User login
Atopic Dermatitis and Sleep Disturbances
Recently one of my keep-up-to-date apps alerted me to a study in Pediatric Dermatology on sleep and atopic dermatitis. When I chased down the abstract it was a shoulder-shrugging-so-what encounter. The authors reported that having a child with atopic dermatitis decreased the odds of a parent getting 7 hours of sleep a night and increased the odds that the parent was also taking sleep-aiding medications. The authors felt their data was meaningful enough to publish based on the size and the cross-sectional nature of their sample. However, anyone who has worked with families with atopic dermatitis shouldn’t be surprised at their findings.
Curious about what other investigators had discovered about the anecdotally obvious relationship between sleep and atopic dermatitis, I dug until I found a rather thorough discussion of the literature published in The Journal of Clinical Immunology Practice. These authors from the University of Rochester Medical School in New York begin by pointing out that, although 47%-80% of children with atopic dermatitis and 33%-90% of adults with atopic dermatitis have disturbed sleep, “literature on this topic remains sparse with most studies evaluating sleep as a secondary outcome using subjective measures.” They further note that sleep is one of the three most problematic symptoms for children with atopic dermatitis and their families.
Characterizing the Sleep Loss
Difficulty falling asleep, frequent and long waking, and excessive daytime sleepiness are the most common symptoms reported. In the few sleep laboratory studies that have been done there has been no significant decrease in sleep duration, which is a bit of a surprise. However, as expected, sleep-onset latency, more wake time after sleep onset, sleep fragmentation, and decreased sleep efficiency have been observed in the atopic dermatitis patients. In other studies of younger children, female gender and lower socioeconomic status seem to be associated with poor sleep quality.
Most studies found that in general the prevalence and severity of sleep disturbances increases with the severity of the disease. As the disease flares, increased bedtime resistance, nocturnal wakings and daytime sleepiness become more likely. These parentally reported associations have also been confirmed by sleep laboratory observations.
The sleep disturbances quickly become a family affair with 60% of siblings and parents reporting disturbed sleep. When the child with atopic dermatitis is having a flareup, nearly 90% of their parents report losing up to 2.5 hours of sleep. Not surprisingly sleep disturbances have been associated with behavioral and emotional problems including decreased happiness, poor cognitive performance, hyperactivity, and inattention. Mothers seem to bear the brunt of the problem and interpersonal conflicts and exhaustion are unfortunately not uncommon.
Probing the Causes
So why are atopic dermatitis patients and their families so prone to the ill effects of disturbed sleep? Although you might think it should be obvious, this review of the “sparse” literature doesn’t provide a satisfying answer. However, the authors provide three possible explanations.
The one with the least supporting evidence is circadian variations in the products of inflammation such as cytokines and their effect on melatonin production. The explanation which I think most of us have already considered is that pruritus disrupts sleep. This is the often-quoted itch-scratch feedback cycle which can release inflammatory mediators (“pruritogens”). However, the investigators have found that many studies report “conflicting results or only weak correlations.”
The third alternative posed by the authors is by far the most appealing and hinges on the assumption that, as with many other chronic conditions, atopic dermatitis renders the patient vulnerable to insomnia. “Nocturnal scratching disrupts sleep and sets the stage for cognitive and behavioral factors that reinforce insomnia as a conditioned response.” In other words, even after the “co-concurring condition” resolves insomnia related sleep behaviors continue. The investigators point to a study supporting this explanation which found that, even after a child’s skin cleared, his/her sleep arousals failed to return to normal suggesting that learned behavior patterns might be playing a role.
It may be a stretch to suggest that poor sleep hygiene might in and of itself cause atopic dermatitis, but it can’t be ruled out. At a minimum the current research suggests that there is a bidirectional relationship between sleep disturbances and atopic dermatitis.
Next Steps
The authors of this study urge that we be more creative in using already-existing portable and relatively low-cost sleep monitoring technology to better define this relationship. While that is a worthwhile avenue for research, I think we who see children (both primary care providers and dermatologists) now have enough evidence to move managing the sleep hygiene of our atopic dermatitis patients to the front burner, along with moisturizers and topical medications, without needing to do costly and time-consuming studies.
This means taking a thorough sleep history. If, in the rare cases where the child’s sleep habits are normal, the parents should be warned that falling off the sleep wagon is likely to exacerbate the child’s skin. If the history reveals an inefficient and dysfunctional bedtime routine or other symptoms of insomnia, advise the parents on how it can be improved. Then follow up at each visit if there has been no improvement. Sleep management can be time-consuming as well but it should be part of every primary care pediatrician’s toolbox. For the dermatologist who doesn’t feel comfortable managing sleep problems, a consultation with a pediatrician or a sleep specialist is in order.
The adult with atopic dermatitis is a somewhat different animal and a formal sleep study may be indicated. Cognitive-behavioral therapy might be helpful for adult population but the investigators could find no trials of its use in patients with atopic dermatitis.
Convincing the parents of an atopic dermatitis patient that their family’s disturbed sleep may not only be the result of his/her itchy skin but may be a preexisting compounding problem may not be an easy sell. I hope if you can be open to the strong possibility that disordered sleep is not just the effect but in some ways may be a likely contributor to your patients’ atopic dermatitis, you may become more effective in managing the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Recently one of my keep-up-to-date apps alerted me to a study in Pediatric Dermatology on sleep and atopic dermatitis. When I chased down the abstract it was a shoulder-shrugging-so-what encounter. The authors reported that having a child with atopic dermatitis decreased the odds of a parent getting 7 hours of sleep a night and increased the odds that the parent was also taking sleep-aiding medications. The authors felt their data was meaningful enough to publish based on the size and the cross-sectional nature of their sample. However, anyone who has worked with families with atopic dermatitis shouldn’t be surprised at their findings.
Curious about what other investigators had discovered about the anecdotally obvious relationship between sleep and atopic dermatitis, I dug until I found a rather thorough discussion of the literature published in The Journal of Clinical Immunology Practice. These authors from the University of Rochester Medical School in New York begin by pointing out that, although 47%-80% of children with atopic dermatitis and 33%-90% of adults with atopic dermatitis have disturbed sleep, “literature on this topic remains sparse with most studies evaluating sleep as a secondary outcome using subjective measures.” They further note that sleep is one of the three most problematic symptoms for children with atopic dermatitis and their families.
Characterizing the Sleep Loss
Difficulty falling asleep, frequent and long waking, and excessive daytime sleepiness are the most common symptoms reported. In the few sleep laboratory studies that have been done there has been no significant decrease in sleep duration, which is a bit of a surprise. However, as expected, sleep-onset latency, more wake time after sleep onset, sleep fragmentation, and decreased sleep efficiency have been observed in the atopic dermatitis patients. In other studies of younger children, female gender and lower socioeconomic status seem to be associated with poor sleep quality.
Most studies found that in general the prevalence and severity of sleep disturbances increases with the severity of the disease. As the disease flares, increased bedtime resistance, nocturnal wakings and daytime sleepiness become more likely. These parentally reported associations have also been confirmed by sleep laboratory observations.
