for individual lesions, not just clinically but histologically. Some dermatologists refer to DN as ‘pre-melanoma,’ which is a negative framing,” he noted.
“We also refer to common nevi as ‘benign,’ which implies that DN are not benign,” he added. “The good news is that regardless of what they are called, the histologic criteria is generally agreed upon. The names can be used interchangeably.”
The bad news, he continued, is that there is less-than-perfect interobserver variability for grading DN lesions and significant variability in the treatment recommendations that pathologists give to clinicians. In one study, a group of pathology experts was asked to review 48 photomicrographs of melanocytic lesions and provide their diagnosis and treatment recommendations based on the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis scheme. For one, which showed a broad lesion with irregular epidermal thinning and thickening, the diagnoses ranged from solar lentigo to melanoma in situ. Treatment recommendations ranged from no treatment to re-excise with appropriate margins.
“This is an extreme example, but it shows you how difficult [establishing a diagnosis] can be,” Dr. Kelley said.
In a more recent study, researchers analyzed interobserver reproducibility in grading 179 DN cases among three observers who applied the 2018 World Health Organization grading criteria. The observers showed moderate to good agreement for most of the architectural features, except for criteria regarding focal continuous basal proliferation of melanocytes, density of non-nested junctional melanocytes, and presence of dyscohesive nests of intraepidermal melanocytes, whereas fair agreement was achieved for the cytological criteria. “So, it sounds to me like there was not a whole lot of agreement,” Dr. Kelley said.
An earlier single-center study titled “Clinicians Are From Mars and Pathologists Are From Venus” found that surgeons misunderstood the pathologist’s report 30% of the time.
In Dr. Kelly’s opinion, management of DNs will be successful if clinicians have a good working relationship with their dermatopathologists, if they biopsy to ensure an adequate, representative specimen, and if that they know what the terminology on the pathology report means and what actions to take. “The biopsy method matters,” he emphasized.
In a 14-year follow-up survey, investigators assessed DN management trends among 703 U.S. dermatologists. One key finding was that 69% of dermatologists in 2015 performed total removals when biopsying DN to achieve clear margins, compared with 86% in 2001.
A subsequent survey of 213 New England–based dermatologists found that the degree of clinical suspicion for melanoma was important in DN biopsy technique, with more respondents favoring shave biopsies for lesions with low suspicion and full-thickness biopsies for highly suspicious lesions.
“Misdiagnosis is more common for melanomas that have been assessed with punch and shave biopsies than with an excisional biopsy,” Dr. Kelley said. “I’m not too much of a stickler. I don’t require everyone to send me a giant excision, but I do want a representative sample.”
What about re-excision of DN considered to be mild or moderate? In 2015, members of the Pigmented Lesion Subcommittee of the Melanoma Prevention Working Group published a consensus statement on DN management recommendations for clinically atypical nevi/DN based on a review of published evidence. The subcommittee members concluded that mildly and moderately DN with clear margins do not need to be re-excised, and that mildly DN biopsied with positive histologic margins without clinical residual pigmentation may be safely observed rather than re-excised.
For moderately DN with positive histologic margins without clinically apparent residual pigmentation, the subcommittee members concluded that observation may be reasonable.
In his own informal analysis, Dr. Kelley compiled data from published studies he could find on DN management and divided them into two groups: the observation group, in which researchers from eight studies biopsied the DN lesion and watched the patients over time to see what happened, and the re-excision group, in which researchers from seven studies biopsied the DN lesion and subsequently re-excised it. There were about 1,500 patients in both groups. No deaths occurred in either group, he said, but 15 patients in the re-excision group developed a melanoma at the site of the original biopsy (1%), compared with 7 in the observation group (0.5%).
Six of seven melanomas in the observation group came from one article conducted at a VA clinic. In the study, 6 of 304 observed DN subsequently developed melanoma at the site of the lesion. “However, five of six that developed melanoma had an original biopsy that was a partial biopsy with grossly positive margins; I think that’s where the problem lies,” Dr. Kelley said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “All five grew lentigo maligna type melanoma, which we know can extend multiple millimeters beyond the clinically apparent lesion.”
The findings support mounting evidence that re-excising mild and moderate DN, regardless of border involvement, may not be necessary. “Currently, most clinicians still re-excise moderate and severe DN involving margins, especially if there is residual pigment,” Dr. Kelley said. “Most re-excise severe DN regardless of margin involvement, but beware if your biopsy was a partial sample of a larger lesion.”
He acknowledged limitations to pathologic studies of DN, including the potential for diagnostic uncertainty. “That doesn’t necessarily mean that the pathologist got the diagnosis wrong. It could be, what is the risk that the portion of tissue not visualized contains melanoma? If you give me a 5 mm sample of a DN, and I cut it into 4-micrometer sections, I’m only looking at less than 1% of the actual nevus. That’s compounded if the pathologist only receives a partial sample.”
Dr. Kelley reported having no relevant disclosures.
AT MELANOMA 2023
Dermoscopy, other modalities for improving melanoma diagnoses reviewed
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
AT MELANOMA 2023
Screen all patients for cannabis use before surgery: Guideline
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
FROM REGIONAL ANETHESIA AND MEDICINE
Surgeon’s license suspension spotlights hazards, ethics of live-streaming surgeries
potentially endangering patients. The surgeon has a large social media following.
In November, the State Medical Board of Ohio temporarily suspended the license of Katherine Roxanne Grawe, MD, who practices in the wealthy Columbus suburb of Powell.
Among other accusations of misconduct, the board stated that “during some videos/live-streams you engage in dialogue to respond to viewers’ online questions while the surgical procedure remains actively ongoing.”
One patient needed emergency treatment following liposuction and was diagnosed with a perforated bowel and serious bacterial infection.
“Despite liposuction being a blind surgery that requires awareness of the tip of the cannula to avoid injury, your attention to the camera meant at those moments you were not looking at the patient or palpating the location of the tip of the cannula,” the medical board said.
Neither Dr. Grawe nor her attorney responded to requests for comment.
Dr. Grawe, known as “Dr. Roxy,” has a popular TikTok account – now set to private – with 841,600 followers and 14.6 million likes. She has another 123,000 followers on her Instagram account, also now private.
The Columbus Dispatch reported that Dr. Grawe had previously been warned to protect patient privacy on social media. The board has yet to make a final decision regarding her license.
According to Columbus TV station WSYX, she said in a TikTok video, “We show our surgeries every single day on Snapchat. Patients get to decide if they want to be part of it. And if you do, you can watch your own surgery.”
The TV station quoted former patients who described surgical complications. One said: “I went to her because, I thought, from all of her social media that she uplifted women. That she helped women empower themselves. But she didn’t.”
Dallas plastic surgeon Rod J. Rohrich, MD, who has written about social-media best practices and has 430,000 followers on Instagram, said in an interview that many surgeons have been reprimanded by state medical boards for being distracted by social media during procedures.
“It is best not to do live-streaming unless it is an educational event to demonstrate techniques and technology with full informed consent of the patient. It should be a very well-rehearsed event for education,” he said.
Nurses also have been disciplined for inappropriate posts on social media. In December 2022, an Atlanta hospital announced that four nurses were no longer on the job after they appeared in a TikTok video in scrubs and revealed their “icks” regarding obstetric care.
“My ick is when you ask me how much the baby weighs,” one worker said in the video, “and it’s still ... in your hands.”
Plastic surgeon Christian J. Vercler, MD, of the University of Michigan, Ann Arbor, who’s studied social-media guidelines for surgeons, said in an interview that plastic surgery content on TikTok has “blown up” in recent years.
“Five years or so ago, it was Snapchat where I saw a lot of inappropriate things posted by surgeons,” Dr. Vercler said in an interview. “That may still be happening on Snapchat, but I actually don’t ever use that platform anymore, and neither do my trainees.”
Dr. Vercler cautioned colleagues to consider their motivations for live-streaming surgery and to think about whether they can fully focus on the patient.
“There are many potential distractions in the OR. We get pages, phone calls, nurses asking us questions, anesthesiologists trying to talk to us. Social media is just one more thing competing for the surgeon’s attention,” he said. “Every surgeon should strive to eliminate unnecessary or unavoidable distractions, so the question becomes, ‘who is best being served by me focusing my attention on recording this operation on someone’s phone so we can post it on social media? Is it the patient?’ ”
Dr. Vercler added, “There are many, many plastic surgeons using social media as the powerful platform that it is to build their brands, to connect with potential patients, and to educate the public about what they do. I believe that most are doing this in a way that is respectful to patients and doesn’t exploit patients for the surgeon’s benefit.
“Unfortunately,” he concluded, “there are some who do see patients as merely instruments by which they can achieve fame, notoriety, and wealth.”
Dr. Rohrich and Dr. Vercler disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
potentially endangering patients. The surgeon has a large social media following.
In November, the State Medical Board of Ohio temporarily suspended the license of Katherine Roxanne Grawe, MD, who practices in the wealthy Columbus suburb of Powell.
Among other accusations of misconduct, the board stated that “during some videos/live-streams you engage in dialogue to respond to viewers’ online questions while the surgical procedure remains actively ongoing.”
One patient needed emergency treatment following liposuction and was diagnosed with a perforated bowel and serious bacterial infection.
“Despite liposuction being a blind surgery that requires awareness of the tip of the cannula to avoid injury, your attention to the camera meant at those moments you were not looking at the patient or palpating the location of the tip of the cannula,” the medical board said.
Neither Dr. Grawe nor her attorney responded to requests for comment.
Dr. Grawe, known as “Dr. Roxy,” has a popular TikTok account – now set to private – with 841,600 followers and 14.6 million likes. She has another 123,000 followers on her Instagram account, also now private.
The Columbus Dispatch reported that Dr. Grawe had previously been warned to protect patient privacy on social media. The board has yet to make a final decision regarding her license.
According to Columbus TV station WSYX, she said in a TikTok video, “We show our surgeries every single day on Snapchat. Patients get to decide if they want to be part of it. And if you do, you can watch your own surgery.”
The TV station quoted former patients who described surgical complications. One said: “I went to her because, I thought, from all of her social media that she uplifted women. That she helped women empower themselves. But she didn’t.”
Dallas plastic surgeon Rod J. Rohrich, MD, who has written about social-media best practices and has 430,000 followers on Instagram, said in an interview that many surgeons have been reprimanded by state medical boards for being distracted by social media during procedures.
“It is best not to do live-streaming unless it is an educational event to demonstrate techniques and technology with full informed consent of the patient. It should be a very well-rehearsed event for education,” he said.
Nurses also have been disciplined for inappropriate posts on social media. In December 2022, an Atlanta hospital announced that four nurses were no longer on the job after they appeared in a TikTok video in scrubs and revealed their “icks” regarding obstetric care.
“My ick is when you ask me how much the baby weighs,” one worker said in the video, “and it’s still ... in your hands.”
Plastic surgeon Christian J. Vercler, MD, of the University of Michigan, Ann Arbor, who’s studied social-media guidelines for surgeons, said in an interview that plastic surgery content on TikTok has “blown up” in recent years.
“Five years or so ago, it was Snapchat where I saw a lot of inappropriate things posted by surgeons,” Dr. Vercler said in an interview. “That may still be happening on Snapchat, but I actually don’t ever use that platform anymore, and neither do my trainees.”
Dr. Vercler cautioned colleagues to consider their motivations for live-streaming surgery and to think about whether they can fully focus on the patient.
“There are many potential distractions in the OR. We get pages, phone calls, nurses asking us questions, anesthesiologists trying to talk to us. Social media is just one more thing competing for the surgeon’s attention,” he said. “Every surgeon should strive to eliminate unnecessary or unavoidable distractions, so the question becomes, ‘who is best being served by me focusing my attention on recording this operation on someone’s phone so we can post it on social media? Is it the patient?’ ”
Dr. Vercler added, “There are many, many plastic surgeons using social media as the powerful platform that it is to build their brands, to connect with potential patients, and to educate the public about what they do. I believe that most are doing this in a way that is respectful to patients and doesn’t exploit patients for the surgeon’s benefit.
“Unfortunately,” he concluded, “there are some who do see patients as merely instruments by which they can achieve fame, notoriety, and wealth.”
Dr. Rohrich and Dr. Vercler disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
potentially endangering patients. The surgeon has a large social media following.
