User login
‘So You Have an Idea…’: A Practical Guide to Tech and Device Development for the Early Career GI
You are in the middle of a busy clinic day and think, “there has to be a better way to do this.” Suddenly, a better way to do something becomes obvious. Maybe it’s a tool that simplifies documentation, a device that improves patient comfort, or an app that bridges a clinical gap. Many physicians, especially early career gastroenterologists, have ideas like this, but few know what to do next.
This article is for the curious innovator at the beginning of their clinical career. It offers practical, real-world guidance on developing a clinical product: whether that be hardware, software, or a hybrid. It outlines what questions to ask, who to consult, and how to protect your work, using personal insights and business principles learned through lived experience.
1. Understand Intellectual Property (IP): Know Its Value and Ownership
What is IP?
Intellectual property refers to your original creations: inventions, designs, software, and more. This is what you want to protect legally through patents, trademarks, or copyrights.
Who owns your idea?
This is the first and most important question to ask. If you are employed (especially by a hospital or academic center), your contract may already give your employer rights to any inventions you create, even those developed in your personal time.
What to ask:
- Does my employment contract include an “assignment of inventions” clause?
- Does the institution claim rights to anything developed with institutional resources?
- Are there moonlighting or external activity policies that affect this?
If you are developing an idea on your personal time, with your own resources, and outside your scope of clinical duties, it might still be considered “theirs” under some contracts. Early legal consultation is critical. A specialized IP attorney can help you understand what you own and how to protect it. This should be done early, ideally before you start building anything.
2. Lawyers Aren’t Optional: They’re Essential Early Partners
You do not need a full legal team, but you do need a lawyer early. An early consultation with an IP attorney can clarify your rights, guide your filing process (e.g. provisional patents), and help you avoid costly missteps.
Do this before sharing your idea publicly, including in academic presentations, pitch competitions, or even on social media. Public disclosure can start a clock ticking for application to protect your IP.
3. Build a Founding Team with Intent
Think of your startup team like a long-term relationship: you’re committing to build something together through uncertainty, tension, and change.
Strong early-stage teams often include:
- The Visionary – understands the clinical need and vision
- The Builder – engineer, developer, or designer
- The Doer – project manager or operations lead
Before forming a company, clearly define:
- Ownership (equity percentages)
- Roles and responsibilities
- Time commitments
- What happens if someone exits
Have these discussions early and document your agreements. Avoid informal “handshake” deals that can lead to serious disputes later.
4. You Don’t Need to Know Everything on Day One
You do not need to know how to write code, build a prototype, or get FDA clearance on day one. Successful innovators are humble learners. Use a Minimum Viable Product (MVP), a simple, functional version of your idea, to test assumptions and gather feedback. Iterate based on what you learn. Do not chase perfection; pursue progress. Consider using online accelerators like Y Combinator’s startup school or AGA’s Center for GI Innovation and Technology.
5. Incubators: Use them Strategically
Incubators can offer mentorship, seed funding, legal support, and technical resources, but they vary widely in value (see Table 1). Many may want equity, and not all offer when you truly need.
Ask Yourself:
- Do I need technical help, business mentorship, or just accountability?
- What does this incubator offer in terms of IP protection, exposure, and connections?
- Do I understand the equity trade-off?
- What services and funding do they provide?
- Do they take equity? How much and when?
- What’s their track record with similar ventures?
- Are their incentives aligned with your vision?
6. Academic Institutions: Partners or Pitfalls?
Universities can provide credibility, resources, and early funding through their tech transfer office (TTO).
Key Questions to Ask:
- Will my IP be managed by the TTO?
- How much say do I have in licensing decisions?
- Are there royalty-sharing agreements in place?
- Can I form a startup while employed here?
You may need to negotiate if you want to commercialize your idea independently.
7. Do it for Purpose, Not Payday
Most founders end up owning only a small percentage of their company by the time a product reaches the market. Do not expect to get rich. Do it because it solves a problem you care about. If it happens to come with a nice paycheck, then that is an added bonus.
Your clinical training and insight give you a unique edge. You already know what’s broken. Use that as your compass.
Conclusion
Innovation isn’t about brilliance, it’s about curiosity, structure, and tenacity (see Table 2). Start small. Protect your work. Choose the right partners. Most importantly, stay anchored in your mission to make GI care better.
Dr. Muratore is based at UNC Rex Digestive Health, Raleigh, North Carolina. She has no conflicts related to this article. Dr. Wechsler is based at the University of North Carolina at Chapel Hill, Chapel Hill, North Carolina. She holds a patent assigned to Trustees of Dartmouth College. Dr. Shah is based at the University of Michigan, Ann Arbor, Michigan. He consults for Ardelyx, Laborie, Neuraxis, Salix, Sanofi, and Takeda and holds a patent with the Regents of the University of Michigan.
You are in the middle of a busy clinic day and think, “there has to be a better way to do this.” Suddenly, a better way to do something becomes obvious. Maybe it’s a tool that simplifies documentation, a device that improves patient comfort, or an app that bridges a clinical gap. Many physicians, especially early career gastroenterologists, have ideas like this, but few know what to do next.
This article is for the curious innovator at the beginning of their clinical career. It offers practical, real-world guidance on developing a clinical product: whether that be hardware, software, or a hybrid. It outlines what questions to ask, who to consult, and how to protect your work, using personal insights and business principles learned through lived experience.
1. Understand Intellectual Property (IP): Know Its Value and Ownership
What is IP?
Intellectual property refers to your original creations: inventions, designs, software, and more. This is what you want to protect legally through patents, trademarks, or copyrights.
Who owns your idea?
This is the first and most important question to ask. If you are employed (especially by a hospital or academic center), your contract may already give your employer rights to any inventions you create, even those developed in your personal time.
What to ask:
- Does my employment contract include an “assignment of inventions” clause?
- Does the institution claim rights to anything developed with institutional resources?
- Are there moonlighting or external activity policies that affect this?
If you are developing an idea on your personal time, with your own resources, and outside your scope of clinical duties, it might still be considered “theirs” under some contracts. Early legal consultation is critical. A specialized IP attorney can help you understand what you own and how to protect it. This should be done early, ideally before you start building anything.
2. Lawyers Aren’t Optional: They’re Essential Early Partners
You do not need a full legal team, but you do need a lawyer early. An early consultation with an IP attorney can clarify your rights, guide your filing process (e.g. provisional patents), and help you avoid costly missteps.
Do this before sharing your idea publicly, including in academic presentations, pitch competitions, or even on social media. Public disclosure can start a clock ticking for application to protect your IP.
3. Build a Founding Team with Intent
Think of your startup team like a long-term relationship: you’re committing to build something together through uncertainty, tension, and change.
Strong early-stage teams often include:
- The Visionary – understands the clinical need and vision
- The Builder – engineer, developer, or designer
- The Doer – project manager or operations lead
Before forming a company, clearly define:
- Ownership (equity percentages)
- Roles and responsibilities
- Time commitments
- What happens if someone exits
Have these discussions early and document your agreements. Avoid informal “handshake” deals that can lead to serious disputes later.
4. You Don’t Need to Know Everything on Day One
You do not need to know how to write code, build a prototype, or get FDA clearance on day one. Successful innovators are humble learners. Use a Minimum Viable Product (MVP), a simple, functional version of your idea, to test assumptions and gather feedback. Iterate based on what you learn. Do not chase perfection; pursue progress. Consider using online accelerators like Y Combinator’s startup school or AGA’s Center for GI Innovation and Technology.
5. Incubators: Use them Strategically
Incubators can offer mentorship, seed funding, legal support, and technical resources, but they vary widely in value (see Table 1). Many may want equity, and not all offer when you truly need.
Ask Yourself:
- Do I need technical help, business mentorship, or just accountability?
- What does this incubator offer in terms of IP protection, exposure, and connections?
- Do I understand the equity trade-off?
- What services and funding do they provide?
- Do they take equity? How much and when?
- What’s their track record with similar ventures?
- Are their incentives aligned with your vision?
6. Academic Institutions: Partners or Pitfalls?
Universities can provide credibility, resources, and early funding through their tech transfer office (TTO).
Key Questions to Ask:
- Will my IP be managed by the TTO?
- How much say do I have in licensing decisions?
- Are there royalty-sharing agreements in place?
- Can I form a startup while employed here?
You may need to negotiate if you want to commercialize your idea independently.
7. Do it for Purpose, Not Payday
Most founders end up owning only a small percentage of their company by the time a product reaches the market. Do not expect to get rich. Do it because it solves a problem you care about. If it happens to come with a nice paycheck, then that is an added bonus.
Your clinical training and insight give you a unique edge. You already know what’s broken. Use that as your compass.
Conclusion
Innovation isn’t about brilliance, it’s about curiosity, structure, and tenacity (see Table 2). Start small. Protect your work. Choose the right partners. Most importantly, stay anchored in your mission to make GI care better.
Dr. Muratore is based at UNC Rex Digestive Health, Raleigh, North Carolina. She has no conflicts related to this article. Dr. Wechsler is based at the University of North Carolina at Chapel Hill, Chapel Hill, North Carolina. She holds a patent assigned to Trustees of Dartmouth College. Dr. Shah is based at the University of Michigan, Ann Arbor, Michigan. He consults for Ardelyx, Laborie, Neuraxis, Salix, Sanofi, and Takeda and holds a patent with the Regents of the University of Michigan.
You are in the middle of a busy clinic day and think, “there has to be a better way to do this.” Suddenly, a better way to do something becomes obvious. Maybe it’s a tool that simplifies documentation, a device that improves patient comfort, or an app that bridges a clinical gap. Many physicians, especially early career gastroenterologists, have ideas like this, but few know what to do next.