The sleep disturbances quickly become a family affair with 60% of siblings and parents reporting disturbed sleep. When the child with atopic dermatitis is having a flareup, nearly 90% of their parents report losing up to 2.5 hours of sleep. Not surprisingly sleep disturbances have been associated with behavioral and emotional problems including decreased happiness, poor cognitive performance, hyperactivity, and inattention. Mothers seem to bear the brunt of the problem and interpersonal conflicts and exhaustion are unfortunately not uncommon.
Probing the Causes
So why are atopic dermatitis patients and their families so prone to the ill effects of disturbed sleep? Although you might think it should be obvious, this review of the “sparse” literature doesn’t provide a satisfying answer. However, the authors provide three possible explanations.
The one with the least supporting evidence is circadian variations in the products of inflammation such as cytokines and their effect on melatonin production. The explanation which I think most of us have already considered is that pruritus disrupts sleep. This is the often-quoted itch-scratch feedback cycle which can release inflammatory mediators (“pruritogens”). However, the investigators have found that many studies report “conflicting results or only weak correlations.”
The third alternative posed by the authors is by far the most appealing and hinges on the assumption that, as with many other chronic conditions, atopic dermatitis renders the patient vulnerable to insomnia. “Nocturnal scratching disrupts sleep and sets the stage for cognitive and behavioral factors that reinforce insomnia as a conditioned response.” In other words, even after the “co-concurring condition” resolves insomnia related sleep behaviors continue. The investigators point to a study supporting this explanation which found that, even after a child’s skin cleared, his/her sleep arousals failed to return to normal suggesting that learned behavior patterns might be playing a role.
It may be a stretch to suggest that poor sleep hygiene might in and of itself cause atopic dermatitis, but it can’t be ruled out. At a minimum the current research suggests that there is a bidirectional relationship between sleep disturbances and atopic dermatitis.
Next Steps
The authors of this study urge that we be more creative in using already-existing portable and relatively low-cost sleep monitoring technology to better define this relationship. While that is a worthwhile avenue for research, I think we who see children (both primary care providers and dermatologists) now have enough evidence to move managing the sleep hygiene of our atopic dermatitis patients to the front burner, along with moisturizers and topical medications, without needing to do costly and time-consuming studies.
This means taking a thorough sleep history. If, in the rare cases where the child’s sleep habits are normal, the parents should be warned that falling off the sleep wagon is likely to exacerbate the child’s skin. If the history reveals an inefficient and dysfunctional bedtime routine or other symptoms of insomnia, advise the parents on how it can be improved. Then follow up at each visit if there has been no improvement. Sleep management can be time-consuming as well but it should be part of every primary care pediatrician’s toolbox. For the dermatologist who doesn’t feel comfortable managing sleep problems, a consultation with a pediatrician or a sleep specialist is in order.
The adult with atopic dermatitis is a somewhat different animal and a formal sleep study may be indicated. Cognitive-behavioral therapy might be helpful for adult population but the investigators could find no trials of its use in patients with atopic dermatitis.
Convincing the parents of an atopic dermatitis patient that their family’s disturbed sleep may not only be the result of his/her itchy skin but may be a preexisting compounding problem may not be an easy sell. I hope if you can be open to the strong possibility that disordered sleep is not just the effect but in some ways may be a likely contributor to your patients’ atopic dermatitis, you may become more effective in managing the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Recently one of my keep-up-to-date apps alerted me to a study in Pediatric Dermatology on sleep and atopic dermatitis. When I chased down the abstract it was a shoulder-shrugging-so-what encounter. The authors reported that having a child with atopic dermatitis decreased the odds of a parent getting 7 hours of sleep a night and increased the odds that the parent was also taking sleep-aiding medications. The authors felt their data was meaningful enough to publish based on the size and the cross-sectional nature of their sample. However, anyone who has worked with families with atopic dermatitis shouldn’t be surprised at their findings.
Curious about what other investigators had discovered about the anecdotally obvious relationship between sleep and atopic dermatitis, I dug until I found a rather thorough discussion of the literature published in The Journal of Clinical Immunology Practice. These authors from the University of Rochester Medical School in New York begin by pointing out that, although 47%-80% of children with atopic dermatitis and 33%-90% of adults with atopic dermatitis have disturbed sleep, “literature on this topic remains sparse with most studies evaluating sleep as a secondary outcome using subjective measures.” They further note that sleep is one of the three most problematic symptoms for children with atopic dermatitis and their families.
Characterizing the Sleep Loss
Difficulty falling asleep, frequent and long waking, and excessive daytime sleepiness are the most common symptoms reported. In the few sleep laboratory studies that have been done there has been no significant decrease in sleep duration, which is a bit of a surprise. However, as expected, sleep-onset latency, more wake time after sleep onset, sleep fragmentation, and decreased sleep efficiency have been observed in the atopic dermatitis patients. In other studies of younger children, female gender and lower socioeconomic status seem to be associated with poor sleep quality.
Most studies found that in general the prevalence and severity of sleep disturbances increases with the severity of the disease. As the disease flares, increased bedtime resistance, nocturnal wakings and daytime sleepiness become more likely. These parentally reported associations have also been confirmed by sleep laboratory observations.
The sleep disturbances quickly become a family affair with 60% of siblings and parents reporting disturbed sleep. When the child with atopic dermatitis is having a flareup, nearly 90% of their parents report losing up to 2.5 hours of sleep. Not surprisingly sleep disturbances have been associated with behavioral and emotional problems including decreased happiness, poor cognitive performance, hyperactivity, and inattention. Mothers seem to bear the brunt of the problem and interpersonal conflicts and exhaustion are unfortunately not uncommon.
Probing the Causes
So why are atopic dermatitis patients and their families so prone to the ill effects of disturbed sleep? Although you might think it should be obvious, this review of the “sparse” literature doesn’t provide a satisfying answer. However, the authors provide three possible explanations.
The one with the least supporting evidence is circadian variations in the products of inflammation such as cytokines and their effect on melatonin production. The explanation which I think most of us have already considered is that pruritus disrupts sleep. This is the often-quoted itch-scratch feedback cycle which can release inflammatory mediators (“pruritogens”). However, the investigators have found that many studies report “conflicting results or only weak correlations.”
The third alternative posed by the authors is by far the most appealing and hinges on the assumption that, as with many other chronic conditions, atopic dermatitis renders the patient vulnerable to insomnia. “Nocturnal scratching disrupts sleep and sets the stage for cognitive and behavioral factors that reinforce insomnia as a conditioned response.” In other words, even after the “co-concurring condition” resolves insomnia related sleep behaviors continue. The investigators point to a study supporting this explanation which found that, even after a child’s skin cleared, his/her sleep arousals failed to return to normal suggesting that learned behavior patterns might be playing a role.
It may be a stretch to suggest that poor sleep hygiene might in and of itself cause atopic dermatitis, but it can’t be ruled out. At a minimum the current research suggests that there is a bidirectional relationship between sleep disturbances and atopic dermatitis.
