User login
Axolotl Salamander Holds Potential for Cosmeceuticals, Wound Healing
For over 200 years, researchers have been captivated by axolotl salamanders (Ambystoma mexicanum) and their remarkable regenerative abilities, seeking to uncover secrets that could revolutionize regenerative medicine, including the scarless healing of wounds.
“The axolotl salamander is the most studied animal ever in science for its neotenic ability to regenerate,” Jill S. Waibel, MD, dermatologist and researcher in Miami, Florida, said in an interview. Neotenic tissue retains a juvenile or immature state throughout an organism’s life. In the case of the axolotl, “it can regenerate limbs, part of its heart, even its brain.”
A 2019 review of several studies on the regenerative abilities of axolotls highlights the importance of gene activity in controlling its skin regeneration. Specifically, growth factors such as fibroblast growth factors, transforming growth factor beta, and Wnt play a key role in guiding how the creature’s skin cells behave during healing and regrowth. The immune response , particularly the actions of macrophages and neutrophils, is also crucial in the early stages of regeneration, as these cells clear away dead tissue and kickstart the healing process.
without harming the animal. In axolotls, Waibel explained, damaged neotenic tissue “still thinks it’s in fetal mode, so if it injures its muscle, bone, nerves, collagen, or skin, everything will redevelop. After a few months in utero, that process stops in humans, but it never stops in the axolotl. The axolotl has scarless healing and immunity because of antimicrobial properties found in the neotenic tissue.”
RegenX scientists have developed a proprietary decellularization process that renders the urodele collagen extract safe and effective for use in humans. “We then harnessed a reservoir of bioactive peptides, which are small proteins that come from the axolotl, but they don’t contain any RNA or DNA that could confer the risk of any diseases or cancer,” she added.
According to Waibel, who is also subsection chief of dermatology at Baptist Hospital and past medical director of the Miami Cancer Institute’s Multidisciplinary Skin Cancer Clinic, genetic analysis of the axolotl revealed genes that have not been seen in humans. The urodele collagen extract also has anti-inflammatory and analgesic properties. “It decreases TNF [tumor necrosis factor] and IL [interleukin]–23 and stimulates regenerative pathways like FETUB (Fetuin-B), which is a gene involved in tissue regeneration,” she said. “We’re exploring these for some products.”
Institutional Review Board–approved human clinical trials at three US sites are nearly complete for evaluating an antiaging hydrating daily serum, an antiaging serum for damaged skin, and a restorative serum to be applied following cosmetic procedures, all containing the extract. The product furthest along is a “super gel” that contains properties of the urodele collagen extract.
In a proof-of-concept study using a third-degree burn model in two pigs, Waibel and colleagues at the University of Miami, found that 3 days after the injury was induced, application of the gel led to 92% reepithelialization of the pig’s skin, compared with only 54% in untreated skin.
Shortly after this study was conducted, a burn patient was referred to Waibel — 4 years after he was struck by lightning while fishing on a boat in Mississippi, an accident that resulted in the loss of his right arm and both legs. During a telemedicine consultation, Waibel noticed open ulcers on his chest. “What are those from?” she asked. “They’re from my accident 4 years ago,” he replied.
After the man flew to Miami for an in-person evaluation, Waibel treated his ulcers with a fractional laser to debride the wound, then applied the gel as part of a proof-of-concept approach, testing its potential in a real-world patient setting. Within 3 weeks, the long-standing ulcerated area had healed completely, marking the first time a human was treated with the super gel.
Looking ahead, the million-dollar question, Waibel noted, is how much healing can be achieved in humans with formulations of axolotl-derived technology. “For example, can we help a spinal cord injury patient? That sounds like a science fiction movie, but there are proteins in genes in this animal that we have turned off that potentially can be turned on in a human,” she said. “It’s very exciting.”
Arisa E. Ortiz, MD, director of Laser and Cosmetic Dermatology at the University of California, San Diego, and current president of the American Society for Laser Medicine and Surgery, who was asked to comment on this work, said that the use of urodele collagen extract derived from axolotl tissue “is an exciting innovation, especially given its unique properties like scarless healing and antimicrobial activity.”