In November, the State Medical Board of Ohio temporarily suspended the license of Katherine Roxanne Grawe, MD, who practices in the wealthy Columbus suburb of Powell.
Among other accusations of misconduct, the board stated that “during some videos/live-streams you engage in dialogue to respond to viewers’ online questions while the surgical procedure remains actively ongoing.”
One patient needed emergency treatment following liposuction and was diagnosed with a perforated bowel and serious bacterial infection.
“Despite liposuction being a blind surgery that requires awareness of the tip of the cannula to avoid injury, your attention to the camera meant at those moments you were not looking at the patient or palpating the location of the tip of the cannula,” the medical board said.
Neither Dr. Grawe nor her attorney responded to requests for comment.
Dr. Grawe, known as “Dr. Roxy,” has a popular TikTok account – now set to private – with 841,600 followers and 14.6 million likes. She has another 123,000 followers on her Instagram account, also now private.
The Columbus Dispatch reported that Dr. Grawe had previously been warned to protect patient privacy on social media. The board has yet to make a final decision regarding her license.
According to Columbus TV station WSYX, she said in a TikTok video, “We show our surgeries every single day on Snapchat. Patients get to decide if they want to be part of it. And if you do, you can watch your own surgery.”
The TV station quoted former patients who described surgical complications. One said: “I went to her because, I thought, from all of her social media that she uplifted women. That she helped women empower themselves. But she didn’t.”
Dallas plastic surgeon Rod J. Rohrich, MD, who has written about social-media best practices and has 430,000 followers on Instagram, said in an interview that many surgeons have been reprimanded by state medical boards for being distracted by social media during procedures.
“It is best not to do live-streaming unless it is an educational event to demonstrate techniques and technology with full informed consent of the patient. It should be a very well-rehearsed event for education,” he said.
Nurses also have been disciplined for inappropriate posts on social media. In December 2022, an Atlanta hospital announced that four nurses were no longer on the job after they appeared in a TikTok video in scrubs and revealed their “icks” regarding obstetric care.
“My ick is when you ask me how much the baby weighs,” one worker said in the video, “and it’s still ... in your hands.”
Plastic surgeon Christian J. Vercler, MD, of the University of Michigan, Ann Arbor, who’s studied social-media guidelines for surgeons, said in an interview that plastic surgery content on TikTok has “blown up” in recent years.
“Five years or so ago, it was Snapchat where I saw a lot of inappropriate things posted by surgeons,” Dr. Vercler said in an interview. “That may still be happening on Snapchat, but I actually don’t ever use that platform anymore, and neither do my trainees.”
Dr. Vercler cautioned colleagues to consider their motivations for live-streaming surgery and to think about whether they can fully focus on the patient.
“There are many potential distractions in the OR. We get pages, phone calls, nurses asking us questions, anesthesiologists trying to talk to us. Social media is just one more thing competing for the surgeon’s attention,” he said. “Every surgeon should strive to eliminate unnecessary or unavoidable distractions, so the question becomes, ‘who is best being served by me focusing my attention on recording this operation on someone’s phone so we can post it on social media? Is it the patient?’ ”
Dr. Vercler added, “There are many, many plastic surgeons using social media as the powerful platform that it is to build their brands, to connect with potential patients, and to educate the public about what they do. I believe that most are doing this in a way that is respectful to patients and doesn’t exploit patients for the surgeon’s benefit.
“Unfortunately,” he concluded, “there are some who do see patients as merely instruments by which they can achieve fame, notoriety, and wealth.”
Dr. Rohrich and Dr. Vercler disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What are the risk factors for Mohs surgery–related anxiety?
confirmed by a health care provider (HCP), results from a single-center survey demonstrated.
“Higher patient-reported anxiety in hospital settings is significantly linked to lower patient satisfaction with the quality of care and higher patient-reported postoperative pain,” corresponding author Ally-Khan Somani, MD, PhD, and colleagues wrote in the study, which was published online in Dermatologic Surgery. “Identifying factors associated with perioperative patient anxiety could improve outcomes and patient satisfaction.”
Dr. Somani, director of dermatologic surgery and cutaneous oncology in the department of dermatology at the University of Indiana, Indianapolis, and coauthors surveyed 145 patients who underwent Mohs micrographic surgery (MMS) at the university from February 2018 to March 2020. They collected patient self-reported demographics, medical history, and administered a 10-point visual analog scale assessment of anxiety at multiple stages. They also sought HCP-perceived assessments of anxiety and used a stepwise regression mode to explore factors that potentially contributed to anxiety outcomes. The mean age of the 145 patients was 63 years, 60% were female, and 77% had no self-reported anxiety confirmed by a prior HCP’s diagnosis.
Two-thirds of patients (66%) received a pre-MMS consultation with the surgeon, 59% had a history of skin cancer removal surgery, and 86% had 1-2 layers removed during the current MMS.
Prior to MMS, the researchers found that significant risk factors for increased anxiety included younger age, female sex, and self-reported history of anxiety confirmed by an HCP (P < .05), while intraoperatively, HCP-perceived patient anxiety increased with younger patient age and more layers removed. Following MMS, patient anxiety increased significantly with more layers removed and higher self-reported preoperative anxiety levels. “Although existing research is divided regarding the efficacy of pre-MMS consultation for anxiety reduction, these findings suggest that patient-reported and HCP-perceived anxiety were not significantly affected by in-person pre-MMS consultation with the surgeon,” Dr. Somani and colleagues wrote. “Thus, routinely recommending consultations may not be the best approach for improving anxiety outcomes.”
They acknowledged certain limitations of their analysis, including its single-center design, enrollment of demographically similar patients, and the fact that no objective measurements of anxiety such as heart rate or blood pressure were taken.
“One of the main benefits of Mohs surgery is that we are able to operate under local anesthesia, but this also means that our patients are acutely aware of everything going on around them,” said Patricia M. Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and was asked to comment on the study.
“I think it is so important that this study is primarily focusing on the patient experience,” she said. “While this study did not find that a pre-op consult impacted patient anxiety levels, I do think we can infer that it is critical to connect with your patients on some level prior to surgery, as it helps you tailor your process to make the day more tolerable for them [such as] playing music, determining the need for an oral anxiolytic, etc.”
Neither the researchers nor Dr. Richey reported having financial disclosures.
confirmed by a health care provider (HCP), results from a single-center survey demonstrated.
“Higher patient-reported anxiety in hospital settings is significantly linked to lower patient satisfaction with the quality of care and higher patient-reported postoperative pain,” corresponding author Ally-Khan Somani, MD, PhD, and colleagues wrote in the study, which was published online in Dermatologic Surgery. “Identifying factors associated with perioperative patient anxiety could improve outcomes and patient satisfaction.”
Dr. Somani, director of dermatologic surgery and cutaneous oncology in the department of dermatology at the University of Indiana, Indianapolis, and coauthors surveyed 145 patients who underwent Mohs micrographic surgery (MMS) at the university from February 2018 to March 2020. They collected patient self-reported demographics, medical history, and administered a 10-point visual analog scale assessment of anxiety at multiple stages. They also sought HCP-perceived assessments of anxiety and used a stepwise regression mode to explore factors that potentially contributed to anxiety outcomes. The mean age of the 145 patients was 63 years, 60% were female, and 77% had no self-reported anxiety confirmed by a prior HCP’s diagnosis.
Two-thirds of patients (66%) received a pre-MMS consultation with the surgeon, 59% had a history of skin cancer removal surgery, and 86% had 1-2 layers removed during the current MMS.
Prior to MMS, the researchers found that significant risk factors for increased anxiety included younger age, female sex, and self-reported history of anxiety confirmed by an HCP (P < .05), while intraoperatively, HCP-perceived patient anxiety increased with younger patient age and more layers removed. Following MMS, patient anxiety increased significantly with more layers removed and higher self-reported preoperative anxiety levels. “Although existing research is divided regarding the efficacy of pre-MMS consultation for anxiety reduction, these findings suggest that patient-reported and HCP-perceived anxiety were not significantly affected by in-person pre-MMS consultation with the surgeon,” Dr. Somani and colleagues wrote. “Thus, routinely recommending consultations may not be the best approach for improving anxiety outcomes.”
They acknowledged certain limitations of their analysis, including its single-center design, enrollment of demographically similar patients, and the fact that no objective measurements of anxiety such as heart rate or blood pressure were taken.
“One of the main benefits of Mohs surgery is that we are able to operate under local anesthesia, but this also means that our patients are acutely aware of everything going on around them,” said Patricia M. Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and was asked to comment on the study.
“I think it is so important that this study is primarily focusing on the patient experience,” she said. “While this study did not find that a pre-op consult impacted patient anxiety levels, I do think we can infer that it is critical to connect with your patients on some level prior to surgery, as it helps you tailor your process to make the day more tolerable for them [such as] playing music, determining the need for an oral anxiolytic, etc.”
Neither the researchers nor Dr. Richey reported having financial disclosures.
confirmed by a health care provider (HCP), results from a single-center survey demonstrated.
“Higher patient-reported anxiety in hospital settings is significantly linked to lower patient satisfaction with the quality of care and higher patient-reported postoperative pain,” corresponding author Ally-Khan Somani, MD, PhD, and colleagues wrote in the study, which was published online in Dermatologic Surgery. “Identifying factors associated with perioperative patient anxiety could improve outcomes and patient satisfaction.”
Dr. Somani, director of dermatologic surgery and cutaneous oncology in the department of dermatology at the University of Indiana, Indianapolis, and coauthors surveyed 145 patients who underwent Mohs micrographic surgery (MMS) at the university from February 2018 to March 2020. They collected patient self-reported demographics, medical history, and administered a 10-point visual analog scale assessment of anxiety at multiple stages. They also sought HCP-perceived assessments of anxiety and used a stepwise regression mode to explore factors that potentially contributed to anxiety outcomes. The mean age of the 145 patients was 63 years, 60% were female, and 77% had no self-reported anxiety confirmed by a prior HCP’s diagnosis.
Two-thirds of patients (66%) received a pre-MMS consultation with the surgeon, 59% had a history of skin cancer removal surgery, and 86% had 1-2 layers removed during the current MMS.
Prior to MMS, the researchers found that significant risk factors for increased anxiety included younger age, female sex, and self-reported history of anxiety confirmed by an HCP (P < .05), while intraoperatively, HCP-perceived patient anxiety increased with younger patient age and more layers removed. Following MMS, patient anxiety increased significantly with more layers removed and higher self-reported preoperative anxiety levels. “Although existing research is divided regarding the efficacy of pre-MMS consultation for anxiety reduction, these findings suggest that patient-reported and HCP-perceived anxiety were not significantly affected by in-person pre-MMS consultation with the surgeon,” Dr. Somani and colleagues wrote. “Thus, routinely recommending consultations may not be the best approach for improving anxiety outcomes.”
They acknowledged certain limitations of their analysis, including its single-center design, enrollment of demographically similar patients, and the fact that no objective measurements of anxiety such as heart rate or blood pressure were taken.
“One of the main benefits of Mohs surgery is that we are able to operate under local anesthesia, but this also means that our patients are acutely aware of everything going on around them,” said Patricia M. Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and was asked to comment on the study.
“I think it is so important that this study is primarily focusing on the patient experience,” she said. “While this study did not find that a pre-op consult impacted patient anxiety levels, I do think we can infer that it is critical to connect with your patients on some level prior to surgery, as it helps you tailor your process to make the day more tolerable for them [such as] playing music, determining the need for an oral anxiolytic, etc.”
Neither the researchers nor Dr. Richey reported having financial disclosures.
FROM DERMATOLOGIC SURGERY
Consider radiologic imaging for high-risk cutaneous SCC, expert advises
DENVER –
In a study published in 2020, Emily Ruiz, MD, MPH, and colleagues identified 87 CSCC tumors in 83 patients who underwent baseline or surveillance imaging primary at the Brigham and Women’s Hospital Mohs Surgery Clinic and the Dana-Farber Cancer Institute High-Risk Skin Cancer Clinic, both in Boston, from Jan. 1, 2017, to June 1, 2019. Of the 87 primary CSCCs, 48 (58%) underwent surveillance imaging. The researchers found that imaging detected additional disease in 26 patients, or 30% of cases, “whether that be nodal metastasis, local invasion beyond what was clinically accepted, or in-transit disease,” Dr. Ruiz, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s, said during the annual meeting of the American Society for Dermatologic Surgery. “But if you look at the 16 nodal metastases in this cohort, all were picked up on imaging and not on clinical exam.”