Next Steps
The authors of this study urge that we be more creative in using already-existing portable and relatively low-cost sleep monitoring technology to better define this relationship. While that is a worthwhile avenue for research, I think we who see children (both primary care providers and dermatologists) now have enough evidence to move managing the sleep hygiene of our atopic dermatitis patients to the front burner, along with moisturizers and topical medications, without needing to do costly and time-consuming studies.
This means taking a thorough sleep history. If, in the rare cases where the child’s sleep habits are normal, the parents should be warned that falling off the sleep wagon is likely to exacerbate the child’s skin. If the history reveals an inefficient and dysfunctional bedtime routine or other symptoms of insomnia, advise the parents on how it can be improved. Then follow up at each visit if there has been no improvement. Sleep management can be time-consuming as well but it should be part of every primary care pediatrician’s toolbox. For the dermatologist who doesn’t feel comfortable managing sleep problems, a consultation with a pediatrician or a sleep specialist is in order.
The adult with atopic dermatitis is a somewhat different animal and a formal sleep study may be indicated. Cognitive-behavioral therapy might be helpful for adult population but the investigators could find no trials of its use in patients with atopic dermatitis.
Convincing the parents of an atopic dermatitis patient that their family’s disturbed sleep may not only be the result of his/her itchy skin but may be a preexisting compounding problem may not be an easy sell. I hope if you can be open to the strong possibility that disordered sleep is not just the effect but in some ways may be a likely contributor to your patients’ atopic dermatitis, you may become more effective in managing the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Vitiligo: Updated Guidelines, New Treatments Reviewed
of the disease, delegates heard at a recent conference, the Dermatology Days of Paris 2024, organized by the French Society of Dermatology.
A Distinct Disease
An estimated 65% of patients with vitiligo in Europe have been told that their disease is untreatable, according to a recent international study, and this figure rises to 75% in France, Julien Seneschal, MD, PhD, professor of dermatology at Bordeaux University Hospital in Bordeaux, France, told the audience during his presentation.
“This is a message we must change,” he said.
The survey also revealed that in France, even when treatment is offered, 80% of patients do not receive appropriate care. However, treatments do exist, and novel approaches are revolutionizing the management of patients, whatever the degree of severity, he explained.
As a specialist in inflammatory and autoimmune skin diseases, he stressed that these advances are important because vitiligo is a distinct disease and not merely a cosmetic issue. When widespread, it has a significant impact on quality of life and can lead to depression, anxiety, and even suicidal thoughts, even though it does not affect life expectancy.
Updated Guidelines
Since October 2023, new international guidelines for vitiligo management have defined a therapeutic algorithm.
“Nowadays, we place the patient at the center of therapeutic decision-making,” Seneschal said. It is essential to educate patients about the disease and take the time to understand their treatment goals.
For patients with mild vitiligo that does not affect quality of life, simple monitoring may suffice.
However, when a decision is made to pursue treatment, its goals should be:
- Halting disease progression and melanocyte loss
- Achieving repigmentation (a process that can take 6-24 months)
- Preventing relapse after treatment discontinuation
For moderate cases affecting less than 10% of the skin surface, localized treatment is recommended. Previously, topical corticosteroids were used for body lesions, while tacrolimus 0.1% (off-label) was often prescribed for the face and neck. However, as of March 2024, tacrolimus has been officially approved for use in patients aged ≥ 2 years.
In more severe, generalized, and/or active cases, oral treatments such as corticosteroids taken twice weekly for 12-24 weeks can stabilize the disease in 80% of cases (off-label use). Other off-label options include methotrexate, cyclosporine, and tetracyclines.
Targeted Therapies
Recent targeted therapies have significantly advanced the treatment of moderate to severe vitiligo. Since January 2024, the Janus kinase 1 (JAK1)/JAK2 inhibitor ruxolitinib cream has been available in community pharmacies after previously being restricted to hospital use, Seneschal said, and can have spectacular results if previous treatments have failed.
Ruxolitinib is approved for patients aged > 12 years with nonsegmental vitiligo and facial involvement, covering up to 10% of the body surface area. Treatment typically lasts 6 months to 1 year.
Key Findings
The cream-formulated drug has been demonstrated effective in reducing inflammation in two phase 3 clinical trials published in the New England Journal of Medicine that demonstrated its efficacy and safety in patients aged ≥ 12 years. The treatment was well tolerated despite some mild acne-like reactions in 8% of patients. It was shown to be very effective on the face, with a reduction of over 75% in facial lesions in more than 50% of patients, and had good effectiveness on the body, with a 50% decrease in lesions in more than 50% of patients on the body, trunk, arms, and legs, excluding hands and feet.
“Areas like the underarms, hands, and feet are more resistant to treatment,” Seneschal noted.
Although some improvement continues after 1 year, disease recurrence is common if treatment is stopped: Only 40% of patients maintain therapeutic benefits in the year following discontinuation.
“It is therefore important to consider the value of continuing treatment in order to achieve better efficacy or to maintain the repigmentation obtained,” Seneschal said.
He stressed that all treatments should be paired with phototherapy, typically narrowband UVB, to accelerate repigmentation. “There is no increased skin cancer risk in vitiligo patients treated with narrowband UVB,” Seneschal said.
New Therapies
Emerging treatments under development, including injectable biologics alone or in combination with phototherapy, show great promise, he said. Oral JAK inhibitors such as ritlecitinib, upadacitinib, and povorcitinib are also under investigation.
In particular, ritlecitinib, a JAK3/TEC pathway inhibitor, has shown significant reductions in affected skin area in severely affected patients in a phase 2b trial. Phase 3 trials are now underway.
On the safety profile of JAK inhibitors, Seneschal said that studies are reassuring but highlighted the need to monitor cardiovascular, thromboembolic, and infectious risks.
“The question of safety is important because vitiligo is a visible but nonsevere condition, and we do not want to expose patients to unnecessary risks,” added Gaëlle Quéreux, MD, PhD, president of the French Society of Dermatology.
This story was translated from Medscape’s French edition using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
of the disease, delegates heard at a recent conference, the Dermatology Days of Paris 2024, organized by the French Society of Dermatology.
A Distinct Disease
An estimated 65% of patients with vitiligo in Europe have been told that their disease is untreatable, according to a recent international study, and this figure rises to 75% in France, Julien Seneschal, MD, PhD, professor of dermatology at Bordeaux University Hospital in Bordeaux, France, told the audience during his presentation.
“This is a message we must change,” he said.
The survey also revealed that in France, even when treatment is offered, 80% of patients do not receive appropriate care. However, treatments do exist, and novel approaches are revolutionizing the management of patients, whatever the degree of severity, he explained.
As a specialist in inflammatory and autoimmune skin diseases, he stressed that these advances are important because vitiligo is a distinct disease and not merely a cosmetic issue. When widespread, it has a significant impact on quality of life and can lead to depression, anxiety, and even suicidal thoughts, even though it does not affect life expectancy.
Updated Guidelines
Since October 2023, new international guidelines for vitiligo management have defined a therapeutic algorithm.
