User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
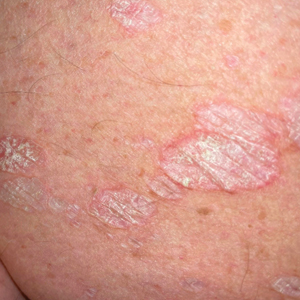
Exploring the Relationship Between Psoriasis and Mobility Among US Adults
Exploring the Relationship Between Psoriasis and Mobility Among US Adults
To the Editor:
Psoriasis is a chronic inflammatory condition that affects individuals in various extracutaneous ways.1 Prior studies have documented a decrease in exercise intensity among patients with psoriasis2; however, few studies have specifically investigated baseline mobility in this population. Baseline mobility denotes an individual’s fundamental ability to walk or move around without assistance of any kind. Impaired mobility—when baseline mobility is compromised—is an aspect of the wider diversity, equity, and inclusion framework that underscores the significance of recognizing challenges and promoting inclusive measures, both at the point of care and in research.3 study sought to analyze the relationship between psoriasis and baseline mobility among US adults (aged 45 to 80 years) utilizing the latest data from the National Health and Nutrition Examination Survey (NHANES) database for psoriasis.4 We used three 2-year cycles of NHANES data to create a 2009-2014 dataset.
The overall NHANES response rate among adults aged 45 to 80 years between 2009 and 2014 was 67.9%. Patients were categorized as having impaired mobility if they responded “yes” to the following question: “Because of a health problem, do you have difficulty walking without using any special equipment?” Psoriasis status was assessed by the following question: “Have you ever been told by a doctor or other health professional that you had psoriasis?” Multivariable logistic regression analyses were performed using Stata/SE 18.0 software (StataCorp LLC) to assess the relationship between psoriasis and impaired mobility. Age, income, education, sex, race, tobacco use, diabetes status, body mass index, and arthritis status were controlled for in our models.
Our analysis initially included 9982 participants; 14 did not respond to questions assessing psoriasis and impaired mobility and were excluded. The prevalence of impaired mobility in patients with psoriasis was 17.1% compared with 10.9% among those without psoriasis (Table 1). There was a significant association between psoriasis and impaired mobility among patients aged 45 to 80 years after adjusting for potential confounding variables (adjusted odds ratio [AOR], 1.54; 95% CI, 1.04- 2.29; P=.032)(Table 2). Analyses of subgroups yielded no statistically significant results.
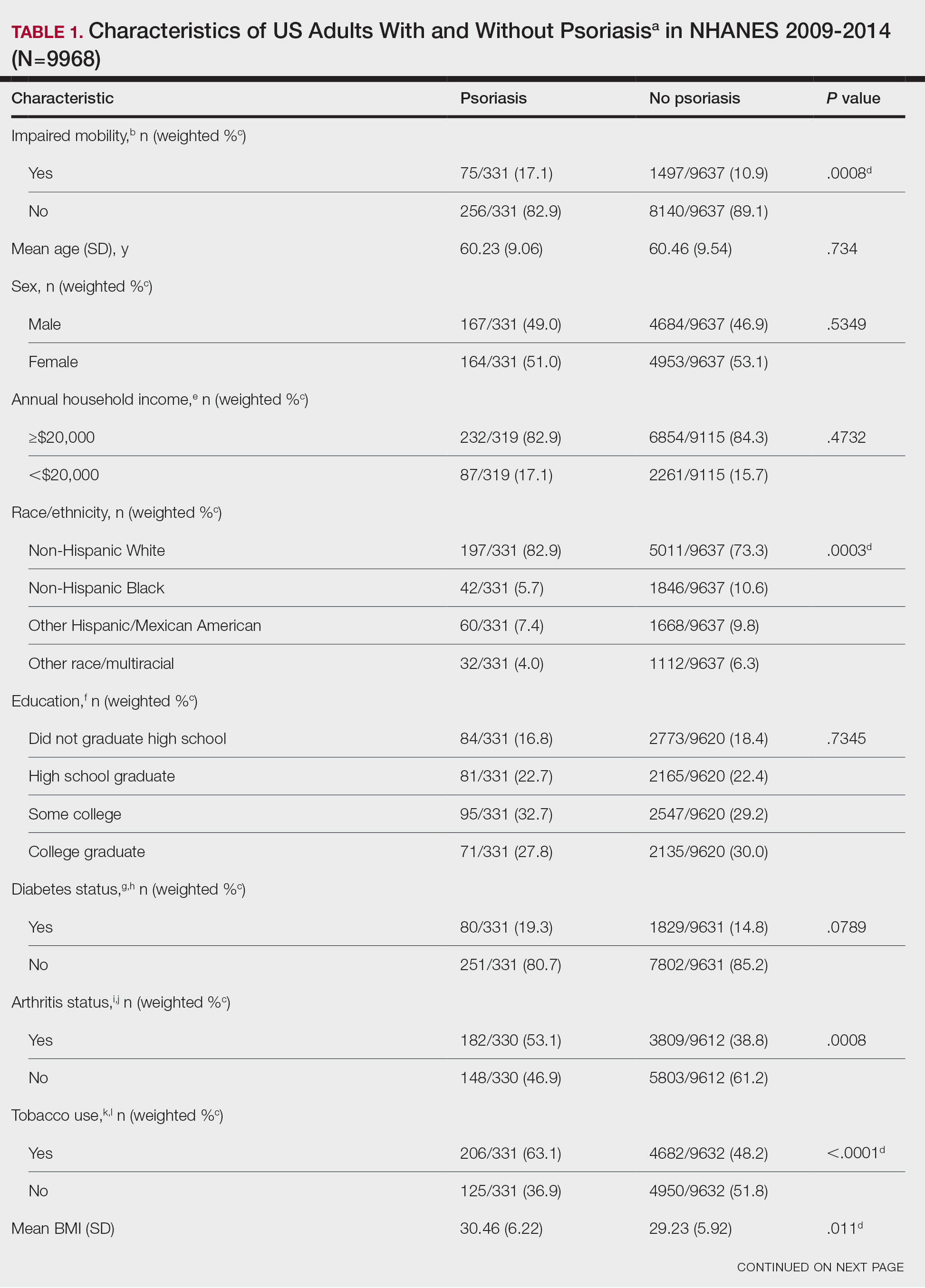

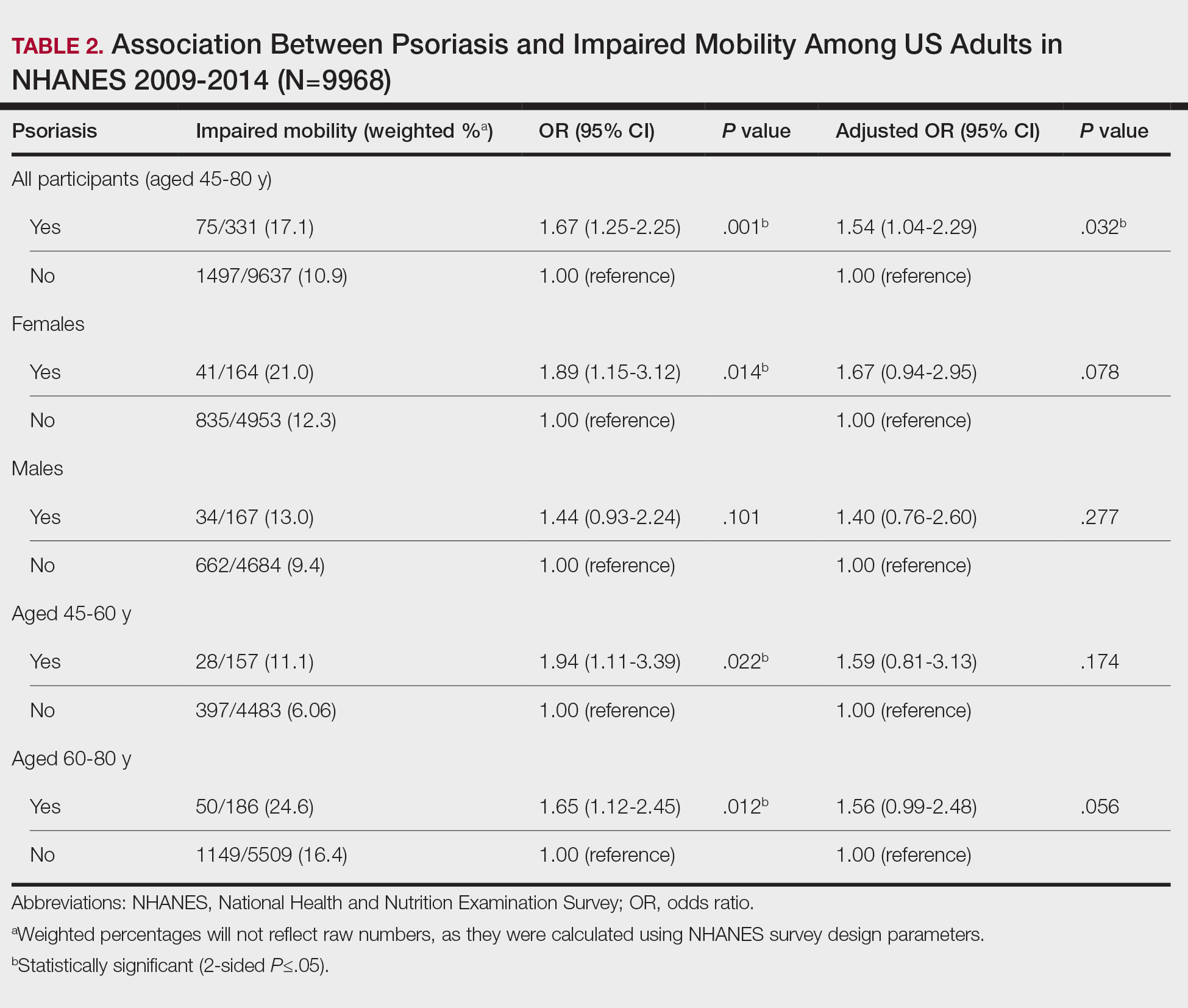
Our study demonstrated a statistically significant difference in mobility between individuals with psoriasis compared with the general population, which remained significant when controlling for arthritis, obesity, and diabetes (P=.032). This may be the result of several influences. First, the location of the psoriasis may impact mobility. Plantar psoriasis—a manifestation on the soles of the feet—can cause discomfort and pain, which can hinder walking and standing.5 Second, a study by Lasselin et al6 found that systemic inflammation contributes to mobility impairment through alterations in gait and posture, which suggests that the inflammatory processes inherent in psoriasis could intrinsically modify walking speed and stride, potentially exacerbating mobility difficulties independent of other comorbid conditions. These findings suggest that psoriasis may disproportionately affect individuals with impaired mobility, independent of comorbid arthritis, obesity, and diabetes.
These findings have broad implications for diversity, equity, and inclusion. They should prompt us to consider the practical challenges faced by this patient population and the ways that we can address barriers to care. Offering telehealth appointments, making primary care referrals for impaired mobility workups, and advising patients of direct-to-home delivery of prescriptions are good places to start.
Limitations to our study include the lack of specificity in the survey question, self-reporting bias, and the inability to control for the psoriasis location. Further investigations are warranted in large, representative US adult populations to assess the implications of impaired mobility in patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi: 10.1016/j.jaad.2018.11.058
- Zheng Q, Sun XY, Miao X, et al. Association between physical activity and risk of prevalent psoriasis: A MOOSE-compliant meta-analysis. Medicine (Baltimore). 2018;97:e11394. doi: 10.1097 /MD.0000000000011394
- Mullin AE, Coe IR, Gooden EA, et al. Inclusion, diversity, equity, and accessibility: from organizational responsibility to leadership competency. Healthc Manage Forum. 2021;34311-315. doi: 10.1177/08404704211038232
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES questionnaires, datasets, and related documentation. Accessed October 21, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Romani M, Biela G, Farr K, et al. Plantar psoriasis: a review of the literature. Clin Podiatr Med Surg. 2021;38:541-552. doi: 10.1016 /j.cpm.2021.06.009
- Lasselin J, Sundelin T, Wayne PM, et al. Biological motion during inflammation in humans. Brain Behav Immun. 2020;84:147-153. doi: 10.1016/j.bbi.2019.11.019
To the Editor:
Psoriasis is a chronic inflammatory condition that affects individuals in various extracutaneous ways.1 Prior studies have documented a decrease in exercise intensity among patients with psoriasis2; however, few studies have specifically investigated baseline mobility in this population. Baseline mobility denotes an individual’s fundamental ability to walk or move around without assistance of any kind. Impaired mobility—when baseline mobility is compromised—is an aspect of the wider diversity, equity, and inclusion framework that underscores the significance of recognizing challenges and promoting inclusive measures, both at the point of care and in research.3 study sought to analyze the relationship between psoriasis and baseline mobility among US adults (aged 45 to 80 years) utilizing the latest data from the National Health and Nutrition Examination Survey (NHANES) database for psoriasis.4 We used three 2-year cycles of NHANES data to create a 2009-2014 dataset.
The overall NHANES response rate among adults aged 45 to 80 years between 2009 and 2014 was 67.9%. Patients were categorized as having impaired mobility if they responded “yes” to the following question: “Because of a health problem, do you have difficulty walking without using any special equipment?” Psoriasis status was assessed by the following question: “Have you ever been told by a doctor or other health professional that you had psoriasis?” Multivariable logistic regression analyses were performed using Stata/SE 18.0 software (StataCorp LLC) to assess the relationship between psoriasis and impaired mobility. Age, income, education, sex, race, tobacco use, diabetes status, body mass index, and arthritis status were controlled for in our models.
Our analysis initially included 9982 participants; 14 did not respond to questions assessing psoriasis and impaired mobility and were excluded. The prevalence of impaired mobility in patients with psoriasis was 17.1% compared with 10.9% among those without psoriasis (Table 1). There was a significant association between psoriasis and impaired mobility among patients aged 45 to 80 years after adjusting for potential confounding variables (adjusted odds ratio [AOR], 1.54; 95% CI, 1.04- 2.29; P=.032)(Table 2). Analyses of subgroups yielded no statistically significant results.



Our study demonstrated a statistically significant difference in mobility between individuals with psoriasis compared with the general population, which remained significant when controlling for arthritis, obesity, and diabetes (P=.032). This may be the result of several influences. First, the location of the psoriasis may impact mobility. Plantar psoriasis—a manifestation on the soles of the feet—can cause discomfort and pain, which can hinder walking and standing.5 Second, a study by Lasselin et al6 found that systemic inflammation contributes to mobility impairment through alterations in gait and posture, which suggests that the inflammatory processes inherent in psoriasis could intrinsically modify walking speed and stride, potentially exacerbating mobility difficulties independent of other comorbid conditions. These findings suggest that psoriasis may disproportionately affect individuals with impaired mobility, independent of comorbid arthritis, obesity, and diabetes.
These findings have broad implications for diversity, equity, and inclusion. They should prompt us to consider the practical challenges faced by this patient population and the ways that we can address barriers to care. Offering telehealth appointments, making primary care referrals for impaired mobility workups, and advising patients of direct-to-home delivery of prescriptions are good places to start.
Limitations to our study include the lack of specificity in the survey question, self-reporting bias, and the inability to control for the psoriasis location. Further investigations are warranted in large, representative US adult populations to assess the implications of impaired mobility in patients with psoriasis.
To the Editor:
Psoriasis is a chronic inflammatory condition that affects individuals in various extracutaneous ways.1 Prior studies have documented a decrease in exercise intensity among patients with psoriasis2; however, few studies have specifically investigated baseline mobility in this population. Baseline mobility denotes an individual’s fundamental ability to walk or move around without assistance of any kind. Impaired mobility—when baseline mobility is compromised—is an aspect of the wider diversity, equity, and inclusion framework that underscores the significance of recognizing challenges and promoting inclusive measures, both at the point of care and in research.3 study sought to analyze the relationship between psoriasis and baseline mobility among US adults (aged 45 to 80 years) utilizing the latest data from the National Health and Nutrition Examination Survey (NHANES) database for psoriasis.4 We used three 2-year cycles of NHANES data to create a 2009-2014 dataset.
The overall NHANES response rate among adults aged 45 to 80 years between 2009 and 2014 was 67.9%. Patients were categorized as having impaired mobility if they responded “yes” to the following question: “Because of a health problem, do you have difficulty walking without using any special equipment?” Psoriasis status was assessed by the following question: “Have you ever been told by a doctor or other health professional that you had psoriasis?” Multivariable logistic regression analyses were performed using Stata/SE 18.0 software (StataCorp LLC) to assess the relationship between psoriasis and impaired mobility. Age, income, education, sex, race, tobacco use, diabetes status, body mass index, and arthritis status were controlled for in our models.
Our analysis initially included 9982 participants; 14 did not respond to questions assessing psoriasis and impaired mobility and were excluded. The prevalence of impaired mobility in patients with psoriasis was 17.1% compared with 10.9% among those without psoriasis (Table 1). There was a significant association between psoriasis and impaired mobility among patients aged 45 to 80 years after adjusting for potential confounding variables (adjusted odds ratio [AOR], 1.54; 95% CI, 1.04- 2.29; P=.032)(Table 2). Analyses of subgroups yielded no statistically significant results.



Our study demonstrated a statistically significant difference in mobility between individuals with psoriasis compared with the general population, which remained significant when controlling for arthritis, obesity, and diabetes (P=.032). This may be the result of several influences. First, the location of the psoriasis may impact mobility. Plantar psoriasis—a manifestation on the soles of the feet—can cause discomfort and pain, which can hinder walking and standing.5 Second, a study by Lasselin et al6 found that systemic inflammation contributes to mobility impairment through alterations in gait and posture, which suggests that the inflammatory processes inherent in psoriasis could intrinsically modify walking speed and stride, potentially exacerbating mobility difficulties independent of other comorbid conditions. These findings suggest that psoriasis may disproportionately affect individuals with impaired mobility, independent of comorbid arthritis, obesity, and diabetes.
These findings have broad implications for diversity, equity, and inclusion. They should prompt us to consider the practical challenges faced by this patient population and the ways that we can address barriers to care. Offering telehealth appointments, making primary care referrals for impaired mobility workups, and advising patients of direct-to-home delivery of prescriptions are good places to start.
Limitations to our study include the lack of specificity in the survey question, self-reporting bias, and the inability to control for the psoriasis location. Further investigations are warranted in large, representative US adult populations to assess the implications of impaired mobility in patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi: 10.1016/j.jaad.2018.11.058
- Zheng Q, Sun XY, Miao X, et al. Association between physical activity and risk of prevalent psoriasis: A MOOSE-compliant meta-analysis. Medicine (Baltimore). 2018;97:e11394. doi: 10.1097 /MD.0000000000011394
- Mullin AE, Coe IR, Gooden EA, et al. Inclusion, diversity, equity, and accessibility: from organizational responsibility to leadership competency. Healthc Manage Forum. 2021;34311-315. doi: 10.1177/08404704211038232
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES questionnaires, datasets, and related documentation. Accessed October 21, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Romani M, Biela G, Farr K, et al. Plantar psoriasis: a review of the literature. Clin Podiatr Med Surg. 2021;38:541-552. doi: 10.1016 /j.cpm.2021.06.009
- Lasselin J, Sundelin T, Wayne PM, et al. Biological motion during inflammation in humans. Brain Behav Immun. 2020;84:147-153. doi: 10.1016/j.bbi.2019.11.019
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi: 10.1016/j.jaad.2018.11.058
- Zheng Q, Sun XY, Miao X, et al. Association between physical activity and risk of prevalent psoriasis: A MOOSE-compliant meta-analysis. Medicine (Baltimore). 2018;97:e11394. doi: 10.1097 /MD.0000000000011394
- Mullin AE, Coe IR, Gooden EA, et al. Inclusion, diversity, equity, and accessibility: from organizational responsibility to leadership competency. Healthc Manage Forum. 2021;34311-315. doi: 10.1177/08404704211038232
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES questionnaires, datasets, and related documentation. Accessed October 21, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Romani M, Biela G, Farr K, et al. Plantar psoriasis: a review of the literature. Clin Podiatr Med Surg. 2021;38:541-552. doi: 10.1016 /j.cpm.2021.06.009
- Lasselin J, Sundelin T, Wayne PM, et al. Biological motion during inflammation in humans. Brain Behav Immun. 2020;84:147-153. doi: 10.1016/j.bbi.2019.11.019
Exploring the Relationship Between Psoriasis and Mobility Among US Adults
Exploring the Relationship Between Psoriasis and Mobility Among US Adults
PRACTICE POINTS
- Mobility issues are more common in patients who have psoriasis than in those who do not.
- It is important to assess patients with psoriasis for mobility issues regardless of age or comorbid conditions such as arthritis, obesity, and diabetes.
- Dermatologists can help patients with psoriasis and impaired mobility overcome potential barriers to care by incorporating telehealth services into their practices and informing patients of direct-to-home delivery of prescriptions.
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
To the Editor:
Cutaneous medical conditions can have a substantial impact on patients’ functioning and quality of life. Many patients with severe skin disease are eligible to receive disability assistance that can provide them with essential income and health care. Previous research has highlighted disability assessment as one of the most important ways physicians can help mitigate the health consequences of poverty.1 Dermatologists can play an important role in the disability assessment process by documenting the facts associated with patients’ skin conditions.
Although skin conditions have a relatively high prevalence, they remain underrepresented in disability claims. Between 1997 and 2004, occupational skin diseases accounted for 12% to 17% of nonfatal work-related illnesses; however, during that same period, skin conditions comprised only 0.21% of disability claims in the United States.2,3 Historically, there has been hesitancy among dermatologists to complete disability paperwork; a 1976 survey of dermatologists cited extensive paperwork, “troublesome patients,” and fee schedule issues as reasons.4 The lack of training regarding disability assessment in medical school and residency also has been noted.5
To characterize modern attitudes toward disability assessments, we conducted a survey of dermatologists across the United States. Our study was reviewed and declared exempt by the institutional review board of the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Torrance, California)(approval #18CR-32242-01). Using convenience sampling, we emailed dermatologists from the Association of Professors of Dermatology and dermatology state societies in all 50 states inviting them to participate in our voluntary and anonymous survey, which was administered using SurveyMonkey. The use of all society mailing lists was approved by the respective owners. The 15-question survey included multiple choice, Likert scale, and free response sections. Summary and descriptive statistics were used to describe respondent demographics and identify any patterns in responses.
For each Likert-based question, participants ranked their degree of agreement with a statement as: 1=strongly disagree, 2=somewhat disagree, 3=neither agree nor disagree/neutral, 4=somewhat agree, and 5=strongly agree. The mean response and standard deviation were reported for each Likert scale prompt. Preplanned 1-sample t testing was used to analyze Likert scale data, in which the mean response for each prompt was compared to a baseline response of 3 (neutral). A P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for MacOS, version 27 (IBM).
Seventy-eight dermatologists agreed to participate, and 70 completed the survey, for a response rate of 89.7% (Table 1). The dermatologists we surveyed practiced in a variety of clinical settings, including academic public hospitals (46.2% [36/78]), academic private hospitals (33.3% [26/78]), and private practices (32.1% [25/78]), and 60.3% (47/78) reported providing disability documentation at some point. Most of the respondents (64.3% [45/70]) did not perform assessments in an average month (Table 2). Medical assessment documentation was provided most frequently for workers’ compensation (50.0% [35/70]), private insurance (27.1% [19/70]), and Social Security Disability Insurance (25.7% [18/70]). Dermatologists overwhelmingly reported no formal training for disability assessment in medical school (94.3% [66/70]), residency (97.1% [68/70]), or clinical practice (81.4% [57/70]).


In the Likert scale prompts, respondents agreed that they were uncertain of their role in disability assessment (mean response, 3.6; P<.001). Moreover, they were uncomfortable providing assessments (mean response, 3.5; P<.001) and felt that they did not have sufficient time to perform them (mean response, 3.6; P<.001). Dermatologists disagreed that they received adequate compensation for performing assessments (mean response, 2.2; P<.001) and felt that they did not have enough time to participate in assessments (mean response, 3.6; P<.001). Respondents generally did not feel distrustful of patients seeking disability assessment (mean response, 2.8; P=.043). Dermatologists neither agreed nor disagreed when asked if they thought that physicians can determine disability status (mean response, 3.2; P=.118). The details of the Likert scale responses are described in Table 3. Respondents also were uncertain as to which dermatologic conditions were eligible for disability. When asked to select which conditions from a list of 10 were eligible per the Social Security Administration listing of disability impairments, only 15.4% (12/70) of respondents correctly identified that all the conditions qualified; these included ichthyosis, pemphigus vulgaris, allergic contact dermatitis, hidradenitis suppurativa, systemic lupus erythematosus, chromoblastomycosis, xeroderma pigmentosum, burns, malignant melanoma, and scleroderma.6