While the results from preclinical and proof-of-concept studies are promising, “a key limitation lies in understanding the extent to which these findings will translate to human applications,” Ortiz said. “Overall, this research contributes significantly to the fields of regenerative medicine and dermatology, offering hope for more effective treatments in the future.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to provide her insights on the topic, said that, if researchers could replicate the axolotl salamander’s ability to regenerate its own limbs and organs, “medicine would be transformed. Rather than transplant another person’s organ with lifelong immunosuppression, a regenerative treatment could program a patient’s own body to create a needed organ.
“On a simpler level,” she continued, “regenerating skin and its underlying structures could hasten wound healing and potentially even treat hair loss. This is not a pipe dream, as Waibel has successfully treated severe ulcers using a super gel containing urodele collagen extract. Urodele collagen is type XII collagen, important in the salamander’s capacity to heal and regenerate.”
Waibel disclosed that she is a scientific adviser to RegenX and is a member of the company’s board of directors. Ortiz and Ko reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
For over 200 years, researchers have been captivated by axolotl salamanders (Ambystoma mexicanum) and their remarkable regenerative abilities, seeking to uncover secrets that could revolutionize regenerative medicine, including the scarless healing of wounds.
“The axolotl salamander is the most studied animal ever in science for its neotenic ability to regenerate,” Jill S. Waibel, MD, dermatologist and researcher in Miami, Florida, said in an interview. Neotenic tissue retains a juvenile or immature state throughout an organism’s life. In the case of the axolotl, “it can regenerate limbs, part of its heart, even its brain.”
A 2019 review of several studies on the regenerative abilities of axolotls highlights the importance of gene activity in controlling its skin regeneration. Specifically, growth factors such as fibroblast growth factors, transforming growth factor beta, and Wnt play a key role in guiding how the creature’s skin cells behave during healing and regrowth. The immune response , particularly the actions of macrophages and neutrophils, is also crucial in the early stages of regeneration, as these cells clear away dead tissue and kickstart the healing process.
without harming the animal. In axolotls, Waibel explained, damaged neotenic tissue “still thinks it’s in fetal mode, so if it injures its muscle, bone, nerves, collagen, or skin, everything will redevelop. After a few months in utero, that process stops in humans, but it never stops in the axolotl. The axolotl has scarless healing and immunity because of antimicrobial properties found in the neotenic tissue.”
RegenX scientists have developed a proprietary decellularization process that renders the urodele collagen extract safe and effective for use in humans. “We then harnessed a reservoir of bioactive peptides, which are small proteins that come from the axolotl, but they don’t contain any RNA or DNA that could confer the risk of any diseases or cancer,” she added.
According to Waibel, who is also subsection chief of dermatology at Baptist Hospital and past medical director of the Miami Cancer Institute’s Multidisciplinary Skin Cancer Clinic, genetic analysis of the axolotl revealed genes that have not been seen in humans. The urodele collagen extract also has anti-inflammatory and analgesic properties. “It decreases TNF [tumor necrosis factor] and IL [interleukin]–23 and stimulates regenerative pathways like FETUB (Fetuin-B), which is a gene involved in tissue regeneration,” she said. “We’re exploring these for some products.”
Institutional Review Board–approved human clinical trials at three US sites are nearly complete for evaluating an antiaging hydrating daily serum, an antiaging serum for damaged skin, and a restorative serum to be applied following cosmetic procedures, all containing the extract. The product furthest along is a “super gel” that contains properties of the urodele collagen extract.
In a proof-of-concept study using a third-degree burn model in two pigs, Waibel and colleagues at the University of Miami, found that 3 days after the injury was induced, application of the gel led to 92% reepithelialization of the pig’s skin, compared with only 54% in untreated skin.
Shortly after this study was conducted, a burn patient was referred to Waibel — 4 years after he was struck by lightning while fishing on a boat in Mississippi, an accident that resulted in the loss of his right arm and both legs. During a telemedicine consultation, Waibel noticed open ulcers on his chest. “What are those from?” she asked. “They’re from my accident 4 years ago,” he replied.
After the man flew to Miami for an in-person evaluation, Waibel treated his ulcers with a fractional laser to debride the wound, then applied the gel as part of a proof-of-concept approach, testing its potential in a real-world patient setting. Within 3 weeks, the long-standing ulcerated area had healed completely, marking the first time a human was treated with the super gel.
Looking ahead, the million-dollar question, Waibel noted, is how much healing can be achieved in humans with formulations of axolotl-derived technology. “For example, can we help a spinal cord injury patient? That sounds like a science fiction movie, but there are proteins in genes in this animal that we have turned off that potentially can be turned on in a human,” she said. “It’s very exciting.”