“Nowadays, we place the patient at the center of therapeutic decision-making,” Seneschal said. It is essential to educate patients about the disease and take the time to understand their treatment goals.
For patients with mild vitiligo that does not affect quality of life, simple monitoring may suffice.
However, when a decision is made to pursue treatment, its goals should be:
- Halting disease progression and melanocyte loss
- Achieving repigmentation (a process that can take 6-24 months)
- Preventing relapse after treatment discontinuation
For moderate cases affecting less than 10% of the skin surface, localized treatment is recommended. Previously, topical corticosteroids were used for body lesions, while tacrolimus 0.1% (off-label) was often prescribed for the face and neck. However, as of March 2024, tacrolimus has been officially approved for use in patients aged ≥ 2 years.
In more severe, generalized, and/or active cases, oral treatments such as corticosteroids taken twice weekly for 12-24 weeks can stabilize the disease in 80% of cases (off-label use). Other off-label options include methotrexate, cyclosporine, and tetracyclines.
Targeted Therapies
Recent targeted therapies have significantly advanced the treatment of moderate to severe vitiligo. Since January 2024, the Janus kinase 1 (JAK1)/JAK2 inhibitor ruxolitinib cream has been available in community pharmacies after previously being restricted to hospital use, Seneschal said, and can have spectacular results if previous treatments have failed.
Ruxolitinib is approved for patients aged > 12 years with nonsegmental vitiligo and facial involvement, covering up to 10% of the body surface area. Treatment typically lasts 6 months to 1 year.
Key Findings
The cream-formulated drug has been demonstrated effective in reducing inflammation in two phase 3 clinical trials published in the New England Journal of Medicine that demonstrated its efficacy and safety in patients aged ≥ 12 years. The treatment was well tolerated despite some mild acne-like reactions in 8% of patients. It was shown to be very effective on the face, with a reduction of over 75% in facial lesions in more than 50% of patients, and had good effectiveness on the body, with a 50% decrease in lesions in more than 50% of patients on the body, trunk, arms, and legs, excluding hands and feet.
“Areas like the underarms, hands, and feet are more resistant to treatment,” Seneschal noted.
Although some improvement continues after 1 year, disease recurrence is common if treatment is stopped: Only 40% of patients maintain therapeutic benefits in the year following discontinuation.
“It is therefore important to consider the value of continuing treatment in order to achieve better efficacy or to maintain the repigmentation obtained,” Seneschal said.
He stressed that all treatments should be paired with phototherapy, typically narrowband UVB, to accelerate repigmentation. “There is no increased skin cancer risk in vitiligo patients treated with narrowband UVB,” Seneschal said.
New Therapies
Emerging treatments under development, including injectable biologics alone or in combination with phototherapy, show great promise, he said. Oral JAK inhibitors such as ritlecitinib, upadacitinib, and povorcitinib are also under investigation.
In particular, ritlecitinib, a JAK3/TEC pathway inhibitor, has shown significant reductions in affected skin area in severely affected patients in a phase 2b trial. Phase 3 trials are now underway.
On the safety profile of JAK inhibitors, Seneschal said that studies are reassuring but highlighted the need to monitor cardiovascular, thromboembolic, and infectious risks.
“The question of safety is important because vitiligo is a visible but nonsevere condition, and we do not want to expose patients to unnecessary risks,” added Gaëlle Quéreux, MD, PhD, president of the French Society of Dermatology.
This story was translated from Medscape’s French edition using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
of the disease, delegates heard at a recent conference, the Dermatology Days of Paris 2024, organized by the French Society of Dermatology.
A Distinct Disease
An estimated 65% of patients with vitiligo in Europe have been told that their disease is untreatable, according to a recent international study, and this figure rises to 75% in France, Julien Seneschal, MD, PhD, professor of dermatology at Bordeaux University Hospital in Bordeaux, France, told the audience during his presentation.
“This is a message we must change,” he said.
The survey also revealed that in France, even when treatment is offered, 80% of patients do not receive appropriate care. However, treatments do exist, and novel approaches are revolutionizing the management of patients, whatever the degree of severity, he explained.
As a specialist in inflammatory and autoimmune skin diseases, he stressed that these advances are important because vitiligo is a distinct disease and not merely a cosmetic issue. When widespread, it has a significant impact on quality of life and can lead to depression, anxiety, and even suicidal thoughts, even though it does not affect life expectancy.
Updated Guidelines
Since October 2023, new international guidelines for vitiligo management have defined a therapeutic algorithm.
“Nowadays, we place the patient at the center of therapeutic decision-making,” Seneschal said. It is essential to educate patients about the disease and take the time to understand their treatment goals.
For patients with mild vitiligo that does not affect quality of life, simple monitoring may suffice.
However, when a decision is made to pursue treatment, its goals should be:
- Halting disease progression and melanocyte loss
- Achieving repigmentation (a process that can take 6-24 months)
- Preventing relapse after treatment discontinuation
For moderate cases affecting less than 10% of the skin surface, localized treatment is recommended. Previously, topical corticosteroids were used for body lesions, while tacrolimus 0.1% (off-label) was often prescribed for the face and neck. However, as of March 2024, tacrolimus has been officially approved for use in patients aged ≥ 2 years.
In more severe, generalized, and/or active cases, oral treatments such as corticosteroids taken twice weekly for 12-24 weeks can stabilize the disease in 80% of cases (off-label use). Other off-label options include methotrexate, cyclosporine, and tetracyclines.
Targeted Therapies
Recent targeted therapies have significantly advanced the treatment of moderate to severe vitiligo. Since January 2024, the Janus kinase 1 (JAK1)/JAK2 inhibitor ruxolitinib cream has been available in community pharmacies after previously being restricted to hospital use, Seneschal said, and can have spectacular results if previous treatments have failed.
Ruxolitinib is approved for patients aged > 12 years with nonsegmental vitiligo and facial involvement, covering up to 10% of the body surface area. Treatment typically lasts 6 months to 1 year.
Key Findings
The cream-formulated drug has been demonstrated effective in reducing inflammation in two phase 3 clinical trials published in the New England Journal of Medicine that demonstrated its efficacy and safety in patients aged ≥ 12 years. The treatment was well tolerated despite some mild acne-like reactions in 8% of patients. It was shown to be very effective on the face, with a reduction of over 75% in facial lesions in more than 50% of patients, and had good effectiveness on the body, with a 50% decrease in lesions in more than 50% of patients on the body, trunk, arms, and legs, excluding hands and feet.
“Areas like the underarms, hands, and feet are more resistant to treatment,” Seneschal noted.
Although some improvement continues after 1 year, disease recurrence is common if treatment is stopped: Only 40% of patients maintain therapeutic benefits in the year following discontinuation.
“It is therefore important to consider the value of continuing treatment in order to achieve better efficacy or to maintain the repigmentation obtained,” Seneschal said.
He stressed that all treatments should be paired with phototherapy, typically narrowband UVB, to accelerate repigmentation. “There is no increased skin cancer risk in vitiligo patients treated with narrowband UVB,” Seneschal said.