In the free-response prompts, respondents frequently described extensive paperwork, inadequate time, and lack of reimbursement as barriers to providing documentation. Often, dermatologists found that the forms were not well matched to the skin conditions they were evaluating and rather had a musculoskeletal focus. Multiple individuals commented on the challenge in assessing the percentage of disability and functional/psychosocial impairment in skin conditions. One respondent noted that workers’ compensation forms ask if the patient is “…permanent and stationary…for most conditions this has no meaning in dermatology.” Some felt hesitant to provide documentation because they had insufficient patient history, especially regarding employment, and opted to defer to primary care providers who might be more familiar with the full patient history.
A dermatologist described their perspective as follows:
“…As a specialist I feel that I don’t have a complete look into all the factors that could contribute to a patient[’]s need to go on disability, and I don’t have experience with filling out disability requests. That being said, if a patient[’]s request for disability was due to a skin disease that I know way more about than [a] primary care [physician] would, I would do the disability assessment.”
Another respondent noted the complexity in “establishing causality” for workers’ compensation. Another dermatologist reported,
“The most frequent challenging situation I encounter is being asked to evaluate for maximum medical improvement after patch testing. If the patient is not fully avoiding contact allergens either at home or at work, then I typically document that they are not at [maximum medical improvement]. The reality is that most frequently it is due to exposure to allergens at home so the line between what is a legitimate worker’s comp[ensation] issue and what is a home life choice is blurry.”
Nevertheless, respondents expressed interest in learning more about disability assessment procedures. Summary guides, lectures, and prefilled paperwork were the most popular initiatives that respondents agreed would be beneficial toward becoming educated regarding disability assessment (78.6%, 58.6%, and 58.6%, respectively)(Table 2). One respondent noted that “previous [internal medicine] history help[ed]” them in performing cutaneous disability assessments.
As with any survey, our study did have some inherent limitations. Only a relatively small sample size was willing to complete the survey. There was a predominance of respondents from California (34.6% [27/78]), as well as those practicing for less than 15 years (58.9% [46/78])(Figure). This could limit generalizability to the national population of dermatologists. In addition, there was potential for recall bias and errors in responding given the self-reported nature of the study. Different individuals may interpret the Likert scale options in various ways, which could skew results unintentionally. However, the survey was largely qualitative in nature, making it a legitimate tool for answering our research questions. Moreover, we were able to hear the perspectives of dermatologists across diverse practice settings, with free response prompts to increase the depth of the survey.
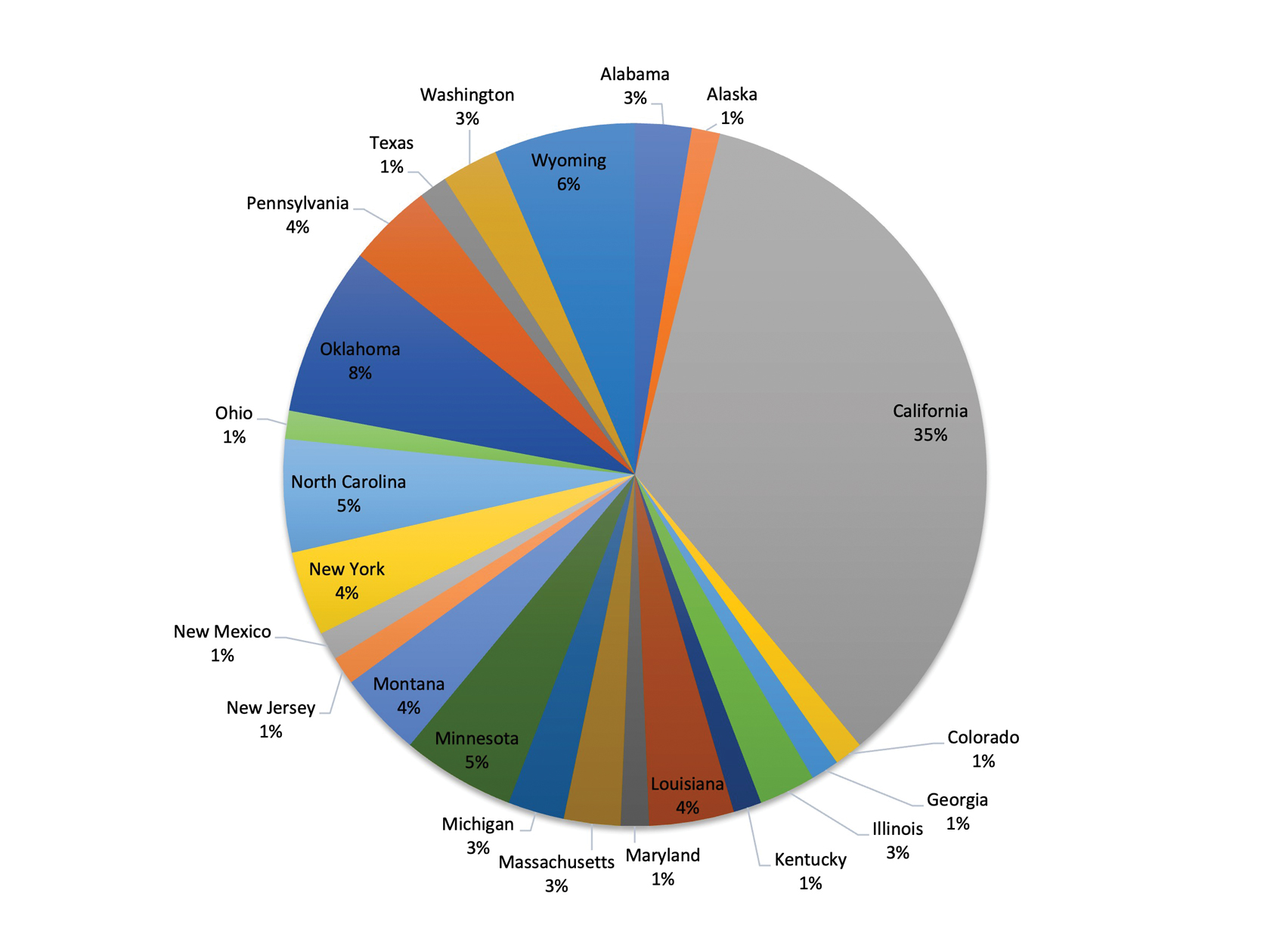
Almost 50 years later, our survey echoes common themes from Adams’ 1976 survey.4 Inadequate compensation, limited time, and burdensome paperwork all continue to hinder dermatologists’ ability to perform disability assessments. Our participants frequently commented that the current disability forms are not congruent with the nature of skin conditions, making it challenging to accurately document the facts.
Moreover, respondents felt uncertain in their role in disability assessment and occasionally noted distrust of patients or insufficient patient history as barriers to completing assessments. They also were unsure if physicians can grant disability status. This is a common misconception among physicians that leads to discomfort in helping with disability assessment.7 The role of physicians in disability assessment is to document the facts of a patient’s illness, not to determine whether they are eligible for benefits. We discovered uncertainty in our respondents’ ability to identify conditions eligible for disability, highlighting an area in need of greater education for physicians.
Despite these obstacles, respondents were interested in learning more about disability assessment and highlighted several practical approaches that could help them better perform this task. As skin specialists, dermatologists are the best-equipped physicians to assess cutaneous conditions and should play a greater role in performing disability assessments, which could be achieved through increased educational initiatives and individual physician motivation.7 We call for greater collaboration and reflection on the importance of disability assistance among dermatologists to increase participation in the disability-assessment process.
- O’Connell JJ, Zevin BD, Quick PD, et al. Documenting disability: simple strategies for medical providers. Health Care for the Homeless Clinicians’ Network. September 2007. Accessed March 31, 2025. https://nhchc.org/wp-content/uploads/2019/08/DocumentingDisability2007.pdf
- US Bureau of Labor Statistics. Injuries, illnesses, and fatalities. Accessed March 31, 2025. https://www.bls.gov/iif/
- Meseguer J. Outcome variation in the Social Security Disability Insurance Program: the role of primary diagnoses. Soc Secur Bull. 2013;73:39-75.
- Adams RM. Attitudes of California dermatologists toward Worker’s Compensation: results of a survey. West J Med. 1976;125:169-175.
- Talmage J, Melhorn J, Hyman M. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
- Social Security Administration. Disability evaluation under Social Security. 8.00 skin disorders - adult. March 31, 2025. https://www.ssa.gov/disability/professionals/bluebook/8.00-Skin-Adult.htm
- Dawson J, Smogorzewski J. Demystifying disability assessments for dermatologists—a call to action. JAMA Dermatol. 2021;157:903-904. doi:10.1001/jamadermatol.2021.1767
To the Editor:
Cutaneous medical conditions can have a substantial impact on patients’ functioning and quality of life. Many patients with severe skin disease are eligible to receive disability assistance that can provide them with essential income and health care. Previous research has highlighted disability assessment as one of the most important ways physicians can help mitigate the health consequences of poverty.1 Dermatologists can play an important role in the disability assessment process by documenting the facts associated with patients’ skin conditions.
Although skin conditions have a relatively high prevalence, they remain underrepresented in disability claims. Between 1997 and 2004, occupational skin diseases accounted for 12% to 17% of nonfatal work-related illnesses; however, during that same period, skin conditions comprised only 0.21% of disability claims in the United States.2,3 Historically, there has been hesitancy among dermatologists to complete disability paperwork; a 1976 survey of dermatologists cited extensive paperwork, “troublesome patients,” and fee schedule issues as reasons.4 The lack of training regarding disability assessment in medical school and residency also has been noted.5
To characterize modern attitudes toward disability assessments, we conducted a survey of dermatologists across the United States. Our study was reviewed and declared exempt by the institutional review board of the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Torrance, California)(approval #18CR-32242-01). Using convenience sampling, we emailed dermatologists from the Association of Professors of Dermatology and dermatology state societies in all 50 states inviting them to participate in our voluntary and anonymous survey, which was administered using SurveyMonkey. The use of all society mailing lists was approved by the respective owners. The 15-question survey included multiple choice, Likert scale, and free response sections. Summary and descriptive statistics were used to describe respondent demographics and identify any patterns in responses.
For each Likert-based question, participants ranked their degree of agreement with a statement as: 1=strongly disagree, 2=somewhat disagree, 3=neither agree nor disagree/neutral, 4=somewhat agree, and 5=strongly agree. The mean response and standard deviation were reported for each Likert scale prompt. Preplanned 1-sample t testing was used to analyze Likert scale data, in which the mean response for each prompt was compared to a baseline response of 3 (neutral). A P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for MacOS, version 27 (IBM).
Seventy-eight dermatologists agreed to participate, and 70 completed the survey, for a response rate of 89.7% (Table 1). The dermatologists we surveyed practiced in a variety of clinical settings, including academic public hospitals (46.2% [36/78]), academic private hospitals (33.3% [26/78]), and private practices (32.1% [25/78]), and 60.3% (47/78) reported providing disability documentation at some point. Most of the respondents (64.3% [45/70]) did not perform assessments in an average month (Table 2). Medical assessment documentation was provided most frequently for workers’ compensation (50.0% [35/70]), private insurance (27.1% [19/70]), and Social Security Disability Insurance (25.7% [18/70]). Dermatologists overwhelmingly reported no formal training for disability assessment in medical school (94.3% [66/70]), residency (97.1% [68/70]), or clinical practice (81.4% [57/70]).


In the Likert scale prompts, respondents agreed that they were uncertain of their role in disability assessment (mean response, 3.6; P<.001). Moreover, they were uncomfortable providing assessments (mean response, 3.5; P<.001) and felt that they did not have sufficient time to perform them (mean response, 3.6; P<.001). Dermatologists disagreed that they received adequate compensation for performing assessments (mean response, 2.2; P<.001) and felt that they did not have enough time to participate in assessments (mean response, 3.6; P<.001). Respondents generally did not feel distrustful of patients seeking disability assessment (mean response, 2.8; P=.043). Dermatologists neither agreed nor disagreed when asked if they thought that physicians can determine disability status (mean response, 3.2; P=.118). The details of the Likert scale responses are described in Table 3. Respondents also were uncertain as to which dermatologic conditions were eligible for disability. When asked to select which conditions from a list of 10 were eligible per the Social Security Administration listing of disability impairments, only 15.4% (12/70) of respondents correctly identified that all the conditions qualified; these included ichthyosis, pemphigus vulgaris, allergic contact dermatitis, hidradenitis suppurativa, systemic lupus erythematosus, chromoblastomycosis, xeroderma pigmentosum, burns, malignant melanoma, and scleroderma.6

In the free-response prompts, respondents frequently described extensive paperwork, inadequate time, and lack of reimbursement as barriers to providing documentation. Often, dermatologists found that the forms were not well matched to the skin conditions they were evaluating and rather had a musculoskeletal focus. Multiple individuals commented on the challenge in assessing the percentage of disability and functional/psychosocial impairment in skin conditions. One respondent noted that workers’ compensation forms ask if the patient is “…permanent and stationary…for most conditions this has no meaning in dermatology.” Some felt hesitant to provide documentation because they had insufficient patient history, especially regarding employment, and opted to defer to primary care providers who might be more familiar with the full patient history.
A dermatologist described their perspective as follows:
“…As a specialist I feel that I don’t have a complete look into all the factors that could contribute to a patient[’]s need to go on disability, and I don’t have experience with filling out disability requests. That being said, if a patient[’]s request for disability was due to a skin disease that I know way more about than [a] primary care [physician] would, I would do the disability assessment.”
Another respondent noted the complexity in “establishing causality” for workers’ compensation. Another dermatologist reported,
“The most frequent challenging situation I encounter is being asked to evaluate for maximum medical improvement after patch testing. If the patient is not fully avoiding contact allergens either at home or at work, then I typically document that they are not at [maximum medical improvement]. The reality is that most frequently it is due to exposure to allergens at home so the line between what is a legitimate worker’s comp[ensation] issue and what is a home life choice is blurry.”
Nevertheless, respondents expressed interest in learning more about disability assessment procedures. Summary guides, lectures, and prefilled paperwork were the most popular initiatives that respondents agreed would be beneficial toward becoming educated regarding disability assessment (78.6%, 58.6%, and 58.6%, respectively)(Table 2). One respondent noted that “previous [internal medicine] history help[ed]” them in performing cutaneous disability assessments.
As with any survey, our study did have some inherent limitations. Only a relatively small sample size was willing to complete the survey. There was a predominance of respondents from California (34.6% [27/78]), as well as those practicing for less than 15 years (58.9% [46/78])(Figure). This could limit generalizability to the national population of dermatologists. In addition, there was potential for recall bias and errors in responding given the self-reported nature of the study. Different individuals may interpret the Likert scale options in various ways, which could skew results unintentionally. However, the survey was largely qualitative in nature, making it a legitimate tool for answering our research questions. Moreover, we were able to hear the perspectives of dermatologists across diverse practice settings, with free response prompts to increase the depth of the survey.

Almost 50 years later, our survey echoes common themes from Adams’ 1976 survey.4 Inadequate compensation, limited time, and burdensome paperwork all continue to hinder dermatologists’ ability to perform disability assessments. Our participants frequently commented that the current disability forms are not congruent with the nature of skin conditions, making it challenging to accurately document the facts.
Moreover, respondents felt uncertain in their role in disability assessment and occasionally noted distrust of patients or insufficient patient history as barriers to completing assessments. They also were unsure if physicians can grant disability status. This is a common misconception among physicians that leads to discomfort in helping with disability assessment.7 The role of physicians in disability assessment is to document the facts of a patient’s illness, not to determine whether they are eligible for benefits. We discovered uncertainty in our respondents’ ability to identify conditions eligible for disability, highlighting an area in need of greater education for physicians.
Despite these obstacles, respondents were interested in learning more about disability assessment and highlighted several practical approaches that could help them better perform this task. As skin specialists, dermatologists are the best-equipped physicians to assess cutaneous conditions and should play a greater role in performing disability assessments, which could be achieved through increased educational initiatives and individual physician motivation.7 We call for greater collaboration and reflection on the importance of disability assistance among dermatologists to increase participation in the disability-assessment process.
To the Editor:
Cutaneous medical conditions can have a substantial impact on patients’ functioning and quality of life. Many patients with severe skin disease are eligible to receive disability assistance that can provide them with essential income and health care. Previous research has highlighted disability assessment as one of the most important ways physicians can help mitigate the health consequences of poverty.1 Dermatologists can play an important role in the disability assessment process by documenting the facts associated with patients’ skin conditions.
Although skin conditions have a relatively high prevalence, they remain underrepresented in disability claims. Between 1997 and 2004, occupational skin diseases accounted for 12% to 17% of nonfatal work-related illnesses; however, during that same period, skin conditions comprised only 0.21% of disability claims in the United States.2,3 Historically, there has been hesitancy among dermatologists to complete disability paperwork; a 1976 survey of dermatologists cited extensive paperwork, “troublesome patients,” and fee schedule issues as reasons.4 The lack of training regarding disability assessment in medical school and residency also has been noted.5
To characterize modern attitudes toward disability assessments, we conducted a survey of dermatologists across the United States. Our study was reviewed and declared exempt by the institutional review board of the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Torrance, California)(approval #18CR-32242-01). Using convenience sampling, we emailed dermatologists from the Association of Professors of Dermatology and dermatology state societies in all 50 states inviting them to participate in our voluntary and anonymous survey, which was administered using SurveyMonkey. The use of all society mailing lists was approved by the respective owners. The 15-question survey included multiple choice, Likert scale, and free response sections. Summary and descriptive statistics were used to describe respondent demographics and identify any patterns in responses.
For each Likert-based question, participants ranked their degree of agreement with a statement as: 1=strongly disagree, 2=somewhat disagree, 3=neither agree nor disagree/neutral, 4=somewhat agree, and 5=strongly agree. The mean response and standard deviation were reported for each Likert scale prompt. Preplanned 1-sample t testing was used to analyze Likert scale data, in which the mean response for each prompt was compared to a baseline response of 3 (neutral). A P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for MacOS, version 27 (IBM).
Seventy-eight dermatologists agreed to participate, and 70 completed the survey, for a response rate of 89.7% (Table 1). The dermatologists we surveyed practiced in a variety of clinical settings, including academic public hospitals (46.2% [36/78]), academic private hospitals (33.3% [26/78]), and private practices (32.1% [25/78]), and 60.3% (47/78) reported providing disability documentation at some point. Most of the respondents (64.3% [45/70]) did not perform assessments in an average month (Table 2). Medical assessment documentation was provided most frequently for workers’ compensation (50.0% [35/70]), private insurance (27.1% [19/70]), and Social Security Disability Insurance (25.7% [18/70]). Dermatologists overwhelmingly reported no formal training for disability assessment in medical school (94.3% [66/70]), residency (97.1% [68/70]), or clinical practice (81.4% [57/70]).


In the Likert scale prompts, respondents agreed that they were uncertain of their role in disability assessment (mean response, 3.6; P<.001). Moreover, they were uncomfortable providing assessments (mean response, 3.5; P<.001) and felt that they did not have sufficient time to perform them (mean response, 3.6; P<.001). Dermatologists disagreed that they received adequate compensation for performing assessments (mean response, 2.2; P<.001) and felt that they did not have enough time to participate in assessments (mean response, 3.6; P<.001). Respondents generally did not feel distrustful of patients seeking disability assessment (mean response, 2.8; P=.043). Dermatologists neither agreed nor disagreed when asked if they thought that physicians can determine disability status (mean response, 3.2; P=.118). The details of the Likert scale responses are described in Table 3. Respondents also were uncertain as to which dermatologic conditions were eligible for disability. When asked to select which conditions from a list of 10 were eligible per the Social Security Administration listing of disability impairments, only 15.4% (12/70) of respondents correctly identified that all the conditions qualified; these included ichthyosis, pemphigus vulgaris, allergic contact dermatitis, hidradenitis suppurativa, systemic lupus erythematosus, chromoblastomycosis, xeroderma pigmentosum, burns, malignant melanoma, and scleroderma.6

In the free-response prompts, respondents frequently described extensive paperwork, inadequate time, and lack of reimbursement as barriers to providing documentation. Often, dermatologists found that the forms were not well matched to the skin conditions they were evaluating and rather had a musculoskeletal focus. Multiple individuals commented on the challenge in assessing the percentage of disability and functional/psychosocial impairment in skin conditions. One respondent noted that workers’ compensation forms ask if the patient is “…permanent and stationary…for most conditions this has no meaning in dermatology.” Some felt hesitant to provide documentation because they had insufficient patient history, especially regarding employment, and opted to defer to primary care providers who might be more familiar with the full patient history.
A dermatologist described their perspective as follows:
“…As a specialist I feel that I don’t have a complete look into all the factors that could contribute to a patient[’]s need to go on disability, and I don’t have experience with filling out disability requests. That being said, if a patient[’]s request for disability was due to a skin disease that I know way more about than [a] primary care [physician] would, I would do the disability assessment.”
Another respondent noted the complexity in “establishing causality” for workers’ compensation. Another dermatologist reported,
“The most frequent challenging situation I encounter is being asked to evaluate for maximum medical improvement after patch testing. If the patient is not fully avoiding contact allergens either at home or at work, then I typically document that they are not at [maximum medical improvement]. The reality is that most frequently it is due to exposure to allergens at home so the line between what is a legitimate worker’s comp[ensation] issue and what is a home life choice is blurry.”
Nevertheless, respondents expressed interest in learning more about disability assessment procedures. Summary guides, lectures, and prefilled paperwork were the most popular initiatives that respondents agreed would be beneficial toward becoming educated regarding disability assessment (78.6%, 58.6%, and 58.6%, respectively)(Table 2). One respondent noted that “previous [internal medicine] history help[ed]” them in performing cutaneous disability assessments.
As with any survey, our study did have some inherent limitations. Only a relatively small sample size was willing to complete the survey. There was a predominance of respondents from California (34.6% [27/78]), as well as those practicing for less than 15 years (58.9% [46/78])(Figure). This could limit generalizability to the national population of dermatologists. In addition, there was potential for recall bias and errors in responding given the self-reported nature of the study. Different individuals may interpret the Likert scale options in various ways, which could skew results unintentionally. However, the survey was largely qualitative in nature, making it a legitimate tool for answering our research questions. Moreover, we were able to hear the perspectives of dermatologists across diverse practice settings, with free response prompts to increase the depth of the survey.

Almost 50 years later, our survey echoes common themes from Adams’ 1976 survey.4 Inadequate compensation, limited time, and burdensome paperwork all continue to hinder dermatologists’ ability to perform disability assessments. Our participants frequently commented that the current disability forms are not congruent with the nature of skin conditions, making it challenging to accurately document the facts.
Moreover, respondents felt uncertain in their role in disability assessment and occasionally noted distrust of patients or insufficient patient history as barriers to completing assessments. They also were unsure if physicians can grant disability status. This is a common misconception among physicians that leads to discomfort in helping with disability assessment.7 The role of physicians in disability assessment is to document the facts of a patient’s illness, not to determine whether they are eligible for benefits. We discovered uncertainty in our respondents’ ability to identify conditions eligible for disability, highlighting an area in need of greater education for physicians.
Despite these obstacles, respondents were interested in learning more about disability assessment and highlighted several practical approaches that could help them better perform this task. As skin specialists, dermatologists are the best-equipped physicians to assess cutaneous conditions and should play a greater role in performing disability assessments, which could be achieved through increased educational initiatives and individual physician motivation.7 We call for greater collaboration and reflection on the importance of disability assistance among dermatologists to increase participation in the disability-assessment process.
- O’Connell JJ, Zevin BD, Quick PD, et al. Documenting disability: simple strategies for medical providers. Health Care for the Homeless Clinicians’ Network. September 2007. Accessed March 31, 2025. https://nhchc.org/wp-content/uploads/2019/08/DocumentingDisability2007.pdf
- US Bureau of Labor Statistics. Injuries, illnesses, and fatalities. Accessed March 31, 2025. https://www.bls.gov/iif/
- Meseguer J. Outcome variation in the Social Security Disability Insurance Program: the role of primary diagnoses. Soc Secur Bull. 2013;73:39-75.
- Adams RM. Attitudes of California dermatologists toward Worker’s Compensation: results of a survey. West J Med. 1976;125:169-175.
- Talmage J, Melhorn J, Hyman M. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
- Social Security Administration. Disability evaluation under Social Security. 8.00 skin disorders - adult. March 31, 2025. https://www.ssa.gov/disability/professionals/bluebook/8.00-Skin-Adult.htm
- Dawson J, Smogorzewski J. Demystifying disability assessments for dermatologists—a call to action. JAMA Dermatol. 2021;157:903-904. doi:10.1001/jamadermatol.2021.1767
- O’Connell JJ, Zevin BD, Quick PD, et al. Documenting disability: simple strategies for medical providers. Health Care for the Homeless Clinicians’ Network. September 2007. Accessed March 31, 2025. https://nhchc.org/wp-content/uploads/2019/08/DocumentingDisability2007.pdf
- US Bureau of Labor Statistics. Injuries, illnesses, and fatalities. Accessed March 31, 2025. https://www.bls.gov/iif/
- Meseguer J. Outcome variation in the Social Security Disability Insurance Program: the role of primary diagnoses. Soc Secur Bull. 2013;73:39-75.
- Adams RM. Attitudes of California dermatologists toward Worker’s Compensation: results of a survey. West J Med. 1976;125:169-175.
- Talmage J, Melhorn J, Hyman M. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
- Social Security Administration. Disability evaluation under Social Security. 8.00 skin disorders - adult. March 31, 2025. https://www.ssa.gov/disability/professionals/bluebook/8.00-Skin-Adult.htm
- Dawson J, Smogorzewski J. Demystifying disability assessments for dermatologists—a call to action. JAMA Dermatol. 2021;157:903-904. doi:10.1001/jamadermatol.2021.1767
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
PRACTICE POINTS
- As experts in skin conditions, dermatologists are most qualified to assist with disability assessment for dermatologic concerns.
- There are several barriers to dermatologists participating in the disability assessment process, including lack of time, compensation, and education on the subject.
- Many dermatologists may be interested in learning more about disability assessment, and education could be provided in the form of summary guides, lectures, and prefilled paperwork.
Analysis of Errors in the Management of Cutaneous Disorders
Analysis of Errors in the Management of Cutaneous Disorders
Humans are inherently prone to errors. The extent and consequences of medical errors were documented in the 2000 publication of To Err is Human: Building a Safer Health System.1 Published research on medical errors in dermatology has emphasized the heuristic issues involved in diagnosis,2-6 essentially approaching the “why?” and “how?” of such errors. By contrast, the current study aimed to elucidate the “what?”—what are the dermatologic conditions most prone to diagnostic and/or management errors? One study published in 1987 approached this question by analyzing patterns of errors for dermatologic conditions in patients referred for specialty care by primary care physicians.7 The current study aimed to update and expand on the findings of this 1987 report by comparing more recent data on the errors made by providers and patients regarding skin conditions.
Methods
Data were collected prospectively from March 18, 2021, through July 25, 2023. Prospective data were obtained by recording the nature of errors noted for all patients seen by a board-certified dermatologist (R.J.P.) during routine outpatient practice in Norfolk, Virginia. This practice is limited to medical dermatology and accepts patients of any age from any referral source, with or without medical insurance. Retrospective data were obtained by review of electronic medical records for all patients seen by the same board-certified dermatologist from June 5, 2020, through March 12, 2021, who previously had been seen by an outside provider or were self-referred. In this study, the term diagnosis is used to describe providers’ explicit or imputed conclusions as to the nature of a dermatosis, and the term interpretation is used to describe patients' conclusions about their own condition. For this study, the patients’ self-made interpretations of their dermatoses were deemed to be correct when they agreed with those made by the dermatologist using standard clinicopathologic criteria supplemented by rapid bedside diagnostic techniques, as detailed in the 1987 study.7
Cases in which diagnostic or therapeutic errors were noted were entered into a spreadsheet that excluded patients’ names or other identifiers. For each noted case of diagnostic or therapeutic error, the following data were entered: patient’s age and sex; the name of the incorrect diagnosis, interpretation, or treatment; and the name of the correct (missed) diagnosis, along with the source of the error (provider or patient). Provider diagnoses were determined from medical records or patient statements or were imputed from the generally accepted indications for prescribed treatments. A provider was deemed to be any practitioner with prescriptive authority. Patients’ interpretations of their conditions were determined by patient statements or were imputed based on the indications for treatments being used. A treatment error was recorded when a diagnosis or interpretation was deemed to be correct, but treatment was deemed to be inappropriate. The same dermatologist (R.J.P) made all determinations as to the nature of the errors and their source.
Diagnostic errors were determined in several situations: (1) if the interpretation made by the patient of their dermatosis differed from the correct diagnosis in the absence of any additional diagnostic documentation, the correct diagnosis was scored as a missed diagnosis and the incorrect interpretation was scored as such; (2) if the provider’s diagnosis in the patient’s medical record differed from the correct diagnosis, both the correct (missed) and incorrect diagnoses were recorded; and (3) if the indication(s) of the medication(s) prescribed by the provider or used by the patient for their condition differed from the correct diagnosis, an imputed diagnosis based on this indication was scored as the incorrect diagnosis and the correct (missed) diagnosis was recorded; for example, an error would be entered into the spreadsheet for a patient using terbinafine cream for what was actually psoriasis. For a medication with multiple active agents, an error would be entered into the spreadsheet only if none of its indications matched the correct diagnosis; for example, if the patient had been prescribed a betamethasone/clotrimazole product, no error would be scored if the correct diagnosis was a steroid-responsive dermatosis, dermatophytosis, candidiasis, or tinea versicolor. For a single medication with multiple indications, no error would be recorded if the correct diagnosis was any of these indications; for example, in a patient who had been prescribed topical ketoconazole, no error would be scored if the correct diagnosis was dermatophytosis, candidiasis, tinea versicolor, or seborrheic dermatitis. Additionally, no error would be recorded if the correct diagnosis was uncertain at the time of initial patient evaluation or during chart review.
Standard spreadsheet functions and the pandas package8 from the Python programming language9 were used to extract relevant data from the spreadsheet (Tables 1-4).