Since publication of these results, Dr. Ruiz routinely considers baseline radiologic imaging in T2b and T3 tumors; borderline T2a tumors (which she said they are now calling “T2a high,” for those who have one risk factor plus another intermediate risk factor),” and T2a tumors in patients who are profoundly immunosuppressed.
“My preference is to always do [the imaging] before treatment unless I’m up-staging them during surgery,” said Dr. Ruiz, who also directs the High-Risk Skin Cancer Clinic at Dana Farber. “We have picked up nodal metastases before surgery, which enables us to create a good therapeutic plan for our patients before we start operating. Then we image them every 6 months or so for about 2 years. Sometimes we will extend that out to 3 years.”
Some clinicians use sentinel lymph node biopsy (SLNB) as a diagnostic test, but there are mixed results about its prognostic significance. A retrospective observational study of 720 patients with CSCC found that SLNB provided no benefit regarding further metastasis or tumor-specific survival, compared with those who received routine observation and follow-up, “but head and neck surgeons in the U.S. are putting together some prospective data from multiple centers,” Dr. Ruiz said. “I think in the coming years, you will have more multicenter data to inform us as to whether to do SLNB or not.”
Surgery may be the mainstay of treatment for resectable SCC, but the emerging role of neoadjuvant therapeutics is changing the way oncologists treat these tumors. For example, in a phase 2 trial recently published in the New England Journal of Medicine, 79 patients with stage II-IV CSCC received up to four doses of immunotherapy with the programmed death receptor–1 (PD-1) blocker cemiplimab administered every 3 weeks. The primary endpoint was a pathologic complete response, defined as the absence of viable tumor cells in the surgical specimen at a central laboratory. The researchers observed that 68% of patients had an objective response.
“These were patients with localized tumors that were either very aggressive or had nodal metastases,” said Dr, Ruiz, who was the site primary investigator at Dana Farber and a coauthor of the NEJM study. “This has altered the way we approach treating our larger tumors that could be resectable but have a lot of disease either locally or in the nodal basin. We think that we can shrink down the tumor and make it easier to resect, but also there is the possibility or improving outcomes.”
At Brigham and Women’s and the Dana Farber, she and her colleagues consider immunotherapy for multiple recurrent tumors that have been previously irradiated; cases of large tumor burden locally or in the nodal basin; tumors that have a complex surgical plan; cases where there is a low likelihood of achieving clear surgical margins; and cases of in-transit disease.
“We use two to four doses of immunotherapy prior to surgery and assess the tumor response after two doses both clinically and radiologically,” she said. “If the tumor continues to grow, we would do surgery sooner.”
The side-effect profile of immunotherapy is another consideration. “Some patients are not appropriate for a neoadjuvant immunotherapy approach, such as transplant patients,” she said.
According to the latest National Comprehensive Cancer Network guidelines, surgery with or without adjuvant radiation is the current standard of care for treating CSCC. These guidelines were developed without much data to support the use of radiation, but a 20-year retrospective cohort study at Brigham and Women’s Hospital and the Cleveland Clinic Foundation found that adjuvant radiation following margin resection in high T-stage CSCC cut the risk of local and locoregional recurrence in half.
“This is something that radiation oncologists have told us for years, but there was no data to support it, so it was nice to see that borne out in clinical data,” said Dr. Ruiz, the study’s lead author. The 10% risk of local recurrence observed in the study “may not be high enough for some of our older patients, so we wanted to see if we could identify a group of high tumors that had higher risk of local recurrence,” she said. They found that patients who had a greater than 20% risk of poor outcome were those with recurrent tumors, those with tumors 6 cm or greater in size, and those with all four BWH risk factors (tumor diameter ≥ 2 cm, poorly differentiated histology, perineural invasion ≥ 0.1 mm, or tumor invasion beyond fat excluding bone invasion).
“Those risks were also cut in half if you added radiation,” she said. “So, the way I now approach counseling patients is, I try to estimate their baseline risk as best I can based on the tumor itself. I tell them that if they want to do adjuvant radiation it would cut the risk in half. Some patients are too frail and want to pass on it, while others are very interested.”
Of patients who did not receive radiation but had a disease recurrence, just under half of tumors were salvageable, about 25% died of their disease, and 23% had persistent disease. “I think this does support using radiation earlier on for the appropriate patient,” Dr. Ruiz said. “I consider the baseline risks [and] balance that with the patient’s comorbidities.”
Limited data exists on adjuvant immunotherapy for CSCC, but two ongoing randomized prospective clinical trials underway are studying the PD-1 inhibitors cemiplimab and pembrolizumab versus placebo. “We don’t have data yet, but prior to randomization, patients undergo surgery with macroscopic gross resection of all disease,” Dr. Ruiz said. “All tumors receive ART [adjuvant radiation therapy] prior to randomization”
Dr. Ruiz disclosed that she is a consultant for Sanofi, Regeneron, Genentech, and Jaunce Therapeutics. She is also a member of the advisory board for Checkpoint Therapeutics and is an investigator for Merck, Sanofi, and Regeneron.
DENVER –
In a study published in 2020, Emily Ruiz, MD, MPH, and colleagues identified 87 CSCC tumors in 83 patients who underwent baseline or surveillance imaging primary at the Brigham and Women’s Hospital Mohs Surgery Clinic and the Dana-Farber Cancer Institute High-Risk Skin Cancer Clinic, both in Boston, from Jan. 1, 2017, to June 1, 2019. Of the 87 primary CSCCs, 48 (58%) underwent surveillance imaging. The researchers found that imaging detected additional disease in 26 patients, or 30% of cases, “whether that be nodal metastasis, local invasion beyond what was clinically accepted, or in-transit disease,” Dr. Ruiz, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s, said during the annual meeting of the American Society for Dermatologic Surgery. “But if you look at the 16 nodal metastases in this cohort, all were picked up on imaging and not on clinical exam.”
Since publication of these results, Dr. Ruiz routinely considers baseline radiologic imaging in T2b and T3 tumors; borderline T2a tumors (which she said they are now calling “T2a high,” for those who have one risk factor plus another intermediate risk factor),” and T2a tumors in patients who are profoundly immunosuppressed.
“My preference is to always do [the imaging] before treatment unless I’m up-staging them during surgery,” said Dr. Ruiz, who also directs the High-Risk Skin Cancer Clinic at Dana Farber. “We have picked up nodal metastases before surgery, which enables us to create a good therapeutic plan for our patients before we start operating. Then we image them every 6 months or so for about 2 years. Sometimes we will extend that out to 3 years.”
Some clinicians use sentinel lymph node biopsy (SLNB) as a diagnostic test, but there are mixed results about its prognostic significance. A retrospective observational study of 720 patients with CSCC found that SLNB provided no benefit regarding further metastasis or tumor-specific survival, compared with those who received routine observation and follow-up, “but head and neck surgeons in the U.S. are putting together some prospective data from multiple centers,” Dr. Ruiz said. “I think in the coming years, you will have more multicenter data to inform us as to whether to do SLNB or not.”
Surgery may be the mainstay of treatment for resectable SCC, but the emerging role of neoadjuvant therapeutics is changing the way oncologists treat these tumors. For example, in a phase 2 trial recently published in the New England Journal of Medicine, 79 patients with stage II-IV CSCC received up to four doses of immunotherapy with the programmed death receptor–1 (PD-1) blocker cemiplimab administered every 3 weeks. The primary endpoint was a pathologic complete response, defined as the absence of viable tumor cells in the surgical specimen at a central laboratory. The researchers observed that 68% of patients had an objective response.
“These were patients with localized tumors that were either very aggressive or had nodal metastases,” said Dr, Ruiz, who was the site primary investigator at Dana Farber and a coauthor of the NEJM study. “This has altered the way we approach treating our larger tumors that could be resectable but have a lot of disease either locally or in the nodal basin. We think that we can shrink down the tumor and make it easier to resect, but also there is the possibility or improving outcomes.”
At Brigham and Women’s and the Dana Farber, she and her colleagues consider immunotherapy for multiple recurrent tumors that have been previously irradiated; cases of large tumor burden locally or in the nodal basin; tumors that have a complex surgical plan; cases where there is a low likelihood of achieving clear surgical margins; and cases of in-transit disease.
“We use two to four doses of immunotherapy prior to surgery and assess the tumor response after two doses both clinically and radiologically,” she said. “If the tumor continues to grow, we would do surgery sooner.”
The side-effect profile of immunotherapy is another consideration. “Some patients are not appropriate for a neoadjuvant immunotherapy approach, such as transplant patients,” she said.
According to the latest National Comprehensive Cancer Network guidelines, surgery with or without adjuvant radiation is the current standard of care for treating CSCC. These guidelines were developed without much data to support the use of radiation, but a 20-year retrospective cohort study at Brigham and Women’s Hospital and the Cleveland Clinic Foundation found that adjuvant radiation following margin resection in high T-stage CSCC cut the risk of local and locoregional recurrence in half.
“This is something that radiation oncologists have told us for years, but there was no data to support it, so it was nice to see that borne out in clinical data,” said Dr. Ruiz, the study’s lead author. The 10% risk of local recurrence observed in the study “may not be high enough for some of our older patients, so we wanted to see if we could identify a group of high tumors that had higher risk of local recurrence,” she said. They found that patients who had a greater than 20% risk of poor outcome were those with recurrent tumors, those with tumors 6 cm or greater in size, and those with all four BWH risk factors (tumor diameter ≥ 2 cm, poorly differentiated histology, perineural invasion ≥ 0.1 mm, or tumor invasion beyond fat excluding bone invasion).
“Those risks were also cut in half if you added radiation,” she said. “So, the way I now approach counseling patients is, I try to estimate their baseline risk as best I can based on the tumor itself. I tell them that if they want to do adjuvant radiation it would cut the risk in half. Some patients are too frail and want to pass on it, while others are very interested.”
Of patients who did not receive radiation but had a disease recurrence, just under half of tumors were salvageable, about 25% died of their disease, and 23% had persistent disease. “I think this does support using radiation earlier on for the appropriate patient,” Dr. Ruiz said. “I consider the baseline risks [and] balance that with the patient’s comorbidities.”
Limited data exists on adjuvant immunotherapy for CSCC, but two ongoing randomized prospective clinical trials underway are studying the PD-1 inhibitors cemiplimab and pembrolizumab versus placebo. “We don’t have data yet, but prior to randomization, patients undergo surgery with macroscopic gross resection of all disease,” Dr. Ruiz said. “All tumors receive ART [adjuvant radiation therapy] prior to randomization”
Dr. Ruiz disclosed that she is a consultant for Sanofi, Regeneron, Genentech, and Jaunce Therapeutics. She is also a member of the advisory board for Checkpoint Therapeutics and is an investigator for Merck, Sanofi, and Regeneron.
DENVER –
In a study published in 2020, Emily Ruiz, MD, MPH, and colleagues identified 87 CSCC tumors in 83 patients who underwent baseline or surveillance imaging primary at the Brigham and Women’s Hospital Mohs Surgery Clinic and the Dana-Farber Cancer Institute High-Risk Skin Cancer Clinic, both in Boston, from Jan. 1, 2017, to June 1, 2019. Of the 87 primary CSCCs, 48 (58%) underwent surveillance imaging. The researchers found that imaging detected additional disease in 26 patients, or 30% of cases, “whether that be nodal metastasis, local invasion beyond what was clinically accepted, or in-transit disease,” Dr. Ruiz, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s, said during the annual meeting of the American Society for Dermatologic Surgery. “But if you look at the 16 nodal metastases in this cohort, all were picked up on imaging and not on clinical exam.”
Since publication of these results, Dr. Ruiz routinely considers baseline radiologic imaging in T2b and T3 tumors; borderline T2a tumors (which she said they are now calling “T2a high,” for those who have one risk factor plus another intermediate risk factor),” and T2a tumors in patients who are profoundly immunosuppressed.
“My preference is to always do [the imaging] before treatment unless I’m up-staging them during surgery,” said Dr. Ruiz, who also directs the High-Risk Skin Cancer Clinic at Dana Farber. “We have picked up nodal metastases before surgery, which enables us to create a good therapeutic plan for our patients before we start operating. Then we image them every 6 months or so for about 2 years. Sometimes we will extend that out to 3 years.”