New Therapies
Emerging treatments under development, including injectable biologics alone or in combination with phototherapy, show great promise, he said. Oral JAK inhibitors such as ritlecitinib, upadacitinib, and povorcitinib are also under investigation.
In particular, ritlecitinib, a JAK3/TEC pathway inhibitor, has shown significant reductions in affected skin area in severely affected patients in a phase 2b trial. Phase 3 trials are now underway.
On the safety profile of JAK inhibitors, Seneschal said that studies are reassuring but highlighted the need to monitor cardiovascular, thromboembolic, and infectious risks.
“The question of safety is important because vitiligo is a visible but nonsevere condition, and we do not want to expose patients to unnecessary risks,” added Gaëlle Quéreux, MD, PhD, president of the French Society of Dermatology.
This story was translated from Medscape’s French edition using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Merkel Cell Carcinoma Less Common, With higher Mortality Than Melanoma
TOPLINE:
that also reported that male gender, older age, and exposure to ultraviolet radiation (UVR) are significant risk factors.
METHODOLOGY:
- Researchers identified 19,444 MCC cases and 646,619 melanoma cases diagnosed between 2000 and 2021 using data from the Surveillance, Epidemiology, and End Results (SEER) Program.
- Ambient UVR exposure data were obtained from the National Aeronautics and Space Administration’s total ozone mapping spectrometer database.
- Risk factors and cancer-specific mortality rates were evaluated for both cancers.
TAKEAWAY:
- Incidence rates per 100,000 person-years of MCC and melanoma were 0.8 and 27.3, respectively.
- Men (adjusted incidence rate ratio [IRR], 1.72 for MCC and 1.23 for melanoma), older age groups (IRR: 2.69 for MCC and 1.62 for melanoma among those 70-79 years; and 5.68 for MCC and 2.26 for melanoma among those 80 years or older) showed higher incidences of MCC and melanoma. Non-Hispanic White individuals were at higher risk for MCC and melanoma than other racial/ethnic groups.
- Exposure to UVR was associated with higher incidences of melanoma (IRR, 1.24-1.49) and MCC (IRR, 1.15-1.20) in non-Hispanic White individuals, particularly on the head and neck. These associations were unclear among racial/ethnic groups.
- Individuals with MCC had a higher risk for cancer-specific mortality than those with melanoma (adjusted hazard ratio [HR], 2.33; 95% CI, 2.26-2.42). Cancer-specific survival for both cancers improved for cases diagnosed during 2012-2021 vs 2004-2011 (MCC: HR, 0.83; 95% CI, 0.78-0.89; melanoma: HR, 0.75; 95% CI, 0.74-0.76).
IN PRACTICE:
“MCC and melanoma are aggressive skin cancers with similar risk factors including male sex, older age, and UV radiation exposure. Clinicians should be alert to diagnosis of these cancers to allow for prompt treatment,” the authors wrote, adding: “It is encouraging that survival for both cancers has increased in recent years, with the largest gains in survival seen in distant stage melanoma, coinciding with the approval of BRAF and PD-1 inhibitors used for distant stage disease,” although mortality for advanced stage tumors “continues to be very high.”
SOURCE:
The study was led by Jacob T. Tribble, BA, National Cancer Institute, Rockville, Maryland. It was published online on January 5 in the Journal of Investigative Dermatology.
LIMITATIONS:
The study relied on SEER’s general staging system rather than the American Joint Committee on Cancer standard, and UVR exposure estimates did not account for individual sun protection behaviors or prior residential history. Race and ethnicity served as a proxy for UVR sensitivity, which may introduce misclassification bias.
DISCLOSURES:
The research was supported by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the American Association for Dental Research, and the Colgate-Palmolive Company. The authors reported no conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also reported that male gender, older age, and exposure to ultraviolet radiation (UVR) are significant risk factors.
METHODOLOGY:
- Researchers identified 19,444 MCC cases and 646,619 melanoma cases diagnosed between 2000 and 2021 using data from the Surveillance, Epidemiology, and End Results (SEER) Program.
- Ambient UVR exposure data were obtained from the National Aeronautics and Space Administration’s total ozone mapping spectrometer database.
- Risk factors and cancer-specific mortality rates were evaluated for both cancers.
TAKEAWAY:
- Incidence rates per 100,000 person-years of MCC and melanoma were 0.8 and 27.3, respectively.
- Men (adjusted incidence rate ratio [IRR], 1.72 for MCC and 1.23 for melanoma), older age groups (IRR: 2.69 for MCC and 1.62 for melanoma among those 70-79 years; and 5.68 for MCC and 2.26 for melanoma among those 80 years or older) showed higher incidences of MCC and melanoma. Non-Hispanic White individuals were at higher risk for MCC and melanoma than other racial/ethnic groups.
- Exposure to UVR was associated with higher incidences of melanoma (IRR, 1.24-1.49) and MCC (IRR, 1.15-1.20) in non-Hispanic White individuals, particularly on the head and neck. These associations were unclear among racial/ethnic groups.
- Individuals with MCC had a higher risk for cancer-specific mortality than those with melanoma (adjusted hazard ratio [HR], 2.33; 95% CI, 2.26-2.42). Cancer-specific survival for both cancers improved for cases diagnosed during 2012-2021 vs 2004-2011 (MCC: HR, 0.83; 95% CI, 0.78-0.89; melanoma: HR, 0.75; 95% CI, 0.74-0.76).
IN PRACTICE:
“MCC and melanoma are aggressive skin cancers with similar risk factors including male sex, older age, and UV radiation exposure. Clinicians should be alert to diagnosis of these cancers to allow for prompt treatment,” the authors wrote, adding: “It is encouraging that survival for both cancers has increased in recent years, with the largest gains in survival seen in distant stage melanoma, coinciding with the approval of BRAF and PD-1 inhibitors used for distant stage disease,” although mortality for advanced stage tumors “continues to be very high.”
SOURCE:
The study was led by Jacob T. Tribble, BA, National Cancer Institute, Rockville, Maryland. It was published online on January 5 in the Journal of Investigative Dermatology.
LIMITATIONS:
The study relied on SEER’s general staging system rather than the American Joint Committee on Cancer standard, and UVR exposure estimates did not account for individual sun protection behaviors or prior residential history. Race and ethnicity served as a proxy for UVR sensitivity, which may introduce misclassification bias.
DISCLOSURES:
The research was supported by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the American Association for Dental Research, and the Colgate-Palmolive Company. The authors reported no conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
that also reported that male gender, older age, and exposure to ultraviolet radiation (UVR) are significant risk factors.
METHODOLOGY:
- Researchers identified 19,444 MCC cases and 646,619 melanoma cases diagnosed between 2000 and 2021 using data from the Surveillance, Epidemiology, and End Results (SEER) Program.
- Ambient UVR exposure data were obtained from the National Aeronautics and Space Administration’s total ozone mapping spectrometer database.
- Risk factors and cancer-specific mortality rates were evaluated for both cancers.