Results
A total of 446 patient visits (182 males, 264 females) were included in the study, in which a total of 486 errors were found in the combined prospective and retrospective portions of the study. These errors involved 1.4% of all patient visits for the study period—specifically, all in routine practice as well as all patient records retrospectively reviewed. The age of the patients ranged from 4 to 95 years; the mean age was 51.5 years for males and 50.8 years for females.
The study results are outlined in Tables 1 through 4. To minimize the amount of data provided with no appreciable effect on the results, cases in which an incorrect or missed diagnosis/interpretation occurred only once (ie, unique case errors) were excluded from the tables. Tables 1 and 2 indicate the numbers and types of incorrect and missed diagnoses.
In the combined patient and provider cases, there were 434 instances in which provider diagnoses and patient interpretations were incorrect, 320 (73.7%) of which involved infectious disorders. By contrast, of the 413 instances of provider and patient missed diagnoses 289 (70.0%) were inflammatory dermatoses. The pattern was similar for patients’ incorrect interpretations compared to the incorrect diagnoses of the medical providers. Patients incorrectly interpreted their dermatoses as infectious in 79.5% (101/127) of cases. Similarly, providers incorrectly diagnosed their patients’ dermatoses as infectious in 75.4% (211/280) of cases (Table 3). For patients’ missed diagnoses, 70.7% (82/116) involved inflammatory dermatoses. For providers’ missed diagnoses, 63.9% (179/280) involved inflammatory dermatoses (Table 4).
Treatment errors in the context of correct diagnoses were uncommon. Fifteen (3.4%) such cases were noted in the 446 error-containing patient visits. In 4 (26.7%) of the 15 cases, potent topical corticosteroids were used long term on inappropriate cutaneous sites (eg, genital, facial, or intertriginous areas). Another 4 (26.7%) cases involved fungal infections: nystatin used for tinea versicolor in 1 case and for dermatophytosis in another, widespread dermatophytosis treated topically, and use of a nonindicated topical antifungal for onychomycosis. Other examples involved inadequate dosing of systemic corticosteroids for extensive acute contact dermatitis, psoriasis treated with systemic corticosteroids, inadequate dosing of medication for seborrheic dermatitis, and treatment with valacyclovir based solely on serologic testing.
Comment
The results of our study indicate that errors in management of cutaneous disorders are overwhelmingly diagnostic in nature, while treatment errors appear to be unusual when the correct diagnosis is made. Both the current study and the 1987 study indicated a notable tendency of providers to incorrectly diagnose infectious disorders and to miss the diagnosis of inflammatory dermatoses.7 The current study extends this finding to include patients’ interpretive errors.
It is notable that many of the incorrect and missed diagnoses can be confirmed or ruled out by rapid bedside techniques, namely potassium hydroxide (KOH) preparation for dermatophytes, candidiasis, and tinea versicolor; wet preparation for scabies and pediculosis; Tzanck preparation for herpes simplex and herpes zoster; and crush preparation for molluscum contagiosum. Notably, 57.8% (281/486) of cases in which error was noted involved disorders for which the use of one of these bedside diagnostic tests could have correctly established a diagnosis or ruled out an incorrect one; thus in an ideal world in which these tests were performed perfectly in all appropriate cases, more than half of the errors detected in this study could have been avoided. Dermatophytosis was involved in 35.8% (174/486) of the error-containing patient encounters in this study; therefore, if only the KOH preparation is considered, more than one-third of all errors documented in this study could have been avoided. Unfortunately, surveys have suggested that among dermatologists in the United States and some other countries, KOH preparations are used infrequently.10-12
Certain limitations were inherent to this study. The data were derived from a single dermatology practice by one physician in one geographic region over a short period of time. These factors may limit the generalizability of the results. Although the goal was to identify all errors made for the patients seen, some errors likely were missed due to incomplete patient history or inaccurate medication listings. There is no absolute way to determine if the diagnoses or the treatments deemed correct by the dermatologist were, in fact, correct. For cases in which a patient’s interpretation or a provider’s diagnosis was imputed from the indication(s) associated with the medication(s) being used, one cannot exclude the possibility that a medication was used appropriately for a nonlabeled or nonstandard indication. The designation of treatment errors may be subject to different interpretations by different clinicians. Despite these limitations, it is likely that the results of this study can be extrapolated to reasonably similar dermatology practices. The apparently persistent and consistent tendency of clinicians to incorrectly diagnose infectious dermatoses and to miss inflammatory conditions has implications for teaching of medical dermatology in the academic and clinical settings. In particular, given that dermatophytosis is the diagnosis involved in the highest number of errors, special emphasis should be placed on this infection in clinician education.
Acknowledgement—The authors would like to acknowledge the essential contributions to this study by Urvi Jain (Virginia Beach, Virginia), particularly for analysis and interpretation of data and for suggestions to improve the manuscript.
- Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, eds. National Academies Press; 2000.
- Lowenstein EJ, Sidlow R, Ko CJ. Visual perception, cognition, and error in dermatologic diagnosis: diagnosis and error. J Am Acad Dermatol. 2019;81:1237-1245.
- Ko CJ, Braverman I, Sidlow R, et al. Visual perception, cognition, and error in dermatologic diagnosis: key cognitive principles. J Am Acad Dermatol. 2019;81:1227-1234.
- Lowenstein EJ. Dermatology and its unique diagnostic heuristics. J Am Acad Dermatol. 2018;78:1239-1240.
- Elston DM. Cognitive bias and medical errors. J Am Acad Dermatol. 2019;81:1249.
- Costa Filho GB, Moura AS, Brandão PR, et al. Effects of deliberate reflection on diagnostic accuracy, confidence and diagnostic calibration in dermatology. Perspect Med Educ. 2019;8:230-236.
- Pariser RJ, Pariser DM. Primary physicians’ errors in handling cutaneous disorders. J Am Acad Dermatol. 1987;17:239-245.
- van Rossum G, Drake FL Jr. Python Reference Manual. Centrum voor Wiskunde en Informatica; 1995.
- The pandas development team. pandas-dev/pandas: Pandas. Zenodo. February 2020. doi:10.5281/zenodo.3509134
- Murphy EC, Friedman AJ. Use of in-office preparations by dermatologists for the diagnosis of cutaneous fungal infections. J Drugs Dermatol. 2019;18:798-802.
- Dhafiri MA, Alhamed AS, Aljughayman MA. Use of potassium hydroxide in dermatology daily practice: a local study from Saudi Arabia. Cureus. 2022;14:E30612. doi:10.7759/cureus .30612.eCollection
- Chandler JD, Yamamoto R, Hay RJ. Use of direct microscopy to diagnose superficial mycoses: a survey of UK dermatology practice. Br J Dermatol. 2023;189:480-481.
Humans are inherently prone to errors. The extent and consequences of medical errors were documented in the 2000 publication of To Err is Human: Building a Safer Health System.1 Published research on medical errors in dermatology has emphasized the heuristic issues involved in diagnosis,2-6 essentially approaching the “why?” and “how?” of such errors. By contrast, the current study aimed to elucidate the “what?”—what are the dermatologic conditions most prone to diagnostic and/or management errors? One study published in 1987 approached this question by analyzing patterns of errors for dermatologic conditions in patients referred for specialty care by primary care physicians.7 The current study aimed to update and expand on the findings of this 1987 report by comparing more recent data on the errors made by providers and patients regarding skin conditions.
Methods
Data were collected prospectively from March 18, 2021, through July 25, 2023. Prospective data were obtained by recording the nature of errors noted for all patients seen by a board-certified dermatologist (R.J.P.) during routine outpatient practice in Norfolk, Virginia. This practice is limited to medical dermatology and accepts patients of any age from any referral source, with or without medical insurance. Retrospective data were obtained by review of electronic medical records for all patients seen by the same board-certified dermatologist from June 5, 2020, through March 12, 2021, who previously had been seen by an outside provider or were self-referred. In this study, the term diagnosis is used to describe providers’ explicit or imputed conclusions as to the nature of a dermatosis, and the term interpretation is used to describe patients' conclusions about their own condition. For this study, the patients’ self-made interpretations of their dermatoses were deemed to be correct when they agreed with those made by the dermatologist using standard clinicopathologic criteria supplemented by rapid bedside diagnostic techniques, as detailed in the 1987 study.7
Cases in which diagnostic or therapeutic errors were noted were entered into a spreadsheet that excluded patients’ names or other identifiers. For each noted case of diagnostic or therapeutic error, the following data were entered: patient’s age and sex; the name of the incorrect diagnosis, interpretation, or treatment; and the name of the correct (missed) diagnosis, along with the source of the error (provider or patient). Provider diagnoses were determined from medical records or patient statements or were imputed from the generally accepted indications for prescribed treatments. A provider was deemed to be any practitioner with prescriptive authority. Patients’ interpretations of their conditions were determined by patient statements or were imputed based on the indications for treatments being used. A treatment error was recorded when a diagnosis or interpretation was deemed to be correct, but treatment was deemed to be inappropriate. The same dermatologist (R.J.P) made all determinations as to the nature of the errors and their source.
Diagnostic errors were determined in several situations: (1) if the interpretation made by the patient of their dermatosis differed from the correct diagnosis in the absence of any additional diagnostic documentation, the correct diagnosis was scored as a missed diagnosis and the incorrect interpretation was scored as such; (2) if the provider’s diagnosis in the patient’s medical record differed from the correct diagnosis, both the correct (missed) and incorrect diagnoses were recorded; and (3) if the indication(s) of the medication(s) prescribed by the provider or used by the patient for their condition differed from the correct diagnosis, an imputed diagnosis based on this indication was scored as the incorrect diagnosis and the correct (missed) diagnosis was recorded; for example, an error would be entered into the spreadsheet for a patient using terbinafine cream for what was actually psoriasis. For a medication with multiple active agents, an error would be entered into the spreadsheet only if none of its indications matched the correct diagnosis; for example, if the patient had been prescribed a betamethasone/clotrimazole product, no error would be scored if the correct diagnosis was a steroid-responsive dermatosis, dermatophytosis, candidiasis, or tinea versicolor. For a single medication with multiple indications, no error would be recorded if the correct diagnosis was any of these indications; for example, in a patient who had been prescribed topical ketoconazole, no error would be scored if the correct diagnosis was dermatophytosis, candidiasis, tinea versicolor, or seborrheic dermatitis. Additionally, no error would be recorded if the correct diagnosis was uncertain at the time of initial patient evaluation or during chart review.
Standard spreadsheet functions and the pandas package8 from the Python programming language9 were used to extract relevant data from the spreadsheet (Tables 1-4).




Results
A total of 446 patient visits (182 males, 264 females) were included in the study, in which a total of 486 errors were found in the combined prospective and retrospective portions of the study. These errors involved 1.4% of all patient visits for the study period—specifically, all in routine practice as well as all patient records retrospectively reviewed. The age of the patients ranged from 4 to 95 years; the mean age was 51.5 years for males and 50.8 years for females.
The study results are outlined in Tables 1 through 4. To minimize the amount of data provided with no appreciable effect on the results, cases in which an incorrect or missed diagnosis/interpretation occurred only once (ie, unique case errors) were excluded from the tables. Tables 1 and 2 indicate the numbers and types of incorrect and missed diagnoses.
In the combined patient and provider cases, there were 434 instances in which provider diagnoses and patient interpretations were incorrect, 320 (73.7%) of which involved infectious disorders. By contrast, of the 413 instances of provider and patient missed diagnoses 289 (70.0%) were inflammatory dermatoses. The pattern was similar for patients’ incorrect interpretations compared to the incorrect diagnoses of the medical providers. Patients incorrectly interpreted their dermatoses as infectious in 79.5% (101/127) of cases. Similarly, providers incorrectly diagnosed their patients’ dermatoses as infectious in 75.4% (211/280) of cases (Table 3). For patients’ missed diagnoses, 70.7% (82/116) involved inflammatory dermatoses. For providers’ missed diagnoses, 63.9% (179/280) involved inflammatory dermatoses (Table 4).
Treatment errors in the context of correct diagnoses were uncommon. Fifteen (3.4%) such cases were noted in the 446 error-containing patient visits. In 4 (26.7%) of the 15 cases, potent topical corticosteroids were used long term on inappropriate cutaneous sites (eg, genital, facial, or intertriginous areas). Another 4 (26.7%) cases involved fungal infections: nystatin used for tinea versicolor in 1 case and for dermatophytosis in another, widespread dermatophytosis treated topically, and use of a nonindicated topical antifungal for onychomycosis. Other examples involved inadequate dosing of systemic corticosteroids for extensive acute contact dermatitis, psoriasis treated with systemic corticosteroids, inadequate dosing of medication for seborrheic dermatitis, and treatment with valacyclovir based solely on serologic testing.
Comment
The results of our study indicate that errors in management of cutaneous disorders are overwhelmingly diagnostic in nature, while treatment errors appear to be unusual when the correct diagnosis is made. Both the current study and the 1987 study indicated a notable tendency of providers to incorrectly diagnose infectious disorders and to miss the diagnosis of inflammatory dermatoses.7 The current study extends this finding to include patients’ interpretive errors.
It is notable that many of the incorrect and missed diagnoses can be confirmed or ruled out by rapid bedside techniques, namely potassium hydroxide (KOH) preparation for dermatophytes, candidiasis, and tinea versicolor; wet preparation for scabies and pediculosis; Tzanck preparation for herpes simplex and herpes zoster; and crush preparation for molluscum contagiosum. Notably, 57.8% (281/486) of cases in which error was noted involved disorders for which the use of one of these bedside diagnostic tests could have correctly established a diagnosis or ruled out an incorrect one; thus in an ideal world in which these tests were performed perfectly in all appropriate cases, more than half of the errors detected in this study could have been avoided. Dermatophytosis was involved in 35.8% (174/486) of the error-containing patient encounters in this study; therefore, if only the KOH preparation is considered, more than one-third of all errors documented in this study could have been avoided. Unfortunately, surveys have suggested that among dermatologists in the United States and some other countries, KOH preparations are used infrequently.10-12
Certain limitations were inherent to this study. The data were derived from a single dermatology practice by one physician in one geographic region over a short period of time. These factors may limit the generalizability of the results. Although the goal was to identify all errors made for the patients seen, some errors likely were missed due to incomplete patient history or inaccurate medication listings. There is no absolute way to determine if the diagnoses or the treatments deemed correct by the dermatologist were, in fact, correct. For cases in which a patient’s interpretation or a provider’s diagnosis was imputed from the indication(s) associated with the medication(s) being used, one cannot exclude the possibility that a medication was used appropriately for a nonlabeled or nonstandard indication. The designation of treatment errors may be subject to different interpretations by different clinicians. Despite these limitations, it is likely that the results of this study can be extrapolated to reasonably similar dermatology practices. The apparently persistent and consistent tendency of clinicians to incorrectly diagnose infectious dermatoses and to miss inflammatory conditions has implications for teaching of medical dermatology in the academic and clinical settings. In particular, given that dermatophytosis is the diagnosis involved in the highest number of errors, special emphasis should be placed on this infection in clinician education.
Acknowledgement—The authors would like to acknowledge the essential contributions to this study by Urvi Jain (Virginia Beach, Virginia), particularly for analysis and interpretation of data and for suggestions to improve the manuscript.
Humans are inherently prone to errors. The extent and consequences of medical errors were documented in the 2000 publication of To Err is Human: Building a Safer Health System.1 Published research on medical errors in dermatology has emphasized the heuristic issues involved in diagnosis,2-6 essentially approaching the “why?” and “how?” of such errors. By contrast, the current study aimed to elucidate the “what?”—what are the dermatologic conditions most prone to diagnostic and/or management errors? One study published in 1987 approached this question by analyzing patterns of errors for dermatologic conditions in patients referred for specialty care by primary care physicians.7 The current study aimed to update and expand on the findings of this 1987 report by comparing more recent data on the errors made by providers and patients regarding skin conditions.
Methods
Data were collected prospectively from March 18, 2021, through July 25, 2023. Prospective data were obtained by recording the nature of errors noted for all patients seen by a board-certified dermatologist (R.J.P.) during routine outpatient practice in Norfolk, Virginia. This practice is limited to medical dermatology and accepts patients of any age from any referral source, with or without medical insurance. Retrospective data were obtained by review of electronic medical records for all patients seen by the same board-certified dermatologist from June 5, 2020, through March 12, 2021, who previously had been seen by an outside provider or were self-referred. In this study, the term diagnosis is used to describe providers’ explicit or imputed conclusions as to the nature of a dermatosis, and the term interpretation is used to describe patients' conclusions about their own condition. For this study, the patients’ self-made interpretations of their dermatoses were deemed to be correct when they agreed with those made by the dermatologist using standard clinicopathologic criteria supplemented by rapid bedside diagnostic techniques, as detailed in the 1987 study.7
Cases in which diagnostic or therapeutic errors were noted were entered into a spreadsheet that excluded patients’ names or other identifiers. For each noted case of diagnostic or therapeutic error, the following data were entered: patient’s age and sex; the name of the incorrect diagnosis, interpretation, or treatment; and the name of the correct (missed) diagnosis, along with the source of the error (provider or patient). Provider diagnoses were determined from medical records or patient statements or were imputed from the generally accepted indications for prescribed treatments. A provider was deemed to be any practitioner with prescriptive authority. Patients’ interpretations of their conditions were determined by patient statements or were imputed based on the indications for treatments being used. A treatment error was recorded when a diagnosis or interpretation was deemed to be correct, but treatment was deemed to be inappropriate. The same dermatologist (R.J.P) made all determinations as to the nature of the errors and their source.
Diagnostic errors were determined in several situations: (1) if the interpretation made by the patient of their dermatosis differed from the correct diagnosis in the absence of any additional diagnostic documentation, the correct diagnosis was scored as a missed diagnosis and the incorrect interpretation was scored as such; (2) if the provider’s diagnosis in the patient’s medical record differed from the correct diagnosis, both the correct (missed) and incorrect diagnoses were recorded; and (3) if the indication(s) of the medication(s) prescribed by the provider or used by the patient for their condition differed from the correct diagnosis, an imputed diagnosis based on this indication was scored as the incorrect diagnosis and the correct (missed) diagnosis was recorded; for example, an error would be entered into the spreadsheet for a patient using terbinafine cream for what was actually psoriasis. For a medication with multiple active agents, an error would be entered into the spreadsheet only if none of its indications matched the correct diagnosis; for example, if the patient had been prescribed a betamethasone/clotrimazole product, no error would be scored if the correct diagnosis was a steroid-responsive dermatosis, dermatophytosis, candidiasis, or tinea versicolor. For a single medication with multiple indications, no error would be recorded if the correct diagnosis was any of these indications; for example, in a patient who had been prescribed topical ketoconazole, no error would be scored if the correct diagnosis was dermatophytosis, candidiasis, tinea versicolor, or seborrheic dermatitis. Additionally, no error would be recorded if the correct diagnosis was uncertain at the time of initial patient evaluation or during chart review.
Standard spreadsheet functions and the pandas package8 from the Python programming language9 were used to extract relevant data from the spreadsheet (Tables 1-4).




Results
A total of 446 patient visits (182 males, 264 females) were included in the study, in which a total of 486 errors were found in the combined prospective and retrospective portions of the study. These errors involved 1.4% of all patient visits for the study period—specifically, all in routine practice as well as all patient records retrospectively reviewed. The age of the patients ranged from 4 to 95 years; the mean age was 51.5 years for males and 50.8 years for females.
The study results are outlined in Tables 1 through 4. To minimize the amount of data provided with no appreciable effect on the results, cases in which an incorrect or missed diagnosis/interpretation occurred only once (ie, unique case errors) were excluded from the tables. Tables 1 and 2 indicate the numbers and types of incorrect and missed diagnoses.
In the combined patient and provider cases, there were 434 instances in which provider diagnoses and patient interpretations were incorrect, 320 (73.7%) of which involved infectious disorders. By contrast, of the 413 instances of provider and patient missed diagnoses 289 (70.0%) were inflammatory dermatoses. The pattern was similar for patients’ incorrect interpretations compared to the incorrect diagnoses of the medical providers. Patients incorrectly interpreted their dermatoses as infectious in 79.5% (101/127) of cases. Similarly, providers incorrectly diagnosed their patients’ dermatoses as infectious in 75.4% (211/280) of cases (Table 3). For patients’ missed diagnoses, 70.7% (82/116) involved inflammatory dermatoses. For providers’ missed diagnoses, 63.9% (179/280) involved inflammatory dermatoses (Table 4).
Treatment errors in the context of correct diagnoses were uncommon. Fifteen (3.4%) such cases were noted in the 446 error-containing patient visits. In 4 (26.7%) of the 15 cases, potent topical corticosteroids were used long term on inappropriate cutaneous sites (eg, genital, facial, or intertriginous areas). Another 4 (26.7%) cases involved fungal infections: nystatin used for tinea versicolor in 1 case and for dermatophytosis in another, widespread dermatophytosis treated topically, and use of a nonindicated topical antifungal for onychomycosis. Other examples involved inadequate dosing of systemic corticosteroids for extensive acute contact dermatitis, psoriasis treated with systemic corticosteroids, inadequate dosing of medication for seborrheic dermatitis, and treatment with valacyclovir based solely on serologic testing.
Comment
The results of our study indicate that errors in management of cutaneous disorders are overwhelmingly diagnostic in nature, while treatment errors appear to be unusual when the correct diagnosis is made. Both the current study and the 1987 study indicated a notable tendency of providers to incorrectly diagnose infectious disorders and to miss the diagnosis of inflammatory dermatoses.7 The current study extends this finding to include patients’ interpretive errors.
It is notable that many of the incorrect and missed diagnoses can be confirmed or ruled out by rapid bedside techniques, namely potassium hydroxide (KOH) preparation for dermatophytes, candidiasis, and tinea versicolor; wet preparation for scabies and pediculosis; Tzanck preparation for herpes simplex and herpes zoster; and crush preparation for molluscum contagiosum. Notably, 57.8% (281/486) of cases in which error was noted involved disorders for which the use of one of these bedside diagnostic tests could have correctly established a diagnosis or ruled out an incorrect one; thus in an ideal world in which these tests were performed perfectly in all appropriate cases, more than half of the errors detected in this study could have been avoided. Dermatophytosis was involved in 35.8% (174/486) of the error-containing patient encounters in this study; therefore, if only the KOH preparation is considered, more than one-third of all errors documented in this study could have been avoided. Unfortunately, surveys have suggested that among dermatologists in the United States and some other countries, KOH preparations are used infrequently.10-12
Certain limitations were inherent to this study. The data were derived from a single dermatology practice by one physician in one geographic region over a short period of time. These factors may limit the generalizability of the results. Although the goal was to identify all errors made for the patients seen, some errors likely were missed due to incomplete patient history or inaccurate medication listings. There is no absolute way to determine if the diagnoses or the treatments deemed correct by the dermatologist were, in fact, correct. For cases in which a patient’s interpretation or a provider’s diagnosis was imputed from the indication(s) associated with the medication(s) being used, one cannot exclude the possibility that a medication was used appropriately for a nonlabeled or nonstandard indication. The designation of treatment errors may be subject to different interpretations by different clinicians. Despite these limitations, it is likely that the results of this study can be extrapolated to reasonably similar dermatology practices. The apparently persistent and consistent tendency of clinicians to incorrectly diagnose infectious dermatoses and to miss inflammatory conditions has implications for teaching of medical dermatology in the academic and clinical settings. In particular, given that dermatophytosis is the diagnosis involved in the highest number of errors, special emphasis should be placed on this infection in clinician education.
Acknowledgement—The authors would like to acknowledge the essential contributions to this study by Urvi Jain (Virginia Beach, Virginia), particularly for analysis and interpretation of data and for suggestions to improve the manuscript.
- Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, eds. National Academies Press; 2000.
- Lowenstein EJ, Sidlow R, Ko CJ. Visual perception, cognition, and error in dermatologic diagnosis: diagnosis and error. J Am Acad Dermatol. 2019;81:1237-1245.
- Ko CJ, Braverman I, Sidlow R, et al. Visual perception, cognition, and error in dermatologic diagnosis: key cognitive principles. J Am Acad Dermatol. 2019;81:1227-1234.
- Lowenstein EJ. Dermatology and its unique diagnostic heuristics. J Am Acad Dermatol. 2018;78:1239-1240.
- Elston DM. Cognitive bias and medical errors. J Am Acad Dermatol. 2019;81:1249.
- Costa Filho GB, Moura AS, Brandão PR, et al. Effects of deliberate reflection on diagnostic accuracy, confidence and diagnostic calibration in dermatology. Perspect Med Educ. 2019;8:230-236.
- Pariser RJ, Pariser DM. Primary physicians’ errors in handling cutaneous disorders. J Am Acad Dermatol. 1987;17:239-245.
- van Rossum G, Drake FL Jr. Python Reference Manual. Centrum voor Wiskunde en Informatica; 1995.
- The pandas development team. pandas-dev/pandas: Pandas. Zenodo. February 2020. doi:10.5281/zenodo.3509134
- Murphy EC, Friedman AJ. Use of in-office preparations by dermatologists for the diagnosis of cutaneous fungal infections. J Drugs Dermatol. 2019;18:798-802.
- Dhafiri MA, Alhamed AS, Aljughayman MA. Use of potassium hydroxide in dermatology daily practice: a local study from Saudi Arabia. Cureus. 2022;14:E30612. doi:10.7759/cureus .30612.eCollection
- Chandler JD, Yamamoto R, Hay RJ. Use of direct microscopy to diagnose superficial mycoses: a survey of UK dermatology practice. Br J Dermatol. 2023;189:480-481.
- Institute of Medicine (US) Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, eds. National Academies Press; 2000.
- Lowenstein EJ, Sidlow R, Ko CJ. Visual perception, cognition, and error in dermatologic diagnosis: diagnosis and error. J Am Acad Dermatol. 2019;81:1237-1245.
- Ko CJ, Braverman I, Sidlow R, et al. Visual perception, cognition, and error in dermatologic diagnosis: key cognitive principles. J Am Acad Dermatol. 2019;81:1227-1234.
- Lowenstein EJ. Dermatology and its unique diagnostic heuristics. J Am Acad Dermatol. 2018;78:1239-1240.
- Elston DM. Cognitive bias and medical errors. J Am Acad Dermatol. 2019;81:1249.
- Costa Filho GB, Moura AS, Brandão PR, et al. Effects of deliberate reflection on diagnostic accuracy, confidence and diagnostic calibration in dermatology. Perspect Med Educ. 2019;8:230-236.
- Pariser RJ, Pariser DM. Primary physicians’ errors in handling cutaneous disorders. J Am Acad Dermatol. 1987;17:239-245.
- van Rossum G, Drake FL Jr. Python Reference Manual. Centrum voor Wiskunde en Informatica; 1995.
- The pandas development team. pandas-dev/pandas: Pandas. Zenodo. February 2020. doi:10.5281/zenodo.3509134
- Murphy EC, Friedman AJ. Use of in-office preparations by dermatologists for the diagnosis of cutaneous fungal infections. J Drugs Dermatol. 2019;18:798-802.
- Dhafiri MA, Alhamed AS, Aljughayman MA. Use of potassium hydroxide in dermatology daily practice: a local study from Saudi Arabia. Cureus. 2022;14:E30612. doi:10.7759/cureus .30612.eCollection
- Chandler JD, Yamamoto R, Hay RJ. Use of direct microscopy to diagnose superficial mycoses: a survey of UK dermatology practice. Br J Dermatol. 2023;189:480-481.
Analysis of Errors in the Management of Cutaneous Disorders
Analysis of Errors in the Management of Cutaneous Disorders
PRACTICE POINTS
- Errors in the management of cutaneous disorders predominantly are due to misdiagnosis rather than treatment oversights.
- There is a tendency among medical providers to incorrectly diagnose dermatoses as infectious disorders and to miss the diagnosis of inflammatory dermatoses.
- A similar pattern of errors occurs for patients’ interpretations of their own skin conditions.
- Use of available rapid bedside diagnostic techniques can reduce the likelihood of errors made by medical providers.
White Atrophic Plaques on the Thighs
White Atrophic Plaques on the Thighs
THE DIAGNOSIS: Lichen Sclerosus
Given the clinical appearance of white atrophic plaques with characteristic wrinkling of the skin, a diagnosis of lichen sclerosus was strongly suspected. At the initial office visit, the patient was prescribed clobetasol 0.05% ointment twice daily for 6 weeks. Histopathology revealed hyperkeratosis, follicular plugging, papillary dermal pallor, and adjacent lymphocytic inflammation, confirming the clinical diagnosis of lichen sclerosus (Figure). The patient then was lost to follow-up.
Lichen sclerosus is a chronic benign dermatologic condition of unknown etiology that is characterized by epidermal atrophy and inflammation and is common in postmenopausal women. It features pale, ivory-colored lesions with partially atrophic skin and a wrinkled cigarette paper appearance.1 The differential for lichen sclerosus is broad, and definitive diagnosis is made via biopsy to rule out potential malignancy and other inflammatory skin diseases.1 Lichen sclerosus is an immune-mediated disorder driven by type 1 T helper cells and regulated by miR-155. There has been an association with extracellular matrix protein 1, a glycoprotein that is found in the dermal-epidermal basement membrane zone, which provides structural integrity to the skin. Autoantibodies against extracellular matrix protein 1 and other antigens in the basement membrane generally are found in anogenital lichen sclerosus; however, their precise roles in the pathogenesis of lichen sclerosus remains unclear.1
The differential diagnoses for lichen sclerosus include psoriasis, tinea corporis, lichen simplex chronicus, and atopic dermatitis. Psoriasis typically manifests as pink plaques with silver scales on the elbows, knees, and scalp in adult patients.2 Our patient’s white plaques may have suggested psoriasis, but the partially atrophic skin with a wrinkled cigarette paper appearance was not compatible with that diagnosis.
Tinea corporis, a superficial fungal infection of the skin, manifests as circular or ovoid lesions with raised erythematous scaly borders, often with central clearing resembling a ring, that can occur anywhere on the body other than the feet, groin, face, scalp, or beard area.3 The fact that our patient previously had tried topical antifungal medications with no relief and that the skin lesions were atrophic rather than ring shaped made the diagnosis of tinea corporis unlikely.
Lichen simplex chronicus is a chronic condition caused by friction or scratching that is characterized by dry, patchy, scaly, and thickened areas of the skin. Typically affecting the head, arms, neck, scalp, and genital region, lichen simplex chronicus manifests with violaceous or hyperpigmented lesions.4 The nonpruritic atrophic plaques on the inner thighs and the presence of white patches on the vaginal area were not indicative of lichen simplex chronicus in our patient.
Atopic dermatitis manifests as pruritic erythematous scaly papules and plaques with secondary excoriation and possible lichenification. In adults, atopic dermatitis commonly appears on flexural surfaces.2 Atopic dermatitis does not manifest with atrophy and skin wrinkling as seen in our patient.
In the management of lichen sclerosus, the standard treatment is potent topical corticosteroids. Alternatively, topical calcineurin inhibitors can be employed; however, due to the unknown nature of the condition’s underlying cause, targeted treatment is challenging. Our case underscores how lichen sclerosus can be misdiagnosed, highlighting the need for more frequent reporting in the literature to enhance early recognition and reduce delays in patient treatment.
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389 /fmed.2023.1106318
- Chovatiya R, Silverberg JI. Pathophysiology of atopic dermatitis and psoriasis: implications for management in children. Children (Basel). 2019;6:108. doi:10.3390/children6100108
- Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291.
- Ju T, Vander Does A, Mohsin N, et al. Lichen simplex chronicus itch: an update. Acta Derm Venereol. 2022;102:adv00796. doi:10.2340 /actadv.v102.4367
THE DIAGNOSIS: Lichen Sclerosus
Given the clinical appearance of white atrophic plaques with characteristic wrinkling of the skin, a diagnosis of lichen sclerosus was strongly suspected. At the initial office visit, the patient was prescribed clobetasol 0.05% ointment twice daily for 6 weeks. Histopathology revealed hyperkeratosis, follicular plugging, papillary dermal pallor, and adjacent lymphocytic inflammation, confirming the clinical diagnosis of lichen sclerosus (Figure). The patient then was lost to follow-up.