This article is for the curious innovator at the beginning of their clinical career. It offers practical, real-world guidance on developing a clinical product: whether that be hardware, software, or a hybrid. It outlines what questions to ask, who to consult, and how to protect your work, using personal insights and business principles learned through lived experience.
1. Understand Intellectual Property (IP): Know Its Value and Ownership
What is IP?
Intellectual property refers to your original creations: inventions, designs, software, and more. This is what you want to protect legally through patents, trademarks, or copyrights.
Who owns your idea?
This is the first and most important question to ask. If you are employed (especially by a hospital or academic center), your contract may already give your employer rights to any inventions you create, even those developed in your personal time.
What to ask:
- Does my employment contract include an “assignment of inventions” clause?
- Does the institution claim rights to anything developed with institutional resources?
- Are there moonlighting or external activity policies that affect this?
If you are developing an idea on your personal time, with your own resources, and outside your scope of clinical duties, it might still be considered “theirs” under some contracts. Early legal consultation is critical. A specialized IP attorney can help you understand what you own and how to protect it. This should be done early, ideally before you start building anything.
2. Lawyers Aren’t Optional: They’re Essential Early Partners
You do not need a full legal team, but you do need a lawyer early. An early consultation with an IP attorney can clarify your rights, guide your filing process (e.g. provisional patents), and help you avoid costly missteps.
Do this before sharing your idea publicly, including in academic presentations, pitch competitions, or even on social media. Public disclosure can start a clock ticking for application to protect your IP.
3. Build a Founding Team with Intent
Think of your startup team like a long-term relationship: you’re committing to build something together through uncertainty, tension, and change.
Strong early-stage teams often include:
- The Visionary – understands the clinical need and vision
- The Builder – engineer, developer, or designer
- The Doer – project manager or operations lead
Before forming a company, clearly define:
- Ownership (equity percentages)
- Roles and responsibilities
- Time commitments
- What happens if someone exits
Have these discussions early and document your agreements. Avoid informal “handshake” deals that can lead to serious disputes later.
4. You Don’t Need to Know Everything on Day One
You do not need to know how to write code, build a prototype, or get FDA clearance on day one. Successful innovators are humble learners. Use a Minimum Viable Product (MVP), a simple, functional version of your idea, to test assumptions and gather feedback. Iterate based on what you learn. Do not chase perfection; pursue progress. Consider using online accelerators like Y Combinator’s startup school or AGA’s Center for GI Innovation and Technology.
5. Incubators: Use them Strategically
Incubators can offer mentorship, seed funding, legal support, and technical resources, but they vary widely in value (see Table 1). Many may want equity, and not all offer when you truly need.
Ask Yourself:
- Do I need technical help, business mentorship, or just accountability?
- What does this incubator offer in terms of IP protection, exposure, and connections?
- Do I understand the equity trade-off?
- What services and funding do they provide?
- Do they take equity? How much and when?
- What’s their track record with similar ventures?
- Are their incentives aligned with your vision?
6. Academic Institutions: Partners or Pitfalls?
Universities can provide credibility, resources, and early funding through their tech transfer office (TTO).
Key Questions to Ask:
- Will my IP be managed by the TTO?
- How much say do I have in licensing decisions?
- Are there royalty-sharing agreements in place?
- Can I form a startup while employed here?
You may need to negotiate if you want to commercialize your idea independently.
7. Do it for Purpose, Not Payday
Most founders end up owning only a small percentage of their company by the time a product reaches the market. Do not expect to get rich. Do it because it solves a problem you care about. If it happens to come with a nice paycheck, then that is an added bonus.
Your clinical training and insight give you a unique edge. You already know what’s broken. Use that as your compass.
Conclusion
Innovation isn’t about brilliance, it’s about curiosity, structure, and tenacity (see Table 2). Start small. Protect your work. Choose the right partners. Most importantly, stay anchored in your mission to make GI care better.
Dr. Muratore is based at UNC Rex Digestive Health, Raleigh, North Carolina. She has no conflicts related to this article. Dr. Wechsler is based at the University of North Carolina at Chapel Hill, Chapel Hill, North Carolina. She holds a patent assigned to Trustees of Dartmouth College. Dr. Shah is based at the University of Michigan, Ann Arbor, Michigan. He consults for Ardelyx, Laborie, Neuraxis, Salix, Sanofi, and Takeda and holds a patent with the Regents of the University of Michigan.
When Your First Job Isn’t Forever: Lessons from My Journey and What Early-Career GIs Need to Know
Introduction
For many of us in gastroenterology, landing that first attending job feels like the ultimate victory lap — the reward for all those years of training. We sign the contract, relocate, and imagine this will be our “forever job.” Reality often plays out differently.
In fact, 43% of physicians change jobs within five years, while 83% changed employers at least once in their careers.1 Even within our field — which is always in demand — turnover is high; 1 in 3 gastroenterologists are planning to leave their current role within two years.2 Why does this happen? More importantly, how do we navigate this transition with clarity and confidence as an early-career GI?
My Story: When I Dared to Change My “Forever Job”
When I signed my first attending contract, I didn’t negotiate a single thing. My priorities were simple: family in Toronto and visa requirements. After a decade of medical school, residency, and fellowship, everything else felt secondary. I was happy to be back home.
The job itself was good — reasonable hours, flexible colleagues, and ample opportunity to enhance my procedural skills. As I started carving out my niche in endobariatrics, the support I needed to grow further was not there. I kept telling myself that this job fulfilled my values and I needed to be patient: “this is my forever job. I am close to my family and that’s what matters.”
Then, during a suturing course at the American Society of Gastrointestinal Endoscopy, I had a casual chat with the course director (now my boss). It took me by surprise, but as the conversation continued, he offered me a job. It was tempting: the chance to build my own endobariatrics program with real institutional backing. The catch? It was in a city I had never been to, with no family or friends around. I politely said “no, thank you, I can’t.” He smiled, gave me his number, and said, “think about it.”
For the first time, I allowed myself to ask, “could I really leave my forever job?”
The Power of a Circle and a Spreadsheet
I leaned on my circle — a close group of fellowship friends who each took a turn being someone’s lifeline. We have monthly Zoom calls to talk about jobs, family, and career aspirations. When I shared my dilemma, I realized I wasn’t alone; one friend was also unhappy with her first job. Suddenly, we were asking one another, “can we really leave?”
I hired a career consultant familiar with physician visa issues — hands down, the best money I ever invested. The job search felt like dating: each interview was a first date; some needed a second or third date before I knew if it could be a match.
After every interview, I’d jump on Zoom with my circle. We’d screen-share my giant Excel spreadsheet — our decision matrix — with columns for everything I cared about:
- Institute
- Administrative Time
- Endobariatric support
- Director Title
- Salary
- On-call
- Vacation
- Proximity to airport
- Cost of living
- RVU percentage
- Endoscopy center buy-in
- Contract duration
- Support staff
- CME
We scored each job, line by line, and not a single job checked all the boxes. As I sat there in a state of decision paralysis, it became clear that this was not a simple decision.
The GI Community: A Small, Supportive World
The GI community is incredibly close-knit and kind-hearted. At every conference, I made a point to chat with as many colleagues as I could, to hear their perspectives on jobs and how they made tough career moves. Those conversations were real — no Google search or Excel sheet could offer the perspective and insight I gained by simply asking and leaning on the GI community.
Meanwhile, the person who had first offered me that job kept checking in, catching up at conferences, and bonding over our love for food and baking. With him, I never felt like I was being ‘interviewed’ — I felt valued. It did not feel like he was trying to fill a position with just anyone to improve the call pool. He genuinely wanted to understand what my goals were and how I envisioned my future. Through those conversations, he reminded me of my original passions, which were sidelined when so immersed in the daily routine.
I’ve learned that feeling valued doesn’t come from grand gestures in recruitment. It’s in the quiet signs of respect, trust, and being seen. He wasn’t looking for just anyone; he was looking for someone whose goals aligned with his group’s and someone in whom he wanted to invest. While others might chase the highest salary, the most flexible schedule, or the strongest ancillary support, I realized I valued something I did not realize that I was lacking until then: mentorship.
What I Learned: There is No Such Thing As “The Perfect Job”
After a full year of spreadsheets, Zoom calls, conference chats, and overthinking, I came to a big realization: there’s no perfect job — there’s no such thing as an ideal “forever job.” The only constant for humans is change. Our circumstances change, our priorities shift, our interests shuffle, and our finances evolve. The best job is simply the one that fits the stage of life you’re in at that given moment. For me, mentorship and growth became my top priorities, even if it meant moving away from family.
What Physicians Value Most in a Second Job
After their first job, early-career gastroenterologists often reevaluate what really matters. Recent surveys highlight four key priorities:
- Work-life balance:
In a 2022 CompHealth Group healthcare survey, 85% of physicians ranked work-life balance as their top job priority.3
- Mentorship and growth:
Nearly 1 in 3 physicians cited lack of mentorship or career advancement as their reason for leaving a first job, per the 2023 MGMA/Jackson Physician Search report.4
- Compensation:
While not always the main reason for leaving, 77% of physicians now list compensation as a top priority — a big jump from prior years.3
- Practice support:
Poor infrastructure, administrative overload, or understaffed teams are common dealbreakers. In the second job, physicians look for well-run practices with solid support staff and reduced burnout risk.5
Conclusion
Welcome the uncertainty, talk to your circle, lean on your community, and use a spreadsheet if you need to — but don’t forget to trust your gut. There’s no forever job or the perfect path, only the next move that feels most true to who you are in that moment.