TAKEAWAY:
- Incidence rates per 100,000 person-years of MCC and melanoma were 0.8 and 27.3, respectively.
- Men (adjusted incidence rate ratio [IRR], 1.72 for MCC and 1.23 for melanoma), older age groups (IRR: 2.69 for MCC and 1.62 for melanoma among those 70-79 years; and 5.68 for MCC and 2.26 for melanoma among those 80 years or older) showed higher incidences of MCC and melanoma. Non-Hispanic White individuals were at higher risk for MCC and melanoma than other racial/ethnic groups.
- Exposure to UVR was associated with higher incidences of melanoma (IRR, 1.24-1.49) and MCC (IRR, 1.15-1.20) in non-Hispanic White individuals, particularly on the head and neck. These associations were unclear among racial/ethnic groups.
- Individuals with MCC had a higher risk for cancer-specific mortality than those with melanoma (adjusted hazard ratio [HR], 2.33; 95% CI, 2.26-2.42). Cancer-specific survival for both cancers improved for cases diagnosed during 2012-2021 vs 2004-2011 (MCC: HR, 0.83; 95% CI, 0.78-0.89; melanoma: HR, 0.75; 95% CI, 0.74-0.76).
IN PRACTICE:
“MCC and melanoma are aggressive skin cancers with similar risk factors including male sex, older age, and UV radiation exposure. Clinicians should be alert to diagnosis of these cancers to allow for prompt treatment,” the authors wrote, adding: “It is encouraging that survival for both cancers has increased in recent years, with the largest gains in survival seen in distant stage melanoma, coinciding with the approval of BRAF and PD-1 inhibitors used for distant stage disease,” although mortality for advanced stage tumors “continues to be very high.”
SOURCE:
The study was led by Jacob T. Tribble, BA, National Cancer Institute, Rockville, Maryland. It was published online on January 5 in the Journal of Investigative Dermatology.
LIMITATIONS:
The study relied on SEER’s general staging system rather than the American Joint Committee on Cancer standard, and UVR exposure estimates did not account for individual sun protection behaviors or prior residential history. Race and ethnicity served as a proxy for UVR sensitivity, which may introduce misclassification bias.
DISCLOSURES:
The research was supported by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the American Association for Dental Research, and the Colgate-Palmolive Company. The authors reported no conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Treatment with Tildrakizumab Effective for Scalp Psoriasis in Phase 3b Study
TOPLINE:
METHODOLOGY:
- A 72-week, multicenter, randomized, double-blind, placebo-controlled phase 3b trial enrolled 231 patients with moderate to severe plaque psoriasis of the scalp.
- Patients were randomly assigned to receive placebo (n = 114) or tildrakizumab (n = 117) until week 16, when patients in the placebo group switched to receive tildrakizumab.
- The primary endpoint, Investigator Global Assessment modified 2011 (IGA) scalp response, was defined as a score of 0 (clear) or 1 (almost clear) or an improvement of at least two points at week 16.
- The treatment was stopped at week 52, and participants were observed for another 20 weeks for safety and tolerability.
TAKEAWAY:
- At week 16, the response rate was higher in the tildrakizumab group than in the placebo group (49.4% vs 7.3%; P < .00001), and it increased to 62.9% and 56.1% (after crossover), respectively, at week 52.
- Psoriasis Scalp Severity Index 90 (PSSI 90) response rates were 60.7% and 4.9% at week 16 in the tildrakizumab and placebo groups, rising to 65.2% and 57.3%, respectively, at week 52.
- More than 80% of the week 16 responders maintained IGA and PSSI 90 responses at week 52.
- More than 50% of patients in both groups experienced adverse events, with no treatment-related serious toxicity.
IN PRACTICE:
“Tildrakizumab maintains improvements in scalp psoriasis for up to 52 weeks,” the authors wrote.
SOURCE:
Howard L. Sofen, MD, University of California, Los Angeles, led the study, which was published online on December 22, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
This study excluded patients with predominantly scalp involvement and minimal whole body psoriasis, who might respond differently to the treatment. Results were obtained under controlled clinical conditions and may not be generalizable to clinical practice.
DISCLOSURES:
This study and analyses were funded by Sun Pharma. Sofen reported serving as a clinical investigator for various pharmaceutical companies, including Sun Pharma. Five authors were current or former employees of Sun Pharma and associated companies. Others also disclosed financial ties outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 72-week, multicenter, randomized, double-blind, placebo-controlled phase 3b trial enrolled 231 patients with moderate to severe plaque psoriasis of the scalp.
- Patients were randomly assigned to receive placebo (n = 114) or tildrakizumab (n = 117) until week 16, when patients in the placebo group switched to receive tildrakizumab.
- The primary endpoint, Investigator Global Assessment modified 2011 (IGA) scalp response, was defined as a score of 0 (clear) or 1 (almost clear) or an improvement of at least two points at week 16.
- The treatment was stopped at week 52, and participants were observed for another 20 weeks for safety and tolerability.
TAKEAWAY:
- At week 16, the response rate was higher in the tildrakizumab group than in the placebo group (49.4% vs 7.3%; P < .00001), and it increased to 62.9% and 56.1% (after crossover), respectively, at week 52.
- Psoriasis Scalp Severity Index 90 (PSSI 90) response rates were 60.7% and 4.9% at week 16 in the tildrakizumab and placebo groups, rising to 65.2% and 57.3%, respectively, at week 52.
- More than 80% of the week 16 responders maintained IGA and PSSI 90 responses at week 52.
- More than 50% of patients in both groups experienced adverse events, with no treatment-related serious toxicity.
IN PRACTICE:
“Tildrakizumab maintains improvements in scalp psoriasis for up to 52 weeks,” the authors wrote.
SOURCE:
Howard L. Sofen, MD, University of California, Los Angeles, led the study, which was published online on December 22, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
This study excluded patients with predominantly scalp involvement and minimal whole body psoriasis, who might respond differently to the treatment. Results were obtained under controlled clinical conditions and may not be generalizable to clinical practice.
DISCLOSURES:
This study and analyses were funded by Sun Pharma. Sofen reported serving as a clinical investigator for various pharmaceutical companies, including Sun Pharma. Five authors were current or former employees of Sun Pharma and associated companies. Others also disclosed financial ties outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 72-week, multicenter, randomized, double-blind, placebo-controlled phase 3b trial enrolled 231 patients with moderate to severe plaque psoriasis of the scalp.
- Patients were randomly assigned to receive placebo (n = 114) or tildrakizumab (n = 117) until week 16, when patients in the placebo group switched to receive tildrakizumab.
- The primary endpoint, Investigator Global Assessment modified 2011 (IGA) scalp response, was defined as a score of 0 (clear) or 1 (almost clear) or an improvement of at least two points at week 16.
- The treatment was stopped at week 52, and participants were observed for another 20 weeks for safety and tolerability.
TAKEAWAY:
- At week 16, the response rate was higher in the tildrakizumab group than in the placebo group (49.4% vs 7.3%; P < .00001), and it increased to 62.9% and 56.1% (after crossover), respectively, at week 52.