Lichen sclerosus is a chronic benign dermatologic condition of unknown etiology that is characterized by epidermal atrophy and inflammation and is common in postmenopausal women. It features pale, ivory-colored lesions with partially atrophic skin and a wrinkled cigarette paper appearance.1 The differential for lichen sclerosus is broad, and definitive diagnosis is made via biopsy to rule out potential malignancy and other inflammatory skin diseases.1 Lichen sclerosus is an immune-mediated disorder driven by type 1 T helper cells and regulated by miR-155. There has been an association with extracellular matrix protein 1, a glycoprotein that is found in the dermal-epidermal basement membrane zone, which provides structural integrity to the skin. Autoantibodies against extracellular matrix protein 1 and other antigens in the basement membrane generally are found in anogenital lichen sclerosus; however, their precise roles in the pathogenesis of lichen sclerosus remains unclear.1
The differential diagnoses for lichen sclerosus include psoriasis, tinea corporis, lichen simplex chronicus, and atopic dermatitis. Psoriasis typically manifests as pink plaques with silver scales on the elbows, knees, and scalp in adult patients.2 Our patient’s white plaques may have suggested psoriasis, but the partially atrophic skin with a wrinkled cigarette paper appearance was not compatible with that diagnosis.
Tinea corporis, a superficial fungal infection of the skin, manifests as circular or ovoid lesions with raised erythematous scaly borders, often with central clearing resembling a ring, that can occur anywhere on the body other than the feet, groin, face, scalp, or beard area.3 The fact that our patient previously had tried topical antifungal medications with no relief and that the skin lesions were atrophic rather than ring shaped made the diagnosis of tinea corporis unlikely.
Lichen simplex chronicus is a chronic condition caused by friction or scratching that is characterized by dry, patchy, scaly, and thickened areas of the skin. Typically affecting the head, arms, neck, scalp, and genital region, lichen simplex chronicus manifests with violaceous or hyperpigmented lesions.4 The nonpruritic atrophic plaques on the inner thighs and the presence of white patches on the vaginal area were not indicative of lichen simplex chronicus in our patient.
Atopic dermatitis manifests as pruritic erythematous scaly papules and plaques with secondary excoriation and possible lichenification. In adults, atopic dermatitis commonly appears on flexural surfaces.2 Atopic dermatitis does not manifest with atrophy and skin wrinkling as seen in our patient.
In the management of lichen sclerosus, the standard treatment is potent topical corticosteroids. Alternatively, topical calcineurin inhibitors can be employed; however, due to the unknown nature of the condition’s underlying cause, targeted treatment is challenging. Our case underscores how lichen sclerosus can be misdiagnosed, highlighting the need for more frequent reporting in the literature to enhance early recognition and reduce delays in patient treatment.
THE DIAGNOSIS: Lichen Sclerosus
Given the clinical appearance of white atrophic plaques with characteristic wrinkling of the skin, a diagnosis of lichen sclerosus was strongly suspected. At the initial office visit, the patient was prescribed clobetasol 0.05% ointment twice daily for 6 weeks. Histopathology revealed hyperkeratosis, follicular plugging, papillary dermal pallor, and adjacent lymphocytic inflammation, confirming the clinical diagnosis of lichen sclerosus (Figure). The patient then was lost to follow-up.

Lichen sclerosus is a chronic benign dermatologic condition of unknown etiology that is characterized by epidermal atrophy and inflammation and is common in postmenopausal women. It features pale, ivory-colored lesions with partially atrophic skin and a wrinkled cigarette paper appearance.1 The differential for lichen sclerosus is broad, and definitive diagnosis is made via biopsy to rule out potential malignancy and other inflammatory skin diseases.1 Lichen sclerosus is an immune-mediated disorder driven by type 1 T helper cells and regulated by miR-155. There has been an association with extracellular matrix protein 1, a glycoprotein that is found in the dermal-epidermal basement membrane zone, which provides structural integrity to the skin. Autoantibodies against extracellular matrix protein 1 and other antigens in the basement membrane generally are found in anogenital lichen sclerosus; however, their precise roles in the pathogenesis of lichen sclerosus remains unclear.1
The differential diagnoses for lichen sclerosus include psoriasis, tinea corporis, lichen simplex chronicus, and atopic dermatitis. Psoriasis typically manifests as pink plaques with silver scales on the elbows, knees, and scalp in adult patients.2 Our patient’s white plaques may have suggested psoriasis, but the partially atrophic skin with a wrinkled cigarette paper appearance was not compatible with that diagnosis.
Tinea corporis, a superficial fungal infection of the skin, manifests as circular or ovoid lesions with raised erythematous scaly borders, often with central clearing resembling a ring, that can occur anywhere on the body other than the feet, groin, face, scalp, or beard area.3 The fact that our patient previously had tried topical antifungal medications with no relief and that the skin lesions were atrophic rather than ring shaped made the diagnosis of tinea corporis unlikely.
Lichen simplex chronicus is a chronic condition caused by friction or scratching that is characterized by dry, patchy, scaly, and thickened areas of the skin. Typically affecting the head, arms, neck, scalp, and genital region, lichen simplex chronicus manifests with violaceous or hyperpigmented lesions.4 The nonpruritic atrophic plaques on the inner thighs and the presence of white patches on the vaginal area were not indicative of lichen simplex chronicus in our patient.
Atopic dermatitis manifests as pruritic erythematous scaly papules and plaques with secondary excoriation and possible lichenification. In adults, atopic dermatitis commonly appears on flexural surfaces.2 Atopic dermatitis does not manifest with atrophy and skin wrinkling as seen in our patient.
In the management of lichen sclerosus, the standard treatment is potent topical corticosteroids. Alternatively, topical calcineurin inhibitors can be employed; however, due to the unknown nature of the condition’s underlying cause, targeted treatment is challenging. Our case underscores how lichen sclerosus can be misdiagnosed, highlighting the need for more frequent reporting in the literature to enhance early recognition and reduce delays in patient treatment.
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389 /fmed.2023.1106318
- Chovatiya R, Silverberg JI. Pathophysiology of atopic dermatitis and psoriasis: implications for management in children. Children (Basel). 2019;6:108. doi:10.3390/children6100108
- Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291.
- Ju T, Vander Does A, Mohsin N, et al. Lichen simplex chronicus itch: an update. Acta Derm Venereol. 2022;102:adv00796. doi:10.2340 /actadv.v102.4367
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389 /fmed.2023.1106318
- Chovatiya R, Silverberg JI. Pathophysiology of atopic dermatitis and psoriasis: implications for management in children. Children (Basel). 2019;6:108. doi:10.3390/children6100108
- Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291.
- Ju T, Vander Does A, Mohsin N, et al. Lichen simplex chronicus itch: an update. Acta Derm Venereol. 2022;102:adv00796. doi:10.2340 /actadv.v102.4367
White Atrophic Plaques on the Thighs
White Atrophic Plaques on the Thighs
A 71-year-old woman presented to the dermatology clinic for evaluation of intense pruritus of the vaginal region and a nonpruritic rash on the inner thighs of 7 months’ duration. Physical examination revealed white atrophic plaques with scaling and a wrinkled appearance on the inner thighs. White atrophic patches also were noted on the vulva. The patient reported that she had tried over-the-counter antifungals with no improvement. A punch biopsy was performed.
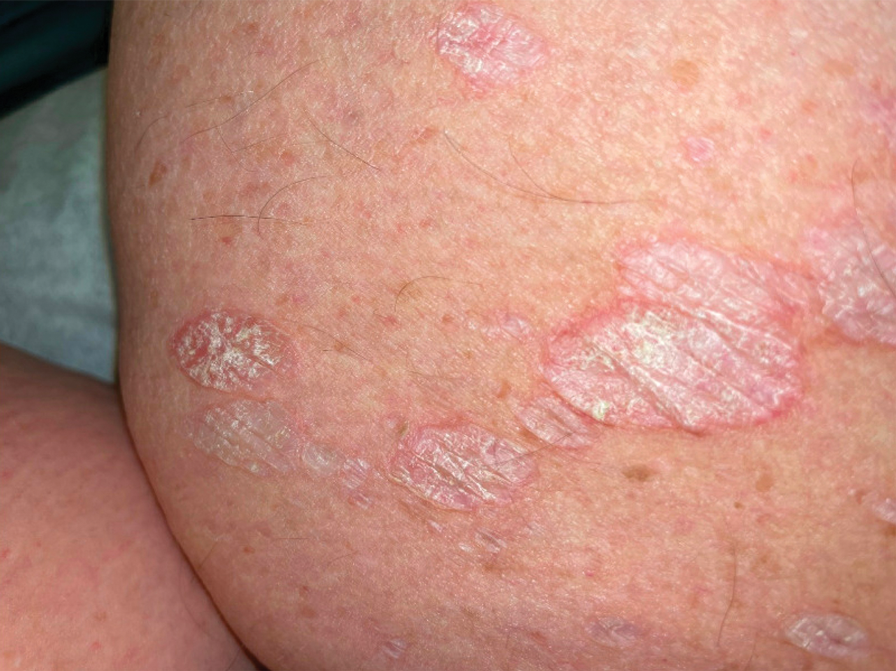
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.
Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 Pseudofolliculitis barbae is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
Pseudofolliculitis barbae is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? em>Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.

Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 Pseudofolliculitis barbae is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
Pseudofolliculitis barbae is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
THE COMPARISON
- A. Pustules, erythematous to violaceous nodules, and hyperpigmented patches on the lower cheek and chin.
- B. Brown papules, pink keloidal papules and nodules, pustules, and hyperpigmented papules on the mandibular area and neck.
- C. Coarse hairs, pustules, and pink papules on the mandibular area and neck.

Pseudofolliculitis barbae (PFB), also known as razor bumps, is a common inflammatory condition characterized by papules and pustules that typically appear in the beard and cheek regions. It occurs when shaved hair regrows and penetrates the skin, leading to irritation and inflammation. While anyone who shaves can develop PFB, it is more prevalent and severe in individuals with naturally tightly coiled, coarse-textured hair.1,2 Pseudofolliculitis barbae is common in individuals who shave frequently due to personal choice or profession, such as members of the US military3,4 and firefighters, who are required to remain clean shaven for safety (eg, ensuring proper fit of a respirator mask).5 Early diagnosis and treatment of PFB are essential to prevent long-term complications such as scarring or hyperpigmentation, which may be more severe in those with darker skin tones.
Epidemiology
Pseudofolliculitis barbae is most common in Black men, affecting 45% to 83% of men of African ancestry.1,2 This condition also can affect individuals of various ethnicities with coarse or curly hair. The spiral shape of the hair increases the likelihood that it will regrow into the skin after shaving.6 Women with hirsutism who shave also can develop PFB.
Key Clinical Features
The papules and pustules seen in PFB may be flesh colored, erythematous, hyperpigmented, brown, or violaceous. Erythema may be less pronounced in darker vs lighter skin tones. Persistent and severe postinflammatory hyperpigmentation may occur, and hypertrophic or keloidal scars may develop in affected areas. Dermoscopy may reveal extrafollicular hair penetration as well as follicular or perifollicular pustules accompanied by hyperkeratosis.
Worth Noting
The most effective management for PFB is to discontinue shaving.1 If shaving is desired or necessary, it is recommended that patients apply lukewarm water to the affected area followed by a generous amount of shaving foam or gel to create a protective antifriction layer that allows the razor to glide more smoothly over the skin and reduces subsequent irritation.2 Using the right razor technology also may help alleviate symptoms. Research has shown that multiblade razors used in conjunction with preshave hair hydration and postshave moisturization do not worsen PFB.2 A recent study found that multiblade razor technology paired with use of a shave foam or gel actually improved skin appearance in patients with PFB.7
It is important to direct patients to shave in the direction of hair growth; however, this may not be possible for individuals with curly or coarse hair, as the hair may grow in many directions.8,9 Patients also should avoid pulling the skin taut while shaving, as doing so allows the hair to be clipped below the surface, where it can repenetrate the skin and cause further irritation. As an alternative to shaving with a razor, patients can use hair clippers to trim beard hair, which leaves behind stubble and interrupts the cycle of retracted hairs under the skin. Nd:YAG laser therapy has demonstrated efficacy in reduction of PFB papules and pustules.9-12 Greater mean improvement in inflammatory papules and reduction in hair density was noted in participants who received Nd:YAG laser plus eflornithine compared with those who received the laser or eflornithine alone.11 Patients should not pluck or dig into the skin to remove any ingrown hairs. If a tweezer is used, the patient should gently lift the tip of the ingrown hair with the tweezer to dislodge it from the skin and prevent plucking out the hair completely.
To help manage inflammation after shaving, topical treatments such as benzoyl peroxide 5%/clindamycin 1% gel can be used.3,13 A low-potency steroid such as topical hydrocortisone 2.5% applied once or twice daily for up to 2 to 3 days may be helpful.1,14 Adjunctive treatments including keratolytics (eg, topical retinoids, hydroxy acids) reduce perifollicular hyperkeratosis.14,15 Agents containing alpha hydroxy acids (eg, glycolic acid) also can decrease the curvature of the hair itself by reducing the sulfhydryl bonds.6 If secondary bacterial infections occur, oral antibiotics (eg, doxycycline) may be necessary.
Health Disparity Highlight
Individuals with darker skin tones are at higher risk for PFB and associated complications. Limited access to dermatology services may further exacerbate these challenges. Individuals with PFB may not seek medical treatment until the condition becomes severe. Clinicians also may underestimate the severity of PFB—particularly in those with darker skin tones—based on erythema alone because it may be less pronounced in darker vs lighter skin tones.16
While permanent hair reduction with laser therapy is a treatment option for PFB, it may be inaccessible to some patients because it can be expensive and is coded as a cosmetic procedure. Additionally, patients may not have access to specialists who are experienced in performing the procedure in those with darker skin tones.9 Some patients also may not want to permanently reduce the amount of hair that grows in the beard area for personal or religious reasons.17
Pseudofolliculitis barbae also has been linked to professional disparities. One study found that members of the US Air Force who had medical shaving waivers experienced longer times to promotion than those with no waiver.18 Delays in promotion may be linked to perceptions of unprofessionalism, exclusion from high-profile duties, and concerns about career progression. While this delay was similar for individuals of all races, the majority of those in the waiver group were Black/African American. In 2021, 4 Black firefighters with PFB were unsuccessful in their bid to get a medical accommodation regarding a New York City Fire Department policy requiring them to be clean shaven where the oxygen mask seals against the skin.5 More research is needed on mask safety and efficiency relative to the length of facial hair. Accommodations or tailored masks for facial hair conditions also are necessary so individuals with PFB can meet job requirements while managing their condition.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? em>Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? em>Dermatol Clin. 2014;32:183-191.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38 (suppl 1):24-27.
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the U.S. military, a review. Mil Med. 2021;186:E52-E57.
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302.
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Moran E, McMichael A, De Souza B, et al. New razor technology improves appearance and quality of life in men with pseudofolliculitis barbae. Cutis. 2022;110:329-334.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin—challenges for shaving. Int J Cosmet Sci. 2016;38 (suppl 1):3-9.
- Ross EV. How would you treat this patient with lasers & EBDs? casebased panel. Presented at: Skin of Color Update; September 13, 2024; New York, NY.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
- Shokeir H, Samy N, Taymour M. Pseudofolliculitis barbae treatment: efficacy of topical eflornithine, long-pulsed Nd-YAG laser versus their combination. J Cosmet Dermatol. 2021;20:3517-3525.
- Amer A, Elsayed A, Gharib K. Evaluation of efficacy and safety of chemical peeling and long-pulse Nd:YAG laser in treatment of pseudofolliculitis barbae. Dermatol Ther. 2021;34:E14859.
- Cook-Bolden FE, Barba A, Halder R, et al. Twice-daily applications of benzoyl peroxide 5%/clindamycin 1% gel versus vehicle in the treatment of pseudofolliculitis barbae. Cutis. 2004;73(6 suppl):18-24.
- Nussbaum D, Friedman A. Pseudofolliculitis barbae: a review of current treatment options. J Drugs Dermatol. 2019;18:246-250.
- Quarles FN, Brody H, Johnson BA, et al. Pseudofolliculitis barbae. Dermatol Ther. 2007;20:133-136.
- McMichael AJ, Frey C. Challenging the tools used to measure cutaneous lupus severity in patients of all skin types. JAMA Dermatol. 2025;161:9-10.
- Okonkwo E, Neal B, Harper HL. Pseudofolliculitis barbae in the military and the need for social awareness. Mil Med. 2021;186:143-144.
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247.
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Beyond the Razor: Managing Pseudofolliculitis Barbae in Skin of Color
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
To the Editor:
Alopecia areata (AA), an autoimmune disease characterized by inflammatory and nonscarring hair loss, can have a considerable impact on quality of life.1 Baricitinib is a Janus kinase inhibitor that recently was approved by the US Food and Drug Administration for treatment of severe AA in adult patients, becoming the only on-label treatment available.2 So far, the most common adverse effects reported in phase 3 trials have been acne, upper respiratory tract infections, headaches, urinary tract infections, and elevated creatine kinase levels.3
At our trichology unit in the dermatology department of a Spanish tertiary-care hospital in Seville, we have successfully used baricitinib to treat 18 patients with severe, therapy-resistant AA. Herein, we present a case of trichilemmal cyst reactivation in one of our patients following successful treatment with baricitinib.
A 53-year-old woman with a history of trichilemmal cysts presented to the dermatology department with total body hair loss of 5 years' duration that was diagnosed as AA universalis (Figure, A). The patient reported that the trichilemmal cysts had shrunk drastically 1 month after complete loss of body hair (Severity of Alopecia Tool [SALT] score, 100)(Figure, B). The largest cyst was surgically removed, and the diagnosis was histologically confirmed by a pathologist. Her mother and sister also had a history of multiple trichilemmal cysts.
The patient previously had failed treatment with oral prednisone 50 mg/d, oral cyclosporine 4 mg/kg/d, oral dexamethasone 4 mg twice weekly, and oral azathioprine 300 mg/wk. Due to the new indication of baricitinib for AA, we opted to start the patient on oral baricitinib 4 mg/d. By week 8 of treatment, she had achieved total hair regrowth (SALT score, 0). This rapid response might indicate a quick-responder phenotype, referring to a subset of patients who exhibit a fast and robust response to treatment (SALT90), generally before week 16, although more evidence is needed.
Notably, we observed the reactivation of 4 trichilemmal cysts on the scalp 6 weeks after starting baricitinib. To our knowledge, this side effect has not previously been reported. We hypothesize that reactivation of the cysts may have been due to the inhibition of the Janus kinase/signal transducer and activator of transcription pathway, which reduces the effects of cytokines and leads to reactivation of hair follicles that were inactive because of inflammation.4 As a result, the outer root sheath of the hair follicle can once again be filled with keratin, thereby reactivating the trichilemmal cysts. Based on our experience with this case, it may be relevant to consider personal and family history of trichilemmal cysts before starting treatment with baricitinib for AA and advise the patient about the possibility of this adverse effect.
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83:761-770. doi:10.1007 /s40265-023-01873-w
- US Food and Drug Administration. FDA approves first systemic treatment for alopecia areata [news release]. July 13, 2022. Accessed March 17, 2025. https://www.prnewswire.com/news-releases/fda-approves-first-systemic-treatment-for-alopecia-areata-301566884.html
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056 /NEJMoa2110343
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
To the Editor:
Alopecia areata (AA), an autoimmune disease characterized by inflammatory and nonscarring hair loss, can have a considerable impact on quality of life.1 Baricitinib is a Janus kinase inhibitor that recently was approved by the US Food and Drug Administration for treatment of severe AA in adult patients, becoming the only on-label treatment available.2 So far, the most common adverse effects reported in phase 3 trials have been acne, upper respiratory tract infections, headaches, urinary tract infections, and elevated creatine kinase levels.3
At our trichology unit in the dermatology department of a Spanish tertiary-care hospital in Seville, we have successfully used baricitinib to treat 18 patients with severe, therapy-resistant AA. Herein, we present a case of trichilemmal cyst reactivation in one of our patients following successful treatment with baricitinib.
A 53-year-old woman with a history of trichilemmal cysts presented to the dermatology department with total body hair loss of 5 years' duration that was diagnosed as AA universalis (Figure, A). The patient reported that the trichilemmal cysts had shrunk drastically 1 month after complete loss of body hair (Severity of Alopecia Tool [SALT] score, 100)(Figure, B). The largest cyst was surgically removed, and the diagnosis was histologically confirmed by a pathologist. Her mother and sister also had a history of multiple trichilemmal cysts.

The patient previously had failed treatment with oral prednisone 50 mg/d, oral cyclosporine 4 mg/kg/d, oral dexamethasone 4 mg twice weekly, and oral azathioprine 300 mg/wk. Due to the new indication of baricitinib for AA, we opted to start the patient on oral baricitinib 4 mg/d. By week 8 of treatment, she had achieved total hair regrowth (SALT score, 0). This rapid response might indicate a quick-responder phenotype, referring to a subset of patients who exhibit a fast and robust response to treatment (SALT90), generally before week 16, although more evidence is needed.
Notably, we observed the reactivation of 4 trichilemmal cysts on the scalp 6 weeks after starting baricitinib. To our knowledge, this side effect has not previously been reported. We hypothesize that reactivation of the cysts may have been due to the inhibition of the Janus kinase/signal transducer and activator of transcription pathway, which reduces the effects of cytokines and leads to reactivation of hair follicles that were inactive because of inflammation.4 As a result, the outer root sheath of the hair follicle can once again be filled with keratin, thereby reactivating the trichilemmal cysts. Based on our experience with this case, it may be relevant to consider personal and family history of trichilemmal cysts before starting treatment with baricitinib for AA and advise the patient about the possibility of this adverse effect.
To the Editor:
Alopecia areata (AA), an autoimmune disease characterized by inflammatory and nonscarring hair loss, can have a considerable impact on quality of life.1 Baricitinib is a Janus kinase inhibitor that recently was approved by the US Food and Drug Administration for treatment of severe AA in adult patients, becoming the only on-label treatment available.2 So far, the most common adverse effects reported in phase 3 trials have been acne, upper respiratory tract infections, headaches, urinary tract infections, and elevated creatine kinase levels.3
At our trichology unit in the dermatology department of a Spanish tertiary-care hospital in Seville, we have successfully used baricitinib to treat 18 patients with severe, therapy-resistant AA. Herein, we present a case of trichilemmal cyst reactivation in one of our patients following successful treatment with baricitinib.
A 53-year-old woman with a history of trichilemmal cysts presented to the dermatology department with total body hair loss of 5 years' duration that was diagnosed as AA universalis (Figure, A). The patient reported that the trichilemmal cysts had shrunk drastically 1 month after complete loss of body hair (Severity of Alopecia Tool [SALT] score, 100)(Figure, B). The largest cyst was surgically removed, and the diagnosis was histologically confirmed by a pathologist. Her mother and sister also had a history of multiple trichilemmal cysts.