Dr. Ismail (@mayyismail) is Assistant Professor of Clinical Medicine (Gastroenterology) at Temple University in Philadelphia, Pennsylvania. She declares no conflicts of interest.
References
1. CHG Healthcare. Survey: 62% of physicians made a career change in the last two years. CHG Healthcare blog. June 10, 2024. Accessed August 5, 2025.
2. Berg S. Physicians in these 10 specialties are less likely to quit. AMA News. Published June 24, 2025. Accessed July 2025.
3. Saley C. Survey: Work/life balance is #1 priority in physicians’ job search. CHG Healthcare Insights. March 10, 2022. Accessed August 2025.
4. Medical Group Management Association; Jackson Physician Search. Early‑Career Physician Recruiting & Retention Playbook. October 23, 2023. Accessed August 2025.
5. Von Rosenvinge EC, et al. A crisis in scope: Recruitment and retention challenges reported by VA gastroenterology section chiefs. Fed Pract. 2024 Aug. doi:10.12788/fp.0504.
Introduction
For many of us in gastroenterology, landing that first attending job feels like the ultimate victory lap — the reward for all those years of training. We sign the contract, relocate, and imagine this will be our “forever job.” Reality often plays out differently.
In fact, 43% of physicians change jobs within five years, while 83% changed employers at least once in their careers.1 Even within our field — which is always in demand — turnover is high; 1 in 3 gastroenterologists are planning to leave their current role within two years.2 Why does this happen? More importantly, how do we navigate this transition with clarity and confidence as an early-career GI?
My Story: When I Dared to Change My “Forever Job”
When I signed my first attending contract, I didn’t negotiate a single thing. My priorities were simple: family in Toronto and visa requirements. After a decade of medical school, residency, and fellowship, everything else felt secondary. I was happy to be back home.
The job itself was good — reasonable hours, flexible colleagues, and ample opportunity to enhance my procedural skills. As I started carving out my niche in endobariatrics, the support I needed to grow further was not there. I kept telling myself that this job fulfilled my values and I needed to be patient: “this is my forever job. I am close to my family and that’s what matters.”
Then, during a suturing course at the American Society of Gastrointestinal Endoscopy, I had a casual chat with the course director (now my boss). It took me by surprise, but as the conversation continued, he offered me a job. It was tempting: the chance to build my own endobariatrics program with real institutional backing. The catch? It was in a city I had never been to, with no family or friends around. I politely said “no, thank you, I can’t.” He smiled, gave me his number, and said, “think about it.”
For the first time, I allowed myself to ask, “could I really leave my forever job?”
The Power of a Circle and a Spreadsheet
I leaned on my circle — a close group of fellowship friends who each took a turn being someone’s lifeline. We have monthly Zoom calls to talk about jobs, family, and career aspirations. When I shared my dilemma, I realized I wasn’t alone; one friend was also unhappy with her first job. Suddenly, we were asking one another, “can we really leave?”
I hired a career consultant familiar with physician visa issues — hands down, the best money I ever invested. The job search felt like dating: each interview was a first date; some needed a second or third date before I knew if it could be a match.
After every interview, I’d jump on Zoom with my circle. We’d screen-share my giant Excel spreadsheet — our decision matrix — with columns for everything I cared about:
- Institute
- Administrative Time
- Endobariatric support
- Director Title
- Salary
- On-call
- Vacation
- Proximity to airport
- Cost of living
- RVU percentage
- Endoscopy center buy-in
- Contract duration
- Support staff
- CME
We scored each job, line by line, and not a single job checked all the boxes. As I sat there in a state of decision paralysis, it became clear that this was not a simple decision.
The GI Community: A Small, Supportive World
The GI community is incredibly close-knit and kind-hearted. At every conference, I made a point to chat with as many colleagues as I could, to hear their perspectives on jobs and how they made tough career moves. Those conversations were real — no Google search or Excel sheet could offer the perspective and insight I gained by simply asking and leaning on the GI community.
Meanwhile, the person who had first offered me that job kept checking in, catching up at conferences, and bonding over our love for food and baking. With him, I never felt like I was being ‘interviewed’ — I felt valued. It did not feel like he was trying to fill a position with just anyone to improve the call pool. He genuinely wanted to understand what my goals were and how I envisioned my future. Through those conversations, he reminded me of my original passions, which were sidelined when so immersed in the daily routine.
I’ve learned that feeling valued doesn’t come from grand gestures in recruitment. It’s in the quiet signs of respect, trust, and being seen. He wasn’t looking for just anyone; he was looking for someone whose goals aligned with his group’s and someone in whom he wanted to invest. While others might chase the highest salary, the most flexible schedule, or the strongest ancillary support, I realized I valued something I did not realize that I was lacking until then: mentorship.
What I Learned: There is No Such Thing As “The Perfect Job”
After a full year of spreadsheets, Zoom calls, conference chats, and overthinking, I came to a big realization: there’s no perfect job — there’s no such thing as an ideal “forever job.” The only constant for humans is change. Our circumstances change, our priorities shift, our interests shuffle, and our finances evolve. The best job is simply the one that fits the stage of life you’re in at that given moment. For me, mentorship and growth became my top priorities, even if it meant moving away from family.
What Physicians Value Most in a Second Job
After their first job, early-career gastroenterologists often reevaluate what really matters. Recent surveys highlight four key priorities:
- Work-life balance:
In a 2022 CompHealth Group healthcare survey, 85% of physicians ranked work-life balance as their top job priority.3
- Mentorship and growth:
Nearly 1 in 3 physicians cited lack of mentorship or career advancement as their reason for leaving a first job, per the 2023 MGMA/Jackson Physician Search report.4
- Compensation:
While not always the main reason for leaving, 77% of physicians now list compensation as a top priority — a big jump from prior years.3
- Practice support:
Poor infrastructure, administrative overload, or understaffed teams are common dealbreakers. In the second job, physicians look for well-run practices with solid support staff and reduced burnout risk.5
Conclusion
Welcome the uncertainty, talk to your circle, lean on your community, and use a spreadsheet if you need to — but don’t forget to trust your gut. There’s no forever job or the perfect path, only the next move that feels most true to who you are in that moment.
Dr. Ismail (@mayyismail) is Assistant Professor of Clinical Medicine (Gastroenterology) at Temple University in Philadelphia, Pennsylvania. She declares no conflicts of interest.
References
1. CHG Healthcare. Survey: 62% of physicians made a career change in the last two years. CHG Healthcare blog. June 10, 2024. Accessed August 5, 2025.
2. Berg S. Physicians in these 10 specialties are less likely to quit. AMA News. Published June 24, 2025. Accessed July 2025.
3. Saley C. Survey: Work/life balance is #1 priority in physicians’ job search. CHG Healthcare Insights. March 10, 2022. Accessed August 2025.
4. Medical Group Management Association; Jackson Physician Search. Early‑Career Physician Recruiting & Retention Playbook. October 23, 2023. Accessed August 2025.
5. Von Rosenvinge EC, et al. A crisis in scope: Recruitment and retention challenges reported by VA gastroenterology section chiefs. Fed Pract. 2024 Aug. doi:10.12788/fp.0504.
Introduction
For many of us in gastroenterology, landing that first attending job feels like the ultimate victory lap — the reward for all those years of training. We sign the contract, relocate, and imagine this will be our “forever job.” Reality often plays out differently.
In fact, 43% of physicians change jobs within five years, while 83% changed employers at least once in their careers.1 Even within our field — which is always in demand — turnover is high; 1 in 3 gastroenterologists are planning to leave their current role within two years.2 Why does this happen? More importantly, how do we navigate this transition with clarity and confidence as an early-career GI?
My Story: When I Dared to Change My “Forever Job”
When I signed my first attending contract, I didn’t negotiate a single thing. My priorities were simple: family in Toronto and visa requirements. After a decade of medical school, residency, and fellowship, everything else felt secondary. I was happy to be back home.
The job itself was good — reasonable hours, flexible colleagues, and ample opportunity to enhance my procedural skills. As I started carving out my niche in endobariatrics, the support I needed to grow further was not there. I kept telling myself that this job fulfilled my values and I needed to be patient: “this is my forever job. I am close to my family and that’s what matters.”
Then, during a suturing course at the American Society of Gastrointestinal Endoscopy, I had a casual chat with the course director (now my boss). It took me by surprise, but as the conversation continued, he offered me a job. It was tempting: the chance to build my own endobariatrics program with real institutional backing. The catch? It was in a city I had never been to, with no family or friends around. I politely said “no, thank you, I can’t.” He smiled, gave me his number, and said, “think about it.”
For the first time, I allowed myself to ask, “could I really leave my forever job?”
The Power of a Circle and a Spreadsheet
I leaned on my circle — a close group of fellowship friends who each took a turn being someone’s lifeline. We have monthly Zoom calls to talk about jobs, family, and career aspirations. When I shared my dilemma, I realized I wasn’t alone; one friend was also unhappy with her first job. Suddenly, we were asking one another, “can we really leave?”
I hired a career consultant familiar with physician visa issues — hands down, the best money I ever invested. The job search felt like dating: each interview was a first date; some needed a second or third date before I knew if it could be a match.
After every interview, I’d jump on Zoom with my circle. We’d screen-share my giant Excel spreadsheet — our decision matrix — with columns for everything I cared about:
- Institute
- Administrative Time
- Endobariatric support
- Director Title
- Salary
- On-call
- Vacation
- Proximity to airport
- Cost of living
- RVU percentage
- Endoscopy center buy-in
- Contract duration
- Support staff
- CME
We scored each job, line by line, and not a single job checked all the boxes. As I sat there in a state of decision paralysis, it became clear that this was not a simple decision.