- Psoriasis Scalp Severity Index 90 (PSSI 90) response rates were 60.7% and 4.9% at week 16 in the tildrakizumab and placebo groups, rising to 65.2% and 57.3%, respectively, at week 52.
- More than 80% of the week 16 responders maintained IGA and PSSI 90 responses at week 52.
- More than 50% of patients in both groups experienced adverse events, with no treatment-related serious toxicity.
IN PRACTICE:
“Tildrakizumab maintains improvements in scalp psoriasis for up to 52 weeks,” the authors wrote.
SOURCE:
Howard L. Sofen, MD, University of California, Los Angeles, led the study, which was published online on December 22, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
This study excluded patients with predominantly scalp involvement and minimal whole body psoriasis, who might respond differently to the treatment. Results were obtained under controlled clinical conditions and may not be generalizable to clinical practice.
DISCLOSURES:
This study and analyses were funded by Sun Pharma. Sofen reported serving as a clinical investigator for various pharmaceutical companies, including Sun Pharma. Five authors were current or former employees of Sun Pharma and associated companies. Others also disclosed financial ties outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Around 5% of US Population Diagnosed With Autoimmune Disease
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Pruritus: Diagnosing and Treating Older Adults
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develops when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
PN can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, selfconsciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
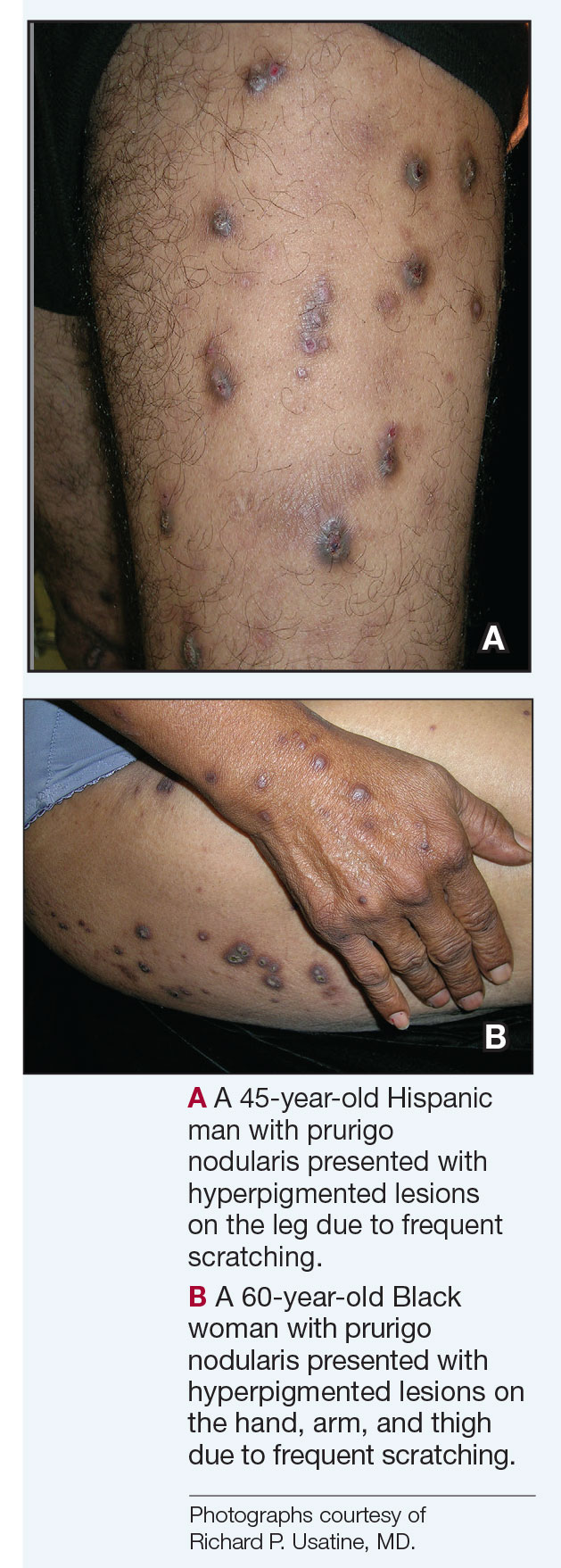
Epidemiology
PN has a prevalence of 72 per 100,000 individuals in the United States, most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.11-13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms. 10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not surprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease. 11,13 Workup for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥ 10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrow-band UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus. 10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 PN is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron– expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multicenter cohort study. J Am Acad Dermatol. 2022;82:487- 490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double- blind, placebo- controlled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develops when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
PN can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, selfconsciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10

Epidemiology
PN has a prevalence of 72 per 100,000 individuals in the United States, most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.11-13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms. 10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not surprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease. 11,13 Workup for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥ 10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrow-band UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus. 10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 PN is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develops when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
PN can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, selfconsciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10

Epidemiology
PN has a prevalence of 72 per 100,000 individuals in the United States, most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.11-13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms. 10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not surprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease. 11,13 Workup for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥ 10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrow-band UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus. 10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 PN is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron– expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multicenter cohort study. J Am Acad Dermatol. 2022;82:487- 490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double- blind, placebo- controlled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron– expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multicenter cohort study. J Am Acad Dermatol. 2022;82:487- 490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double- blind, placebo- controlled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Cardiac Risks of Newer Psoriasis Biologics vs. TNF Inhibitors Compared
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Online CBT for Patients with AD: Self-Guided vs. Clinician-Guided Intervention Compared
TOPLINE:
A brief on the Patient-Oriented Eczema Measure (POEM).
METHODOLOGY:
- Researchers conducted a single-blind randomized clinical noninferiority trial at Karolinska Institutet in Stockholm, Sweden, enrolling 168 adults with AD (mean age, 39 years; 84.5% women) from November 2022 to April 2023.
- Participants were randomly assigned to either a 12-week self-guided online CBT intervention (n = 86) without clinician support or a comprehensive 12-week clinician-guided online CBT program (n = 82).
- The primary outcome was the change in POEM score from baseline; reduction of 4 or more points was considered a response, and the predefined noninferiority margin was 3 points.
TAKEAWAY:
- The clinician-guided group improved by 4.20 points on POEM, while the self-guided group improved by 4.60 points, with an estimated mean difference in change of 0.36 points, which was below noninferiority margin.
- Clinicians spent a mean of 36 minutes on treatment guidance and an additional 14 minutes on assessments in the clinician-guided group, whereas they spent only 15.8 minutes on assessments in the self-guided group.
- Both groups demonstrated significant improvements in quality of life, sleep, depressive mood, pruritus, and stress, with no serious adverse events being reported.
- Completion rates were higher in the self-guided group with 81% of participants completing five or more modules, compared with 67% in the clinician-guided group.
IN PRACTICE:
“Overall, the findings support a self-guided intervention as a noninferior and cost-effective alternative to a previously evaluated clinician-guided treatment,” the authors wrote. “Because psychological interventions are rare in dermatological care, this study is an important step toward implementation of CBT for people with AD. The effectiveness of CBT interventions in primary and dermatological specialist care should be investigated.”