Arisa E. Ortiz, MD, director of Laser and Cosmetic Dermatology at the University of California, San Diego, and current president of the American Society for Laser Medicine and Surgery, who was asked to comment on this work, said that the use of urodele collagen extract derived from axolotl tissue “is an exciting innovation, especially given its unique properties like scarless healing and antimicrobial activity.”
While the results from preclinical and proof-of-concept studies are promising, “a key limitation lies in understanding the extent to which these findings will translate to human applications,” Ortiz said. “Overall, this research contributes significantly to the fields of regenerative medicine and dermatology, offering hope for more effective treatments in the future.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to provide her insights on the topic, said that, if researchers could replicate the axolotl salamander’s ability to regenerate its own limbs and organs, “medicine would be transformed. Rather than transplant another person’s organ with lifelong immunosuppression, a regenerative treatment could program a patient’s own body to create a needed organ.
“On a simpler level,” she continued, “regenerating skin and its underlying structures could hasten wound healing and potentially even treat hair loss. This is not a pipe dream, as Waibel has successfully treated severe ulcers using a super gel containing urodele collagen extract. Urodele collagen is type XII collagen, important in the salamander’s capacity to heal and regenerate.”
Waibel disclosed that she is a scientific adviser to RegenX and is a member of the company’s board of directors. Ortiz and Ko reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
For over 200 years, researchers have been captivated by axolotl salamanders (Ambystoma mexicanum) and their remarkable regenerative abilities, seeking to uncover secrets that could revolutionize regenerative medicine, including the scarless healing of wounds.
“The axolotl salamander is the most studied animal ever in science for its neotenic ability to regenerate,” Jill S. Waibel, MD, dermatologist and researcher in Miami, Florida, said in an interview. Neotenic tissue retains a juvenile or immature state throughout an organism’s life. In the case of the axolotl, “it can regenerate limbs, part of its heart, even its brain.”
A 2019 review of several studies on the regenerative abilities of axolotls highlights the importance of gene activity in controlling its skin regeneration. Specifically, growth factors such as fibroblast growth factors, transforming growth factor beta, and Wnt play a key role in guiding how the creature’s skin cells behave during healing and regrowth. The immune response , particularly the actions of macrophages and neutrophils, is also crucial in the early stages of regeneration, as these cells clear away dead tissue and kickstart the healing process.
without harming the animal. In axolotls, Waibel explained, damaged neotenic tissue “still thinks it’s in fetal mode, so if it injures its muscle, bone, nerves, collagen, or skin, everything will redevelop. After a few months in utero, that process stops in humans, but it never stops in the axolotl. The axolotl has scarless healing and immunity because of antimicrobial properties found in the neotenic tissue.”
RegenX scientists have developed a proprietary decellularization process that renders the urodele collagen extract safe and effective for use in humans. “We then harnessed a reservoir of bioactive peptides, which are small proteins that come from the axolotl, but they don’t contain any RNA or DNA that could confer the risk of any diseases or cancer,” she added.
According to Waibel, who is also subsection chief of dermatology at Baptist Hospital and past medical director of the Miami Cancer Institute’s Multidisciplinary Skin Cancer Clinic, genetic analysis of the axolotl revealed genes that have not been seen in humans. The urodele collagen extract also has anti-inflammatory and analgesic properties. “It decreases TNF [tumor necrosis factor] and IL [interleukin]–23 and stimulates regenerative pathways like FETUB (Fetuin-B), which is a gene involved in tissue regeneration,” she said. “We’re exploring these for some products.”
Institutional Review Board–approved human clinical trials at three US sites are nearly complete for evaluating an antiaging hydrating daily serum, an antiaging serum for damaged skin, and a restorative serum to be applied following cosmetic procedures, all containing the extract. The product furthest along is a “super gel” that contains properties of the urodele collagen extract.
In a proof-of-concept study using a third-degree burn model in two pigs, Waibel and colleagues at the University of Miami, found that 3 days after the injury was induced, application of the gel led to 92% reepithelialization of the pig’s skin, compared with only 54% in untreated skin.