The GI Community: A Small, Supportive World
The GI community is incredibly close-knit and kind-hearted. At every conference, I made a point to chat with as many colleagues as I could, to hear their perspectives on jobs and how they made tough career moves. Those conversations were real — no Google search or Excel sheet could offer the perspective and insight I gained by simply asking and leaning on the GI community.
Meanwhile, the person who had first offered me that job kept checking in, catching up at conferences, and bonding over our love for food and baking. With him, I never felt like I was being ‘interviewed’ — I felt valued. It did not feel like he was trying to fill a position with just anyone to improve the call pool. He genuinely wanted to understand what my goals were and how I envisioned my future. Through those conversations, he reminded me of my original passions, which were sidelined when so immersed in the daily routine.
I’ve learned that feeling valued doesn’t come from grand gestures in recruitment. It’s in the quiet signs of respect, trust, and being seen. He wasn’t looking for just anyone; he was looking for someone whose goals aligned with his group’s and someone in whom he wanted to invest. While others might chase the highest salary, the most flexible schedule, or the strongest ancillary support, I realized I valued something I did not realize that I was lacking until then: mentorship.
What I Learned: There is No Such Thing As “The Perfect Job”
After a full year of spreadsheets, Zoom calls, conference chats, and overthinking, I came to a big realization: there’s no perfect job — there’s no such thing as an ideal “forever job.” The only constant for humans is change. Our circumstances change, our priorities shift, our interests shuffle, and our finances evolve. The best job is simply the one that fits the stage of life you’re in at that given moment. For me, mentorship and growth became my top priorities, even if it meant moving away from family.
What Physicians Value Most in a Second Job
After their first job, early-career gastroenterologists often reevaluate what really matters. Recent surveys highlight four key priorities:
- Work-life balance:
In a 2022 CompHealth Group healthcare survey, 85% of physicians ranked work-life balance as their top job priority.3
- Mentorship and growth:
Nearly 1 in 3 physicians cited lack of mentorship or career advancement as their reason for leaving a first job, per the 2023 MGMA/Jackson Physician Search report.4
- Compensation:
While not always the main reason for leaving, 77% of physicians now list compensation as a top priority — a big jump from prior years.3
- Practice support:
Poor infrastructure, administrative overload, or understaffed teams are common dealbreakers. In the second job, physicians look for well-run practices with solid support staff and reduced burnout risk.5
Conclusion
Welcome the uncertainty, talk to your circle, lean on your community, and use a spreadsheet if you need to — but don’t forget to trust your gut. There’s no forever job or the perfect path, only the next move that feels most true to who you are in that moment.
Dr. Ismail (@mayyismail) is Assistant Professor of Clinical Medicine (Gastroenterology) at Temple University in Philadelphia, Pennsylvania. She declares no conflicts of interest.
References
1. CHG Healthcare. Survey: 62% of physicians made a career change in the last two years. CHG Healthcare blog. June 10, 2024. Accessed August 5, 2025.
2. Berg S. Physicians in these 10 specialties are less likely to quit. AMA News. Published June 24, 2025. Accessed July 2025.
3. Saley C. Survey: Work/life balance is #1 priority in physicians’ job search. CHG Healthcare Insights. March 10, 2022. Accessed August 2025.
4. Medical Group Management Association; Jackson Physician Search. Early‑Career Physician Recruiting & Retention Playbook. October 23, 2023. Accessed August 2025.
5. Von Rosenvinge EC, et al. A crisis in scope: Recruitment and retention challenges reported by VA gastroenterology section chiefs. Fed Pract. 2024 Aug. doi:10.12788/fp.0504.
Developing the Next Generation of GI Leaders

In this episode of Private Practice Perspectives, Dr. Naresh Gunaratnam, current president and board chair of Digestive Health Physician Association, speaks with Dr. Larry Kim, current president of AGA, about .

In this episode of Private Practice Perspectives, Dr. Naresh Gunaratnam, current president and board chair of Digestive Health Physician Association, speaks with Dr. Larry Kim, current president of AGA, about .

In this episode of Private Practice Perspectives, Dr. Naresh Gunaratnam, current president and board chair of Digestive Health Physician Association, speaks with Dr. Larry Kim, current president of AGA, about .
Approach to Weight Management in GI Practice
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
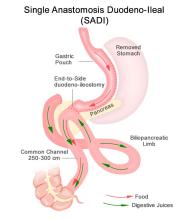
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
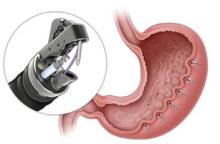
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Medical Liability for the Gastroenterologist
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
While nearly 75% of physicians in low-risk specialties and 99% of physicians in high-risk specialties may face a malpractice claim in their careers,1 malpractice is rarely discussed openly in medical school, residency, fellowship, or even amongst colleagues. Indeed, one study suggested that more than 10% of practicing gastroenterologists may face a malpractice claim,2 with gastroenterologists expected to spend 10-15% of their careers with an outstanding malpractice claim3 as cases may take 27-29 months to resolve on average.4
Believing that if a physician is sued, one must have done something “wrong” or that speaking about one’s experience may implicate a colleague, creates an intense stigma and isolation that only serves to aggravate the “second victim syndrome” (SVS) that is well documented in the surgical literature.2 Herein,
What is Malpractice? Why Do Physicians Get Sued?
Malpractice is defined as negligence on the part of a physician which causes physical or emotional damage to the patient. This requires a variety of legal issues to be evaluated (e.g. breach of duty between the physicians and patient, breach of standard of care), that often center around the question: would a “reasonable, careful, and prudent” doctor behave in the same manner in the same circumstance?
While some fields of medicine lend themselves better to algorithmic applications of highly evidence-based guidelines, many aspects of GI care and endoscopic practice are highly physician/patient-specific, dependent on local expertise, and based on low-quality evidence. As a result, an assessment of negligence may be quite subjective, depending on the expert retained by a plaintiff. Conflicting expert testimony on what professional custom is and whether practice deviates may hinge on technical details that may or may not be appreciated by a lay jury.
Plaintiffs must prove both that they have sustained an injury and that the injury (emotional or physical) was due to the physician’s negligence. While this may be straightforward in a “slip-and-fall” tort claim, medical malpractice claims usually involve sick patients with multiple comorbidities, where assigning causality to a single intervention/misinterpretation/missed opportunity is difficult to weigh against competing causes of adverse outcomes. Assessing a specific liability requires that the plaintiff prove this to a “more likely than not” standard which may be part of the reason why only 30% of cases are closed with indemnity payments, a figure that has not changed significantly in the past decade.4
While the perception amongst physicians is that tort legislation is ever increasing, data from the National Practitioner Data Bank (NPDB) demonstrates that the number of paid claims against physicians has decreased by 75% in the last 20 years.5 This may reflect a progressive improvement in the quality of care delivered or success of “tort reform” on the state level to limit damages and “nuisance” lawsuits. However, another more problematic possibility is that with the corporatization of medicine, an untold number of physicians may be removed from litigation as a named party, with their institution shielding them from reporting. While the number of cases may or may not be declining, the average indemnity payment appears to be rising to $330,000 on average,4 with one study suggesting a significant growth in paid claims in gastroenterology.6
Historically, studies of closed malpractice claims have demonstrated that 59% involved diagnostic errors involving a cancer diagnosis,7 though why this actually happens may be for a wide variety of reasons including errors in the development of a differential diagnosis, ordering of an appropriate diagnostic test, interpretation of the diagnostic test, or follow-up of an abnormality identified.
What are the Intended/Unintended Consequences of Litigation?
The objective of our tort system is to compensate patients for economic damages (medical costs and lost wages) and non-economic damages (pain and suffering), and to ideally deter negligent behavior of providers. Interestingly, data from the NPDB have suggested that approximately 1% of all physicians account for 32% of all paid claims, with the same study showing that among physicians with paid claims, 4% had at least 3.8
While certain fields are obviously more prone to litigation, the risk of additional claims on a physician with 3 prior claims was more than 3 times that of physicians with 1 lifetime claim. One would assume that the system was built to drive out a small proportion of “bad actors.” Indeed, similar data from the NPDB has demonstrated that the number of claims against physicians was associated both with their leaving the practice of medicine and relocating to smaller practice settings.9
Another frequent question is whether the threat of litigation drives “defensive medicine” (i.e. medical care that is not beneficial) or avoidance medicine (i.e. excluding high risk patients and procedures from ones’ practice). These behaviors have been well documented in physicians around the world,10 as well as several surveys of gastroenterologists specifically suggesting regular ordering of unnecessary imaging/endoscopy and referrals of patients to specialists that may not be necessary.11,12
However, does defensive medicine work: does spending more prevent you from being the target of a lawsuit? In an observational study in Florida from 2000-2009, researchers demonstrated that across specialties, greater average spending by physicians was associated with a reduced risk of incurring a malpractice claim. Indeed, the likelihood of a top quintile spending internist having a malpractice incident vs a bottom quintile spending internist was 0.3% vs 1.5%.13
Approximately 10.4-43.3% of physicians may experience SVS, experiencing trauma after an adverse patient event/medical error, manifesting with psychological trauma (shame, guilt, anxiety) and cognitive limitations (burnout, stress).2 Significant emotional consequences are common on the part of the physician and have well-documented stages to recovery,14 which if ignored may lead to long-term detrimental mental/emotional health of the physician and their future patients.