The patient previously had failed treatment with oral prednisone 50 mg/d, oral cyclosporine 4 mg/kg/d, oral dexamethasone 4 mg twice weekly, and oral azathioprine 300 mg/wk. Due to the new indication of baricitinib for AA, we opted to start the patient on oral baricitinib 4 mg/d. By week 8 of treatment, she had achieved total hair regrowth (SALT score, 0). This rapid response might indicate a quick-responder phenotype, referring to a subset of patients who exhibit a fast and robust response to treatment (SALT90), generally before week 16, although more evidence is needed.
Notably, we observed the reactivation of 4 trichilemmal cysts on the scalp 6 weeks after starting baricitinib. To our knowledge, this side effect has not previously been reported. We hypothesize that reactivation of the cysts may have been due to the inhibition of the Janus kinase/signal transducer and activator of transcription pathway, which reduces the effects of cytokines and leads to reactivation of hair follicles that were inactive because of inflammation.4 As a result, the outer root sheath of the hair follicle can once again be filled with keratin, thereby reactivating the trichilemmal cysts. Based on our experience with this case, it may be relevant to consider personal and family history of trichilemmal cysts before starting treatment with baricitinib for AA and advise the patient about the possibility of this adverse effect.
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83:761-770. doi:10.1007 /s40265-023-01873-w
- US Food and Drug Administration. FDA approves first systemic treatment for alopecia areata [news release]. July 13, 2022. Accessed March 17, 2025. https://www.prnewswire.com/news-releases/fda-approves-first-systemic-treatment-for-alopecia-areata-301566884.html
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056 /NEJMoa2110343
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83:761-770. doi:10.1007 /s40265-023-01873-w
- US Food and Drug Administration. FDA approves first systemic treatment for alopecia areata [news release]. July 13, 2022. Accessed March 17, 2025. https://www.prnewswire.com/news-releases/fda-approves-first-systemic-treatment-for-alopecia-areata-301566884.html
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056 /NEJMoa2110343
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
PRACTICE POINTS
- The rapid growth of trichilemmal cysts may serve as an indicator of a quick-responder phenotype to baricitinib in cases of alopecia areata (AA), although more evidence is needed.
- It is imperative to consider personal and family history of trichilemmal cysts prior to initiating baricitinib treatment for AA.
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
Most medical students applying to dermatology residency programs in the United States will participate in an away rotation at an outside institution. Prior to COVID-19–related restrictions, 86.7% of dermatology applicants from the class of 2020 reported completing one or more away rotations for their application cycle.1,2 This requirement can be considerably costly, especially since most programs do not offer financial support for travel, living expenses, or housing during these visiting experiences.3 Underrepresented in medicine (URiM) students may be particularly disadvantaged with regard to the financial obligations that come with away rotations.4,5 Visiting scholarships for URiM students can mitigate these challenges, creating opportunities for increasing diversity in dermatology. When medical students begin the residency application process, the Visiting Student Learning Opportunities (VSLO) program of the Association of American Medical Colleges (AAMC) is the most widely used third-party service for submitting applications. For many URiM students, an unforeseen challenge when applying to dermatology residency programs is the lack of an easily accessible and up-to-date search tool to find programs that offer funding, resulting in more time spent searching and thereby complicating the application process. The VSLO released the Visiting Scholars Resources Database, a search tool that aims to compile opportunities for additional support—academic professional, and/or financial—to address this issue. Additionally, the Funded Away Rotations for Minority Medical Students (FARMS) database is an independent directory of programs that offer stipends to URiM students. In this study, we evaluated the efficacy of the VLSO’s Visiting Scholars Resources Database search tool and the FARMS database in identifying funded dermatology rotations for URiM students.
Overview of Online Search Tools
We used the AAMC’s Electronic Residency Application Service Directory to identify 141 programs offering dermatology residency positions. We then conducted a Google search using each program name with the phrase underrepresented in medicine dermatology away rotation to identify any opportunities noted in the Google results offering scholarship funding for URiM students. If there were no Google results for a webpage discussing URiM away rotation opportunities for a certain program, the individual program’s website search box was queried using the terms URiM, scholarship, and funding. If there were no relevant results, the webpages associated with the dermatology department, away rotations, and diversity and inclusion on the respective institution’s website were reviewed to confirm no indication of funded URiM opportunities. Of the 141 dermatology programs we evaluated, we identified 56 (39.7%) that offered funded away rotations for URiM students.
For comparison, we conducted a search of the VSLO’s Visiting Scholars Resources Database to identify programs that listed dermatology, all (specialties), or any (specialties) under the Specialty column that also had a financial resource for URiM students. Our search of the VSLO database yielded only 12 (21.4%) of the 56 funded away rotations we identified via our initial Google and program website search. Program listings tagged for dermatology also were retrieved from the FARMS database, of which only 17 (30.4%) of the 56 funded away rotations we previously identified were included. All queries were performed from October 24 to October 26, 2024 (Figure).
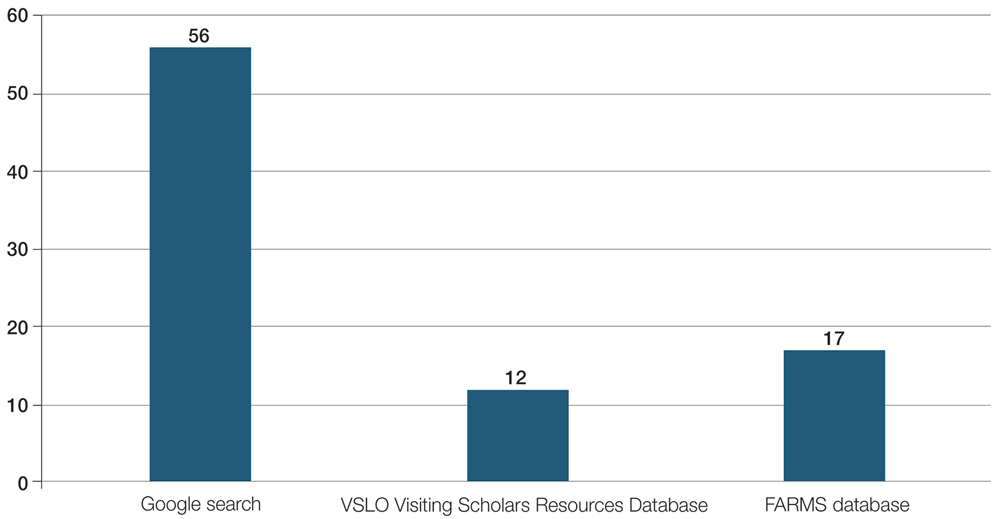
Comment
The 2023-2024 AAMC Report on Residents indicated that 54.9% (800/1455) of active US dermatology medical residents identified as White, 27.5% (400/1455) identified as Asian, 8.9% (129/1455) identified as Hispanic, and 8.7% (126/1455) identified as Black or African American.6 By comparison, 19.5% of the general US population identifies as Hispanic and 13.7% identifies as Black.7 Within the field of dermatology, the proportion of Black dermatology academic faculty in the US is estimated to comprise only 18.7% of all active Black dermatologists.8,9 With a growing population of minority US citizens, the dermatology workforce is lagging in representation across all minority populations, especially when it comes to Hispanic and Black individuals. To increase the diversity of the US dermatology workforce, residency programs must prioritize recruitment of URiM students and support their retention as future faculty.
Reports in the literature suggest that clinical grades, US Medical Licensing Examination scores, letters of recommendation/ networking, and the risk of not matching are among the primary concerns that URiM students face as potential barriers to applying for dermatology residency.4 Meanwhile, dermatology program directors ranked diversity characteristics, perceived interest in the program, personal prior knowledge of an applicant, and audition rotation in their department as important considerations for interviewing applicants.10 As a result, URiM students may have the diverse characteristics that program directors are looking for, but obtaining away rotations and establishing mentors at other institutions may be challenging due to the burden of accruing additional costs for visiting rotations.2,10,11 Other reports have indicated that expanding funded dermatology visiting rotations and promoting national programs such as the American Academy of Dermatology Diversity Mentorship Program (https://www.aad.org/member/career/awards/diversity) or the Skin of Color Society Observership Grant (https://skinofcolorsociety.org/what-we-do/mentorship/observership-grant) can be alternative routes for mentorship and networking.3
Our review demonstrated that, of the 141 dermatology residency programs we identified, only around 40% offer funded rotations for URiM students; however, the current databases that applicants use to find these opportunities do not adequately present the number of available options. A search of the VSLO database—the most widely used third-party database for applying to dermatology away rotations—yielded only 12 (21.4%) of the rotations that we identified in our initial Google search. Similarly, a search of the FARMS database yielded only 17 (30.4%) of the dermatology rotations we previously identified. Aside from missing more than half of the available funded dermatology away rotations, the search process was further complicated by the reliance of the 2 databases on user input rather than presenting all programs offering funded opportunities for dermatology applicants without the need to enter additional information. As of October 26, 2024, there were only 22 inputs for Visiting Scholars Resources across all specialties and programs in the VLSO system.
Our findings indicate a clear need for a reliable and accurate database that captures all funded dermatology rotations for prospective URiM applicants because of the strong emphasis on visiting rotations for application success. Our team created a Google spreadsheet compiling dermatology visiting student health equity and inclusion scholarships from inputs we found in our search. We shared this resource via the Association of Professors of Dermatology listserve so program members could verify the opportunities we compiled to create an accurate and updated resource for finding funded dermatology rotations. The program verification process was conducted by having residency program directors or their respective program coordinators mark “yes” on the spreadsheet to confirm the funded rotation is being offered by their program. Our spreadsheet will continue to be updated yearly through cooperation with participating programs to verify their funded electives and through partnership with the AAMC to include our database in their Visiting Students Resources Database that will be released each year within VLSO as applications open for the following season.
The main limitation of our review is that we presume the information provided in the VSLO and FARMS databases has not changed or been updated to include more programs since our initial search period. Additionally, the information available on dermatology residency program websites limits the data on the total programs obtained, as some website links may not be updated or may be invalid for online web user access. The benefit to creating and continually updating our Dermatology Visiting Student Health Equity and Inclusion Scholarship Database spreadsheet will be to ensure that programs regularly verify their offered funded electives and capture the true total of funded rotations offered for URiM students across the country. We also acknowledge that we did not investigate how URiM student attendance at funded rotations affected their outcomes in matching dermatology programs for residency; however, given the importance of away rotations, which positively influence the ability of URiM students to receive interviews, it is understood that these opportunities are viewed as widely beneficial.
Final Thoughts
The current online search tools that URiM students can use to find funded away rotations in dermatology exclude many of the available opportunities. We aimed to provide an updated and centralized resource for students via the shared spreadsheet we created for residency program directors, but further measures to centralize the most up-to-date information on visiting programs offering scholarships to URiM students would be beneficial.

- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540. doi:10.1016/j.clindermatol.2022.05.001
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 1, 2024. https://students-residents.aamc.org/media/9496/download
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis. J Am Acad Dermatol. 2023;88: 941-943. doi:10.1016/j.jaad.2022.11.018
- Chen A, Shinkai K. Rethinking how we select dermatology applicants —turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001 /jamadermatol.2016.4683
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001 /jamadermatol.2018.4813
- Association of American Medical Colleges. Table B5. Number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. 2023-24 active residents. Accessed March 8, 2025. https://www.aamc.org/data-reports/students-residents/data/report-residents/2024/table-b5-md-residents-race-ethnicity-and-specialty
- United States Census Bureau. QuickFacts: United States. population estimates, July 1, 2024 (V2024). Accessed February 27, 2025. https://www.census.gov/quickfacts/fact/table/US/PST045221
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Gonzalez S, Syder N, Mckenzie SA, et al. Racial diversity in academic dermatology: a cross-sectional analysis of Black academic dermatology faculty in the United States. J Am Acad Dermatol. 2024;90:182-184. doi:10.1016/j.jaad.2023.09.027
- National Resident Matching Program, Data Release and Research Committee. Results of the 2021 NRMP Program Director Survey, 2021. August 2021. Accessed March 9, 2025. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186 /s12909-016-0805-z
Most medical students applying to dermatology residency programs in the United States will participate in an away rotation at an outside institution. Prior to COVID-19–related restrictions, 86.7% of dermatology applicants from the class of 2020 reported completing one or more away rotations for their application cycle.1,2 This requirement can be considerably costly, especially since most programs do not offer financial support for travel, living expenses, or housing during these visiting experiences.3 Underrepresented in medicine (URiM) students may be particularly disadvantaged with regard to the financial obligations that come with away rotations.4,5 Visiting scholarships for URiM students can mitigate these challenges, creating opportunities for increasing diversity in dermatology. When medical students begin the residency application process, the Visiting Student Learning Opportunities (VSLO) program of the Association of American Medical Colleges (AAMC) is the most widely used third-party service for submitting applications. For many URiM students, an unforeseen challenge when applying to dermatology residency programs is the lack of an easily accessible and up-to-date search tool to find programs that offer funding, resulting in more time spent searching and thereby complicating the application process. The VSLO released the Visiting Scholars Resources Database, a search tool that aims to compile opportunities for additional support—academic professional, and/or financial—to address this issue. Additionally, the Funded Away Rotations for Minority Medical Students (FARMS) database is an independent directory of programs that offer stipends to URiM students. In this study, we evaluated the efficacy of the VLSO’s Visiting Scholars Resources Database search tool and the FARMS database in identifying funded dermatology rotations for URiM students.
Overview of Online Search Tools
We used the AAMC’s Electronic Residency Application Service Directory to identify 141 programs offering dermatology residency positions. We then conducted a Google search using each program name with the phrase underrepresented in medicine dermatology away rotation to identify any opportunities noted in the Google results offering scholarship funding for URiM students. If there were no Google results for a webpage discussing URiM away rotation opportunities for a certain program, the individual program’s website search box was queried using the terms URiM, scholarship, and funding. If there were no relevant results, the webpages associated with the dermatology department, away rotations, and diversity and inclusion on the respective institution’s website were reviewed to confirm no indication of funded URiM opportunities. Of the 141 dermatology programs we evaluated, we identified 56 (39.7%) that offered funded away rotations for URiM students.
For comparison, we conducted a search of the VSLO’s Visiting Scholars Resources Database to identify programs that listed dermatology, all (specialties), or any (specialties) under the Specialty column that also had a financial resource for URiM students. Our search of the VSLO database yielded only 12 (21.4%) of the 56 funded away rotations we identified via our initial Google and program website search. Program listings tagged for dermatology also were retrieved from the FARMS database, of which only 17 (30.4%) of the 56 funded away rotations we previously identified were included. All queries were performed from October 24 to October 26, 2024 (Figure).

Comment
The 2023-2024 AAMC Report on Residents indicated that 54.9% (800/1455) of active US dermatology medical residents identified as White, 27.5% (400/1455) identified as Asian, 8.9% (129/1455) identified as Hispanic, and 8.7% (126/1455) identified as Black or African American.6 By comparison, 19.5% of the general US population identifies as Hispanic and 13.7% identifies as Black.7 Within the field of dermatology, the proportion of Black dermatology academic faculty in the US is estimated to comprise only 18.7% of all active Black dermatologists.8,9 With a growing population of minority US citizens, the dermatology workforce is lagging in representation across all minority populations, especially when it comes to Hispanic and Black individuals. To increase the diversity of the US dermatology workforce, residency programs must prioritize recruitment of URiM students and support their retention as future faculty.
Reports in the literature suggest that clinical grades, US Medical Licensing Examination scores, letters of recommendation/ networking, and the risk of not matching are among the primary concerns that URiM students face as potential barriers to applying for dermatology residency.4 Meanwhile, dermatology program directors ranked diversity characteristics, perceived interest in the program, personal prior knowledge of an applicant, and audition rotation in their department as important considerations for interviewing applicants.10 As a result, URiM students may have the diverse characteristics that program directors are looking for, but obtaining away rotations and establishing mentors at other institutions may be challenging due to the burden of accruing additional costs for visiting rotations.2,10,11 Other reports have indicated that expanding funded dermatology visiting rotations and promoting national programs such as the American Academy of Dermatology Diversity Mentorship Program (https://www.aad.org/member/career/awards/diversity) or the Skin of Color Society Observership Grant (https://skinofcolorsociety.org/what-we-do/mentorship/observership-grant) can be alternative routes for mentorship and networking.3
Our review demonstrated that, of the 141 dermatology residency programs we identified, only around 40% offer funded rotations for URiM students; however, the current databases that applicants use to find these opportunities do not adequately present the number of available options. A search of the VSLO database—the most widely used third-party database for applying to dermatology away rotations—yielded only 12 (21.4%) of the rotations that we identified in our initial Google search. Similarly, a search of the FARMS database yielded only 17 (30.4%) of the dermatology rotations we previously identified. Aside from missing more than half of the available funded dermatology away rotations, the search process was further complicated by the reliance of the 2 databases on user input rather than presenting all programs offering funded opportunities for dermatology applicants without the need to enter additional information. As of October 26, 2024, there were only 22 inputs for Visiting Scholars Resources across all specialties and programs in the VLSO system.
Our findings indicate a clear need for a reliable and accurate database that captures all funded dermatology rotations for prospective URiM applicants because of the strong emphasis on visiting rotations for application success. Our team created a Google spreadsheet compiling dermatology visiting student health equity and inclusion scholarships from inputs we found in our search. We shared this resource via the Association of Professors of Dermatology listserve so program members could verify the opportunities we compiled to create an accurate and updated resource for finding funded dermatology rotations. The program verification process was conducted by having residency program directors or their respective program coordinators mark “yes” on the spreadsheet to confirm the funded rotation is being offered by their program. Our spreadsheet will continue to be updated yearly through cooperation with participating programs to verify their funded electives and through partnership with the AAMC to include our database in their Visiting Students Resources Database that will be released each year within VLSO as applications open for the following season.
The main limitation of our review is that we presume the information provided in the VSLO and FARMS databases has not changed or been updated to include more programs since our initial search period. Additionally, the information available on dermatology residency program websites limits the data on the total programs obtained, as some website links may not be updated or may be invalid for online web user access. The benefit to creating and continually updating our Dermatology Visiting Student Health Equity and Inclusion Scholarship Database spreadsheet will be to ensure that programs regularly verify their offered funded electives and capture the true total of funded rotations offered for URiM students across the country. We also acknowledge that we did not investigate how URiM student attendance at funded rotations affected their outcomes in matching dermatology programs for residency; however, given the importance of away rotations, which positively influence the ability of URiM students to receive interviews, it is understood that these opportunities are viewed as widely beneficial.
Final Thoughts
The current online search tools that URiM students can use to find funded away rotations in dermatology exclude many of the available opportunities. We aimed to provide an updated and centralized resource for students via the shared spreadsheet we created for residency program directors, but further measures to centralize the most up-to-date information on visiting programs offering scholarships to URiM students would be beneficial.

Most medical students applying to dermatology residency programs in the United States will participate in an away rotation at an outside institution. Prior to COVID-19–related restrictions, 86.7% of dermatology applicants from the class of 2020 reported completing one or more away rotations for their application cycle.1,2 This requirement can be considerably costly, especially since most programs do not offer financial support for travel, living expenses, or housing during these visiting experiences.3 Underrepresented in medicine (URiM) students may be particularly disadvantaged with regard to the financial obligations that come with away rotations.4,5 Visiting scholarships for URiM students can mitigate these challenges, creating opportunities for increasing diversity in dermatology. When medical students begin the residency application process, the Visiting Student Learning Opportunities (VSLO) program of the Association of American Medical Colleges (AAMC) is the most widely used third-party service for submitting applications. For many URiM students, an unforeseen challenge when applying to dermatology residency programs is the lack of an easily accessible and up-to-date search tool to find programs that offer funding, resulting in more time spent searching and thereby complicating the application process. The VSLO released the Visiting Scholars Resources Database, a search tool that aims to compile opportunities for additional support—academic professional, and/or financial—to address this issue. Additionally, the Funded Away Rotations for Minority Medical Students (FARMS) database is an independent directory of programs that offer stipends to URiM students. In this study, we evaluated the efficacy of the VLSO’s Visiting Scholars Resources Database search tool and the FARMS database in identifying funded dermatology rotations for URiM students.
Overview of Online Search Tools
We used the AAMC’s Electronic Residency Application Service Directory to identify 141 programs offering dermatology residency positions. We then conducted a Google search using each program name with the phrase underrepresented in medicine dermatology away rotation to identify any opportunities noted in the Google results offering scholarship funding for URiM students. If there were no Google results for a webpage discussing URiM away rotation opportunities for a certain program, the individual program’s website search box was queried using the terms URiM, scholarship, and funding. If there were no relevant results, the webpages associated with the dermatology department, away rotations, and diversity and inclusion on the respective institution’s website were reviewed to confirm no indication of funded URiM opportunities. Of the 141 dermatology programs we evaluated, we identified 56 (39.7%) that offered funded away rotations for URiM students.
For comparison, we conducted a search of the VSLO’s Visiting Scholars Resources Database to identify programs that listed dermatology, all (specialties), or any (specialties) under the Specialty column that also had a financial resource for URiM students. Our search of the VSLO database yielded only 12 (21.4%) of the 56 funded away rotations we identified via our initial Google and program website search. Program listings tagged for dermatology also were retrieved from the FARMS database, of which only 17 (30.4%) of the 56 funded away rotations we previously identified were included. All queries were performed from October 24 to October 26, 2024 (Figure).

Comment
The 2023-2024 AAMC Report on Residents indicated that 54.9% (800/1455) of active US dermatology medical residents identified as White, 27.5% (400/1455) identified as Asian, 8.9% (129/1455) identified as Hispanic, and 8.7% (126/1455) identified as Black or African American.6 By comparison, 19.5% of the general US population identifies as Hispanic and 13.7% identifies as Black.7 Within the field of dermatology, the proportion of Black dermatology academic faculty in the US is estimated to comprise only 18.7% of all active Black dermatologists.8,9 With a growing population of minority US citizens, the dermatology workforce is lagging in representation across all minority populations, especially when it comes to Hispanic and Black individuals. To increase the diversity of the US dermatology workforce, residency programs must prioritize recruitment of URiM students and support their retention as future faculty.
Reports in the literature suggest that clinical grades, US Medical Licensing Examination scores, letters of recommendation/ networking, and the risk of not matching are among the primary concerns that URiM students face as potential barriers to applying for dermatology residency.4 Meanwhile, dermatology program directors ranked diversity characteristics, perceived interest in the program, personal prior knowledge of an applicant, and audition rotation in their department as important considerations for interviewing applicants.10 As a result, URiM students may have the diverse characteristics that program directors are looking for, but obtaining away rotations and establishing mentors at other institutions may be challenging due to the burden of accruing additional costs for visiting rotations.2,10,11 Other reports have indicated that expanding funded dermatology visiting rotations and promoting national programs such as the American Academy of Dermatology Diversity Mentorship Program (https://www.aad.org/member/career/awards/diversity) or the Skin of Color Society Observership Grant (https://skinofcolorsociety.org/what-we-do/mentorship/observership-grant) can be alternative routes for mentorship and networking.3
Our review demonstrated that, of the 141 dermatology residency programs we identified, only around 40% offer funded rotations for URiM students; however, the current databases that applicants use to find these opportunities do not adequately present the number of available options. A search of the VSLO database—the most widely used third-party database for applying to dermatology away rotations—yielded only 12 (21.4%) of the rotations that we identified in our initial Google search. Similarly, a search of the FARMS database yielded only 17 (30.4%) of the dermatology rotations we previously identified. Aside from missing more than half of the available funded dermatology away rotations, the search process was further complicated by the reliance of the 2 databases on user input rather than presenting all programs offering funded opportunities for dermatology applicants without the need to enter additional information. As of October 26, 2024, there were only 22 inputs for Visiting Scholars Resources across all specialties and programs in the VLSO system.
Our findings indicate a clear need for a reliable and accurate database that captures all funded dermatology rotations for prospective URiM applicants because of the strong emphasis on visiting rotations for application success. Our team created a Google spreadsheet compiling dermatology visiting student health equity and inclusion scholarships from inputs we found in our search. We shared this resource via the Association of Professors of Dermatology listserve so program members could verify the opportunities we compiled to create an accurate and updated resource for finding funded dermatology rotations. The program verification process was conducted by having residency program directors or their respective program coordinators mark “yes” on the spreadsheet to confirm the funded rotation is being offered by their program. Our spreadsheet will continue to be updated yearly through cooperation with participating programs to verify their funded electives and through partnership with the AAMC to include our database in their Visiting Students Resources Database that will be released each year within VLSO as applications open for the following season.
The main limitation of our review is that we presume the information provided in the VSLO and FARMS databases has not changed or been updated to include more programs since our initial search period. Additionally, the information available on dermatology residency program websites limits the data on the total programs obtained, as some website links may not be updated or may be invalid for online web user access. The benefit to creating and continually updating our Dermatology Visiting Student Health Equity and Inclusion Scholarship Database spreadsheet will be to ensure that programs regularly verify their offered funded electives and capture the true total of funded rotations offered for URiM students across the country. We also acknowledge that we did not investigate how URiM student attendance at funded rotations affected their outcomes in matching dermatology programs for residency; however, given the importance of away rotations, which positively influence the ability of URiM students to receive interviews, it is understood that these opportunities are viewed as widely beneficial.
Final Thoughts
The current online search tools that URiM students can use to find funded away rotations in dermatology exclude many of the available opportunities. We aimed to provide an updated and centralized resource for students via the shared spreadsheet we created for residency program directors, but further measures to centralize the most up-to-date information on visiting programs offering scholarships to URiM students would be beneficial.

- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540. doi:10.1016/j.clindermatol.2022.05.001
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 1, 2024. https://students-residents.aamc.org/media/9496/download
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis. J Am Acad Dermatol. 2023;88: 941-943. doi:10.1016/j.jaad.2022.11.018
- Chen A, Shinkai K. Rethinking how we select dermatology applicants —turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001 /jamadermatol.2016.4683
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001 /jamadermatol.2018.4813
- Association of American Medical Colleges. Table B5. Number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. 2023-24 active residents. Accessed March 8, 2025. https://www.aamc.org/data-reports/students-residents/data/report-residents/2024/table-b5-md-residents-race-ethnicity-and-specialty
- United States Census Bureau. QuickFacts: United States. population estimates, July 1, 2024 (V2024). Accessed February 27, 2025. https://www.census.gov/quickfacts/fact/table/US/PST045221
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Gonzalez S, Syder N, Mckenzie SA, et al. Racial diversity in academic dermatology: a cross-sectional analysis of Black academic dermatology faculty in the United States. J Am Acad Dermatol. 2024;90:182-184. doi:10.1016/j.jaad.2023.09.027
- National Resident Matching Program, Data Release and Research Committee. Results of the 2021 NRMP Program Director Survey, 2021. August 2021. Accessed March 9, 2025. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186 /s12909-016-0805-z
- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540. doi:10.1016/j.clindermatol.2022.05.001
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 1, 2024. https://students-residents.aamc.org/media/9496/download
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis. J Am Acad Dermatol. 2023;88: 941-943. doi:10.1016/j.jaad.2022.11.018
- Chen A, Shinkai K. Rethinking how we select dermatology applicants —turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001 /jamadermatol.2016.4683
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001 /jamadermatol.2018.4813
- Association of American Medical Colleges. Table B5. Number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. 2023-24 active residents. Accessed March 8, 2025. https://www.aamc.org/data-reports/students-residents/data/report-residents/2024/table-b5-md-residents-race-ethnicity-and-specialty
- United States Census Bureau. QuickFacts: United States. population estimates, July 1, 2024 (V2024). Accessed February 27, 2025. https://www.census.gov/quickfacts/fact/table/US/PST045221
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Gonzalez S, Syder N, Mckenzie SA, et al. Racial diversity in academic dermatology: a cross-sectional analysis of Black academic dermatology faculty in the United States. J Am Acad Dermatol. 2024;90:182-184. doi:10.1016/j.jaad.2023.09.027
- National Resident Matching Program, Data Release and Research Committee. Results of the 2021 NRMP Program Director Survey, 2021. August 2021. Accessed March 9, 2025. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186 /s12909-016-0805-z
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
PRACTICE POINTS
- Many funded away rotations are not listed on the most widely used databases for applying to dermatology residency programs.
- Underrepresented in medicine students who are seeking funded dermatology away rotations would benefit from a centralized, comprehensive, and well-organized database to improve equity of opportunity in the dermatology rotation application search process and further diversify the specialty.
- There are limited data assessing outcomes associated with participation in funded rotation and residency match outcomes.
Plaque With Central Ulceration on the Abdomen
Plaque With Central Ulceration on the Abdomen
THE DIAGNOSIS: Plaquelike Myofibroblastic Tumor
An incisional biopsy of the plaque demonstrated a hypercellular proliferation of bland spindle cells in the dermis that infiltrated the subcutis. The overlying epidermis was mildly acanthotic with both ulceration and follicular induction. There was trapping of individual adipocytes in a honeycomb pattern with foci of erythrocyte extravasation, microvesiculation, and widened fibrous septa (Figure 1). Immunohistochemistry was positive for vimentin, actin, and smooth muscle actin (SMA)(Figure 2A). Variable positivity for Factor XIIIa antibodies was noted. CD68 staining was focal positive, suggesting fibrohistiocytic lineage. Expression of CD31, CD34, S100, and anaplastic lymphoma kinase was negative, and Ki-67 was present in less than 10% of cells (Figure 2B).
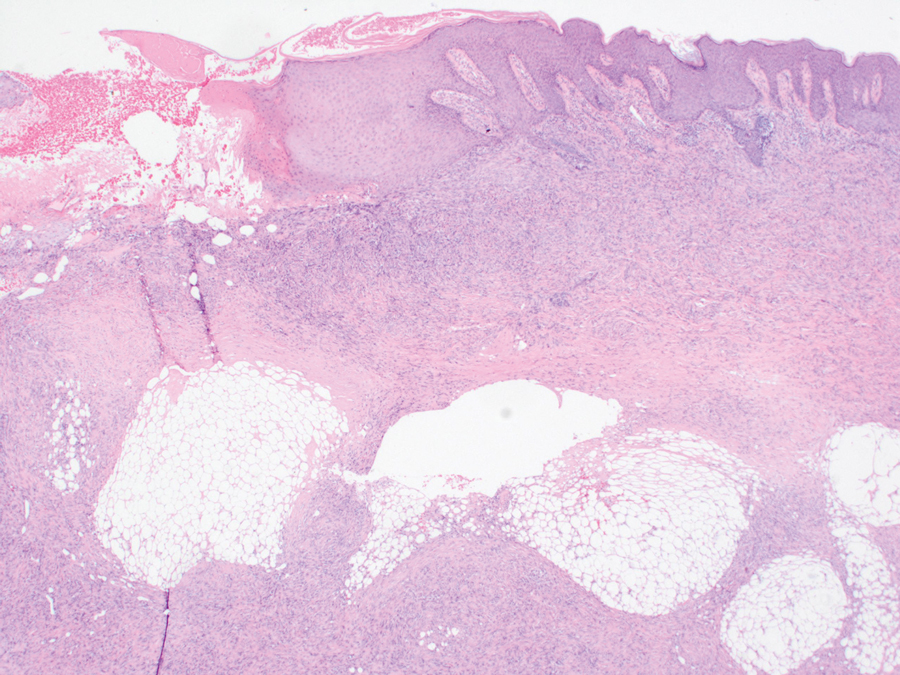
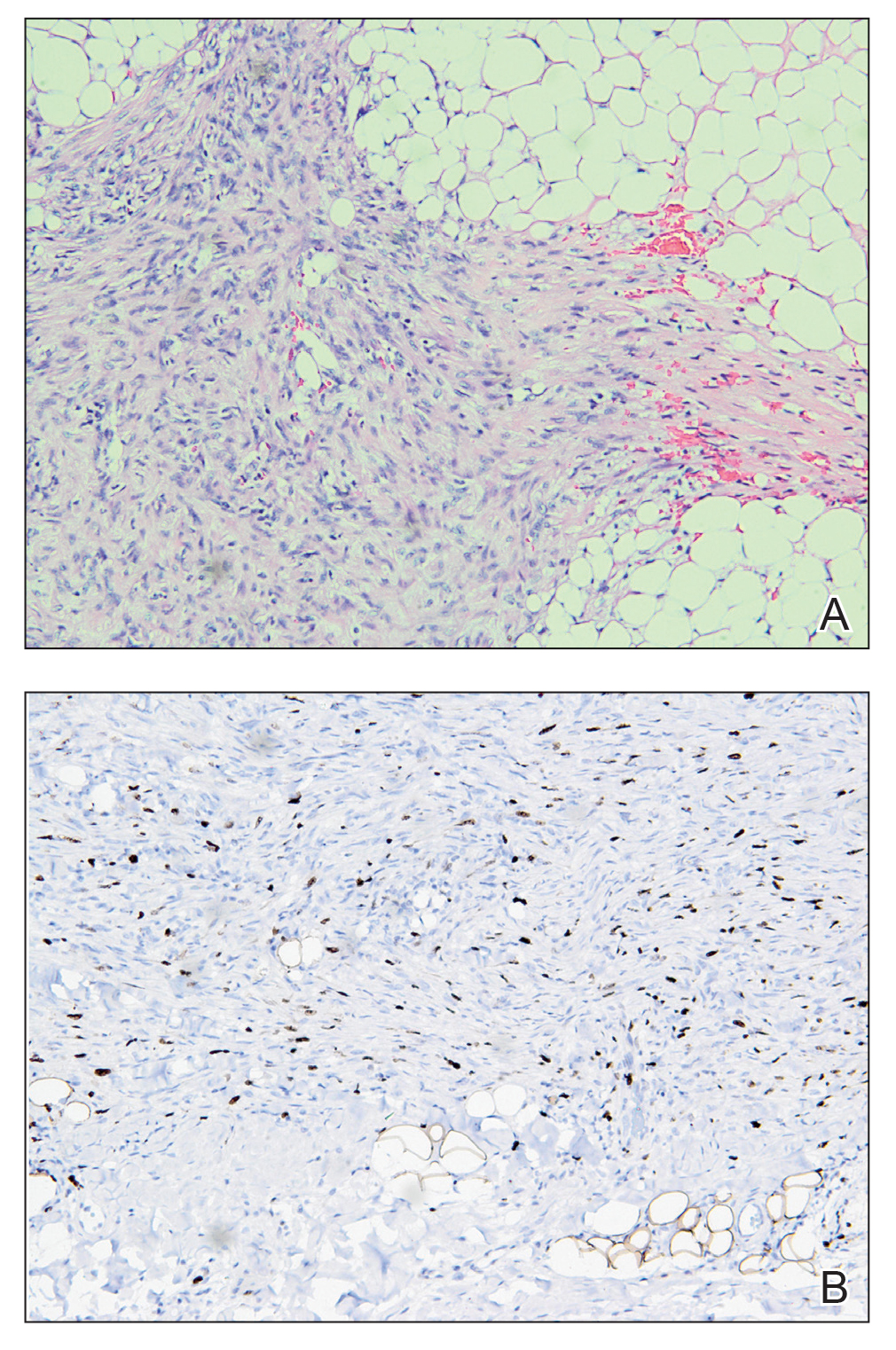
We reviewed the case in conjunction with a soft-tissue pathologist (Y.L.), and based on the clinical and immunophenotypic features, a diagnosis of plaquelike myofibroblastic tumor (PLMT) was made. The patient’s parents refused further treatment, and there was no sign of disease progression at 6-month follow-up.
Plaquelike myofibroblastic tumor is an unusual pediatric dermal tumor that was first described by Clarke et al1 in 2007. Clinical manifestation of PLMT on the right abdomen was unique in our patient, as the lesions typically present as indurated plaques on the lower back, but the central ulceration in our case resembled a report by Marqueling et al.2 Ulceration and induration of PLMT developing at 8 months of age can suggest an aggressive disease course corresponding with deep infiltration and is seen mostly in children.
The histopathologic features of PLMT include an acanthotic epidermis and follicular induction, which also are characteristic of dermatofibroma (DF). The proliferation of spindle cells extended deep into the fat with foci of erythrocyte extravasation and microvesiculation of the stroma similar to nodular fasciitis and proliferative fasciitis. The presentation of infiltrating and expanding fibrous septae and trapping of individual adipocytes in a honeycomb pattern is similar to dermatofibrosarcoma protuberans (DFSP). Most cases of PLMT are positive for SMA. Factor XIIIa typically is variably positive, and in one report, 31% (4/13) of cases showed positive staining for calponin.3 Rapid growth, ulceration, and recurrence emphasize that PLMT can be locally aggressive, similar to DFSP.4
The main differential diagnoses include DF and its variants, dermatomyofibroma, DFSP, and proliferative fasciitis.3,5 In the cases mentioned above, microscopic features were similar with a relatively well-circumscribed proliferation of spindle cells arranged in short fascicles through the entire reticular dermis, and the overlying epidermis was acanthotic.
Dermatofibroma commonly manifests in adults as a minor nodular lesion (commonly <1 cm), and usually is located on the legs. It has several clinical and histologic variants, including multiple clustered DF (MCDF)—a rare condition that has been reported in children and young adults and generally appears in the first and second decades of life. Of the reported cases of MCDF, immunohistochemical staining for SMA was performed in 8 cases. All these cases showed negative or minimal staining.3-5 Smooth muscle actin staining in DFs is negative, or weak and patchy, unlike in PLMT where it is diffuse, uniform, and strong.
Dermatofibrosarcoma protuberans typically occurs in young adults and manifests as dermal and subcutaneous nodular/multinodular or plaquelike masses, with rare congenital cases. Immunohistochemical staining for CD34, which typically is firmly and diffusely positive, is the most reliable marker of DFSP.6 Factor XIIIA in DFSP typically is negative for focal staining, mainly at periphery or in scattered dendritic cells. The prognosis of DFSP generally is excellent, with local recurrences in up to 30% of cases and extremely low metastatic potential (essentially only in cases with fibrosarcomatous transformation).6 Dermatomyofibroma is another rare benign dermal myofibroblastic tumor that typically manifests with indurated hyperpigmented or erythematous plaques or nodules on the shoulders and torso.6 This condition occurs mainly in adolescents and young adults, unlike PLMT. The most striking features of dermatomyofibroma are the horizontal orientation of the spindle cell nuclei and the pattern of the proliferation concerning the adnexal structures, especially hair follicles. The hair follicles have a normal appearance, and the proliferation extends up to each follicle, then continues to the other side without any displacement of the follicle. Tumor cells are variably positive for SMA in dermatomyofibromas and are negative for muscle-specific actin, desmin, S100, CD34, and Factor XIIIA.6
Immunohistochemistry can be very useful in differentiating PLMT from other conditions. Neoplastic cells stain positively for CD34 but not for Factor XIIIa and SMA in cases of DFSP. Dermatofibroma and its variants always present with collagen trapping at the periphery of the lesions and may demonstrate foamy macrophages, hemosiderin, or plasma cells FXIIIA(+), CD34(-), and variable SMA reactivity. This positivity usually is less prominent in DF than in PLMT. Neoplastic cells in dermatomyofibroma often stain positive for calponin, but only focally for SMA. The clinical features of dermatomyofibroma include early onset, large size, multiple nodules, and plaquelike morphology. Moulonguet et al4 hypothesized that, although MCDF and PLMT appear to show some distinctive clinical and histologic features, they also show similarities that could suggest they form part of the myofibroblastic spectrum. Furthermore, Moradi et al7 also considered them as part of the same disease spectrum because of their overlapping clinical, histologic, and immunohistochemical features.
The microscopic features in our case are notable, as the lesion demonstrated overlying acanthosis and follicular induction, resembling DF. The stroma contained microvesicular changes and erythrocyte extravasation, characteristic of nodular or proliferative fasciitis. Additionally, densely packed spindle cells infiltrated deep into the subcutaneous adipose tissue, similar to DFSP.2,3 Our findings expand on the reported histopathologic spectrum of this tumor to date.
- Clarke JT, Clarke LE, Miller C, et al. Plaque-like myofibroblastic tumor of infancy. Pediatr Dermatol. 2007;24:E83-E87. doi:10.1111 /j.1525-1470.2007.00449.x
- Marqueling AL, Dasher D, Friedlander SF, et al. Plaque-like myofibroblastic tumor: report of three cases. Pediatr Dermatol. 2013;30:600-607. doi:10.1111/pde.12185
- Sekar T, Mushtaq J, AlBadry W, et al. Plaque-like myofibroblastic tumor: a series of 2 cases of this unusual dermal tumor which occurs in infancy and early childhood. Pediatr Dev Pathol. 2018;21:444-448. doi: 10.1177/1093526617746807
- Moulonguet I, Biaggi A, Eschard C, et al. Plaque-like myofibroblastic tumor: report of 4 cases. Am J Dermatopathol. 2017;39:767-772. doi: 10.1097/DAD.0000000000000869
- Virdi A, Baraldi C, Barisani A, et al. Plaque-like myofibroblastic tumor, a rare entity of childhood: possible pitfalls in differential diagnosis. J Cutan Pathol. 2019;46:389-392. doi:10.1111/cup.13441
- Cassarino DS. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2021.
- Moradi S, Mnayer L, Earle J, et al. Plaque-like dermatofibroma: case report of a rare entity. Dermatopathology (Basel). 2021;8:337-341. doi:10.3390/dermatopathology8030038
THE DIAGNOSIS: Plaquelike Myofibroblastic Tumor
An incisional biopsy of the plaque demonstrated a hypercellular proliferation of bland spindle cells in the dermis that infiltrated the subcutis. The overlying epidermis was mildly acanthotic with both ulceration and follicular induction. There was trapping of individual adipocytes in a honeycomb pattern with foci of erythrocyte extravasation, microvesiculation, and widened fibrous septa (Figure 1). Immunohistochemistry was positive for vimentin, actin, and smooth muscle actin (SMA)(Figure 2A). Variable positivity for Factor XIIIa antibodies was noted. CD68 staining was focal positive, suggesting fibrohistiocytic lineage. Expression of CD31, CD34, S100, and anaplastic lymphoma kinase was negative, and Ki-67 was present in less than 10% of cells (Figure 2B).


We reviewed the case in conjunction with a soft-tissue pathologist (Y.L.), and based on the clinical and immunophenotypic features, a diagnosis of plaquelike myofibroblastic tumor (PLMT) was made. The patient’s parents refused further treatment, and there was no sign of disease progression at 6-month follow-up.
Plaquelike myofibroblastic tumor is an unusual pediatric dermal tumor that was first described by Clarke et al1 in 2007. Clinical manifestation of PLMT on the right abdomen was unique in our patient, as the lesions typically present as indurated plaques on the lower back, but the central ulceration in our case resembled a report by Marqueling et al.2 Ulceration and induration of PLMT developing at 8 months of age can suggest an aggressive disease course corresponding with deep infiltration and is seen mostly in children.
The histopathologic features of PLMT include an acanthotic epidermis and follicular induction, which also are characteristic of dermatofibroma (DF). The proliferation of spindle cells extended deep into the fat with foci of erythrocyte extravasation and microvesiculation of the stroma similar to nodular fasciitis and proliferative fasciitis. The presentation of infiltrating and expanding fibrous septae and trapping of individual adipocytes in a honeycomb pattern is similar to dermatofibrosarcoma protuberans (DFSP). Most cases of PLMT are positive for SMA. Factor XIIIa typically is variably positive, and in one report, 31% (4/13) of cases showed positive staining for calponin.3 Rapid growth, ulceration, and recurrence emphasize that PLMT can be locally aggressive, similar to DFSP.4
The main differential diagnoses include DF and its variants, dermatomyofibroma, DFSP, and proliferative fasciitis.3,5 In the cases mentioned above, microscopic features were similar with a relatively well-circumscribed proliferation of spindle cells arranged in short fascicles through the entire reticular dermis, and the overlying epidermis was acanthotic.
Dermatofibroma commonly manifests in adults as a minor nodular lesion (commonly <1 cm), and usually is located on the legs. It has several clinical and histologic variants, including multiple clustered DF (MCDF)—a rare condition that has been reported in children and young adults and generally appears in the first and second decades of life. Of the reported cases of MCDF, immunohistochemical staining for SMA was performed in 8 cases. All these cases showed negative or minimal staining.3-5 Smooth muscle actin staining in DFs is negative, or weak and patchy, unlike in PLMT where it is diffuse, uniform, and strong.
Dermatofibrosarcoma protuberans typically occurs in young adults and manifests as dermal and subcutaneous nodular/multinodular or plaquelike masses, with rare congenital cases. Immunohistochemical staining for CD34, which typically is firmly and diffusely positive, is the most reliable marker of DFSP.6 Factor XIIIA in DFSP typically is negative for focal staining, mainly at periphery or in scattered dendritic cells. The prognosis of DFSP generally is excellent, with local recurrences in up to 30% of cases and extremely low metastatic potential (essentially only in cases with fibrosarcomatous transformation).6 Dermatomyofibroma is another rare benign dermal myofibroblastic tumor that typically manifests with indurated hyperpigmented or erythematous plaques or nodules on the shoulders and torso.6 This condition occurs mainly in adolescents and young adults, unlike PLMT. The most striking features of dermatomyofibroma are the horizontal orientation of the spindle cell nuclei and the pattern of the proliferation concerning the adnexal structures, especially hair follicles. The hair follicles have a normal appearance, and the proliferation extends up to each follicle, then continues to the other side without any displacement of the follicle. Tumor cells are variably positive for SMA in dermatomyofibromas and are negative for muscle-specific actin, desmin, S100, CD34, and Factor XIIIA.6
Immunohistochemistry can be very useful in differentiating PLMT from other conditions. Neoplastic cells stain positively for CD34 but not for Factor XIIIa and SMA in cases of DFSP. Dermatofibroma and its variants always present with collagen trapping at the periphery of the lesions and may demonstrate foamy macrophages, hemosiderin, or plasma cells FXIIIA(+), CD34(-), and variable SMA reactivity. This positivity usually is less prominent in DF than in PLMT. Neoplastic cells in dermatomyofibroma often stain positive for calponin, but only focally for SMA. The clinical features of dermatomyofibroma include early onset, large size, multiple nodules, and plaquelike morphology. Moulonguet et al4 hypothesized that, although MCDF and PLMT appear to show some distinctive clinical and histologic features, they also show similarities that could suggest they form part of the myofibroblastic spectrum. Furthermore, Moradi et al7 also considered them as part of the same disease spectrum because of their overlapping clinical, histologic, and immunohistochemical features.
The microscopic features in our case are notable, as the lesion demonstrated overlying acanthosis and follicular induction, resembling DF. The stroma contained microvesicular changes and erythrocyte extravasation, characteristic of nodular or proliferative fasciitis. Additionally, densely packed spindle cells infiltrated deep into the subcutaneous adipose tissue, similar to DFSP.2,3 Our findings expand on the reported histopathologic spectrum of this tumor to date.
THE DIAGNOSIS: Plaquelike Myofibroblastic Tumor
An incisional biopsy of the plaque demonstrated a hypercellular proliferation of bland spindle cells in the dermis that infiltrated the subcutis. The overlying epidermis was mildly acanthotic with both ulceration and follicular induction. There was trapping of individual adipocytes in a honeycomb pattern with foci of erythrocyte extravasation, microvesiculation, and widened fibrous septa (Figure 1). Immunohistochemistry was positive for vimentin, actin, and smooth muscle actin (SMA)(Figure 2A). Variable positivity for Factor XIIIa antibodies was noted. CD68 staining was focal positive, suggesting fibrohistiocytic lineage. Expression of CD31, CD34, S100, and anaplastic lymphoma kinase was negative, and Ki-67 was present in less than 10% of cells (Figure 2B).


We reviewed the case in conjunction with a soft-tissue pathologist (Y.L.), and based on the clinical and immunophenotypic features, a diagnosis of plaquelike myofibroblastic tumor (PLMT) was made. The patient’s parents refused further treatment, and there was no sign of disease progression at 6-month follow-up.
Plaquelike myofibroblastic tumor is an unusual pediatric dermal tumor that was first described by Clarke et al1 in 2007. Clinical manifestation of PLMT on the right abdomen was unique in our patient, as the lesions typically present as indurated plaques on the lower back, but the central ulceration in our case resembled a report by Marqueling et al.2 Ulceration and induration of PLMT developing at 8 months of age can suggest an aggressive disease course corresponding with deep infiltration and is seen mostly in children.
The histopathologic features of PLMT include an acanthotic epidermis and follicular induction, which also are characteristic of dermatofibroma (DF). The proliferation of spindle cells extended deep into the fat with foci of erythrocyte extravasation and microvesiculation of the stroma similar to nodular fasciitis and proliferative fasciitis. The presentation of infiltrating and expanding fibrous septae and trapping of individual adipocytes in a honeycomb pattern is similar to dermatofibrosarcoma protuberans (DFSP). Most cases of PLMT are positive for SMA. Factor XIIIa typically is variably positive, and in one report, 31% (4/13) of cases showed positive staining for calponin.3 Rapid growth, ulceration, and recurrence emphasize that PLMT can be locally aggressive, similar to DFSP.4
The main differential diagnoses include DF and its variants, dermatomyofibroma, DFSP, and proliferative fasciitis.3,5 In the cases mentioned above, microscopic features were similar with a relatively well-circumscribed proliferation of spindle cells arranged in short fascicles through the entire reticular dermis, and the overlying epidermis was acanthotic.
Dermatofibroma commonly manifests in adults as a minor nodular lesion (commonly <1 cm), and usually is located on the legs. It has several clinical and histologic variants, including multiple clustered DF (MCDF)—a rare condition that has been reported in children and young adults and generally appears in the first and second decades of life. Of the reported cases of MCDF, immunohistochemical staining for SMA was performed in 8 cases. All these cases showed negative or minimal staining.3-5 Smooth muscle actin staining in DFs is negative, or weak and patchy, unlike in PLMT where it is diffuse, uniform, and strong.
Dermatofibrosarcoma protuberans typically occurs in young adults and manifests as dermal and subcutaneous nodular/multinodular or plaquelike masses, with rare congenital cases. Immunohistochemical staining for CD34, which typically is firmly and diffusely positive, is the most reliable marker of DFSP.6 Factor XIIIA in DFSP typically is negative for focal staining, mainly at periphery or in scattered dendritic cells. The prognosis of DFSP generally is excellent, with local recurrences in up to 30% of cases and extremely low metastatic potential (essentially only in cases with fibrosarcomatous transformation).6 Dermatomyofibroma is another rare benign dermal myofibroblastic tumor that typically manifests with indurated hyperpigmented or erythematous plaques or nodules on the shoulders and torso.6 This condition occurs mainly in adolescents and young adults, unlike PLMT. The most striking features of dermatomyofibroma are the horizontal orientation of the spindle cell nuclei and the pattern of the proliferation concerning the adnexal structures, especially hair follicles. The hair follicles have a normal appearance, and the proliferation extends up to each follicle, then continues to the other side without any displacement of the follicle. Tumor cells are variably positive for SMA in dermatomyofibromas and are negative for muscle-specific actin, desmin, S100, CD34, and Factor XIIIA.6
Immunohistochemistry can be very useful in differentiating PLMT from other conditions. Neoplastic cells stain positively for CD34 but not for Factor XIIIa and SMA in cases of DFSP. Dermatofibroma and its variants always present with collagen trapping at the periphery of the lesions and may demonstrate foamy macrophages, hemosiderin, or plasma cells FXIIIA(+), CD34(-), and variable SMA reactivity. This positivity usually is less prominent in DF than in PLMT. Neoplastic cells in dermatomyofibroma often stain positive for calponin, but only focally for SMA. The clinical features of dermatomyofibroma include early onset, large size, multiple nodules, and plaquelike morphology. Moulonguet et al4 hypothesized that, although MCDF and PLMT appear to show some distinctive clinical and histologic features, they also show similarities that could suggest they form part of the myofibroblastic spectrum. Furthermore, Moradi et al7 also considered them as part of the same disease spectrum because of their overlapping clinical, histologic, and immunohistochemical features.
The microscopic features in our case are notable, as the lesion demonstrated overlying acanthosis and follicular induction, resembling DF. The stroma contained microvesicular changes and erythrocyte extravasation, characteristic of nodular or proliferative fasciitis. Additionally, densely packed spindle cells infiltrated deep into the subcutaneous adipose tissue, similar to DFSP.2,3 Our findings expand on the reported histopathologic spectrum of this tumor to date.
- Clarke JT, Clarke LE, Miller C, et al. Plaque-like myofibroblastic tumor of infancy. Pediatr Dermatol. 2007;24:E83-E87. doi:10.1111 /j.1525-1470.2007.00449.x
- Marqueling AL, Dasher D, Friedlander SF, et al. Plaque-like myofibroblastic tumor: report of three cases. Pediatr Dermatol. 2013;30:600-607. doi:10.1111/pde.12185
- Sekar T, Mushtaq J, AlBadry W, et al. Plaque-like myofibroblastic tumor: a series of 2 cases of this unusual dermal tumor which occurs in infancy and early childhood. Pediatr Dev Pathol. 2018;21:444-448. doi: 10.1177/1093526617746807
- Moulonguet I, Biaggi A, Eschard C, et al. Plaque-like myofibroblastic tumor: report of 4 cases. Am J Dermatopathol. 2017;39:767-772. doi: 10.1097/DAD.0000000000000869
- Virdi A, Baraldi C, Barisani A, et al. Plaque-like myofibroblastic tumor, a rare entity of childhood: possible pitfalls in differential diagnosis. J Cutan Pathol. 2019;46:389-392. doi:10.1111/cup.13441
- Cassarino DS. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2021.
- Moradi S, Mnayer L, Earle J, et al. Plaque-like dermatofibroma: case report of a rare entity. Dermatopathology (Basel). 2021;8:337-341. doi:10.3390/dermatopathology8030038
- Clarke JT, Clarke LE, Miller C, et al. Plaque-like myofibroblastic tumor of infancy. Pediatr Dermatol. 2007;24:E83-E87. doi:10.1111 /j.1525-1470.2007.00449.x
- Marqueling AL, Dasher D, Friedlander SF, et al. Plaque-like myofibroblastic tumor: report of three cases. Pediatr Dermatol. 2013;30:600-607. doi:10.1111/pde.12185
- Sekar T, Mushtaq J, AlBadry W, et al. Plaque-like myofibroblastic tumor: a series of 2 cases of this unusual dermal tumor which occurs in infancy and early childhood. Pediatr Dev Pathol. 2018;21:444-448. doi: 10.1177/1093526617746807
- Moulonguet I, Biaggi A, Eschard C, et al. Plaque-like myofibroblastic tumor: report of 4 cases. Am J Dermatopathol. 2017;39:767-772. doi: 10.1097/DAD.0000000000000869
- Virdi A, Baraldi C, Barisani A, et al. Plaque-like myofibroblastic tumor, a rare entity of childhood: possible pitfalls in differential diagnosis. J Cutan Pathol. 2019;46:389-392. doi:10.1111/cup.13441
- Cassarino DS. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2021.
- Moradi S, Mnayer L, Earle J, et al. Plaque-like dermatofibroma: case report of a rare entity. Dermatopathology (Basel). 2021;8:337-341. doi:10.3390/dermatopathology8030038
Plaque With Central Ulceration on the Abdomen
Plaque With Central Ulceration on the Abdomen
A 14-month-old girl presented to the dermatology department with a firm asymptomatic lesion on the abdomen of 6 months’ duration. The lesion started as a flesh-colored papule and developed slowly into an indurated plaque that darkened in color. The patient had no history of trauma to the area. Physical examination revealed a dark reddish–brown, indurated, irregularly shaped plaque with central ulceration and elevated borders on the right abdomen. The plaque measured 2×3 cm with a few smaller satellite nodules distributed along the periphery. Abdominal ultrasonography revealed a multinodular proliferation in the dermis and subcutis of the right abdomen.
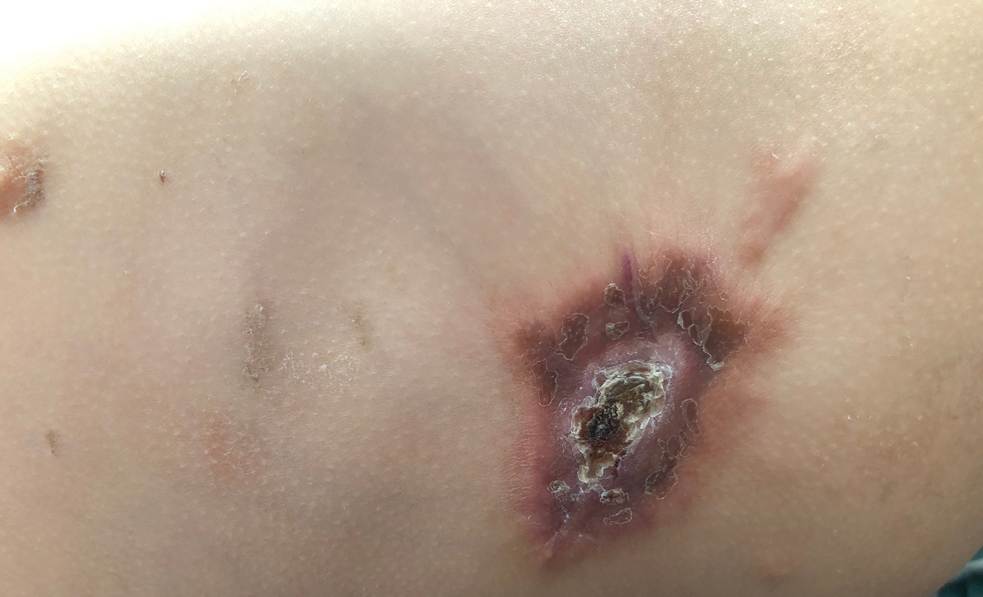
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
The yellow fly (Diachlorus ferrugatus) is a flying biting insect belonging to the order Diptera, family Tabanidae, which also includes deer flies (genus Chrysops) and horse flies (genus Tabanus).1 They are different from stinging insects of the order Hymenoptera (bees, wasps, yellow jackets, and hornets). As the name suggests, the yellow fly has a distinct yellow appearance, and adult yellow flies have a body length of approximately 1 cm.1,2 Distinguishing features of the yellow fly include prominently dark forelegs (the remaining legs are yellow), dark purple to black eyes with 2 fluorescent green lines, and a yellow abdomen with black hairs along the lateral regions and a broad central yellow stripe.1-3 Their wings have longitudinal black veins with clear spaces in between and a conspicuous brown patch at the apex (Figure 1A). In comparison, horse flies are darker and larger (Figure 1B), and deer flies are similar in shape but have stripes on the abdomen and thorax and mottled wings with dark patches near the apex (Figure 1C).1