Shortly after this study was conducted, a burn patient was referred to Waibel — 4 years after he was struck by lightning while fishing on a boat in Mississippi, an accident that resulted in the loss of his right arm and both legs. During a telemedicine consultation, Waibel noticed open ulcers on his chest. “What are those from?” she asked. “They’re from my accident 4 years ago,” he replied.
After the man flew to Miami for an in-person evaluation, Waibel treated his ulcers with a fractional laser to debride the wound, then applied the gel as part of a proof-of-concept approach, testing its potential in a real-world patient setting. Within 3 weeks, the long-standing ulcerated area had healed completely, marking the first time a human was treated with the super gel.
Looking ahead, the million-dollar question, Waibel noted, is how much healing can be achieved in humans with formulations of axolotl-derived technology. “For example, can we help a spinal cord injury patient? That sounds like a science fiction movie, but there are proteins in genes in this animal that we have turned off that potentially can be turned on in a human,” she said. “It’s very exciting.”
Arisa E. Ortiz, MD, director of Laser and Cosmetic Dermatology at the University of California, San Diego, and current president of the American Society for Laser Medicine and Surgery, who was asked to comment on this work, said that the use of urodele collagen extract derived from axolotl tissue “is an exciting innovation, especially given its unique properties like scarless healing and antimicrobial activity.”
While the results from preclinical and proof-of-concept studies are promising, “a key limitation lies in understanding the extent to which these findings will translate to human applications,” Ortiz said. “Overall, this research contributes significantly to the fields of regenerative medicine and dermatology, offering hope for more effective treatments in the future.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to provide her insights on the topic, said that, if researchers could replicate the axolotl salamander’s ability to regenerate its own limbs and organs, “medicine would be transformed. Rather than transplant another person’s organ with lifelong immunosuppression, a regenerative treatment could program a patient’s own body to create a needed organ.
“On a simpler level,” she continued, “regenerating skin and its underlying structures could hasten wound healing and potentially even treat hair loss. This is not a pipe dream, as Waibel has successfully treated severe ulcers using a super gel containing urodele collagen extract. Urodele collagen is type XII collagen, important in the salamander’s capacity to heal and regenerate.”
Waibel disclosed that she is a scientific adviser to RegenX and is a member of the company’s board of directors. Ortiz and Ko reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
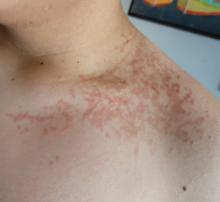
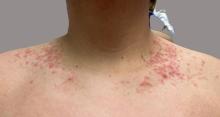
Allergic Contact Dermatitis: New Culprits
New allergens responsible for contact dermatitis emerge regularly. During the Dermatology Days of Paris 2024 conference, Angèle Soria, MD, PhD, a dermatologist at Tenon Hospital in Paris, France, outlined four major categories driving this trend. Among them are (meth)acrylates found in nail cosmetics used in salons or do-it-yourself false nail kits that can be bought online.
Isothiazolinones
a preservative used in many cosmetics; (meth)acrylates; essential oils; and epoxy resins used in industry and leisure activities.
Around 15 years ago, parabens, commonly used as preservatives in cosmetics, were identified as endocrine disruptors. In response, they were largely replaced by newer preservatives, notably MI. However, this led to a proliferation of allergic contact dermatitis in Europe between 2010 and 2013.
“About 10% of the population that we tested showed allergies to these preservatives, primarily found in cosmetics,” explained Soria. Since 2015, the use of MI in leave-on cosmetics has been prohibited in Europe and its concentration restricted in rinse-off products. However, cosmetics sold online from outside Europe may not comply with these regulations.
MI is also present in water-based paints to prevent mold. “A few years ago, we started seeing patients with facial angioedema, sometimes combined with asthma, caused by these isothiazolinone preservatives, including in patients who are not professional painters,” said Soria. More recently, attention has shifted to MI’s presence in household cleaning products. A 2020 Spanish study found MI in 76% of 34 analyzed cleaning products.
MI-based fungicides are also used to treat leather during transport, which can lead to contact allergies among professionals and consumers alike. Additionally, MI has been identified in children’s toys, including slime gels, and in florists’ gel cubes used to preserve flowers.
“We are therefore surrounded by these preservatives, which are no longer only in cosmetics,” warned the dermatologist.
(Meth)acrylates
Another major allergen category is (meth)acrylates, responsible for many cases of allergic contact dermatitis. Acrylates and their derivatives are widely used in everyday items. They are low–molecular weight monomers, sensitizing on contact with the skin. Their polymerized forms include materials like Plexiglas.