Some clinicians use sentinel lymph node biopsy (SLNB) as a diagnostic test, but there are mixed results about its prognostic significance. A retrospective observational study of 720 patients with CSCC found that SLNB provided no benefit regarding further metastasis or tumor-specific survival, compared with those who received routine observation and follow-up, “but head and neck surgeons in the U.S. are putting together some prospective data from multiple centers,” Dr. Ruiz said. “I think in the coming years, you will have more multicenter data to inform us as to whether to do SLNB or not.”
Surgery may be the mainstay of treatment for resectable SCC, but the emerging role of neoadjuvant therapeutics is changing the way oncologists treat these tumors. For example, in a phase 2 trial recently published in the New England Journal of Medicine, 79 patients with stage II-IV CSCC received up to four doses of immunotherapy with the programmed death receptor–1 (PD-1) blocker cemiplimab administered every 3 weeks. The primary endpoint was a pathologic complete response, defined as the absence of viable tumor cells in the surgical specimen at a central laboratory. The researchers observed that 68% of patients had an objective response.
“These were patients with localized tumors that were either very aggressive or had nodal metastases,” said Dr, Ruiz, who was the site primary investigator at Dana Farber and a coauthor of the NEJM study. “This has altered the way we approach treating our larger tumors that could be resectable but have a lot of disease either locally or in the nodal basin. We think that we can shrink down the tumor and make it easier to resect, but also there is the possibility or improving outcomes.”
At Brigham and Women’s and the Dana Farber, she and her colleagues consider immunotherapy for multiple recurrent tumors that have been previously irradiated; cases of large tumor burden locally or in the nodal basin; tumors that have a complex surgical plan; cases where there is a low likelihood of achieving clear surgical margins; and cases of in-transit disease.
“We use two to four doses of immunotherapy prior to surgery and assess the tumor response after two doses both clinically and radiologically,” she said. “If the tumor continues to grow, we would do surgery sooner.”
The side-effect profile of immunotherapy is another consideration. “Some patients are not appropriate for a neoadjuvant immunotherapy approach, such as transplant patients,” she said.
According to the latest National Comprehensive Cancer Network guidelines, surgery with or without adjuvant radiation is the current standard of care for treating CSCC. These guidelines were developed without much data to support the use of radiation, but a 20-year retrospective cohort study at Brigham and Women’s Hospital and the Cleveland Clinic Foundation found that adjuvant radiation following margin resection in high T-stage CSCC cut the risk of local and locoregional recurrence in half.
“This is something that radiation oncologists have told us for years, but there was no data to support it, so it was nice to see that borne out in clinical data,” said Dr. Ruiz, the study’s lead author. The 10% risk of local recurrence observed in the study “may not be high enough for some of our older patients, so we wanted to see if we could identify a group of high tumors that had higher risk of local recurrence,” she said. They found that patients who had a greater than 20% risk of poor outcome were those with recurrent tumors, those with tumors 6 cm or greater in size, and those with all four BWH risk factors (tumor diameter ≥ 2 cm, poorly differentiated histology, perineural invasion ≥ 0.1 mm, or tumor invasion beyond fat excluding bone invasion).
“Those risks were also cut in half if you added radiation,” she said. “So, the way I now approach counseling patients is, I try to estimate their baseline risk as best I can based on the tumor itself. I tell them that if they want to do adjuvant radiation it would cut the risk in half. Some patients are too frail and want to pass on it, while others are very interested.”
Of patients who did not receive radiation but had a disease recurrence, just under half of tumors were salvageable, about 25% died of their disease, and 23% had persistent disease. “I think this does support using radiation earlier on for the appropriate patient,” Dr. Ruiz said. “I consider the baseline risks [and] balance that with the patient’s comorbidities.”
Limited data exists on adjuvant immunotherapy for CSCC, but two ongoing randomized prospective clinical trials underway are studying the PD-1 inhibitors cemiplimab and pembrolizumab versus placebo. “We don’t have data yet, but prior to randomization, patients undergo surgery with macroscopic gross resection of all disease,” Dr. Ruiz said. “All tumors receive ART [adjuvant radiation therapy] prior to randomization”
Dr. Ruiz disclosed that she is a consultant for Sanofi, Regeneron, Genentech, and Jaunce Therapeutics. She is also a member of the advisory board for Checkpoint Therapeutics and is an investigator for Merck, Sanofi, and Regeneron.
AT ASDS 2022
Update on high-grade vulvar interepithelial neoplasia
Vulvar squamous cell carcinomas (VSCC) comprise approximately 90% of all vulvar malignancies. Unlike cervical SCC, which are predominantly human papilloma virus (HPV) positive, only a minority of VSCC are HPV positive – on the order of 15%-25% of cases. Most cases occur in the setting of lichen sclerosus and are HPV negative.
Lichen sclerosus is a chronic inflammatory dermatitis typically involving the anogenital area, which in some cases can become seriously distorted (e.g. atrophy of the labia minora, clitoral phimosis, and introital stenosis). Although most cases are diagnosed in postmenopausal women, LS can affect women of any age. The true prevalence of lichen sclerosus is unknown. Recent studies have shown a prevalence of 1 in 60; among older women, it can even be as high as 1 in 30. While lichen sclerosus is a pruriginous condition, it is often asymptomatic. It is not considered a premalignant condition. The diagnosis is clinical; however, suspicious lesions (erosions/ulcerations, hyperkeratosis, pigmented areas, ecchymosis, warty or papular lesions), particularly when recalcitrant to adequate first-line therapy, should be biopsied.
VSCC arises from precursor lesions or high-grade vulvar intraepithelial neoplasia (VIN). The 2015 International Society for the Study of Vulvovaginal Disease nomenclature classifies high-grade VIN into high-grade squamous intraepithelial lesion (HSIL) and differentiated VIN (dVIN). Most patients with high-grade VIN are diagnosed with HSIL or usual type VIN. A preponderance of these lesions (75%-85%) are HPV positive, predominantly HPV 16. Vulvar HSIL (vHSIL) lesions affect younger women. The lesions tend to be multifocal and extensive. On the other hand, dVIN typically affects older women and commonly develops as a solitary lesion. While dVIN accounts for only a small subset of patients with high-grade VIN, these lesions are HPV negative and associated with lichen sclerosus.
Both disease entities, vHSIL and dVIN, are increasing in incidence. There is a higher risk and shortened period of progression to cancer in patients with dVIN compared to HSIL. The cancer risk of vHSIL is relatively low. The 10-year cumulative VSCC risk reported in the literature is 10.3%; 9.7% for vHSIL and 50% for dVIN. Patients with vHSIL could benefit from less aggressive treatment modalities.
Patients present with a constellation of signs such as itching, pain, burning, bleeding, and discharge. Chronic symptoms portend HPV-independent lesions associated with lichen sclerosus while episodic signs are suggestive of HPV-positive lesions.
The recurrence risk of high-grade VIN is 46%-70%. Risk factors for recurrence include age greater than 50, immunosuppression, metasynchronous HSIL, and multifocal lesions. Recurrences occur in up to 50% of women who have undergone surgery. For those who undergo surgical treatment for high-grade VIN, recurrence is more common in the setting of positive margins, underlying lichen sclerosis, persistent HPV infection, and immunosuppression.
Management of high-grade VIN is determined by the lesion characteristics, patient characteristics, and medical expertise. Given the risk of progression of high-grade VIN to cancer and risk of underlying cancer, surgical therapy is typically recommended. The treatment of choice is surgical excision in cases of dVIN. Surgical treatments include CO2 laser ablation, wide local excision, and vulvectomy. Women who undergo surgical treatment for vHSIL have about a 50% chance of the condition recurring 1 year later, irrespective of whether treatment is by surgical excision or laser vaporization.
Since surgery can be associated with disfigurement and sexual dysfunction, alternatives to surgery should be considered in cases of vHSIL. The potential for effect on sexual function should be part of preoperative counseling and treatment. Women treated for VIN often experience increased inhibition of sexual excitement and increased inhibition of orgasm. One study found that in women undergoing vulvar excision for VIN, the impairment was found to be psychological in nature. Overall, the studies of sexual effect from treatment of VIN have found that women do not return to their pretreatment sexual function. However, the optimal management of vHSIL has not been determined. Nonsurgical options include topical therapies (imiquimod, 5-fluorouracil, cidofovir, and interferon) and nonpharmacologic treatments, such as photodynamic therapy.
Imiquimod, a topical immune modulator, is the most studied pharmacologic treatment of vHSIL. The drug induces secretion of cytokines, creating an immune response that clears the HPV infection. Imiquimod is safe and well tolerated. The clinical response rate varies between 35% and 81%. A recent study demonstrated the efficacy of imiquimod and the treatment was found to be noninferior to surgery. Adverse events differed, with local pain following surgical treatment and local pruritus and erythema associated with imiquimod use. Some patients did not respond to imiquimod; it was thought by the authors of the study that specific immunological factors affect the clinical response.
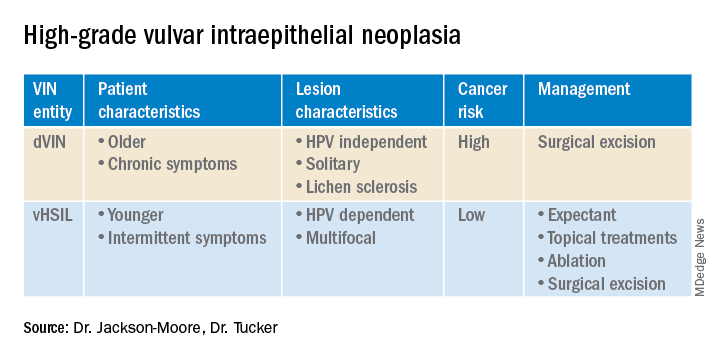
In conclusion, high-grade VIN is a heterogeneous disease made up of two distinct disease entities with rising incidence. In contrast to dVIN, the cancer risk is low for patients with vHSIL. Treatment should be driven by the clinical characteristics of the vulvar lesions, patients’ preferences, sexual activity, and compliance. Future directions include risk stratification of patients with vHSIL who are most likely to benefit from topical treatments, thus reducing overtreatment. Molecular biomarkers that could identify dVIN at an early stage are needed.
Dr. Jackson-Moore is associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
Cendejas BR et al. Am J Obstet Gynecol. 2015 Mar;212(3):291-7.
Lebreton M et al. J Gynecol Obstet Hum Reprod. 2020 Nov;49(9):101801.
Thuijs NB et al. Int J Cancer. 2021 Jan 1;148(1):90-8. doi: 10.1002/ijc.33198. .
Trutnovsky G et al. Lancet. 2022 May 7;399(10337):1790-8. Erratum in: Lancet. 2022 Oct 8;400(10359):1194.
Vulvar squamous cell carcinomas (VSCC) comprise approximately 90% of all vulvar malignancies. Unlike cervical SCC, which are predominantly human papilloma virus (HPV) positive, only a minority of VSCC are HPV positive – on the order of 15%-25% of cases. Most cases occur in the setting of lichen sclerosus and are HPV negative.
Lichen sclerosus is a chronic inflammatory dermatitis typically involving the anogenital area, which in some cases can become seriously distorted (e.g. atrophy of the labia minora, clitoral phimosis, and introital stenosis). Although most cases are diagnosed in postmenopausal women, LS can affect women of any age. The true prevalence of lichen sclerosus is unknown. Recent studies have shown a prevalence of 1 in 60; among older women, it can even be as high as 1 in 30. While lichen sclerosus is a pruriginous condition, it is often asymptomatic. It is not considered a premalignant condition. The diagnosis is clinical; however, suspicious lesions (erosions/ulcerations, hyperkeratosis, pigmented areas, ecchymosis, warty or papular lesions), particularly when recalcitrant to adequate first-line therapy, should be biopsied.
VSCC arises from precursor lesions or high-grade vulvar intraepithelial neoplasia (VIN). The 2015 International Society for the Study of Vulvovaginal Disease nomenclature classifies high-grade VIN into high-grade squamous intraepithelial lesion (HSIL) and differentiated VIN (dVIN). Most patients with high-grade VIN are diagnosed with HSIL or usual type VIN. A preponderance of these lesions (75%-85%) are HPV positive, predominantly HPV 16. Vulvar HSIL (vHSIL) lesions affect younger women. The lesions tend to be multifocal and extensive. On the other hand, dVIN typically affects older women and commonly develops as a solitary lesion. While dVIN accounts for only a small subset of patients with high-grade VIN, these lesions are HPV negative and associated with lichen sclerosus.