SOURCE:
The study was led by Dorian Kern, PhD, Division of Psychology, Karolinska Institutet, and was published online in JAMA Dermatology.
LIMITATIONS:
High data loss for secondary measurements could affect interpretation of these results. The study relied solely on self-reported measures. The predominance of women participants and the Swedish-language requirement may have limited participation from migrant populations, which could hinder the broader implementation of the study’s findings.
DISCLOSURES:
The study was supported by the Swedish Ministry of Health and Social Affairs. Kern reported receiving grants from the Swedish Ministry of Health and Social Affairs during the conduct of the study. Other authors also reported authorships and royalties, personal fees, grants, or held stocks in DahliaQomit.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A brief on the Patient-Oriented Eczema Measure (POEM).
METHODOLOGY:
- Researchers conducted a single-blind randomized clinical noninferiority trial at Karolinska Institutet in Stockholm, Sweden, enrolling 168 adults with AD (mean age, 39 years; 84.5% women) from November 2022 to April 2023.
- Participants were randomly assigned to either a 12-week self-guided online CBT intervention (n = 86) without clinician support or a comprehensive 12-week clinician-guided online CBT program (n = 82).
- The primary outcome was the change in POEM score from baseline; reduction of 4 or more points was considered a response, and the predefined noninferiority margin was 3 points.
TAKEAWAY:
- The clinician-guided group improved by 4.20 points on POEM, while the self-guided group improved by 4.60 points, with an estimated mean difference in change of 0.36 points, which was below noninferiority margin.
- Clinicians spent a mean of 36 minutes on treatment guidance and an additional 14 minutes on assessments in the clinician-guided group, whereas they spent only 15.8 minutes on assessments in the self-guided group.
- Both groups demonstrated significant improvements in quality of life, sleep, depressive mood, pruritus, and stress, with no serious adverse events being reported.
- Completion rates were higher in the self-guided group with 81% of participants completing five or more modules, compared with 67% in the clinician-guided group.
IN PRACTICE:
“Overall, the findings support a self-guided intervention as a noninferior and cost-effective alternative to a previously evaluated clinician-guided treatment,” the authors wrote. “Because psychological interventions are rare in dermatological care, this study is an important step toward implementation of CBT for people with AD. The effectiveness of CBT interventions in primary and dermatological specialist care should be investigated.”
SOURCE:
The study was led by Dorian Kern, PhD, Division of Psychology, Karolinska Institutet, and was published online in JAMA Dermatology.
LIMITATIONS:
High data loss for secondary measurements could affect interpretation of these results. The study relied solely on self-reported measures. The predominance of women participants and the Swedish-language requirement may have limited participation from migrant populations, which could hinder the broader implementation of the study’s findings.
DISCLOSURES:
The study was supported by the Swedish Ministry of Health and Social Affairs. Kern reported receiving grants from the Swedish Ministry of Health and Social Affairs during the conduct of the study. Other authors also reported authorships and royalties, personal fees, grants, or held stocks in DahliaQomit.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A brief on the Patient-Oriented Eczema Measure (POEM).
METHODOLOGY:
- Researchers conducted a single-blind randomized clinical noninferiority trial at Karolinska Institutet in Stockholm, Sweden, enrolling 168 adults with AD (mean age, 39 years; 84.5% women) from November 2022 to April 2023.
- Participants were randomly assigned to either a 12-week self-guided online CBT intervention (n = 86) without clinician support or a comprehensive 12-week clinician-guided online CBT program (n = 82).
- The primary outcome was the change in POEM score from baseline; reduction of 4 or more points was considered a response, and the predefined noninferiority margin was 3 points.
TAKEAWAY:
- The clinician-guided group improved by 4.20 points on POEM, while the self-guided group improved by 4.60 points, with an estimated mean difference in change of 0.36 points, which was below noninferiority margin.
- Clinicians spent a mean of 36 minutes on treatment guidance and an additional 14 minutes on assessments in the clinician-guided group, whereas they spent only 15.8 minutes on assessments in the self-guided group.
- Both groups demonstrated significant improvements in quality of life, sleep, depressive mood, pruritus, and stress, with no serious adverse events being reported.
- Completion rates were higher in the self-guided group with 81% of participants completing five or more modules, compared with 67% in the clinician-guided group.
IN PRACTICE:
“Overall, the findings support a self-guided intervention as a noninferior and cost-effective alternative to a previously evaluated clinician-guided treatment,” the authors wrote. “Because psychological interventions are rare in dermatological care, this study is an important step toward implementation of CBT for people with AD. The effectiveness of CBT interventions in primary and dermatological specialist care should be investigated.”
SOURCE:
The study was led by Dorian Kern, PhD, Division of Psychology, Karolinska Institutet, and was published online in JAMA Dermatology.
LIMITATIONS:
High data loss for secondary measurements could affect interpretation of these results. The study relied solely on self-reported measures. The predominance of women participants and the Swedish-language requirement may have limited participation from migrant populations, which could hinder the broader implementation of the study’s findings.
DISCLOSURES:
The study was supported by the Swedish Ministry of Health and Social Affairs. Kern reported receiving grants from the Swedish Ministry of Health and Social Affairs during the conduct of the study. Other authors also reported authorships and royalties, personal fees, grants, or held stocks in DahliaQomit.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Central Line Skin Reactions in Children: Survey Addresses Treatment Protocols in Use
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A and reported varying management approaches.
METHODOLOGY:
- Researchers developed and administered a 14-item Qualtrics survey to 107 dermatologists providing pediatric inpatient care through the Society for Pediatric Dermatology’s Inpatient Dermatology Section and Section Chief email lists.
- A total of 35 dermatologists (33%) from multiple institutions responded to the survey; most respondents (94%) specialized in pediatric dermatology.
- Researchers assessed management of CLD-associated adverse skin reactions.
TAKEAWAY:
- All respondents reported receiving CLD-related consults, but 66% indicated there was no personal or institutional standardized approach for managing CLD-associated skin reactions.
- Respondents said most reactions were in children aged 1-12 years (19 or 76% of 25 respondents) compared with those aged < 1 year (3 or 12% of 25 respondents).
- Management strategies included switching to alternative products, applying topical corticosteroids, and performing patch testing for allergies.
IN PRACTICE:
“Insights derived from this study, including variation in clinician familiarity with reaction patterns, underscore the necessity of a standardized protocol for classifying and managing cutaneous CLD reactions in pediatric patients,” the authors wrote. “Further investigation is needed to better characterize CLD-associated allergic CD [contact dermatitis], irritant CD, and skin infections, as well as at-risk populations, to better inform clinical approaches,” they added.
SOURCE:
The study was led by Carly Mulinda, Columbia University College of Physicians and Surgeons, New York, and was published online on December 16 in Pediatric Dermatology.
LIMITATIONS:
The authors noted variable respondent awareness of institutional CLD and potential recency bias as key limitations of the study.
DISCLOSURES:
Study funding source was not declared. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