The Tabanidae family comprises 4455 species belonging to 137 genera and is notorious for bites that result in localized pain, swelling, itching, and discomfort.4 While some Tabanidae species are mechanical or biologic vectors of pathogens (eg, Loa loa, equine infectious anemia virus, Trypanosoma species, cattle and sheep anthrax and tularemia), yellow flies do not appear to play a considerable role in disease transmission.4,5 Nonetheless, their bites can cause discomfort and create a nuisance for individuals residing within their distribution areas as well as for agricultural livestock, contributing to lower weight gain and milk production.1
Yellow flies are a commonly occurring species in the southeastern United States; their distribution spans several states, including New Jersey, Florida, and Texas.1,2 In Florida, specifically, yellow flies exhibit a seasonal pattern, with peak activity typically occurring from April through June.6-9 Activity levels are heightened around sunset as well as sunrise.1,9 Tabanids can be found in forests, parks, and gardens—particularly those that contain waterways such as freshwater lakes and streams—and typically stay near shaded woodlands that are prone to flooding.9
Tabanids go through the life cycle stages of egg, larva, pupa, and adult; the life cycle typically spans 1 year, with the adults living 30 to 60 days.1 Mating occurs soon after adults emerge from the pupal case in the soil.1,10 Females then are attracted to large dark moving objects and will feed on blood to develop eggs.2,10 Only female members of the Tabanidae family have modifications of the mouth parts that allow wounding of the skin (Figure 2). Their bites introduce saliva to the skin containing anticoagulants and other likely allergens. The tongue is used to lap between 20 to 600 microliters of blood.11 Males feed primarily on pollen and nectar.10 Most tabanid bites result in transient wheal-and-flare reactions, but some can result in more severe allergic reactions such as in our reported case.10 Rarely, anaphylactic reactions have been documented.10,12

Case Report
A 48-year-old man presented with swelling of the left hand following a yellow fly bite to the wrist 30 minutes prior while he worked outside at a ranch in central Florida (Figure 3). The patient was afebrile and reported no respiratory or gastrointestinal symptoms. The left hand and forearm were warm to the touch and appeared red and edematous (Figure 4). He was not tachycardic and did not appear to be in any distress. The patient reported that he had worked on the ranch for several years, and during that time had noted he was developing worsening localized reactions to yellow fly bites. He had visually identified the offending insect prior to the current presentation and had trapped some flies in previous incidents. Recently he had experienced rapid swelling at the bite sites but had never experienced respiratory difficulties or signs of systemic allergic reactions. He previously had used topical steroids when bites resulted in mild wheal-and-flare reactions, but he reported that these were no longer effective.
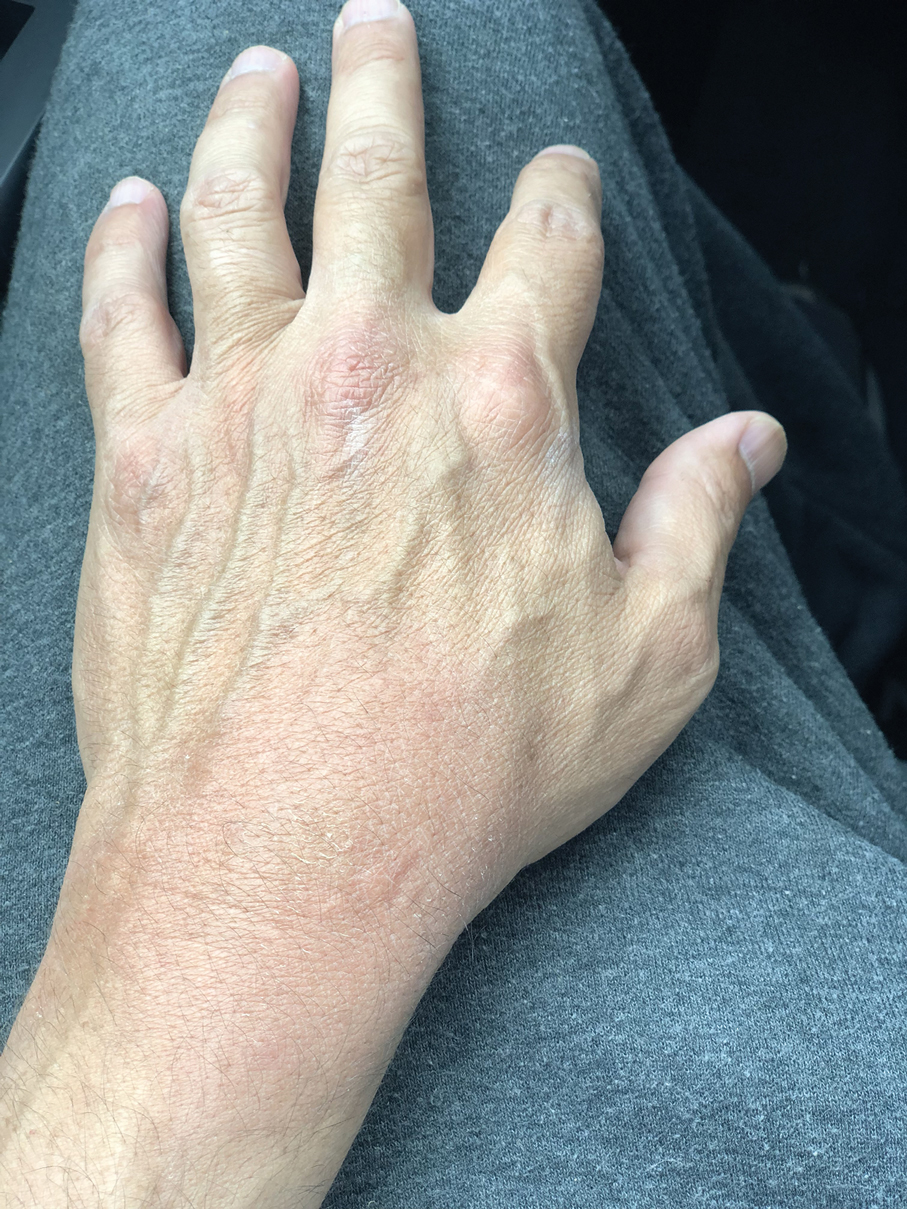

Management of the current bite reaction included oral prednisone tapered over 1 week from 40 mg to 10 mg daily as well as oral cetirizine 10 mg daily. Although bacterial cellulitis was considered in the differential diagnosis, no oral antibiotics were prescribed given the patient’s history of similar clinical presentations following yellow fly bites. His symptoms resolved within a few hours of his dose of prednisone. Incidentally, our patient has been able to control the progression of subsequent hypersensitivity reactions to yellow fly bites with a single 20-mg dose of prednisone administered at the onset of the bite.
Comment
In general, blood-feeding (hematophagous) insects rarely cause anaphylaxis and are more likely to cause cutaneous hypersensitivity reactions, possibly due to the small amount of antigen injected from a bite.13,14 The immediate wheal-and-flare reaction is an IgE-mediated type 1 immune reaction compared to a less common type 4 T-cell mediated delayed hypersensitivity reaction.14,15 There are many protein allergens in the saliva of biting insects that are not well characterized. Relevant allergens include a 69 kDa salivary gland protein as well as a Tab y 1 (anticoagulant), Tab y 2 (hyaluronidase), and Tab y 5 (antigen 5–related venom protein).11,15-17 Some of these proteins have structural homology between insects of different orders and can cause cross-reactivity in patients who also are allergic to Hymenoptera stings (wasp-horsefly syndrome).12,16
Our patient’s cutaneous reaction was localized and clinically manifested with rapidly progressive erythema and edema at the bite location. He did not exhibit signs of a systemic reaction such as angioedema, respiratory or gastrointestinal symptoms, tachycardia, or hypotension. Management of affected patients depends on the extent of the reaction and may include oral or parenteral antihistamines as well as oral steroids for more severe edema.11 Anaphylactic reactions generally respond to subcutaneous epinephrine.15 It would be prudent for patients with a relevant anaphylactic history to carry an autoinjectable epinephrine pen in case of difficulty breathing or general malaise following a bite. Besides avoidance of insect bites, personal protection methods include wearing long-sleeved shirts and pants and using insect repellents containing diethyl toluamide (DEET), citronella, or geraniol.1
At present, diagnosis of cutaneous reactions to yellow fly bites is best made based on the patient’s personal history.14 If the offending fly is trapped, it can be identified. As most patients cannot differentiate between insects, it may be helpful for dermatologists to know that a small amount of blood at the bite site is suggestive of a fly bite rather than a sting from a member of the order Hymenoptera. Currently, there are no consistently useful extracts for intradermal skin testing.11 Although there are several commercially available serum-specific IgE tests for suspected horse fly reactions, their usefulness is doubtful without further information on sensitivity and specificity as well as the allergen utilized.11,18,19 The use of allergen immunotherapy to induce hyposensitization in patients who experience cutaneous reactions is not standardized and poses some risks including severe allergic reactions requiring facilities for resuscitation, variability of response patterns, and supporting evidence is weak.11
Final Thoughts
Cutaneous reactions to yellow fly bites rarely are described in the dermatology literature. The salivary proteins implicated in inducing an allergic response and cross-reactivity of D ferrugatus with other biting and stinging insects as well as the natural course of immune reactions over time need to be further characterized.
- Squitier JM. Deer flies, yellow flies, and horse flies, Chrysops, Diachlorus and Tabanus spp. (Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN155
- Fairchild GB, Weems HB Jr, Fasulo TR. Yellow fly, Diachlorus ferrugatus (Fabricius)(Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN595
- Mullens BA. Horse flies and deer flies (Tabanidae). In: Mullen G, Durden L. Med Vet Entomol. Elsevier Science; 2009:327-344.
- Akhoundi M, Sereno D, Marteau A, et al. Who bites me? A tentative discriminative key to diagnose hematophagous ectoparasites biting using clinical manifestations. Diagnostics (Basel). 2020;10:308.
- Cheng TC. General Parasitology. 2nd ed. Elsevier Science; 2021:660.
- Wells K, Varnadoe C, Dorman D, et al. Survey of the distribution and seasonal activity of yellow flies (Diptera: Tabanidae) in Florida, USA. J Vector Ecol. 2019;44:235-242.
- Hribar LJ, Leppla NC, Beshear RJ, et al. Seasonal abundance of Diachlorus ferrugatus (Diptera: Tabanidae) in Monroe County, Florida. Florida Scientist. 2003;66:52-54.
- Fairchild GB, Weems HV. Diachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular. 1973;139.
- Cilek JE, Schreiber ET. Diel host-seeking activity of adult Diachlorus ferrugatus (F.) (Diptera: Tabanidae) in Northwestern Florida. J Entomol Sci. 1999;34:462-466.
- Sean S. Tabanids (horseflies). Dermatol Online J. 1999;5:6.
- Whyte AF, Popeseu FD, Carlson J. Tabanidae insect (horsefly and deerfly) allergy in humans: a review of the literature. Clin Exp Allergy. 2020;50:886-893.
- Buonomo A, Rizzi A, Aruanno A, et al. Anaphylaxis after horsefly sting: a strange case of wasp-horsefly syndrome. Postepi Dermatol Alergol. 2021;2:331-332.
- Freye HB, Litwin C. Coexistent anaphylaxis to Diptera and Hymenoptera. Ann Allergy Asthma Immunol. 1996 76:270-272.
- Hemmer W, Wantke F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol Select. 2020;4:97-104.
- Ewan PW. Allergy to insect stings: a review. J R Soc Med. 1985;78:234-239.
- Ma D, Li Y, Dong J, et al. Purification and characterization of two new allergens from the salivary glands of the horsefly Tabanus yao. Allergy. 2011;66:101-109.
- Hemmer W, Focke M, Vieluf D, et al. Anaphylaxis induced by horsefly bites: identification of a 69 kd IgE-binding protein from Chrysops spp. (Diptera: Tabanidae) by western blot analysis. J Allergy Clin Immunol. 1998;101:134-136.
- Mayo Clinic Laboratories. Test catalog: horse fly. Accessed March 11, 2025. https://www.mayocliniclabs.com/search?q=horse%20fly
- HealthLabs.com. Horsefly allergy test. Accessed March 11, 2025. https://www.healthlabs.com/horsefly-allergy-testing
The yellow fly (Diachlorus ferrugatus) is a flying biting insect belonging to the order Diptera, family Tabanidae, which also includes deer flies (genus Chrysops) and horse flies (genus Tabanus).1 They are different from stinging insects of the order Hymenoptera (bees, wasps, yellow jackets, and hornets). As the name suggests, the yellow fly has a distinct yellow appearance, and adult yellow flies have a body length of approximately 1 cm.1,2 Distinguishing features of the yellow fly include prominently dark forelegs (the remaining legs are yellow), dark purple to black eyes with 2 fluorescent green lines, and a yellow abdomen with black hairs along the lateral regions and a broad central yellow stripe.1-3 Their wings have longitudinal black veins with clear spaces in between and a conspicuous brown patch at the apex (Figure 1A). In comparison, horse flies are darker and larger (Figure 1B), and deer flies are similar in shape but have stripes on the abdomen and thorax and mottled wings with dark patches near the apex (Figure 1C).1

The Tabanidae family comprises 4455 species belonging to 137 genera and is notorious for bites that result in localized pain, swelling, itching, and discomfort.4 While some Tabanidae species are mechanical or biologic vectors of pathogens (eg, Loa loa, equine infectious anemia virus, Trypanosoma species, cattle and sheep anthrax and tularemia), yellow flies do not appear to play a considerable role in disease transmission.4,5 Nonetheless, their bites can cause discomfort and create a nuisance for individuals residing within their distribution areas as well as for agricultural livestock, contributing to lower weight gain and milk production.1
Yellow flies are a commonly occurring species in the southeastern United States; their distribution spans several states, including New Jersey, Florida, and Texas.1,2 In Florida, specifically, yellow flies exhibit a seasonal pattern, with peak activity typically occurring from April through June.6-9 Activity levels are heightened around sunset as well as sunrise.1,9 Tabanids can be found in forests, parks, and gardens—particularly those that contain waterways such as freshwater lakes and streams—and typically stay near shaded woodlands that are prone to flooding.9
Tabanids go through the life cycle stages of egg, larva, pupa, and adult; the life cycle typically spans 1 year, with the adults living 30 to 60 days.1 Mating occurs soon after adults emerge from the pupal case in the soil.1,10 Females then are attracted to large dark moving objects and will feed on blood to develop eggs.2,10 Only female members of the Tabanidae family have modifications of the mouth parts that allow wounding of the skin (Figure 2). Their bites introduce saliva to the skin containing anticoagulants and other likely allergens. The tongue is used to lap between 20 to 600 microliters of blood.11 Males feed primarily on pollen and nectar.10 Most tabanid bites result in transient wheal-and-flare reactions, but some can result in more severe allergic reactions such as in our reported case.10 Rarely, anaphylactic reactions have been documented.10,12

Case Report
A 48-year-old man presented with swelling of the left hand following a yellow fly bite to the wrist 30 minutes prior while he worked outside at a ranch in central Florida (Figure 3). The patient was afebrile and reported no respiratory or gastrointestinal symptoms. The left hand and forearm were warm to the touch and appeared red and edematous (Figure 4). He was not tachycardic and did not appear to be in any distress. The patient reported that he had worked on the ranch for several years, and during that time had noted he was developing worsening localized reactions to yellow fly bites. He had visually identified the offending insect prior to the current presentation and had trapped some flies in previous incidents. Recently he had experienced rapid swelling at the bite sites but had never experienced respiratory difficulties or signs of systemic allergic reactions. He previously had used topical steroids when bites resulted in mild wheal-and-flare reactions, but he reported that these were no longer effective.


Management of the current bite reaction included oral prednisone tapered over 1 week from 40 mg to 10 mg daily as well as oral cetirizine 10 mg daily. Although bacterial cellulitis was considered in the differential diagnosis, no oral antibiotics were prescribed given the patient’s history of similar clinical presentations following yellow fly bites. His symptoms resolved within a few hours of his dose of prednisone. Incidentally, our patient has been able to control the progression of subsequent hypersensitivity reactions to yellow fly bites with a single 20-mg dose of prednisone administered at the onset of the bite.
Comment
In general, blood-feeding (hematophagous) insects rarely cause anaphylaxis and are more likely to cause cutaneous hypersensitivity reactions, possibly due to the small amount of antigen injected from a bite.13,14 The immediate wheal-and-flare reaction is an IgE-mediated type 1 immune reaction compared to a less common type 4 T-cell mediated delayed hypersensitivity reaction.14,15 There are many protein allergens in the saliva of biting insects that are not well characterized. Relevant allergens include a 69 kDa salivary gland protein as well as a Tab y 1 (anticoagulant), Tab y 2 (hyaluronidase), and Tab y 5 (antigen 5–related venom protein).11,15-17 Some of these proteins have structural homology between insects of different orders and can cause cross-reactivity in patients who also are allergic to Hymenoptera stings (wasp-horsefly syndrome).12,16
Our patient’s cutaneous reaction was localized and clinically manifested with rapidly progressive erythema and edema at the bite location. He did not exhibit signs of a systemic reaction such as angioedema, respiratory or gastrointestinal symptoms, tachycardia, or hypotension. Management of affected patients depends on the extent of the reaction and may include oral or parenteral antihistamines as well as oral steroids for more severe edema.11 Anaphylactic reactions generally respond to subcutaneous epinephrine.15 It would be prudent for patients with a relevant anaphylactic history to carry an autoinjectable epinephrine pen in case of difficulty breathing or general malaise following a bite. Besides avoidance of insect bites, personal protection methods include wearing long-sleeved shirts and pants and using insect repellents containing diethyl toluamide (DEET), citronella, or geraniol.1
At present, diagnosis of cutaneous reactions to yellow fly bites is best made based on the patient’s personal history.14 If the offending fly is trapped, it can be identified. As most patients cannot differentiate between insects, it may be helpful for dermatologists to know that a small amount of blood at the bite site is suggestive of a fly bite rather than a sting from a member of the order Hymenoptera. Currently, there are no consistently useful extracts for intradermal skin testing.11 Although there are several commercially available serum-specific IgE tests for suspected horse fly reactions, their usefulness is doubtful without further information on sensitivity and specificity as well as the allergen utilized.11,18,19 The use of allergen immunotherapy to induce hyposensitization in patients who experience cutaneous reactions is not standardized and poses some risks including severe allergic reactions requiring facilities for resuscitation, variability of response patterns, and supporting evidence is weak.11
Final Thoughts
Cutaneous reactions to yellow fly bites rarely are described in the dermatology literature. The salivary proteins implicated in inducing an allergic response and cross-reactivity of D ferrugatus with other biting and stinging insects as well as the natural course of immune reactions over time need to be further characterized.
The yellow fly (Diachlorus ferrugatus) is a flying biting insect belonging to the order Diptera, family Tabanidae, which also includes deer flies (genus Chrysops) and horse flies (genus Tabanus).1 They are different from stinging insects of the order Hymenoptera (bees, wasps, yellow jackets, and hornets). As the name suggests, the yellow fly has a distinct yellow appearance, and adult yellow flies have a body length of approximately 1 cm.1,2 Distinguishing features of the yellow fly include prominently dark forelegs (the remaining legs are yellow), dark purple to black eyes with 2 fluorescent green lines, and a yellow abdomen with black hairs along the lateral regions and a broad central yellow stripe.1-3 Their wings have longitudinal black veins with clear spaces in between and a conspicuous brown patch at the apex (Figure 1A). In comparison, horse flies are darker and larger (Figure 1B), and deer flies are similar in shape but have stripes on the abdomen and thorax and mottled wings with dark patches near the apex (Figure 1C).1

The Tabanidae family comprises 4455 species belonging to 137 genera and is notorious for bites that result in localized pain, swelling, itching, and discomfort.4 While some Tabanidae species are mechanical or biologic vectors of pathogens (eg, Loa loa, equine infectious anemia virus, Trypanosoma species, cattle and sheep anthrax and tularemia), yellow flies do not appear to play a considerable role in disease transmission.4,5 Nonetheless, their bites can cause discomfort and create a nuisance for individuals residing within their distribution areas as well as for agricultural livestock, contributing to lower weight gain and milk production.1
Yellow flies are a commonly occurring species in the southeastern United States; their distribution spans several states, including New Jersey, Florida, and Texas.1,2 In Florida, specifically, yellow flies exhibit a seasonal pattern, with peak activity typically occurring from April through June.6-9 Activity levels are heightened around sunset as well as sunrise.1,9 Tabanids can be found in forests, parks, and gardens—particularly those that contain waterways such as freshwater lakes and streams—and typically stay near shaded woodlands that are prone to flooding.9
Tabanids go through the life cycle stages of egg, larva, pupa, and adult; the life cycle typically spans 1 year, with the adults living 30 to 60 days.1 Mating occurs soon after adults emerge from the pupal case in the soil.1,10 Females then are attracted to large dark moving objects and will feed on blood to develop eggs.2,10 Only female members of the Tabanidae family have modifications of the mouth parts that allow wounding of the skin (Figure 2). Their bites introduce saliva to the skin containing anticoagulants and other likely allergens. The tongue is used to lap between 20 to 600 microliters of blood.11 Males feed primarily on pollen and nectar.10 Most tabanid bites result in transient wheal-and-flare reactions, but some can result in more severe allergic reactions such as in our reported case.10 Rarely, anaphylactic reactions have been documented.10,12

Case Report
A 48-year-old man presented with swelling of the left hand following a yellow fly bite to the wrist 30 minutes prior while he worked outside at a ranch in central Florida (Figure 3). The patient was afebrile and reported no respiratory or gastrointestinal symptoms. The left hand and forearm were warm to the touch and appeared red and edematous (Figure 4). He was not tachycardic and did not appear to be in any distress. The patient reported that he had worked on the ranch for several years, and during that time had noted he was developing worsening localized reactions to yellow fly bites. He had visually identified the offending insect prior to the current presentation and had trapped some flies in previous incidents. Recently he had experienced rapid swelling at the bite sites but had never experienced respiratory difficulties or signs of systemic allergic reactions. He previously had used topical steroids when bites resulted in mild wheal-and-flare reactions, but he reported that these were no longer effective.