Specifically, in one study, 80.8% of physicians who had a closed malpractice claim reported significant emotional distress (regardless of the legal outcome), with frequent reports of mood symptoms that affected professional conduct.15 Recognizing these effects and implementing peer counseling and institutional support may help to expedite recovery and mitigate future adverse career outcomes.14
Anatomy/Timeline of a Liability Lawsuit
Medical malpractice cases are heard in state courts, in the jurisdictions where the care was provided. From the time an event occurs to when a jury verdict may be rendered may take 4-5 years or more depending on the local statute of limitations, discovery process, backlog of the local case docket, and specific circumstances of the case. The length of time is important to consider given the likelihood that a physician may advance in training or move practice locations during the course of litigation. Several common myths surrounding this process are summarized in the accompanying box, titled “Myths Surrounding Medical Liability Litigation.”
The plaintiff faces a statute of limitations to file a lawsuit that may range from 1-6 years depending on the state. The first indication that legal action may be pending will generally be a plaintiff’s formal request for medical records. After these records are reviewed, the plaintiff’s attorney will consult one or more experts (often credentialed in the same specialty) to assess if the case is viable and to ultimately form the basis of an affidavit of merit from a plaintiff expert.
Once the lawsuit is filed, the physician(s) named will be assigned an attorney by their employer/insurance company. A state medical board malpractice questionnaire will generally follow that will seek to independently evaluate the alleged malpractice with interrogatives to determine if censure is warranted. There is a formal response to the plaintiff’s petition by the defense and then the discovery phase begins where both sides depose the defendants/plaintiffs and retain medical experts that are favorable to their arguments.
In choosing potential “experts,” physicians must ensure that they are willing/able to be present for a potential trial, do not have any personal/professional/academic conflicts with the defendants, and are willing to provide compelling testimony to a jury. A pre-trial conference and trial date is set which may be >12 months away depending on the local docket. While the amount of time a trial may take is variable, it may be up to 5-7 days that the defendants are expected to be in court in addition to days where depositions are being taken.
During the discovery process, dismissal of the physician from the lawsuit is pursued. In addition, settlement negotiations generally proceed in parallel with discovery process and may result in a pre-trial/pre-verdict settlement. Once a verdict is reached, whether for the plaintiff or the defendant, the case may be appealed, and the trial preparation process may be repeated.
Conclusions
Awareness of the medical liability process is critical for trainees and attendings alike, given the high likelihood of litigation in a gastroenterologist’s career. Specific considerations like local tort law and malpractice coverage are important to be familiar. Ongoing health services research help to shape our understanding on the intended and unintended consequences of litigation on medicine, though detailed data on outcomes/settlements are limited by confidentiality agreements, which may hamper efforts to improve patient safety.
Dr. Das is associate professor of medicine in the Division of Gastroenterology at Washington University School of Medicine, St. Louis, Missouri. He has served as a consultant for Olympus, but has no other relevant conflicts.
References
1. Jena AB, et al. Malpractice Risk According to Physician Specialty. N Engl J Med. 2011 Aug. doi: 10.1056/NEJMsa1012370.
2. Chong RIH, et al. Scoping review of the second victim syndrome among surgeons: Understanding the impact, responses, and support systems. Am J Surg 2024 Mar. doi: 10.1016/j.amjsurg.2023.09.045.
3. Seabury S, et al. On Average, Physicians Spend Nearly 11 Percent Of Their 40-Year Careers With An Open, Unresolved Malpractice Claim. Health Aff Proj Hope. 2013 Jan. doi: 10.1377/hlthaff.2012.0967.
4. CRICO Strategies. Medical Malpractice in America: A 10-Year Asessment with Insights. 2018. Accessed Apr 28, 2025.
5. Studdert DM, Hall MA. Medical Malpractice Law — Doctrine and Dynamics. N Engl J Med 2022 Oct. doi: 10.1056/NEJMp2201675.
6. Schaffer AC, et al. Rates and Characteristics of Paid Malpractice Claims Among US Physicians by Specialty, 1992-2014. JAMA Intern Med. 2017 May. doi: 10.1001/jamainternmed.2017.0311.
7. Gandhi TK, et al. Missed and Delayed Diagnoses in the Ambulatory Setting: A Study of Closed Malpractice Claims. Ann Intern Med. 2006 Oct. doi: 10.7326/0003-4819-145-7-200610030-00006.
8. Studdert DM, et al. Prevalence and Characteristics of Physicians Prone to Malpractice Claims. N Engl J Med. 2016 Jan. doi: 10.1056/NEJMsa1506137.
9. Studdert DM, et al. Changes in Practice among Physicians with Malpractice Claims. N Engl J Med. 2019 Mar. doi: 10.1056/NEJMsa1809981.
10. Ries NM, Jansen J. Physicians’ views and experiences of defensive medicine: An international review of empirical research. Health Policy. 2021 May. doi: 10.1016/j.healthpol.2021.02.005.
11. Hiyama T, et al. Defensive medicine practices among gastroenterologists in Japan. World J Gastroenterol. 2006 Dec. doi: 10.3748/wjg.v12.i47.7671.
12. Elli L, et al. Defensive medicine practices among gastroenterologists in Lombardy: Between lawsuits and the economic crisis. Dig Liver Dis. 2013 Jun. doi: 10.1016/j.dld.2013.01.004.
13. Jena AB, et al. Physician spending and subsequent risk of malpractice claims: observational study. BMJ. 2015 Nov. doi: 10.1136/bmj.h5516.
14. Scott SD, et al. The natural history of recovery for the healthcare provider “second victim” after adverse patient events. BMJ Qual Saf. 2009 Oct. doi: 10.1136/qshc.2009.032870.
15. Gómez-Durán EL, et al. Physicians as second victims after a malpractice claim: An important issue in need of attention. J Healthc Qual Res. 2018 Oct. doi: 10.1016/j.jhqr.2018.06.002.
Remembering Why We Are In Medicine
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Journal Highlights: May-July 2025
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Positioning Yourself For Success in Private Practice

In this video, Peter Naas, MD, of Gastroenterology Associates in Greenville, South Carolina, shares insights on how young physicians can best position themselves for a successful career in private practice gastroenterology.

In this video, Peter Naas, MD, of Gastroenterology Associates in Greenville, South Carolina, shares insights on how young physicians can best position themselves for a successful career in private practice gastroenterology.

In this video, Peter Naas, MD, of Gastroenterology Associates in Greenville, South Carolina, shares insights on how young physicians can best position themselves for a successful career in private practice gastroenterology.
GLP-1 Receptor Agonist Use in Gastrointestinal Endoscopy: A Review of Current Evidence and Guidelines
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has increased over the past several years and has become a cornerstone in both diabetes and weight loss management, particularly because of its unique combination of glucose control, weight reduction potential, and cardiac and metabolic benefits. However, increased use of these agents presents a dilemma in gastrointestinal endoscopy as it pertains to their safety and management during the periprocedural period.
highlighting gaps and future directions.
Pharmacology and Mechanisms of Action
GLP-1 RAs have several mechanisms of action that make them relevant in gastrointestinal endoscopy. These medications modulate glucose control via enhancement of glucose-dependent insulin secretion and reduction of postprandial glucagon, which promotes satiety and delays gastric emptying. This delay in gastric emptying mediated by vagal pathways has been postulated to increase gastric residuals, posing a risk for aspiration during anesthesia.1
It is important to also consider the pharmacokinetics of GLP-1 RAs, as some have shorter half-lives on the order of several hours, like exenatide, while others, like semaglutide, are dosed weekly. Additionally, common side effects of GLP-1 RAs include nausea, vomiting, bloating, and early satiety, which pose challenges for patients undergoing endoscopic procedures.
Current Guidelines
Various societies have published guidelines on the periprocedural use of GLP-1 RAs. The American Society of Anesthesiologist (ASA) in 2023 presented early recommendations to hold GLP-1 RAs either day of procedure or week prior depending on pharmacokinetics, because of the risk of delayed gastric emptying and increased potential for aspiration.2 Soon thereafter, a multi-gastroenterology society guideline was released stating more data is needed to decide if GLP-1 RAs need to be held prior to endoscopic procedures.3
In early 2024, the American Gastroenterological Association (AGA) published a rapid clinical update that advocated for a more individualized approach, particularly in light of limited overall data for GLP-1 RAs and endoscopic procedures.4 In asymptomatic patients who follow typical fasting protocols for procedures, it is generally safe to proceed with endoscopy without holding GLP-1 RAs. In symptomatic patients (nausea, abdominal distension, etc), the AGA advises additional precautions, including performing transabdominal ultrasound if feasible to assess retained gastric contents. The AGA also suggests placing a patient on a clear liquid diet the day prior to the procedure — rather than holding GLP-1 RAs — as another reasonable strategy.
The guidelines continue to evolve with newer multi-society guidelines establishing best practices. While initially in 2023 the ASA did recommend holding these medications prior to endoscopy, the initial guidance was based on expert opinion with limited evidence. Newer multi-society guidance published jointly by the ASA along with various gastroenterology societies, including the AGA in December 2024, takes a more nuanced approach.5
The newer guidelines include two main recommendations:
1. Periprocedural management of GLP-1 RAs should be a joint decision among the procedural, anesthesia, and prescribing team balancing metabolic needs vs patient risks.
- In a low-risk patient, one that is asymptomatic and on standard dosing, among other factors, the guidance states that GLP-1 RAs can be continued.
- In higher-risk patients, the original guidance of holding a day or a week prior to endoscopic procedures should be followed.