Both disease entities, vHSIL and dVIN, are increasing in incidence. There is a higher risk and shortened period of progression to cancer in patients with dVIN compared to HSIL. The cancer risk of vHSIL is relatively low. The 10-year cumulative VSCC risk reported in the literature is 10.3%; 9.7% for vHSIL and 50% for dVIN. Patients with vHSIL could benefit from less aggressive treatment modalities.
Patients present with a constellation of signs such as itching, pain, burning, bleeding, and discharge. Chronic symptoms portend HPV-independent lesions associated with lichen sclerosus while episodic signs are suggestive of HPV-positive lesions.
The recurrence risk of high-grade VIN is 46%-70%. Risk factors for recurrence include age greater than 50, immunosuppression, metasynchronous HSIL, and multifocal lesions. Recurrences occur in up to 50% of women who have undergone surgery. For those who undergo surgical treatment for high-grade VIN, recurrence is more common in the setting of positive margins, underlying lichen sclerosis, persistent HPV infection, and immunosuppression.
Management of high-grade VIN is determined by the lesion characteristics, patient characteristics, and medical expertise. Given the risk of progression of high-grade VIN to cancer and risk of underlying cancer, surgical therapy is typically recommended. The treatment of choice is surgical excision in cases of dVIN. Surgical treatments include CO2 laser ablation, wide local excision, and vulvectomy. Women who undergo surgical treatment for vHSIL have about a 50% chance of the condition recurring 1 year later, irrespective of whether treatment is by surgical excision or laser vaporization.
Since surgery can be associated with disfigurement and sexual dysfunction, alternatives to surgery should be considered in cases of vHSIL. The potential for effect on sexual function should be part of preoperative counseling and treatment. Women treated for VIN often experience increased inhibition of sexual excitement and increased inhibition of orgasm. One study found that in women undergoing vulvar excision for VIN, the impairment was found to be psychological in nature. Overall, the studies of sexual effect from treatment of VIN have found that women do not return to their pretreatment sexual function. However, the optimal management of vHSIL has not been determined. Nonsurgical options include topical therapies (imiquimod, 5-fluorouracil, cidofovir, and interferon) and nonpharmacologic treatments, such as photodynamic therapy.
Imiquimod, a topical immune modulator, is the most studied pharmacologic treatment of vHSIL. The drug induces secretion of cytokines, creating an immune response that clears the HPV infection. Imiquimod is safe and well tolerated. The clinical response rate varies between 35% and 81%. A recent study demonstrated the efficacy of imiquimod and the treatment was found to be noninferior to surgery. Adverse events differed, with local pain following surgical treatment and local pruritus and erythema associated with imiquimod use. Some patients did not respond to imiquimod; it was thought by the authors of the study that specific immunological factors affect the clinical response.

In conclusion, high-grade VIN is a heterogeneous disease made up of two distinct disease entities with rising incidence. In contrast to dVIN, the cancer risk is low for patients with vHSIL. Treatment should be driven by the clinical characteristics of the vulvar lesions, patients’ preferences, sexual activity, and compliance. Future directions include risk stratification of patients with vHSIL who are most likely to benefit from topical treatments, thus reducing overtreatment. Molecular biomarkers that could identify dVIN at an early stage are needed.
Dr. Jackson-Moore is associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
Cendejas BR et al. Am J Obstet Gynecol. 2015 Mar;212(3):291-7.
Lebreton M et al. J Gynecol Obstet Hum Reprod. 2020 Nov;49(9):101801.
Thuijs NB et al. Int J Cancer. 2021 Jan 1;148(1):90-8. doi: 10.1002/ijc.33198. .
Trutnovsky G et al. Lancet. 2022 May 7;399(10337):1790-8. Erratum in: Lancet. 2022 Oct 8;400(10359):1194.
Vulvar squamous cell carcinomas (VSCC) comprise approximately 90% of all vulvar malignancies. Unlike cervical SCC, which are predominantly human papilloma virus (HPV) positive, only a minority of VSCC are HPV positive – on the order of 15%-25% of cases. Most cases occur in the setting of lichen sclerosus and are HPV negative.
Lichen sclerosus is a chronic inflammatory dermatitis typically involving the anogenital area, which in some cases can become seriously distorted (e.g. atrophy of the labia minora, clitoral phimosis, and introital stenosis). Although most cases are diagnosed in postmenopausal women, LS can affect women of any age. The true prevalence of lichen sclerosus is unknown. Recent studies have shown a prevalence of 1 in 60; among older women, it can even be as high as 1 in 30. While lichen sclerosus is a pruriginous condition, it is often asymptomatic. It is not considered a premalignant condition. The diagnosis is clinical; however, suspicious lesions (erosions/ulcerations, hyperkeratosis, pigmented areas, ecchymosis, warty or papular lesions), particularly when recalcitrant to adequate first-line therapy, should be biopsied.
VSCC arises from precursor lesions or high-grade vulvar intraepithelial neoplasia (VIN). The 2015 International Society for the Study of Vulvovaginal Disease nomenclature classifies high-grade VIN into high-grade squamous intraepithelial lesion (HSIL) and differentiated VIN (dVIN). Most patients with high-grade VIN are diagnosed with HSIL or usual type VIN. A preponderance of these lesions (75%-85%) are HPV positive, predominantly HPV 16. Vulvar HSIL (vHSIL) lesions affect younger women. The lesions tend to be multifocal and extensive. On the other hand, dVIN typically affects older women and commonly develops as a solitary lesion. While dVIN accounts for only a small subset of patients with high-grade VIN, these lesions are HPV negative and associated with lichen sclerosus.
Both disease entities, vHSIL and dVIN, are increasing in incidence. There is a higher risk and shortened period of progression to cancer in patients with dVIN compared to HSIL. The cancer risk of vHSIL is relatively low. The 10-year cumulative VSCC risk reported in the literature is 10.3%; 9.7% for vHSIL and 50% for dVIN. Patients with vHSIL could benefit from less aggressive treatment modalities.
Patients present with a constellation of signs such as itching, pain, burning, bleeding, and discharge. Chronic symptoms portend HPV-independent lesions associated with lichen sclerosus while episodic signs are suggestive of HPV-positive lesions.
The recurrence risk of high-grade VIN is 46%-70%. Risk factors for recurrence include age greater than 50, immunosuppression, metasynchronous HSIL, and multifocal lesions. Recurrences occur in up to 50% of women who have undergone surgery. For those who undergo surgical treatment for high-grade VIN, recurrence is more common in the setting of positive margins, underlying lichen sclerosis, persistent HPV infection, and immunosuppression.
Management of high-grade VIN is determined by the lesion characteristics, patient characteristics, and medical expertise. Given the risk of progression of high-grade VIN to cancer and risk of underlying cancer, surgical therapy is typically recommended. The treatment of choice is surgical excision in cases of dVIN. Surgical treatments include CO2 laser ablation, wide local excision, and vulvectomy. Women who undergo surgical treatment for vHSIL have about a 50% chance of the condition recurring 1 year later, irrespective of whether treatment is by surgical excision or laser vaporization.
Since surgery can be associated with disfigurement and sexual dysfunction, alternatives to surgery should be considered in cases of vHSIL. The potential for effect on sexual function should be part of preoperative counseling and treatment. Women treated for VIN often experience increased inhibition of sexual excitement and increased inhibition of orgasm. One study found that in women undergoing vulvar excision for VIN, the impairment was found to be psychological in nature. Overall, the studies of sexual effect from treatment of VIN have found that women do not return to their pretreatment sexual function. However, the optimal management of vHSIL has not been determined. Nonsurgical options include topical therapies (imiquimod, 5-fluorouracil, cidofovir, and interferon) and nonpharmacologic treatments, such as photodynamic therapy.
Imiquimod, a topical immune modulator, is the most studied pharmacologic treatment of vHSIL. The drug induces secretion of cytokines, creating an immune response that clears the HPV infection. Imiquimod is safe and well tolerated. The clinical response rate varies between 35% and 81%. A recent study demonstrated the efficacy of imiquimod and the treatment was found to be noninferior to surgery. Adverse events differed, with local pain following surgical treatment and local pruritus and erythema associated with imiquimod use. Some patients did not respond to imiquimod; it was thought by the authors of the study that specific immunological factors affect the clinical response.

In conclusion, high-grade VIN is a heterogeneous disease made up of two distinct disease entities with rising incidence. In contrast to dVIN, the cancer risk is low for patients with vHSIL. Treatment should be driven by the clinical characteristics of the vulvar lesions, patients’ preferences, sexual activity, and compliance. Future directions include risk stratification of patients with vHSIL who are most likely to benefit from topical treatments, thus reducing overtreatment. Molecular biomarkers that could identify dVIN at an early stage are needed.
Dr. Jackson-Moore is associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
Cendejas BR et al. Am J Obstet Gynecol. 2015 Mar;212(3):291-7.
Lebreton M et al. J Gynecol Obstet Hum Reprod. 2020 Nov;49(9):101801.
Thuijs NB et al. Int J Cancer. 2021 Jan 1;148(1):90-8. doi: 10.1002/ijc.33198. .
Trutnovsky G et al. Lancet. 2022 May 7;399(10337):1790-8. Erratum in: Lancet. 2022 Oct 8;400(10359):1194.
Combining treatment options for scar revision often a useful approach
DENVER –
“It’s important to manage expectations,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual meeting of the American Society for Dermatologic Surgery. “I tell them I can improve their scar and make it look less noticeable, but I can’t make it look like normal skin. It’s going to require multiple treatments. It’s not a one-time thing; it’s going to take several months to see the full benefit. And, it’s an investment of time and money.”
Nonablative, ablative, and fractional resurfacing stimulates dermal fibroblasts to replace lost collagen and elastin. Traditional lasers offer impressive clinical results for scars but are associated with significant preprocedural discomfort, prolonged recovery, and a significant risk of side effects, Dr. Ortiz said, while nonablative lasers are more tolerable with shorter recovery times.
Multiple sessions are required, and results are often less clinically impressive. “It’s often difficult for patients to have a lot of downtime with each treatment so often I prefer to use the nonablative laser, especially for acne scarring,” she said.
Mounting evidence suggests that the sooner scars are treated after they are formed, the better. That may not be feasible for patients with a long history of acne scars, but for surgical scars, Dr. Ortiz prefers to start treatment on the day of suture removal. “Whenever I do that, I always get better results,” she said.
Outcomes may also improve by combining different treatment options, but the type of scar drives the type of modality to consider. There are red scars from postinflammatory erythema, hyperpigmented scars, hypopigmented scars, atrophic scars, hypertrophic scars, spread scars, pin cushion scars, and keloid scars, “which are the most difficult to treat,” she said. “When I’m using a combination approach, I start with the redness component of the scar, because you don’t want to exacerbate nonspecific erythema, or it’ll be difficult to see where the redness is. So, I always use vascular laser first, then a pigment-specific laser, followed by resurfacing, and augmentation with filler if needed.”
Red scars generally fade with time, but that can take several months to more than a year. “If you use a laser, that can speed up the recovery,” said Dr. Ortiz, who is the vice president of the American Society for Laser Medicine and Surgery. “A vascular laser will work, such as KTP, or intense pulsed light. Studies favor a low fluence and a short pulse duration. Pulsed dye laser (PDL) penetrates deeper than KTP, so theoretically you get a bit of collagen remodeling because it can increase TGF-beta [transforming growth factor–beta], so theoretically, PDL is a little bit better than KTP for red scars, but both will work.”
In a comparative study, researchers used purpuric and nonpurpuric parameters to treat surgical scars but found no significant differences between the two treatment settings. “I tend to stick to short pulse duration and low fluence settings,” said Dr. Ortiz, who was not affiliated with the study.
A separate, single-blinded, split scar study, which compared the efficacy of KTP to 595 nm PDL in reduction of erythema in surgical scars, found no significant difference between the two approaches. A review of available therapeutic lasers for acne scarring found that the thermal energy delivered by KTP extends only to the papillary dermis, making it useful for postinflammatory erythema without significant effects on collagen remodeling.