“We are currently witnessing an epidemic of contact dermatitis in the general population, mainly due to nail cosmetics, such as semipermanent nail polishes and at-home false nail kits,” reported Soria. Nail cosmetics account for 97% of new sensitization cases involving (meth)acrylates. These allergens often cause severe dermatitis, prompting the European Union to mandate labeling in 2020, warning that these products are “for professional use only” and can “cause allergic reactions.”
Beyond nail cosmetics, these allergens are also found in dental products (such as trays), ECG electrodes, prosthetics, glucose sensors, surgical adhesives, and some electronic devices like earbuds and phone screens. Notably, patients sensitized to acrylates via nail kits may experience reactions during dental treatments involving acrylates.
Investigating Essential Oil Use
Essential oils, distinct from vegetable oils like almond or argan, are another known allergen. Often considered risk-free due to their “natural” label, these products are widely used topically, orally, or via inhalation for various purposes, such as treating respiratory infections or creating relaxing atmospheres. However, essential oils contain fragrant molecules like terpenes, which can become highly allergenic over time, especially after repeated exposure.
Soria emphasized the importance of asking patients about their use of essential oils, especially tea tree and lavender oils, which are commonly used but rarely mentioned by patients unless prompted.
Epoxy Resins in Recreational Use
Epoxy resins are a growing cause of contact allergies, not just in professional settings such as aeronautics and construction work but also increasingly in recreational activities. Soria highlighted the case of a 12-year-old girl hospitalized for severe facial edema after engaging in resin crafts inspired by TikTok. For 6 months, she had been creating resin objects, such as bowls and cutting boards, using vinyl gloves and a Filtering FacePiece 2 mask under adult supervision.
“The growing popularity and online availability of epoxy resins mean that allergic reactions should now be considered even in nonprofessional contexts,” warned Soria.
Clinical Approach
When dermatologists suspect allergic contact dermatitis, the first step is to treat the condition with corticosteroid creams. This is followed by a detailed patient interview to identify suspected allergens in products they’ve used.
Patch testing is then conducted to confirm the allergen. Small chambers containing potential allergens are applied to the upper back for 48 hours without removal. Results are read 2-5 days later, with some cases requiring a 7-day follow-up.
The patient’s occupation is an important factor, as certain professions, such as hairdressing, healthcare, or beauty therapy, are known to trigger allergic contact dermatitis. Similarly, certain hobbies may also play a role.
A thorough approach ensures accurate diagnosis and targeted prevention strategies.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New allergens responsible for contact dermatitis emerge regularly. During the Dermatology Days of Paris 2024 conference, Angèle Soria, MD, PhD, a dermatologist at Tenon Hospital in Paris, France, outlined four major categories driving this trend. Among them are (meth)acrylates found in nail cosmetics used in salons or do-it-yourself false nail kits that can be bought online.
Isothiazolinones
a preservative used in many cosmetics; (meth)acrylates; essential oils; and epoxy resins used in industry and leisure activities.
Around 15 years ago, parabens, commonly used as preservatives in cosmetics, were identified as endocrine disruptors. In response, they were largely replaced by newer preservatives, notably MI. However, this led to a proliferation of allergic contact dermatitis in Europe between 2010 and 2013.
“About 10% of the population that we tested showed allergies to these preservatives, primarily found in cosmetics,” explained Soria. Since 2015, the use of MI in leave-on cosmetics has been prohibited in Europe and its concentration restricted in rinse-off products. However, cosmetics sold online from outside Europe may not comply with these regulations.
MI is also present in water-based paints to prevent mold. “A few years ago, we started seeing patients with facial angioedema, sometimes combined with asthma, caused by these isothiazolinone preservatives, including in patients who are not professional painters,” said Soria. More recently, attention has shifted to MI’s presence in household cleaning products. A 2020 Spanish study found MI in 76% of 34 analyzed cleaning products.
MI-based fungicides are also used to treat leather during transport, which can lead to contact allergies among professionals and consumers alike. Additionally, MI has been identified in children’s toys, including slime gels, and in florists’ gel cubes used to preserve flowers.
“We are therefore surrounded by these preservatives, which are no longer only in cosmetics,” warned the dermatologist.