2. Periprocedural management of GLP-1 RAs should attempt to minimize the aspiration risks loosely associated with delayed gastric emptying.
- Consider a 24-hour clear liquid diet a day prior to the procedure and transabdominal ultrasound to check gastric contents.
- It is acknowledged that this guidance is based on limited evidence and will be evolving as new medications and data are released.
Recent Clinical Studies
Although there is very little data to guide clinicians, several recent studies have been published that can direct clinical decision-making as guidelines continue to be refined and updated.
A multicenter trial of approximately 800 patients undergoing upper endoscopy found a significant difference in rates of retained gastric contents between those that underwent endoscopy who did and did not follow the ASA guidance on periprocedural management of GLP-1 RAs (12.7% vs 4.4%; P < .0001). However, there were no significant differences in rates of aborted procedures or unplanned intubations.
Furthermore, a multivariable analysis was performed controlling for GLP-1 RA type and other factors, which found the likelihood of gastric retention increased by 36% for every 1% increase in hemoglobin A1c. This study suggests that a more individualized approach to holding GLP-1 RA would be applicable rather than a universal periprocedural hold.6
More recently, a single-center study of nearly 600 patients undergoing upper endoscopy showed that while there were slightly increased rates of retained gastric contents (OR 3.80; P = .003) and aborted procedures (1.3% vs 0%; P = .02), the rates of adverse anesthesia events (hypoxia, etc) were similar between the groups and no cases of pulmonary aspiration were noted.7
One single-center study of 57 patients evaluated the safety of GLP-1 RAs in those undergoing endoscopic sleeve gastrectomy. GLP-1 RAs were continued on all patients, but all adhered to a liquid only diet for at least 24 hours prior to the procedure. There were no instances of retained gastric solids, aspiration, or hypoxia. This study suggests that with a 24-hour clear liquid diet and routine NPO recommendations prior to endoscopy, it would be safe to continue GLP-1 RAs. This study provides rationale for the AGA recommendation for a clear liquid diet 24 hours prior to endoscopic procedures for those on GLP-1 RAs.8
A study looking at those who underwent emergency surgery and endoscopy with claims data of use of GLP-1 RAs found an overall incidence of postoperative respiratory complications of 3.5% for those with GLP-1 RAs fill history vs 4.0% for those without (P = .12). Approximately 800 of the 24,000 patients identified had undergone endoscopic procedures for GI bleeding or food impaction. The study overall showed that preoperative use of GLP-1 RAs in patients undergoing surgery or endoscopy, evaluated as a combined group, was not associated with an increased risk of pulmonary complications.9
Lastly, a systematic review and meta-analysis that included 15 studies that quantified gastric emptying using various methods, including gastric emptying scintigraphy and acetaminophen absorption test, found that there was a quantifiable delay in gastric emptying of about 36 minutes, compared to placebo (P < .01), in patients using GLP-1 RAs. However, compared to standard periprocedural fasting, this delay is clinically insignificant and standard fasting protocols would still be appropriate for patients on GLP-1 RAs.10
These studies taken together suggest that while GLP-1 RAs can mildly increase the likelihood of retained gastric contents, there is no statistically significant increase in the risk of aspiration or other anesthesia complications. Furthermore, while decreased gastric emptying is a known effect of GLP-1 RAs, this effect may not be clinically significant in the context of standard periprocedural fasting protocols particularly when combined with a 24-hour clear liquid diet. These findings support at a minimum a more patient-specific strategy for periprocedural management of GLP-1 RAs.
Clinical Implications
These most recent studies, as well as prior studies and guidelines by various societies lead to a dilemma among endoscopists on proper patient counseling on GLP-1 RAs use before endoscopic procedures. Clinicians must balance the metabolic benefits of GLP-1 RAs with potential endoscopic complications and risks.
Holding therapy theoretically decreases aspiration risk and pulmonary complications, though evidence remains low to support this. Holding medication, however, affects glycemic control leading to potential rebound hyperglycemia which may impact and delay plans for endoscopy. With growing indications for the use of GLP-1 RAs, a more tailored patient-centered treatment plan may be required, especially with consideration of procedure indication and comorbidities.
Currently, practice patterns at different institutions vary widely, making standardization much more difficult. Some centers have opted to follow ASA guidelines of holding these medications up to 1 week prior to procedures, while others have continued therapy with no pre-procedural adjustments. This leaves endoscopists to deal with the downstream effects of inconvenience to patients, care delays, and financial considerations if procedures are postponed related to GLP-1 RAs use.
Future Directions
Future studies are needed to make further evidence-based recommendations. Studies should focus on stratifying risks and recommendations based on procedure type (EGD, colonoscopy, etc). More widespread implementation of gastric ultrasound can assist in real-time decision-making, albeit this would require expertise and dedicated time within the pre-procedural workflow. Randomized controlled trials comparing outcomes of patients who continue GLP-1 RAs vs those who discontinue stratified by baseline risk will be instrumental for making concrete guidelines that provide clarity on periprocedural management of GLP-1 RAs.
Conclusion
The periprocedural management of GLP-1 RAs remains a controversial topic that presents unique challenges in endoscopy. Several guidelines have been released by various stakeholders including anesthesiologists, gastroenterologists, and other prescribing providers. Clinical data remains limited with no robust evidence available to suggest that gastric emptying delays caused by GLP-1 RAs prior to endoscopic procedures significantly increases risk of aspiration, pulmonary complications, or other comorbidities. Evolving multi-society guidelines will be important to establish more consistent practices with reassessment of the data as new studies emerge. A multidisciplinary, individualized patient approach may be the best strategy for managing GLP-1 RAs for patients undergoing endoscopic procedures.
Dr. Sekar and Dr. Asamoah are based in the department of gastroenterology at MedStar Georgetown University Hospital, Washington, D.C. Dr. Sekar reports no conflicts of interest in regard to this article. Dr. Asamoah serves on the Johnson & Johnson advisory board for inflammatory bowel disease–related therapies.
References
1. Halim MA et al. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018 Feb. doi: 10.1210/jc.2017-02006.
2. American Society of Anesthesiologists. American Society of Anesthesiologists releases consensus-based guidance on preoperative use of GLP-1 receptor agonists. 2023 Jun 20. www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
3. American Gastroenterological Association. GI multi-society statement regarding GLP-1 agonists and endoscopy. 2023 Jul 25. gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/.
4. Hashash JG et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr. doi: 10.1016/j.cgh.2023.11.002.
5. Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multi-society Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
6. Phan J et al. Glucagon-Like Peptide Receptor Agonists Use Before Endoscopy Is Associated With Low Retained Gastric Contents: A Multicenter Cross-Sectional Analysis. Am J Gastroenterol. 2025 Mar. doi: 10.14309/ajg.0000000000002969.
7. Panchal S et al. Endoscopy and Anesthesia Outcomes Associated With Glucagon-like Peptide-1 Receptor Agonist use in Patients Undergoing Outpatient Upper Endoscopy. Gastrointest Endosc. 2025 Aug. doi:10.1016/j.gie.2025.01.004.
8. Maselli DB et al. Safe Continuation of glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes Surg. 2024 Jul. doi: 10.1007/s11695-024-07278-2.
9. Dixit AA et al. Preoperative GLP-1 Receptor Agonist Use and Risk of Postoperative Respiratory Complications. JAMA. 2024 Apr. doi: 10.1001/jama.2024.5003.
10. Hiramoto B et al. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A systematic review and meta-analysis with insights for periprocedural management. Am J Gastroenterol. 2024 Jun. doi: 10.14309/ajg.0000000000002820.
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has increased over the past several years and has become a cornerstone in both diabetes and weight loss management, particularly because of its unique combination of glucose control, weight reduction potential, and cardiac and metabolic benefits. However, increased use of these agents presents a dilemma in gastrointestinal endoscopy as it pertains to their safety and management during the periprocedural period.
highlighting gaps and future directions.
Pharmacology and Mechanisms of Action
GLP-1 RAs have several mechanisms of action that make them relevant in gastrointestinal endoscopy. These medications modulate glucose control via enhancement of glucose-dependent insulin secretion and reduction of postprandial glucagon, which promotes satiety and delays gastric emptying. This delay in gastric emptying mediated by vagal pathways has been postulated to increase gastric residuals, posing a risk for aspiration during anesthesia.1
It is important to also consider the pharmacokinetics of GLP-1 RAs, as some have shorter half-lives on the order of several hours, like exenatide, while others, like semaglutide, are dosed weekly. Additionally, common side effects of GLP-1 RAs include nausea, vomiting, bloating, and early satiety, which pose challenges for patients undergoing endoscopic procedures.
Current Guidelines
Various societies have published guidelines on the periprocedural use of GLP-1 RAs. The American Society of Anesthesiologist (ASA) in 2023 presented early recommendations to hold GLP-1 RAs either day of procedure or week prior depending on pharmacokinetics, because of the risk of delayed gastric emptying and increased potential for aspiration.2 Soon thereafter, a multi-gastroenterology society guideline was released stating more data is needed to decide if GLP-1 RAs need to be held prior to endoscopic procedures.3
In early 2024, the American Gastroenterological Association (AGA) published a rapid clinical update that advocated for a more individualized approach, particularly in light of limited overall data for GLP-1 RAs and endoscopic procedures.4 In asymptomatic patients who follow typical fasting protocols for procedures, it is generally safe to proceed with endoscopy without holding GLP-1 RAs. In symptomatic patients (nausea, abdominal distension, etc), the AGA advises additional precautions, including performing transabdominal ultrasound if feasible to assess retained gastric contents. The AGA also suggests placing a patient on a clear liquid diet the day prior to the procedure — rather than holding GLP-1 RAs — as another reasonable strategy.