Hyperpigmentation
Use of concomitant bleaching cream can also help as a preventive strategy for hyperpigmentation. But one study of 100 patients found that pretreatment with a bleaching regimen prior to undergoing CO2 laser resurfacing made no significant difference in hyperpigmentation compared with those who received no pretreatment regimen.
When Dr. Ortiz is concerned about hyperpigmentation after laser treatment, she prescribes post-treatment tranexamic acid 325 mg twice daily for 6 months or longer. “I don’t do any kind of workup or labs, but I do not prescribe it if a patient has increased risk of clotting,” she said. Those at increased risk include smokers, those on birth control pills, those on hormonal supplementation, those with a current malignancy, and those with a history of a cerebrovascular accident or deep vein thrombosis.
Hypopigmented, atrophic scars
In Dr. Ortiz’s clinical experience, hypopigmented scars respond well to treatment with the 1550 nonablative laser. “The idea is that you’re removing some of the scarred collagen and it allows the melanocytes to migrate in and repigment,” she said. Following laser treatment, consider applying topical bimatoprost 0.03% twice daily for at least 3 months to optimize results, she added.
For atrophic scars, options include subcision, laser treatment, radiofrequency microneedling, fillers, or biostimulators. “I caution against using permanent fillers because there is a higher risk of granuloma formation,” Dr. Ortiz said. “I tend to use hyaluronic acid fillers, which have a low G prime. I inject superficially.”
She shared a technique she learned from Mathew Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston. It entails spreading the skin with one’s fingers for a scar, especially an acne scar. “If it improves when you spread the skin, then you know it’s amenable to laser treatment,” Dr. Ortiz said. “But if it doesn’t improve when you spread the skin, it probably needs a little subcision. Insert an 18- or 20-gauge tribeveled hypodermic needle or an 18-gauge Nokor under the scar to sever the fibrous components that anchor the scar. This can take more than one treatment. I’ll often do this immediately before resurfacing.”
For hypertrophic scars, consider laser-assisted drug delivery, which creates vertical channels that assist the delivery of topically applied drugs into the skin. “You never want to use something that isn’t meant to be injected into the skin because you can get a granulomatous reaction,” she warned. “I often use topical triamcinolone acetonide, 5-FU, or poly-l-lactic acid.”
Dr. Ortiz noted that botulinum toxin type A may be helpful for scars, despite the paucity of evidence regarding specific mechanisms of action. “There is some thought that it can modulate TGF-beta,” she said. “It also may modulate collagen deposition. Currently we’re looking into Botox alone for keloid scars. The initial results look just okay.”
Dr. Ortiz disclosed that she has received consulting fees from Alastin, Cutera, and Sciton, and honoraria from BTL and Procter & Gamble. She is also a member of the advisory board for Aerolase, Allergan, Bausch Health, Endo, Galderma, Rodan + Fields, and Sciton, and has received equipment from BTL, Sciton, and SmartGraft.
DENVER –
“It’s important to manage expectations,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual meeting of the American Society for Dermatologic Surgery. “I tell them I can improve their scar and make it look less noticeable, but I can’t make it look like normal skin. It’s going to require multiple treatments. It’s not a one-time thing; it’s going to take several months to see the full benefit. And, it’s an investment of time and money.”
Nonablative, ablative, and fractional resurfacing stimulates dermal fibroblasts to replace lost collagen and elastin. Traditional lasers offer impressive clinical results for scars but are associated with significant preprocedural discomfort, prolonged recovery, and a significant risk of side effects, Dr. Ortiz said, while nonablative lasers are more tolerable with shorter recovery times.
Multiple sessions are required, and results are often less clinically impressive. “It’s often difficult for patients to have a lot of downtime with each treatment so often I prefer to use the nonablative laser, especially for acne scarring,” she said.
Mounting evidence suggests that the sooner scars are treated after they are formed, the better. That may not be feasible for patients with a long history of acne scars, but for surgical scars, Dr. Ortiz prefers to start treatment on the day of suture removal. “Whenever I do that, I always get better results,” she said.
Outcomes may also improve by combining different treatment options, but the type of scar drives the type of modality to consider. There are red scars from postinflammatory erythema, hyperpigmented scars, hypopigmented scars, atrophic scars, hypertrophic scars, spread scars, pin cushion scars, and keloid scars, “which are the most difficult to treat,” she said. “When I’m using a combination approach, I start with the redness component of the scar, because you don’t want to exacerbate nonspecific erythema, or it’ll be difficult to see where the redness is. So, I always use vascular laser first, then a pigment-specific laser, followed by resurfacing, and augmentation with filler if needed.”
Red scars generally fade with time, but that can take several months to more than a year. “If you use a laser, that can speed up the recovery,” said Dr. Ortiz, who is the vice president of the American Society for Laser Medicine and Surgery. “A vascular laser will work, such as KTP, or intense pulsed light. Studies favor a low fluence and a short pulse duration. Pulsed dye laser (PDL) penetrates deeper than KTP, so theoretically you get a bit of collagen remodeling because it can increase TGF-beta [transforming growth factor–beta], so theoretically, PDL is a little bit better than KTP for red scars, but both will work.”
In a comparative study, researchers used purpuric and nonpurpuric parameters to treat surgical scars but found no significant differences between the two treatment settings. “I tend to stick to short pulse duration and low fluence settings,” said Dr. Ortiz, who was not affiliated with the study.
A separate, single-blinded, split scar study, which compared the efficacy of KTP to 595 nm PDL in reduction of erythema in surgical scars, found no significant difference between the two approaches. A review of available therapeutic lasers for acne scarring found that the thermal energy delivered by KTP extends only to the papillary dermis, making it useful for postinflammatory erythema without significant effects on collagen remodeling.
Hyperpigmentation
Use of concomitant bleaching cream can also help as a preventive strategy for hyperpigmentation. But one study of 100 patients found that pretreatment with a bleaching regimen prior to undergoing CO2 laser resurfacing made no significant difference in hyperpigmentation compared with those who received no pretreatment regimen.
When Dr. Ortiz is concerned about hyperpigmentation after laser treatment, she prescribes post-treatment tranexamic acid 325 mg twice daily for 6 months or longer. “I don’t do any kind of workup or labs, but I do not prescribe it if a patient has increased risk of clotting,” she said. Those at increased risk include smokers, those on birth control pills, those on hormonal supplementation, those with a current malignancy, and those with a history of a cerebrovascular accident or deep vein thrombosis.
Hypopigmented, atrophic scars
In Dr. Ortiz’s clinical experience, hypopigmented scars respond well to treatment with the 1550 nonablative laser. “The idea is that you’re removing some of the scarred collagen and it allows the melanocytes to migrate in and repigment,” she said. Following laser treatment, consider applying topical bimatoprost 0.03% twice daily for at least 3 months to optimize results, she added.
For atrophic scars, options include subcision, laser treatment, radiofrequency microneedling, fillers, or biostimulators. “I caution against using permanent fillers because there is a higher risk of granuloma formation,” Dr. Ortiz said. “I tend to use hyaluronic acid fillers, which have a low G prime. I inject superficially.”
She shared a technique she learned from Mathew Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston. It entails spreading the skin with one’s fingers for a scar, especially an acne scar. “If it improves when you spread the skin, then you know it’s amenable to laser treatment,” Dr. Ortiz said. “But if it doesn’t improve when you spread the skin, it probably needs a little subcision. Insert an 18- or 20-gauge tribeveled hypodermic needle or an 18-gauge Nokor under the scar to sever the fibrous components that anchor the scar. This can take more than one treatment. I’ll often do this immediately before resurfacing.”
For hypertrophic scars, consider laser-assisted drug delivery, which creates vertical channels that assist the delivery of topically applied drugs into the skin. “You never want to use something that isn’t meant to be injected into the skin because you can get a granulomatous reaction,” she warned. “I often use topical triamcinolone acetonide, 5-FU, or poly-l-lactic acid.”
Dr. Ortiz noted that botulinum toxin type A may be helpful for scars, despite the paucity of evidence regarding specific mechanisms of action. “There is some thought that it can modulate TGF-beta,” she said. “It also may modulate collagen deposition. Currently we’re looking into Botox alone for keloid scars. The initial results look just okay.”
Dr. Ortiz disclosed that she has received consulting fees from Alastin, Cutera, and Sciton, and honoraria from BTL and Procter & Gamble. She is also a member of the advisory board for Aerolase, Allergan, Bausch Health, Endo, Galderma, Rodan + Fields, and Sciton, and has received equipment from BTL, Sciton, and SmartGraft.
DENVER –
“It’s important to manage expectations,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual meeting of the American Society for Dermatologic Surgery. “I tell them I can improve their scar and make it look less noticeable, but I can’t make it look like normal skin. It’s going to require multiple treatments. It’s not a one-time thing; it’s going to take several months to see the full benefit. And, it’s an investment of time and money.”
Nonablative, ablative, and fractional resurfacing stimulates dermal fibroblasts to replace lost collagen and elastin. Traditional lasers offer impressive clinical results for scars but are associated with significant preprocedural discomfort, prolonged recovery, and a significant risk of side effects, Dr. Ortiz said, while nonablative lasers are more tolerable with shorter recovery times.
Multiple sessions are required, and results are often less clinically impressive. “It’s often difficult for patients to have a lot of downtime with each treatment so often I prefer to use the nonablative laser, especially for acne scarring,” she said.
Mounting evidence suggests that the sooner scars are treated after they are formed, the better. That may not be feasible for patients with a long history of acne scars, but for surgical scars, Dr. Ortiz prefers to start treatment on the day of suture removal. “Whenever I do that, I always get better results,” she said.
Outcomes may also improve by combining different treatment options, but the type of scar drives the type of modality to consider. There are red scars from postinflammatory erythema, hyperpigmented scars, hypopigmented scars, atrophic scars, hypertrophic scars, spread scars, pin cushion scars, and keloid scars, “which are the most difficult to treat,” she said. “When I’m using a combination approach, I start with the redness component of the scar, because you don’t want to exacerbate nonspecific erythema, or it’ll be difficult to see where the redness is. So, I always use vascular laser first, then a pigment-specific laser, followed by resurfacing, and augmentation with filler if needed.”
Red scars generally fade with time, but that can take several months to more than a year. “If you use a laser, that can speed up the recovery,” said Dr. Ortiz, who is the vice president of the American Society for Laser Medicine and Surgery. “A vascular laser will work, such as KTP, or intense pulsed light. Studies favor a low fluence and a short pulse duration. Pulsed dye laser (PDL) penetrates deeper than KTP, so theoretically you get a bit of collagen remodeling because it can increase TGF-beta [transforming growth factor–beta], so theoretically, PDL is a little bit better than KTP for red scars, but both will work.”
In a comparative study, researchers used purpuric and nonpurpuric parameters to treat surgical scars but found no significant differences between the two treatment settings. “I tend to stick to short pulse duration and low fluence settings,” said Dr. Ortiz, who was not affiliated with the study.
A separate, single-blinded, split scar study, which compared the efficacy of KTP to 595 nm PDL in reduction of erythema in surgical scars, found no significant difference between the two approaches. A review of available therapeutic lasers for acne scarring found that the thermal energy delivered by KTP extends only to the papillary dermis, making it useful for postinflammatory erythema without significant effects on collagen remodeling.
Hyperpigmentation
Use of concomitant bleaching cream can also help as a preventive strategy for hyperpigmentation. But one study of 100 patients found that pretreatment with a bleaching regimen prior to undergoing CO2 laser resurfacing made no significant difference in hyperpigmentation compared with those who received no pretreatment regimen.
When Dr. Ortiz is concerned about hyperpigmentation after laser treatment, she prescribes post-treatment tranexamic acid 325 mg twice daily for 6 months or longer. “I don’t do any kind of workup or labs, but I do not prescribe it if a patient has increased risk of clotting,” she said. Those at increased risk include smokers, those on birth control pills, those on hormonal supplementation, those with a current malignancy, and those with a history of a cerebrovascular accident or deep vein thrombosis.
Hypopigmented, atrophic scars
In Dr. Ortiz’s clinical experience, hypopigmented scars respond well to treatment with the 1550 nonablative laser. “The idea is that you’re removing some of the scarred collagen and it allows the melanocytes to migrate in and repigment,” she said. Following laser treatment, consider applying topical bimatoprost 0.03% twice daily for at least 3 months to optimize results, she added.