Management of the current bite reaction included oral prednisone tapered over 1 week from 40 mg to 10 mg daily as well as oral cetirizine 10 mg daily. Although bacterial cellulitis was considered in the differential diagnosis, no oral antibiotics were prescribed given the patient’s history of similar clinical presentations following yellow fly bites. His symptoms resolved within a few hours of his dose of prednisone. Incidentally, our patient has been able to control the progression of subsequent hypersensitivity reactions to yellow fly bites with a single 20-mg dose of prednisone administered at the onset of the bite.
Comment
In general, blood-feeding (hematophagous) insects rarely cause anaphylaxis and are more likely to cause cutaneous hypersensitivity reactions, possibly due to the small amount of antigen injected from a bite.13,14 The immediate wheal-and-flare reaction is an IgE-mediated type 1 immune reaction compared to a less common type 4 T-cell mediated delayed hypersensitivity reaction.14,15 There are many protein allergens in the saliva of biting insects that are not well characterized. Relevant allergens include a 69 kDa salivary gland protein as well as a Tab y 1 (anticoagulant), Tab y 2 (hyaluronidase), and Tab y 5 (antigen 5–related venom protein).11,15-17 Some of these proteins have structural homology between insects of different orders and can cause cross-reactivity in patients who also are allergic to Hymenoptera stings (wasp-horsefly syndrome).12,16
Our patient’s cutaneous reaction was localized and clinically manifested with rapidly progressive erythema and edema at the bite location. He did not exhibit signs of a systemic reaction such as angioedema, respiratory or gastrointestinal symptoms, tachycardia, or hypotension. Management of affected patients depends on the extent of the reaction and may include oral or parenteral antihistamines as well as oral steroids for more severe edema.11 Anaphylactic reactions generally respond to subcutaneous epinephrine.15 It would be prudent for patients with a relevant anaphylactic history to carry an autoinjectable epinephrine pen in case of difficulty breathing or general malaise following a bite. Besides avoidance of insect bites, personal protection methods include wearing long-sleeved shirts and pants and using insect repellents containing diethyl toluamide (DEET), citronella, or geraniol.1
At present, diagnosis of cutaneous reactions to yellow fly bites is best made based on the patient’s personal history.14 If the offending fly is trapped, it can be identified. As most patients cannot differentiate between insects, it may be helpful for dermatologists to know that a small amount of blood at the bite site is suggestive of a fly bite rather than a sting from a member of the order Hymenoptera. Currently, there are no consistently useful extracts for intradermal skin testing.11 Although there are several commercially available serum-specific IgE tests for suspected horse fly reactions, their usefulness is doubtful without further information on sensitivity and specificity as well as the allergen utilized.11,18,19 The use of allergen immunotherapy to induce hyposensitization in patients who experience cutaneous reactions is not standardized and poses some risks including severe allergic reactions requiring facilities for resuscitation, variability of response patterns, and supporting evidence is weak.11
Final Thoughts
Cutaneous reactions to yellow fly bites rarely are described in the dermatology literature. The salivary proteins implicated in inducing an allergic response and cross-reactivity of D ferrugatus with other biting and stinging insects as well as the natural course of immune reactions over time need to be further characterized.
- Squitier JM. Deer flies, yellow flies, and horse flies, Chrysops, Diachlorus and Tabanus spp. (Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN155
- Fairchild GB, Weems HB Jr, Fasulo TR. Yellow fly, Diachlorus ferrugatus (Fabricius)(Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN595
- Mullens BA. Horse flies and deer flies (Tabanidae). In: Mullen G, Durden L. Med Vet Entomol. Elsevier Science; 2009:327-344.
- Akhoundi M, Sereno D, Marteau A, et al. Who bites me? A tentative discriminative key to diagnose hematophagous ectoparasites biting using clinical manifestations. Diagnostics (Basel). 2020;10:308.
- Cheng TC. General Parasitology. 2nd ed. Elsevier Science; 2021:660.
- Wells K, Varnadoe C, Dorman D, et al. Survey of the distribution and seasonal activity of yellow flies (Diptera: Tabanidae) in Florida, USA. J Vector Ecol. 2019;44:235-242.
- Hribar LJ, Leppla NC, Beshear RJ, et al. Seasonal abundance of Diachlorus ferrugatus (Diptera: Tabanidae) in Monroe County, Florida. Florida Scientist. 2003;66:52-54.
- Fairchild GB, Weems HV. Diachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular. 1973;139.
- Cilek JE, Schreiber ET. Diel host-seeking activity of adult Diachlorus ferrugatus (F.) (Diptera: Tabanidae) in Northwestern Florida. J Entomol Sci. 1999;34:462-466.
- Sean S. Tabanids (horseflies). Dermatol Online J. 1999;5:6.
- Whyte AF, Popeseu FD, Carlson J. Tabanidae insect (horsefly and deerfly) allergy in humans: a review of the literature. Clin Exp Allergy. 2020;50:886-893.
- Buonomo A, Rizzi A, Aruanno A, et al. Anaphylaxis after horsefly sting: a strange case of wasp-horsefly syndrome. Postepi Dermatol Alergol. 2021;2:331-332.
- Freye HB, Litwin C. Coexistent anaphylaxis to Diptera and Hymenoptera. Ann Allergy Asthma Immunol. 1996 76:270-272.
- Hemmer W, Wantke F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol Select. 2020;4:97-104.
- Ewan PW. Allergy to insect stings: a review. J R Soc Med. 1985;78:234-239.
- Ma D, Li Y, Dong J, et al. Purification and characterization of two new allergens from the salivary glands of the horsefly Tabanus yao. Allergy. 2011;66:101-109.
- Hemmer W, Focke M, Vieluf D, et al. Anaphylaxis induced by horsefly bites: identification of a 69 kd IgE-binding protein from Chrysops spp. (Diptera: Tabanidae) by western blot analysis. J Allergy Clin Immunol. 1998;101:134-136.
- Mayo Clinic Laboratories. Test catalog: horse fly. Accessed March 11, 2025. https://www.mayocliniclabs.com/search?q=horse%20fly
- HealthLabs.com. Horsefly allergy test. Accessed March 11, 2025. https://www.healthlabs.com/horsefly-allergy-testing
- Squitier JM. Deer flies, yellow flies, and horse flies, Chrysops, Diachlorus and Tabanus spp. (Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN155
- Fairchild GB, Weems HB Jr, Fasulo TR. Yellow fly, Diachlorus ferrugatus (Fabricius)(Insecta: Diptera: Tabanidae). University of Florida. Accessed March 11, 2025. https://edis.ifas.ufl.edu/publication/IN595
- Mullens BA. Horse flies and deer flies (Tabanidae). In: Mullen G, Durden L. Med Vet Entomol. Elsevier Science; 2009:327-344.
- Akhoundi M, Sereno D, Marteau A, et al. Who bites me? A tentative discriminative key to diagnose hematophagous ectoparasites biting using clinical manifestations. Diagnostics (Basel). 2020;10:308.
- Cheng TC. General Parasitology. 2nd ed. Elsevier Science; 2021:660.
- Wells K, Varnadoe C, Dorman D, et al. Survey of the distribution and seasonal activity of yellow flies (Diptera: Tabanidae) in Florida, USA. J Vector Ecol. 2019;44:235-242.
- Hribar LJ, Leppla NC, Beshear RJ, et al. Seasonal abundance of Diachlorus ferrugatus (Diptera: Tabanidae) in Monroe County, Florida. Florida Scientist. 2003;66:52-54.
- Fairchild GB, Weems HV. Diachlorus ferrugatus (Fabricius), a fierce biting fly (Diptera: Tabanidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular. 1973;139.
- Cilek JE, Schreiber ET. Diel host-seeking activity of adult Diachlorus ferrugatus (F.) (Diptera: Tabanidae) in Northwestern Florida. J Entomol Sci. 1999;34:462-466.
- Sean S. Tabanids (horseflies). Dermatol Online J. 1999;5:6.
- Whyte AF, Popeseu FD, Carlson J. Tabanidae insect (horsefly and deerfly) allergy in humans: a review of the literature. Clin Exp Allergy. 2020;50:886-893.
- Buonomo A, Rizzi A, Aruanno A, et al. Anaphylaxis after horsefly sting: a strange case of wasp-horsefly syndrome. Postepi Dermatol Alergol. 2021;2:331-332.
- Freye HB, Litwin C. Coexistent anaphylaxis to Diptera and Hymenoptera. Ann Allergy Asthma Immunol. 1996 76:270-272.
- Hemmer W, Wantke F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol Select. 2020;4:97-104.
- Ewan PW. Allergy to insect stings: a review. J R Soc Med. 1985;78:234-239.
- Ma D, Li Y, Dong J, et al. Purification and characterization of two new allergens from the salivary glands of the horsefly Tabanus yao. Allergy. 2011;66:101-109.
- Hemmer W, Focke M, Vieluf D, et al. Anaphylaxis induced by horsefly bites: identification of a 69 kd IgE-binding protein from Chrysops spp. (Diptera: Tabanidae) by western blot analysis. J Allergy Clin Immunol. 1998;101:134-136.
- Mayo Clinic Laboratories. Test catalog: horse fly. Accessed March 11, 2025. https://www.mayocliniclabs.com/search?q=horse%20fly
- HealthLabs.com. Horsefly allergy test. Accessed March 11, 2025. https://www.healthlabs.com/horsefly-allergy-testing
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
Managing Cutaneous Reactions to Yellow Fly (Diachlorus ferrugatus) Bites
PRACTICE POINTS
- Diachlorus ferrugatus, commonly known as the yellow fly, belongs to the Tabanidae family of insects that also includes deer flies and horse flies.
- The female yellow fly can instill a painful bite in humans and can cause local and systemic allergic reactions.
- Medical management of yellow fly bites is dictated by the severity of the reaction.
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15
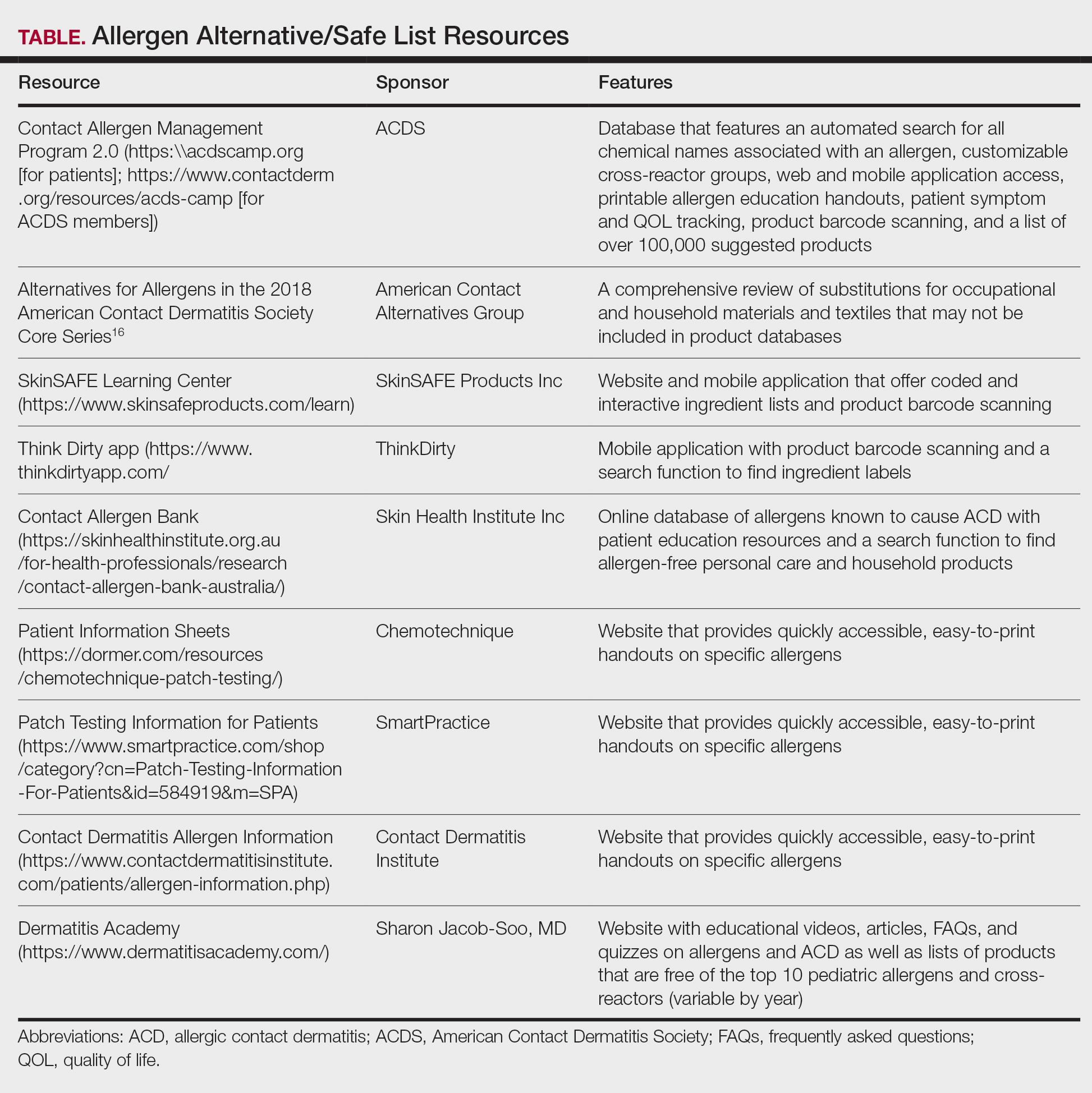
Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.
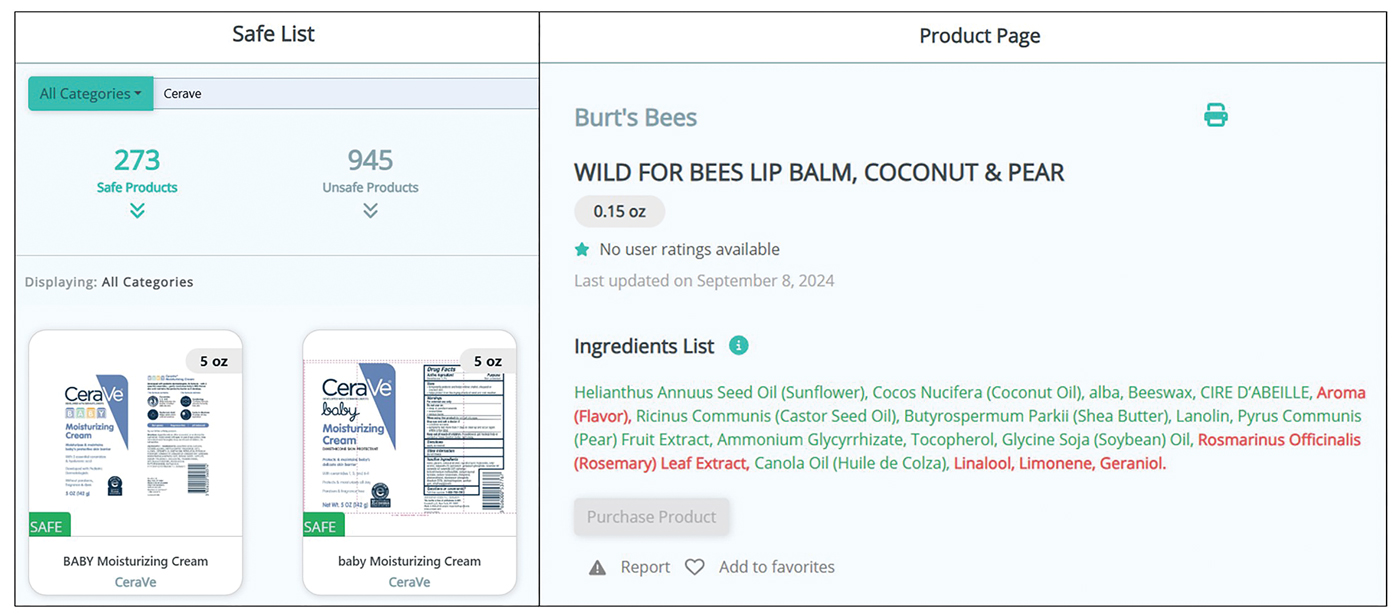
Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.
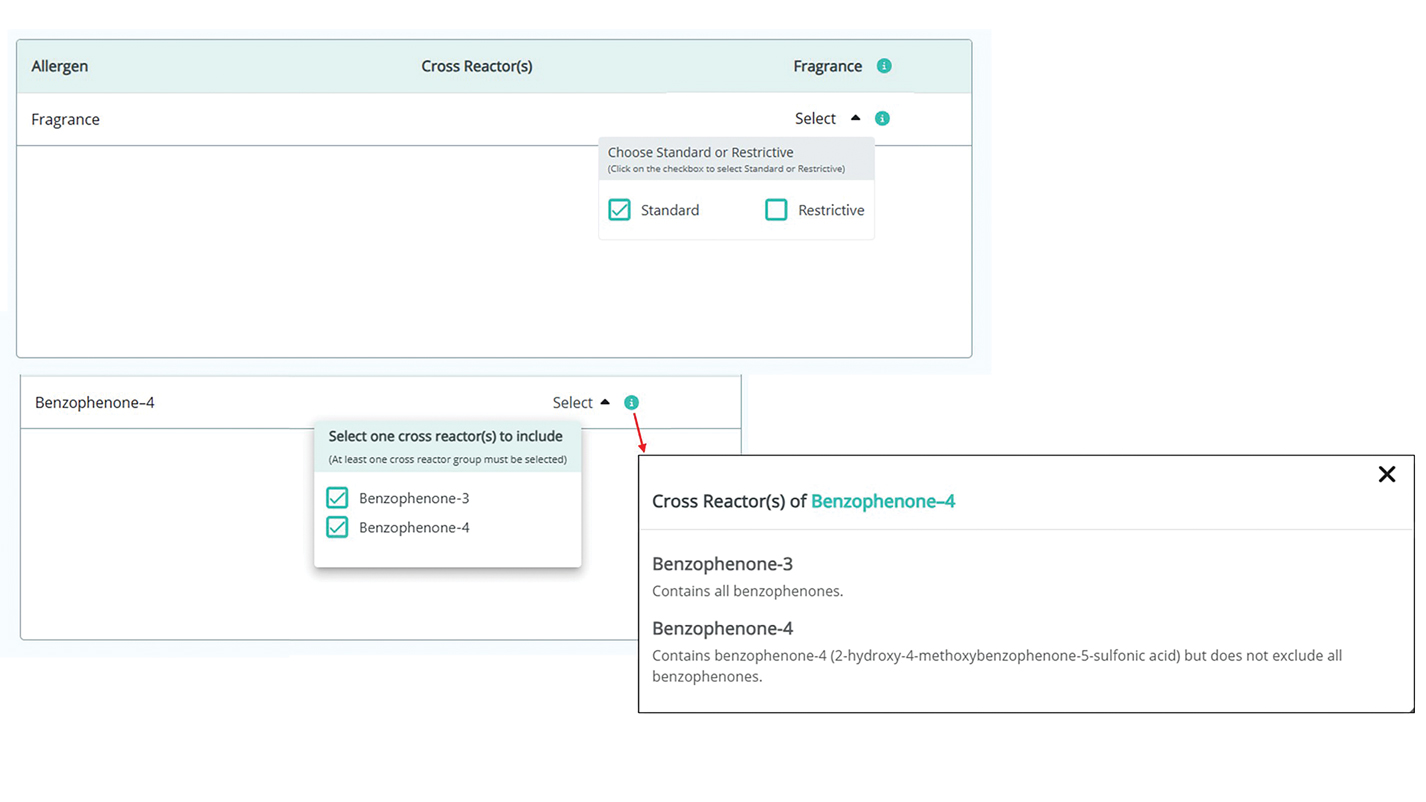
Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
While patch testing is the gold standard to diagnose type IV cutaneous hypersensitivity reactions, interpreting results can feel like trying to decipher a secret code, leaving patients feeling disempowered in avoiding their triggers. To truly manage allergic contact dermatitis (ACD), patients need comprehensive education on which allergens to avoid and ways to spot potential sources of exposure, including counseling, written guidelines, and lists of product alternatives.1 Patients who can recall and avoid their triggers experience greater improvement in clinical and quality-of-life scores.2 However, several studies have demonstrated that patients have difficulty recalling their allergens, even with longitudinal reminders.2-5 Quality-of-life and clinical outcomes also are not necessarily improved by successful allergen recall alone, as patients have reported limited success in actually avoiding allergens, highlighting the complexity of navigating exposures in daily life.2,6 To address these challenges, we examine common pitfalls patients encounter when avoiding allergens, highlight the benefits of utilizing safe lists and databases for allergen management, and introduce the updated Contact Allergen Management Program (CAMP) 2.0 as an optimal tool for long-term management of ACD.
Allergen Avoidance Pitfalls
Simply reading ingredient labels to avoid allergens is only marginally effective, as patients need to identify and interpret multiple chemical names as well as cross-reactors and related compounds to achieve success. Some allergens, such as fragrances or manufacturing impurities, are not explicitly identified on product labels. Even patients who can practice diligent label reading may struggle to find information on household or occupational products when full ingredient disclosure is not required.
Many of the allergens included in the American Contact Dermatitis Society (ACDS) Core 90 Series have alternative chemical aliases, and many have related compounds.6 For example, individuals with contact allergy to formaldehyde or a formaldehyde releaser usually need to avoid multiple other formaldehyde-releasing chemicals. Patients who test positive to amidoamine or dimethylaminopropylamine also must avoid the surfactant cocamidopropyl betaine—not because it is a cross-reactor, but because it is an impurity in the synthetic pathway.
Fragrance is one of the most common causes of ACD but can be challenging to avoid. Patients with allergies to fragrance or specific compounds (eg, limonene, linalool hydroperoxides) need to be savvy enough to navigate a broad spectrum of synthetic and botanical fragrance additives. Avoiding products that contain “fragrance” or “parfum” is simple enough, but patients also may need to recognize more than 3000 chemical names to identify individual fragrance ingredients that may be listed separately.7 Further, some fragrances are added for alternative purposes—preservative, medicinal, or emulsification—in which case products may deceptively tout themselves as being “fragrance free” yet still contain a fragrance allergen. This is made even more complex considering additional additives that commonly may cross-react with individual fragrance compounds; balsam of Peru, for example, is a botanical amalgam containing more than 250 compounds, including several fragrance components, making it an excellent indicator of fragrance allergy.8 While balsam of Peru and its fragrance constituents will almost never be listed on a product label, it cross-reacts with several benzyl derivatives commonly used in cosmetic formulations, such as benzyl alcohol, benzyl acetate, benzoic acid, benzyl benzoate, and benzyl cinnamate.9,10
Given that ACD is a common reason for patients to seek dermatologic care, it is crucial for clinicians to equip themselves with effective strategies to support patients after patch testing.11 This includes efficient translation of patch test results into practical advice while avoiding the oversimplified suggestion to read product labels; however, education alone cannot address the complexities of managing ACD, which is where contact allergen databases come into play.
An Essential Tool: Patient Allergen Databases and Safe Lists
Contact allergen databases are like a trusty sidekick for patients and clinicians, providing easily accessible information and tools to support allergen avoidance and improve ACD outcomes. While there are several existing resources, the ACDS launched its CAMP database in 2011 for ACDS members and their patients.12 The CAMP allows clinicians to easily generate personalized safe lists for household, medicament, and personal care products, facilitating seamless patient access both online and via a mobile application. The database also includes allergen-specific handouts to guide patient education.13 A key highlight of the CAMP is automated management of cross-reactors, which allows patients to choose products without having to memorize complex cross-reactor algorithms and helps avoid overly restrictive safe lists (Table).12-15

Other databases and online resources provide similar features, such as resources for patient education or finding safe products. The 2018 Alternatives for Allergens report is a vital adjunctive resource for guiding patients to suitable allergen-free products not included in commonly accessible product databases such as occupational materials, medical adhesives, shoes, or textiles.16
Introduction of CAMP 2.0
The latest version, CAMP 2.0, was launched in late 2024. The fully revamped database has a catalog of more than 100,000 products and comes packed with features that address many of the limitations found in the original CAMP. How does CAMP 2.0 work? The clinician inputs the patient’s allergens and makes choices about cross-reactor groups, and CAMP 2.0 outputs a list of allergen-free products that the patient can use when shopping for personal care products and the clinician can use for prescribing medicaments. The new user experience is intended to be more informative and engaging for all parties.
The CAMP 2.0 interface offers frequent product updates and streamlined database navigation, including enhanced search functions, barcode scanning, and a new mobile application for Apple and Android users. The mobile application also allows patients to track their symptoms and quality of life over time. With this additional functionality, there also is an extensive section for frequently asked questions and tutorials to help patients understand and utilize these features effectively.
Patients no longer have to wonder if a product that is not listed on their safe list is actually unsafe or just missing from the database. Several new features, including color-coded ingredient lists and organization of search codes into “safe” and “unsafe” product lists (Figure 1), help increase product transparency. These features can facilitate patient recognition of allergen names and cross-reactors in selected products. Future updates will include product purchasing through the mobile application and more educational handouts, including Spanish translations and dietary guidelines for systemic contact dermatitis.

Patient Experience—Once patients complete patch testing with an ACDS member, they can access the CAMP 2.0 database for free via web-based or a mobile application. After setting up an account, patients gain immediate access to their allergen information, product database, and educational resources about ACD and CAMP 2.0. Patients can search for specific products using text or barcode scanning or browse through categorized lists of medical, household, and personal care items. Each product page contains the product name and brand along with a color-coded ingredient list to help patients identify safe and unsafe ingredients at a glance (Figure 1). Products not currently included in the database can be requested using the “Add Product” feature. Additional patient engagement features include options to mark favorite products, write reviews, and track quality of life over time.
Physician Experience—The updated version includes several tutorials and frequently asked questions on how to improve ACD management and make the most of the new CAMP 2.0 tools and features. Generating patient allergen codes has been streamlined with an “Allergen Search” feature, allowing providers to quickly search and add or remove allergens to patients’ safe lists. Cross-reactor groups may be selectively added or removed for greater transparency and specificity in creating a patient safe list (Figure 2). Allergen codes now can be edited over time and are available for patient use via alphanumeric text or QR code format, which easily can be printed on a handout with instructions to help patients get acquainted with the system. For patient counseling, updated education handouts are available in the patient’s app and may be printed to provide supportive written educational material.

Approach to Long-Term Follow-up
When it comes to getting the most from patch testing, ongoing allergen avoidance is crucial. Patients may not see improvement unless they understand what ACD is and what needs to be done to improve it as well as become familiar with the names and common sources of their triggers.17 Clinicians can use CAMP 2.0 to facilitate patient improvement after patch testing, focusing on 3 key areas: continued patient education, patients’ ongoing progress in avoiding allergens, and monitored clinical improvement.
A solid understanding of ACD, such as its delayed (ie, 24-72 hours) onset after exposure, the need for allergen avoidance for at least 4 to 6 weeks before seeing improvement, and correlation of identified allergens with daily exposures, plays a major role in patient success. The CAMP 2.0 patch testing basics section is an excellent resource for patient-friendly explanations on patch testing and ACD. This resource, as well as allergen education handouts, may be reviewed at follow-up visits to continue to solidify patient learning.
Patients often have questions about allergen avoidance, such as occupational exposures, the suitability of specific products, or specific allergen names. These discussions are helpful for gauging how well patients are equipped to avoid their triggers as well as any hurdles they may be facing. If a patient still is experiencing flares after 6 to 8 weeks of safe-list adherence, it is important to take a thorough history of product use, daily exposures, and the patterns of distribution on the skin. Possible allergen exposures via topical medications also should be considered.18,19 Cross-checking products with a patient’s CAMP 2.0 safe list and correlating exposures with the continued ACD distribution are effective strategies to troubleshoot for unknown exposures to allergens.
Final Thoughts
Helping patients avoid allergens is essential to long-term management of ACD. The CAMP 2.0 safe list is an essential tool and a comprehensive reference for both patients and clinicians. With CAMP 2.0, allergen avoidance has never been more interactive or accessible.
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
- Tam I, Yu J. Allergic contact dermatitis in children: recommendations for patch testing. Curr Allergy Asthma Rep. 2020;20:41. doi:10.1007 /s11882-020-00939-z
- Dizdarevic A, Troensegaard W, Uldahl A, et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br J Dermatol. 2021;184:43-49. doi:10.1111/bjd.19119
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the Department of Dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220. doi:10.1111/j.1600-0536.2011.02039.x
- Scalf LA, Genebriera J, Davis MDP, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932. doi:10.1016/j.jaad.2006.11.034
- Mossing K, Dizdarevic A, Svensson Å, et al. Impact on quality of life of an intervention providing additional information to patients with allergic contact dermatitis; a randomized clinical trial. J Eur Acad Dermatol Venereol. 2022;36:2166-2171. doi:10.1111/jdv.18412
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Ingredient Breakdown: Fragrance. Think Dirty® Shop Clean. Accessed January 9, 2025. https://www.thinkdirtyapp.com/ingredient-breakdown-fragrance-3a8ef28f296a/
- Guarneri F, Corazza M, Stingeni L, et al. Myroxylon pereirae (balsam of Peru): still worth testing? Contact Dermatitis. 2021;85:269-273. doi:10.1111/cod.13839
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Balsam of Peru: past and future. Allergic Contact Dermatitis Society; 2024. https://www.contactderm.org/UserFiles/members/Balsam_of_Peru___Past_and_Future.2.pdf
- Tramontana M, Hansel K, Bianchi L, et al. Advancing the understanding of allergic contact dermatitis: from pathophysiology to novel therapeutic approaches. Front Med. 2023;10. doi:10.3389 /fmed.2023.1184289
- McNamara D. ACDS launches Contact Allergen Management Program (CAMP). Internal Med News. March 7, 2011. Accessed December 31, 2024. https://www.mdedge.com/content/acds-launches-contact-allergen-management-program-camp-0
- Haque MZ, Rehman R, Guan L, et al. Recommendations to optimize patient education for allergic contact dermatitis: our approach. Contact Dermatitis. 2023;88:423-424. doi:10.1111/cod.14269
- Kist JM, el-Azhary RA, Hentz JG, et al. The Contact Allergen Replacement Database and treatment of allergic contact dermatitis. Arch Dermatol. 2004;140:1448-1450. doi:0.1001/archderm.140.12.1448
- El-Azhary RA, Yiannias JA. A new patient education approach in contact allergic dermatitis: the Contact Allergen Replacement Database (CARD). Int J Dermatol. 2004;43:278-280. doi:10.1111 /j.1365-4632.2004.01843.x
- Scheman A, Hylwa-Deufel S, Jacob SE, et al. Alternatives for allergens in the 2018 American Contact Dermatitis Society Core Series: report by the American Contact Alternatives Group. Dermatitis. 2019;30:87-105. doi:10.1097/DER.0000000000000453
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054. doi:10.1016/j.jaad.2015.02.1144
- Ng A, Atwater AR, Reeder M. Contact allergy to topical medicaments, part 1: a double-edged sword. Cutis. 2021;108:271-275. doi:10.12788 /cutis.0390
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313. doi:10.1111 /j.1600-0536.2009.01542.x
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
Simplifying Allergic Contact Dermatitis Management with the Contact Allergen Management Program 2.0
PRACTICE POINTS
- Comprehensive patient education is critical for appropriate allergen avoidance after patch testing, and allergen databases and product safe lists are invaluable tools to complement clinical guidance.
- The updated Contact Allergen Management Program 2.0 offers an updated approach to patient guidance, including a database of more than 100,000 products and an easy-to-use platform to find safe, allergen-free products.
- Interactive learning resources, product pages, and quality-of-life tracking tools can help equip patients with information to encourage further autonomy in allergen avoidance.