The guidelines continue to evolve with newer multi-society guidelines establishing best practices. While initially in 2023 the ASA did recommend holding these medications prior to endoscopy, the initial guidance was based on expert opinion with limited evidence. Newer multi-society guidance published jointly by the ASA along with various gastroenterology societies, including the AGA in December 2024, takes a more nuanced approach.5
The newer guidelines include two main recommendations:
1. Periprocedural management of GLP-1 RAs should be a joint decision among the procedural, anesthesia, and prescribing team balancing metabolic needs vs patient risks.
- In a low-risk patient, one that is asymptomatic and on standard dosing, among other factors, the guidance states that GLP-1 RAs can be continued.
- In higher-risk patients, the original guidance of holding a day or a week prior to endoscopic procedures should be followed.
2. Periprocedural management of GLP-1 RAs should attempt to minimize the aspiration risks loosely associated with delayed gastric emptying.
- Consider a 24-hour clear liquid diet a day prior to the procedure and transabdominal ultrasound to check gastric contents.
- It is acknowledged that this guidance is based on limited evidence and will be evolving as new medications and data are released.
Recent Clinical Studies
Although there is very little data to guide clinicians, several recent studies have been published that can direct clinical decision-making as guidelines continue to be refined and updated.
A multicenter trial of approximately 800 patients undergoing upper endoscopy found a significant difference in rates of retained gastric contents between those that underwent endoscopy who did and did not follow the ASA guidance on periprocedural management of GLP-1 RAs (12.7% vs 4.4%; P < .0001). However, there were no significant differences in rates of aborted procedures or unplanned intubations.
Furthermore, a multivariable analysis was performed controlling for GLP-1 RA type and other factors, which found the likelihood of gastric retention increased by 36% for every 1% increase in hemoglobin A1c. This study suggests that a more individualized approach to holding GLP-1 RA would be applicable rather than a universal periprocedural hold.6
More recently, a single-center study of nearly 600 patients undergoing upper endoscopy showed that while there were slightly increased rates of retained gastric contents (OR 3.80; P = .003) and aborted procedures (1.3% vs 0%; P = .02), the rates of adverse anesthesia events (hypoxia, etc) were similar between the groups and no cases of pulmonary aspiration were noted.7
One single-center study of 57 patients evaluated the safety of GLP-1 RAs in those undergoing endoscopic sleeve gastrectomy. GLP-1 RAs were continued on all patients, but all adhered to a liquid only diet for at least 24 hours prior to the procedure. There were no instances of retained gastric solids, aspiration, or hypoxia. This study suggests that with a 24-hour clear liquid diet and routine NPO recommendations prior to endoscopy, it would be safe to continue GLP-1 RAs. This study provides rationale for the AGA recommendation for a clear liquid diet 24 hours prior to endoscopic procedures for those on GLP-1 RAs.8
A study looking at those who underwent emergency surgery and endoscopy with claims data of use of GLP-1 RAs found an overall incidence of postoperative respiratory complications of 3.5% for those with GLP-1 RAs fill history vs 4.0% for those without (P = .12). Approximately 800 of the 24,000 patients identified had undergone endoscopic procedures for GI bleeding or food impaction. The study overall showed that preoperative use of GLP-1 RAs in patients undergoing surgery or endoscopy, evaluated as a combined group, was not associated with an increased risk of pulmonary complications.9
Lastly, a systematic review and meta-analysis that included 15 studies that quantified gastric emptying using various methods, including gastric emptying scintigraphy and acetaminophen absorption test, found that there was a quantifiable delay in gastric emptying of about 36 minutes, compared to placebo (P < .01), in patients using GLP-1 RAs. However, compared to standard periprocedural fasting, this delay is clinically insignificant and standard fasting protocols would still be appropriate for patients on GLP-1 RAs.10
These studies taken together suggest that while GLP-1 RAs can mildly increase the likelihood of retained gastric contents, there is no statistically significant increase in the risk of aspiration or other anesthesia complications. Furthermore, while decreased gastric emptying is a known effect of GLP-1 RAs, this effect may not be clinically significant in the context of standard periprocedural fasting protocols particularly when combined with a 24-hour clear liquid diet. These findings support at a minimum a more patient-specific strategy for periprocedural management of GLP-1 RAs.
Clinical Implications
These most recent studies, as well as prior studies and guidelines by various societies lead to a dilemma among endoscopists on proper patient counseling on GLP-1 RAs use before endoscopic procedures. Clinicians must balance the metabolic benefits of GLP-1 RAs with potential endoscopic complications and risks.
Holding therapy theoretically decreases aspiration risk and pulmonary complications, though evidence remains low to support this. Holding medication, however, affects glycemic control leading to potential rebound hyperglycemia which may impact and delay plans for endoscopy. With growing indications for the use of GLP-1 RAs, a more tailored patient-centered treatment plan may be required, especially with consideration of procedure indication and comorbidities.
Currently, practice patterns at different institutions vary widely, making standardization much more difficult. Some centers have opted to follow ASA guidelines of holding these medications up to 1 week prior to procedures, while others have continued therapy with no pre-procedural adjustments. This leaves endoscopists to deal with the downstream effects of inconvenience to patients, care delays, and financial considerations if procedures are postponed related to GLP-1 RAs use.
Future Directions
Future studies are needed to make further evidence-based recommendations. Studies should focus on stratifying risks and recommendations based on procedure type (EGD, colonoscopy, etc). More widespread implementation of gastric ultrasound can assist in real-time decision-making, albeit this would require expertise and dedicated time within the pre-procedural workflow. Randomized controlled trials comparing outcomes of patients who continue GLP-1 RAs vs those who discontinue stratified by baseline risk will be instrumental for making concrete guidelines that provide clarity on periprocedural management of GLP-1 RAs.
Conclusion
The periprocedural management of GLP-1 RAs remains a controversial topic that presents unique challenges in endoscopy. Several guidelines have been released by various stakeholders including anesthesiologists, gastroenterologists, and other prescribing providers. Clinical data remains limited with no robust evidence available to suggest that gastric emptying delays caused by GLP-1 RAs prior to endoscopic procedures significantly increases risk of aspiration, pulmonary complications, or other comorbidities. Evolving multi-society guidelines will be important to establish more consistent practices with reassessment of the data as new studies emerge. A multidisciplinary, individualized patient approach may be the best strategy for managing GLP-1 RAs for patients undergoing endoscopic procedures.
Dr. Sekar and Dr. Asamoah are based in the department of gastroenterology at MedStar Georgetown University Hospital, Washington, D.C. Dr. Sekar reports no conflicts of interest in regard to this article. Dr. Asamoah serves on the Johnson & Johnson advisory board for inflammatory bowel disease–related therapies.
References
1. Halim MA et al. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018 Feb. doi: 10.1210/jc.2017-02006.
2. American Society of Anesthesiologists. American Society of Anesthesiologists releases consensus-based guidance on preoperative use of GLP-1 receptor agonists. 2023 Jun 20. www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
3. American Gastroenterological Association. GI multi-society statement regarding GLP-1 agonists and endoscopy. 2023 Jul 25. gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/.
4. Hashash JG et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr. doi: 10.1016/j.cgh.2023.11.002.
5. Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multi-society Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
6. Phan J et al. Glucagon-Like Peptide Receptor Agonists Use Before Endoscopy Is Associated With Low Retained Gastric Contents: A Multicenter Cross-Sectional Analysis. Am J Gastroenterol. 2025 Mar. doi: 10.14309/ajg.0000000000002969.
7. Panchal S et al. Endoscopy and Anesthesia Outcomes Associated With Glucagon-like Peptide-1 Receptor Agonist use in Patients Undergoing Outpatient Upper Endoscopy. Gastrointest Endosc. 2025 Aug. doi:10.1016/j.gie.2025.01.004.
8. Maselli DB et al. Safe Continuation of glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes Surg. 2024 Jul. doi: 10.1007/s11695-024-07278-2.
9. Dixit AA et al. Preoperative GLP-1 Receptor Agonist Use and Risk of Postoperative Respiratory Complications. JAMA. 2024 Apr. doi: 10.1001/jama.2024.5003.
10. Hiramoto B et al. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A systematic review and meta-analysis with insights for periprocedural management. Am J Gastroenterol. 2024 Jun. doi: 10.14309/ajg.0000000000002820.
The use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) has increased over the past several years and has become a cornerstone in both diabetes and weight loss management, particularly because of its unique combination of glucose control, weight reduction potential, and cardiac and metabolic benefits. However, increased use of these agents presents a dilemma in gastrointestinal endoscopy as it pertains to their safety and management during the periprocedural period.
highlighting gaps and future directions.
Pharmacology and Mechanisms of Action
GLP-1 RAs have several mechanisms of action that make them relevant in gastrointestinal endoscopy. These medications modulate glucose control via enhancement of glucose-dependent insulin secretion and reduction of postprandial glucagon, which promotes satiety and delays gastric emptying. This delay in gastric emptying mediated by vagal pathways has been postulated to increase gastric residuals, posing a risk for aspiration during anesthesia.1
It is important to also consider the pharmacokinetics of GLP-1 RAs, as some have shorter half-lives on the order of several hours, like exenatide, while others, like semaglutide, are dosed weekly. Additionally, common side effects of GLP-1 RAs include nausea, vomiting, bloating, and early satiety, which pose challenges for patients undergoing endoscopic procedures.