For atrophic scars, options include subcision, laser treatment, radiofrequency microneedling, fillers, or biostimulators. “I caution against using permanent fillers because there is a higher risk of granuloma formation,” Dr. Ortiz said. “I tend to use hyaluronic acid fillers, which have a low G prime. I inject superficially.”
She shared a technique she learned from Mathew Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston. It entails spreading the skin with one’s fingers for a scar, especially an acne scar. “If it improves when you spread the skin, then you know it’s amenable to laser treatment,” Dr. Ortiz said. “But if it doesn’t improve when you spread the skin, it probably needs a little subcision. Insert an 18- or 20-gauge tribeveled hypodermic needle or an 18-gauge Nokor under the scar to sever the fibrous components that anchor the scar. This can take more than one treatment. I’ll often do this immediately before resurfacing.”
For hypertrophic scars, consider laser-assisted drug delivery, which creates vertical channels that assist the delivery of topically applied drugs into the skin. “You never want to use something that isn’t meant to be injected into the skin because you can get a granulomatous reaction,” she warned. “I often use topical triamcinolone acetonide, 5-FU, or poly-l-lactic acid.”
Dr. Ortiz noted that botulinum toxin type A may be helpful for scars, despite the paucity of evidence regarding specific mechanisms of action. “There is some thought that it can modulate TGF-beta,” she said. “It also may modulate collagen deposition. Currently we’re looking into Botox alone for keloid scars. The initial results look just okay.”
Dr. Ortiz disclosed that she has received consulting fees from Alastin, Cutera, and Sciton, and honoraria from BTL and Procter & Gamble. She is also a member of the advisory board for Aerolase, Allergan, Bausch Health, Endo, Galderma, Rodan + Fields, and Sciton, and has received equipment from BTL, Sciton, and SmartGraft.
AT ASDS 2022
Anatomic site influences ropivacaine duration during dermatologic surgery
DENVER – , results from a single-center study showed.
Ropivacaine is a long-acting anesthetic that may be used as a substitute for the more commonly local anesthetics such as lidocaine or bupivacaine in dermatologic surgery, lead study author Kira Minkis, MD, PhD, told this news organization following the annual meeting of the American Society for Dermatologic Surgery, where the study results were presented during an oral abstract session. By comparison, ropivacaine has been reported to have a faster onset, similar duration in the range of 6-14 hours, less pain upon injection, and inherent vasoconstrictive properties.
“With tumescent anesthesia, studies have previously shown that the rate and absorption of anesthetics is influenced by the site of administration,” said Dr. Minkis, director of Mohs and dermatologic surgery at Weill Cornell Medicine, New York. “In studies comparing absorption of local anesthetics in tumescent anesthesia by regions that differ in vascularity, peak serum concentrations are greater and rise more rapidly after use in the head and neck compared to the trunk and extremities. However, no studies to date have compared the duration of ropivacaine in highly vascularized tissue or compared duration between regions that differ in vascularity.” The aim of the study, she noted, was to characterize the difference in duration of ropivacaine’s effects between anatomic regions of rich and comparably poor vascularity, such as the face and extremities, respectively.
Dr. Minkis and her colleagues recruited 17 women and 12 men with a mean age of 72 years who underwent Mohs surgery on the nose or the shin at Weill Cornell Medicine. Patients were anesthetized at each site with a subcutaneous injection of 0.5 mL of ropivacaine, 0.2%. Sensation was determined by pinprick prior to injection, at baseline, and every 15 minutes until sensation returned or surgery concluded. The primary endpoint was time to return of pinprick sensation.
The researchers found that the duration of ropivacaine was significantly shorter on the nose (a median of 60 minutes) than on the shin (a median of 210 minutes). In fact, the upper limit of the range of duration at the shin was not determinable because 22 of the 29 (76%) of participants did not regain sensation on the shin prior to leaving the surgical suite and concluding the study. The proportion of study participants who regained sensation within 1 hour was 76% among those who were treated on the nose vs. 3% of those who were treated on the shin (P < .0001).
“With durations of up to 6-14 hours reported, our results indicate a strikingly shorter duration of local anesthesia in highly vascularized tissue,” Dr. Minkis said. “The brevity of local anesthesia is even more surprising given the intrinsic vasoconstrictive properties of ropivacaine. Often, we co-administer epinephrine to achieve vasoconstriction and reduce local blood flow, thus prolonging local concentrations of the anesthetic with the added benefit of reducing bleeding during surgery. The short duration we’ve observed in our study is emphasized in using a potent, long-acting local anesthetic with vasoconstrictive properties that otherwise should attenuate the effects of high local vascularity.”
In other findings, patients with history of hypertension were more likely to regain sensation on the nose by 60 minutes but this did not reach statistical significance (P = .079). Other comorbidities including underlying anxiety/depression, diabetes, and kidney disease did not significantly impact duration of ropivacaine action on the nose. The same held true for patients who were treated on the shin.
“We highlight an inconsistency between the reported duration of a long-lasting local anesthetic and the short-lived anesthesia experienced by our patients in a highly vascularized region,” Dr. Minkis said. “In practice, adjunctive use of a long-acting anesthetic to prolong anesthesia is common, which may provide relief from multiple injections of shorter-acting lidocaine. However, the duration of Mohs surgery can be unpredictable. Extended wait times between stages may exceed the duration we’ve observed in this study.”
In addition, she continued, “pain is frequently reported on postoperative days 0 to 3, leading some to recommend the use of long-acting local anesthetics to prevent overprescription or a gap in pain coverage. This emphasizes a gap in effective pain control, but also an opportunity to improve our patients’ surgical and recovery experiences.”
Impact on practice
Keith L. Duffy, MD, associate professor of dermatology at the University of Utah, Salt Lake City, who was asked to comment on the study, said that in light of current local anesthetic shortages and back orders, “we dermatologic surgeons have been experimenting with different anesthetics and concentrations that we can use in our patients. Ropivacaine may become the anesthetic of choice for many of our practices given its inherent properties.”
The duration of anesthetic effects by anatomic location in this study is “actually more impressive than I would have suspected as a practicing Mohs surgeon. The results of this study will immediately impact my Mohs surgery clinic,” he said, adding that he hoped that Dr. Minkis and others “will expand on this study to include more patients, different anesthetics, and more anatomic locations.”
Dr. Minkis acknowledged certain limitations of the study, including its single-center design and the fact that there were too few observations of medical and clinical characteristics for subgroup analysis.
She and Dr. Duffy reported having no financial disclosures.
DENVER – , results from a single-center study showed.
Ropivacaine is a long-acting anesthetic that may be used as a substitute for the more commonly local anesthetics such as lidocaine or bupivacaine in dermatologic surgery, lead study author Kira Minkis, MD, PhD, told this news organization following the annual meeting of the American Society for Dermatologic Surgery, where the study results were presented during an oral abstract session. By comparison, ropivacaine has been reported to have a faster onset, similar duration in the range of 6-14 hours, less pain upon injection, and inherent vasoconstrictive properties.
“With tumescent anesthesia, studies have previously shown that the rate and absorption of anesthetics is influenced by the site of administration,” said Dr. Minkis, director of Mohs and dermatologic surgery at Weill Cornell Medicine, New York. “In studies comparing absorption of local anesthetics in tumescent anesthesia by regions that differ in vascularity, peak serum concentrations are greater and rise more rapidly after use in the head and neck compared to the trunk and extremities. However, no studies to date have compared the duration of ropivacaine in highly vascularized tissue or compared duration between regions that differ in vascularity.” The aim of the study, she noted, was to characterize the difference in duration of ropivacaine’s effects between anatomic regions of rich and comparably poor vascularity, such as the face and extremities, respectively.
Dr. Minkis and her colleagues recruited 17 women and 12 men with a mean age of 72 years who underwent Mohs surgery on the nose or the shin at Weill Cornell Medicine. Patients were anesthetized at each site with a subcutaneous injection of 0.5 mL of ropivacaine, 0.2%. Sensation was determined by pinprick prior to injection, at baseline, and every 15 minutes until sensation returned or surgery concluded. The primary endpoint was time to return of pinprick sensation.
The researchers found that the duration of ropivacaine was significantly shorter on the nose (a median of 60 minutes) than on the shin (a median of 210 minutes). In fact, the upper limit of the range of duration at the shin was not determinable because 22 of the 29 (76%) of participants did not regain sensation on the shin prior to leaving the surgical suite and concluding the study. The proportion of study participants who regained sensation within 1 hour was 76% among those who were treated on the nose vs. 3% of those who were treated on the shin (P < .0001).
“With durations of up to 6-14 hours reported, our results indicate a strikingly shorter duration of local anesthesia in highly vascularized tissue,” Dr. Minkis said. “The brevity of local anesthesia is even more surprising given the intrinsic vasoconstrictive properties of ropivacaine. Often, we co-administer epinephrine to achieve vasoconstriction and reduce local blood flow, thus prolonging local concentrations of the anesthetic with the added benefit of reducing bleeding during surgery. The short duration we’ve observed in our study is emphasized in using a potent, long-acting local anesthetic with vasoconstrictive properties that otherwise should attenuate the effects of high local vascularity.”
In other findings, patients with history of hypertension were more likely to regain sensation on the nose by 60 minutes but this did not reach statistical significance (P = .079). Other comorbidities including underlying anxiety/depression, diabetes, and kidney disease did not significantly impact duration of ropivacaine action on the nose. The same held true for patients who were treated on the shin.
“We highlight an inconsistency between the reported duration of a long-lasting local anesthetic and the short-lived anesthesia experienced by our patients in a highly vascularized region,” Dr. Minkis said. “In practice, adjunctive use of a long-acting anesthetic to prolong anesthesia is common, which may provide relief from multiple injections of shorter-acting lidocaine. However, the duration of Mohs surgery can be unpredictable. Extended wait times between stages may exceed the duration we’ve observed in this study.”
In addition, she continued, “pain is frequently reported on postoperative days 0 to 3, leading some to recommend the use of long-acting local anesthetics to prevent overprescription or a gap in pain coverage. This emphasizes a gap in effective pain control, but also an opportunity to improve our patients’ surgical and recovery experiences.”
Impact on practice
Keith L. Duffy, MD, associate professor of dermatology at the University of Utah, Salt Lake City, who was asked to comment on the study, said that in light of current local anesthetic shortages and back orders, “we dermatologic surgeons have been experimenting with different anesthetics and concentrations that we can use in our patients. Ropivacaine may become the anesthetic of choice for many of our practices given its inherent properties.”
The duration of anesthetic effects by anatomic location in this study is “actually more impressive than I would have suspected as a practicing Mohs surgeon. The results of this study will immediately impact my Mohs surgery clinic,” he said, adding that he hoped that Dr. Minkis and others “will expand on this study to include more patients, different anesthetics, and more anatomic locations.”
Dr. Minkis acknowledged certain limitations of the study, including its single-center design and the fact that there were too few observations of medical and clinical characteristics for subgroup analysis.
She and Dr. Duffy reported having no financial disclosures.
DENVER – , results from a single-center study showed.
Ropivacaine is a long-acting anesthetic that may be used as a substitute for the more commonly local anesthetics such as lidocaine or bupivacaine in dermatologic surgery, lead study author Kira Minkis, MD, PhD, told this news organization following the annual meeting of the American Society for Dermatologic Surgery, where the study results were presented during an oral abstract session. By comparison, ropivacaine has been reported to have a faster onset, similar duration in the range of 6-14 hours, less pain upon injection, and inherent vasoconstrictive properties.
“With tumescent anesthesia, studies have previously shown that the rate and absorption of anesthetics is influenced by the site of administration,” said Dr. Minkis, director of Mohs and dermatologic surgery at Weill Cornell Medicine, New York. “In studies comparing absorption of local anesthetics in tumescent anesthesia by regions that differ in vascularity, peak serum concentrations are greater and rise more rapidly after use in the head and neck compared to the trunk and extremities. However, no studies to date have compared the duration of ropivacaine in highly vascularized tissue or compared duration between regions that differ in vascularity.” The aim of the study, she noted, was to characterize the difference in duration of ropivacaine’s effects between anatomic regions of rich and comparably poor vascularity, such as the face and extremities, respectively.
Dr. Minkis and her colleagues recruited 17 women and 12 men with a mean age of 72 years who underwent Mohs surgery on the nose or the shin at Weill Cornell Medicine. Patients were anesthetized at each site with a subcutaneous injection of 0.5 mL of ropivacaine, 0.2%. Sensation was determined by pinprick prior to injection, at baseline, and every 15 minutes until sensation returned or surgery concluded. The primary endpoint was time to return of pinprick sensation.
The researchers found that the duration of ropivacaine was significantly shorter on the nose (a median of 60 minutes) than on the shin (a median of 210 minutes). In fact, the upper limit of the range of duration at the shin was not determinable because 22 of the 29 (76%) of participants did not regain sensation on the shin prior to leaving the surgical suite and concluding the study. The proportion of study participants who regained sensation within 1 hour was 76% among those who were treated on the nose vs. 3% of those who were treated on the shin (P < .0001).
“With durations of up to 6-14 hours reported, our results indicate a strikingly shorter duration of local anesthesia in highly vascularized tissue,” Dr. Minkis said. “The brevity of local anesthesia is even more surprising given the intrinsic vasoconstrictive properties of ropivacaine. Often, we co-administer epinephrine to achieve vasoconstriction and reduce local blood flow, thus prolonging local concentrations of the anesthetic with the added benefit of reducing bleeding during surgery. The short duration we’ve observed in our study is emphasized in using a potent, long-acting local anesthetic with vasoconstrictive properties that otherwise should attenuate the effects of high local vascularity.”
In other findings, patients with history of hypertension were more likely to regain sensation on the nose by 60 minutes but this did not reach statistical significance (P = .079). Other comorbidities including underlying anxiety/depression, diabetes, and kidney disease did not significantly impact duration of ropivacaine action on the nose. The same held true for patients who were treated on the shin.
“We highlight an inconsistency between the reported duration of a long-lasting local anesthetic and the short-lived anesthesia experienced by our patients in a highly vascularized region,” Dr. Minkis said. “In practice, adjunctive use of a long-acting anesthetic to prolong anesthesia is common, which may provide relief from multiple injections of shorter-acting lidocaine. However, the duration of Mohs surgery can be unpredictable. Extended wait times between stages may exceed the duration we’ve observed in this study.”
In addition, she continued, “pain is frequently reported on postoperative days 0 to 3, leading some to recommend the use of long-acting local anesthetics to prevent overprescription or a gap in pain coverage. This emphasizes a gap in effective pain control, but also an opportunity to improve our patients’ surgical and recovery experiences.”
Impact on practice
Keith L. Duffy, MD, associate professor of dermatology at the University of Utah, Salt Lake City, who was asked to comment on the study, said that in light of current local anesthetic shortages and back orders, “we dermatologic surgeons have been experimenting with different anesthetics and concentrations that we can use in our patients. Ropivacaine may become the anesthetic of choice for many of our practices given its inherent properties.”
The duration of anesthetic effects by anatomic location in this study is “actually more impressive than I would have suspected as a practicing Mohs surgeon. The results of this study will immediately impact my Mohs surgery clinic,” he said, adding that he hoped that Dr. Minkis and others “will expand on this study to include more patients, different anesthetics, and more anatomic locations.”
Dr. Minkis acknowledged certain limitations of the study, including its single-center design and the fact that there were too few observations of medical and clinical characteristics for subgroup analysis.
She and Dr. Duffy reported having no financial disclosures.
AT ASDS 2022
Combination of energy-based treatments found to improve Becker’s nevi
Denver – out to 40 weeks, results of a small retrospective case series demonstrated.
During an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery, presenting author Shelby L. Kubicki, MD, said that NAFR and LHR target the clinically bothersome Becker’s nevi features of hyperpigmentation and hypertrichosis via different mechanisms. “NAFR creates microcolumns of thermal injury in the skin, which improves hyperpigmentation,” explained Dr. Kubicki, a 3rd-year dermatology resident at University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston.
“LHR targets follicular melanocytes, which are located more deeply in the dermis,” she said. “This improves hypertrichosis and likely prevents recurrence of hyperpigmentation by targeting these melanocytes that are not reached by NAFR.”
Dr. Kubicki and her colleagues retrospectively reviewed 12 patients with Becker’s nevus who underwent a mean of 5.3 NAFR treatments at a single dermatology practice at intervals that ranged between 1 and 4 months. The long-pulsed 755-nm alexandrite laser was used for study participants with skin types I-III, while the long-pulsed 1,064-nm Nd: YAG laser was used for those with skin types IV-VI. Ten of the 12 patients underwent concomitant LHR with one of the two devices and three independent physicians used a 5-point visual analog scale (VAS) to rate clinical photographs. All patients completed a strict pre- and postoperative regimen with either 4% hydroquinone or topical 3% tranexamic acid and broad-spectrum sunscreen and postoperative treatment with a midpotency topical corticosteroid for 3 days.
The study is the largest known case series of therapy combining 1,550-nm NAFR and LHR for Becker’s nevus patients with skin types III-VI.
After comparing VAS scores at baseline and follow-up, physicians rated the cosmetic appearance of Becker’s nevus as improving by a range of 51%-75%. Two patients did not undergo LHR: one male patient with Becker’s nevus in his beard region, for whom LHR was undesirable, and a second patient with atrichotic Becker’s nevus. These two patients demonstrated improvements in VAS scores of 26%-50% and 76%-99%, respectively.
No long-term adverse events were observed during follow-up, which ranged from 6 to 40 weeks. “We do want more long-term follow-up,” Dr. Kubicki said, noting that there are more data on some patients to extend the follow-up.
She and her coinvestigators concluded that the results show that treatment with a combination of NAFR and LHR safely addresses both hyperpigmentation and hypertrichosis in Becker’s nevi. “In addition, LHR likely prevents recurrence of hyperpigmentation by targeting follicular melanocytes,” she said. “In our study, we did have one patient experience recurrence of a Becker’s nevus during follow-up, but [the rest] did not, which we considered a success.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on the study, characterized Becker’s nevus as a difficult-to-treat condition that is made even more difficult to treat in skin types III-VI.
“Combining laser hair removal using appropriate wavelengths with 1,550-nm nonablative fractional resurfacing yielded good clinical results with few recurrences,” he said in an interview with this news organization. “Though it was a small series, it definitely is an interesting option for practicing dermatologists who encounter patients interested in improving the appearance of a Becker’s nevus.”
The researchers reported having no relevant disclosures.
Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory boards for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
Denver – out to 40 weeks, results of a small retrospective case series demonstrated.
During an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery, presenting author Shelby L. Kubicki, MD, said that NAFR and LHR target the clinically bothersome Becker’s nevi features of hyperpigmentation and hypertrichosis via different mechanisms. “NAFR creates microcolumns of thermal injury in the skin, which improves hyperpigmentation,” explained Dr. Kubicki, a 3rd-year dermatology resident at University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston.
“LHR targets follicular melanocytes, which are located more deeply in the dermis,” she said. “This improves hypertrichosis and likely prevents recurrence of hyperpigmentation by targeting these melanocytes that are not reached by NAFR.”
Dr. Kubicki and her colleagues retrospectively reviewed 12 patients with Becker’s nevus who underwent a mean of 5.3 NAFR treatments at a single dermatology practice at intervals that ranged between 1 and 4 months. The long-pulsed 755-nm alexandrite laser was used for study participants with skin types I-III, while the long-pulsed 1,064-nm Nd: YAG laser was used for those with skin types IV-VI. Ten of the 12 patients underwent concomitant LHR with one of the two devices and three independent physicians used a 5-point visual analog scale (VAS) to rate clinical photographs. All patients completed a strict pre- and postoperative regimen with either 4% hydroquinone or topical 3% tranexamic acid and broad-spectrum sunscreen and postoperative treatment with a midpotency topical corticosteroid for 3 days.
The study is the largest known case series of therapy combining 1,550-nm NAFR and LHR for Becker’s nevus patients with skin types III-VI.
After comparing VAS scores at baseline and follow-up, physicians rated the cosmetic appearance of Becker’s nevus as improving by a range of 51%-75%. Two patients did not undergo LHR: one male patient with Becker’s nevus in his beard region, for whom LHR was undesirable, and a second patient with atrichotic Becker’s nevus. These two patients demonstrated improvements in VAS scores of 26%-50% and 76%-99%, respectively.
No long-term adverse events were observed during follow-up, which ranged from 6 to 40 weeks. “We do want more long-term follow-up,” Dr. Kubicki said, noting that there are more data on some patients to extend the follow-up.
She and her coinvestigators concluded that the results show that treatment with a combination of NAFR and LHR safely addresses both hyperpigmentation and hypertrichosis in Becker’s nevi. “In addition, LHR likely prevents recurrence of hyperpigmentation by targeting follicular melanocytes,” she said. “In our study, we did have one patient experience recurrence of a Becker’s nevus during follow-up, but [the rest] did not, which we considered a success.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on the study, characterized Becker’s nevus as a difficult-to-treat condition that is made even more difficult to treat in skin types III-VI.
“Combining laser hair removal using appropriate wavelengths with 1,550-nm nonablative fractional resurfacing yielded good clinical results with few recurrences,” he said in an interview with this news organization. “Though it was a small series, it definitely is an interesting option for practicing dermatologists who encounter patients interested in improving the appearance of a Becker’s nevus.”
The researchers reported having no relevant disclosures.
Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory boards for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
Denver – out to 40 weeks, results of a small retrospective case series demonstrated.
During an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery, presenting author Shelby L. Kubicki, MD, said that NAFR and LHR target the clinically bothersome Becker’s nevi features of hyperpigmentation and hypertrichosis via different mechanisms. “NAFR creates microcolumns of thermal injury in the skin, which improves hyperpigmentation,” explained Dr. Kubicki, a 3rd-year dermatology resident at University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston.
“LHR targets follicular melanocytes, which are located more deeply in the dermis,” she said. “This improves hypertrichosis and likely prevents recurrence of hyperpigmentation by targeting these melanocytes that are not reached by NAFR.”
Dr. Kubicki and her colleagues retrospectively reviewed 12 patients with Becker’s nevus who underwent a mean of 5.3 NAFR treatments at a single dermatology practice at intervals that ranged between 1 and 4 months. The long-pulsed 755-nm alexandrite laser was used for study participants with skin types I-III, while the long-pulsed 1,064-nm Nd: YAG laser was used for those with skin types IV-VI. Ten of the 12 patients underwent concomitant LHR with one of the two devices and three independent physicians used a 5-point visual analog scale (VAS) to rate clinical photographs. All patients completed a strict pre- and postoperative regimen with either 4% hydroquinone or topical 3% tranexamic acid and broad-spectrum sunscreen and postoperative treatment with a midpotency topical corticosteroid for 3 days.
The study is the largest known case series of therapy combining 1,550-nm NAFR and LHR for Becker’s nevus patients with skin types III-VI.
After comparing VAS scores at baseline and follow-up, physicians rated the cosmetic appearance of Becker’s nevus as improving by a range of 51%-75%. Two patients did not undergo LHR: one male patient with Becker’s nevus in his beard region, for whom LHR was undesirable, and a second patient with atrichotic Becker’s nevus. These two patients demonstrated improvements in VAS scores of 26%-50% and 76%-99%, respectively.
No long-term adverse events were observed during follow-up, which ranged from 6 to 40 weeks. “We do want more long-term follow-up,” Dr. Kubicki said, noting that there are more data on some patients to extend the follow-up.
She and her coinvestigators concluded that the results show that treatment with a combination of NAFR and LHR safely addresses both hyperpigmentation and hypertrichosis in Becker’s nevi. “In addition, LHR likely prevents recurrence of hyperpigmentation by targeting follicular melanocytes,” she said. “In our study, we did have one patient experience recurrence of a Becker’s nevus during follow-up, but [the rest] did not, which we considered a success.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on the study, characterized Becker’s nevus as a difficult-to-treat condition that is made even more difficult to treat in skin types III-VI.
“Combining laser hair removal using appropriate wavelengths with 1,550-nm nonablative fractional resurfacing yielded good clinical results with few recurrences,” he said in an interview with this news organization. “Though it was a small series, it definitely is an interesting option for practicing dermatologists who encounter patients interested in improving the appearance of a Becker’s nevus.”
The researchers reported having no relevant disclosures.
Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory boards for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
AT ASDS 2022