Current Guidelines
Various societies have published guidelines on the periprocedural use of GLP-1 RAs. The American Society of Anesthesiologist (ASA) in 2023 presented early recommendations to hold GLP-1 RAs either day of procedure or week prior depending on pharmacokinetics, because of the risk of delayed gastric emptying and increased potential for aspiration.2 Soon thereafter, a multi-gastroenterology society guideline was released stating more data is needed to decide if GLP-1 RAs need to be held prior to endoscopic procedures.3
In early 2024, the American Gastroenterological Association (AGA) published a rapid clinical update that advocated for a more individualized approach, particularly in light of limited overall data for GLP-1 RAs and endoscopic procedures.4 In asymptomatic patients who follow typical fasting protocols for procedures, it is generally safe to proceed with endoscopy without holding GLP-1 RAs. In symptomatic patients (nausea, abdominal distension, etc), the AGA advises additional precautions, including performing transabdominal ultrasound if feasible to assess retained gastric contents. The AGA also suggests placing a patient on a clear liquid diet the day prior to the procedure — rather than holding GLP-1 RAs — as another reasonable strategy.
The guidelines continue to evolve with newer multi-society guidelines establishing best practices. While initially in 2023 the ASA did recommend holding these medications prior to endoscopy, the initial guidance was based on expert opinion with limited evidence. Newer multi-society guidance published jointly by the ASA along with various gastroenterology societies, including the AGA in December 2024, takes a more nuanced approach.5
The newer guidelines include two main recommendations:
1. Periprocedural management of GLP-1 RAs should be a joint decision among the procedural, anesthesia, and prescribing team balancing metabolic needs vs patient risks.
- In a low-risk patient, one that is asymptomatic and on standard dosing, among other factors, the guidance states that GLP-1 RAs can be continued.
- In higher-risk patients, the original guidance of holding a day or a week prior to endoscopic procedures should be followed.
2. Periprocedural management of GLP-1 RAs should attempt to minimize the aspiration risks loosely associated with delayed gastric emptying.
- Consider a 24-hour clear liquid diet a day prior to the procedure and transabdominal ultrasound to check gastric contents.
- It is acknowledged that this guidance is based on limited evidence and will be evolving as new medications and data are released.
Recent Clinical Studies
Although there is very little data to guide clinicians, several recent studies have been published that can direct clinical decision-making as guidelines continue to be refined and updated.
A multicenter trial of approximately 800 patients undergoing upper endoscopy found a significant difference in rates of retained gastric contents between those that underwent endoscopy who did and did not follow the ASA guidance on periprocedural management of GLP-1 RAs (12.7% vs 4.4%; P < .0001). However, there were no significant differences in rates of aborted procedures or unplanned intubations.
Furthermore, a multivariable analysis was performed controlling for GLP-1 RA type and other factors, which found the likelihood of gastric retention increased by 36% for every 1% increase in hemoglobin A1c. This study suggests that a more individualized approach to holding GLP-1 RA would be applicable rather than a universal periprocedural hold.6
More recently, a single-center study of nearly 600 patients undergoing upper endoscopy showed that while there were slightly increased rates of retained gastric contents (OR 3.80; P = .003) and aborted procedures (1.3% vs 0%; P = .02), the rates of adverse anesthesia events (hypoxia, etc) were similar between the groups and no cases of pulmonary aspiration were noted.7
One single-center study of 57 patients evaluated the safety of GLP-1 RAs in those undergoing endoscopic sleeve gastrectomy. GLP-1 RAs were continued on all patients, but all adhered to a liquid only diet for at least 24 hours prior to the procedure. There were no instances of retained gastric solids, aspiration, or hypoxia. This study suggests that with a 24-hour clear liquid diet and routine NPO recommendations prior to endoscopy, it would be safe to continue GLP-1 RAs. This study provides rationale for the AGA recommendation for a clear liquid diet 24 hours prior to endoscopic procedures for those on GLP-1 RAs.8
A study looking at those who underwent emergency surgery and endoscopy with claims data of use of GLP-1 RAs found an overall incidence of postoperative respiratory complications of 3.5% for those with GLP-1 RAs fill history vs 4.0% for those without (P = .12). Approximately 800 of the 24,000 patients identified had undergone endoscopic procedures for GI bleeding or food impaction. The study overall showed that preoperative use of GLP-1 RAs in patients undergoing surgery or endoscopy, evaluated as a combined group, was not associated with an increased risk of pulmonary complications.9
Lastly, a systematic review and meta-analysis that included 15 studies that quantified gastric emptying using various methods, including gastric emptying scintigraphy and acetaminophen absorption test, found that there was a quantifiable delay in gastric emptying of about 36 minutes, compared to placebo (P < .01), in patients using GLP-1 RAs. However, compared to standard periprocedural fasting, this delay is clinically insignificant and standard fasting protocols would still be appropriate for patients on GLP-1 RAs.10
These studies taken together suggest that while GLP-1 RAs can mildly increase the likelihood of retained gastric contents, there is no statistically significant increase in the risk of aspiration or other anesthesia complications. Furthermore, while decreased gastric emptying is a known effect of GLP-1 RAs, this effect may not be clinically significant in the context of standard periprocedural fasting protocols particularly when combined with a 24-hour clear liquid diet. These findings support at a minimum a more patient-specific strategy for periprocedural management of GLP-1 RAs.
Clinical Implications
These most recent studies, as well as prior studies and guidelines by various societies lead to a dilemma among endoscopists on proper patient counseling on GLP-1 RAs use before endoscopic procedures. Clinicians must balance the metabolic benefits of GLP-1 RAs with potential endoscopic complications and risks.
Holding therapy theoretically decreases aspiration risk and pulmonary complications, though evidence remains low to support this. Holding medication, however, affects glycemic control leading to potential rebound hyperglycemia which may impact and delay plans for endoscopy. With growing indications for the use of GLP-1 RAs, a more tailored patient-centered treatment plan may be required, especially with consideration of procedure indication and comorbidities.
Currently, practice patterns at different institutions vary widely, making standardization much more difficult. Some centers have opted to follow ASA guidelines of holding these medications up to 1 week prior to procedures, while others have continued therapy with no pre-procedural adjustments. This leaves endoscopists to deal with the downstream effects of inconvenience to patients, care delays, and financial considerations if procedures are postponed related to GLP-1 RAs use.
Future Directions
Future studies are needed to make further evidence-based recommendations. Studies should focus on stratifying risks and recommendations based on procedure type (EGD, colonoscopy, etc). More widespread implementation of gastric ultrasound can assist in real-time decision-making, albeit this would require expertise and dedicated time within the pre-procedural workflow. Randomized controlled trials comparing outcomes of patients who continue GLP-1 RAs vs those who discontinue stratified by baseline risk will be instrumental for making concrete guidelines that provide clarity on periprocedural management of GLP-1 RAs.
Conclusion
The periprocedural management of GLP-1 RAs remains a controversial topic that presents unique challenges in endoscopy. Several guidelines have been released by various stakeholders including anesthesiologists, gastroenterologists, and other prescribing providers. Clinical data remains limited with no robust evidence available to suggest that gastric emptying delays caused by GLP-1 RAs prior to endoscopic procedures significantly increases risk of aspiration, pulmonary complications, or other comorbidities. Evolving multi-society guidelines will be important to establish more consistent practices with reassessment of the data as new studies emerge. A multidisciplinary, individualized patient approach may be the best strategy for managing GLP-1 RAs for patients undergoing endoscopic procedures.
Dr. Sekar and Dr. Asamoah are based in the department of gastroenterology at MedStar Georgetown University Hospital, Washington, D.C. Dr. Sekar reports no conflicts of interest in regard to this article. Dr. Asamoah serves on the Johnson & Johnson advisory board for inflammatory bowel disease–related therapies.
References
1. Halim MA et al. Glucagon-Like Peptide-1 Inhibits Prandial Gastrointestinal Motility Through Myenteric Neuronal Mechanisms in Humans. J Clin Endocrinol Metab. 2018 Feb. doi: 10.1210/jc.2017-02006.
2. American Society of Anesthesiologists. American Society of Anesthesiologists releases consensus-based guidance on preoperative use of GLP-1 receptor agonists. 2023 Jun 20. www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
3. American Gastroenterological Association. GI multi-society statement regarding GLP-1 agonists and endoscopy. 2023 Jul 25. gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/.
4. Hashash JG et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr. doi: 10.1016/j.cgh.2023.11.002.
5. Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multi-society Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
6. Phan J et al. Glucagon-Like Peptide Receptor Agonists Use Before Endoscopy Is Associated With Low Retained Gastric Contents: A Multicenter Cross-Sectional Analysis. Am J Gastroenterol. 2025 Mar. doi: 10.14309/ajg.0000000000002969.
7. Panchal S et al. Endoscopy and Anesthesia Outcomes Associated With Glucagon-like Peptide-1 Receptor Agonist use in Patients Undergoing Outpatient Upper Endoscopy. Gastrointest Endosc. 2025 Aug. doi:10.1016/j.gie.2025.01.004.
8. Maselli DB et al. Safe Continuation of glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes Surg. 2024 Jul. doi: 10.1007/s11695-024-07278-2.
9. Dixit AA et al. Preoperative GLP-1 Receptor Agonist Use and Risk of Postoperative Respiratory Complications. JAMA. 2024 Apr. doi: 10.1001/jama.2024.5003.
10. Hiramoto B et al. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A systematic review and meta-analysis with insights for periprocedural management. Am J Gastroenterol. 2024 Jun. doi: 10.14309/ajg.0000000000002820.
Top 5 Tips for Becoming an Effective Gastroenterology Consultant
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.