(Meth)acrylates
Another major allergen category is (meth)acrylates, responsible for many cases of allergic contact dermatitis. Acrylates and their derivatives are widely used in everyday items. They are low–molecular weight monomers, sensitizing on contact with the skin. Their polymerized forms include materials like Plexiglas.
“We are currently witnessing an epidemic of contact dermatitis in the general population, mainly due to nail cosmetics, such as semipermanent nail polishes and at-home false nail kits,” reported Soria. Nail cosmetics account for 97% of new sensitization cases involving (meth)acrylates. These allergens often cause severe dermatitis, prompting the European Union to mandate labeling in 2020, warning that these products are “for professional use only” and can “cause allergic reactions.”
Beyond nail cosmetics, these allergens are also found in dental products (such as trays), ECG electrodes, prosthetics, glucose sensors, surgical adhesives, and some electronic devices like earbuds and phone screens. Notably, patients sensitized to acrylates via nail kits may experience reactions during dental treatments involving acrylates.
Investigating Essential Oil Use
Essential oils, distinct from vegetable oils like almond or argan, are another known allergen. Often considered risk-free due to their “natural” label, these products are widely used topically, orally, or via inhalation for various purposes, such as treating respiratory infections or creating relaxing atmospheres. However, essential oils contain fragrant molecules like terpenes, which can become highly allergenic over time, especially after repeated exposure.
Soria emphasized the importance of asking patients about their use of essential oils, especially tea tree and lavender oils, which are commonly used but rarely mentioned by patients unless prompted.
Epoxy Resins in Recreational Use
Epoxy resins are a growing cause of contact allergies, not just in professional settings such as aeronautics and construction work but also increasingly in recreational activities. Soria highlighted the case of a 12-year-old girl hospitalized for severe facial edema after engaging in resin crafts inspired by TikTok. For 6 months, she had been creating resin objects, such as bowls and cutting boards, using vinyl gloves and a Filtering FacePiece 2 mask under adult supervision.
“The growing popularity and online availability of epoxy resins mean that allergic reactions should now be considered even in nonprofessional contexts,” warned Soria.
Clinical Approach
When dermatologists suspect allergic contact dermatitis, the first step is to treat the condition with corticosteroid creams. This is followed by a detailed patient interview to identify suspected allergens in products they’ve used.
Patch testing is then conducted to confirm the allergen. Small chambers containing potential allergens are applied to the upper back for 48 hours without removal. Results are read 2-5 days later, with some cases requiring a 7-day follow-up.
The patient’s occupation is an important factor, as certain professions, such as hairdressing, healthcare, or beauty therapy, are known to trigger allergic contact dermatitis. Similarly, certain hobbies may also play a role.
A thorough approach ensures accurate diagnosis and targeted prevention strategies.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New allergens responsible for contact dermatitis emerge regularly. During the Dermatology Days of Paris 2024 conference, Angèle Soria, MD, PhD, a dermatologist at Tenon Hospital in Paris, France, outlined four major categories driving this trend. Among them are (meth)acrylates found in nail cosmetics used in salons or do-it-yourself false nail kits that can be bought online.
Isothiazolinones
a preservative used in many cosmetics; (meth)acrylates; essential oils; and epoxy resins used in industry and leisure activities.
Around 15 years ago, parabens, commonly used as preservatives in cosmetics, were identified as endocrine disruptors. In response, they were largely replaced by newer preservatives, notably MI. However, this led to a proliferation of allergic contact dermatitis in Europe between 2010 and 2013.
“About 10% of the population that we tested showed allergies to these preservatives, primarily found in cosmetics,” explained Soria. Since 2015, the use of MI in leave-on cosmetics has been prohibited in Europe and its concentration restricted in rinse-off products. However, cosmetics sold online from outside Europe may not comply with these regulations.
MI is also present in water-based paints to prevent mold. “A few years ago, we started seeing patients with facial angioedema, sometimes combined with asthma, caused by these isothiazolinone preservatives, including in patients who are not professional painters,” said Soria. More recently, attention has shifted to MI’s presence in household cleaning products. A 2020 Spanish study found MI in 76% of 34 analyzed cleaning products.
MI-based fungicides are also used to treat leather during transport, which can lead to contact allergies among professionals and consumers alike. Additionally, MI has been identified in children’s toys, including slime gels, and in florists’ gel cubes used to preserve flowers.
“We are therefore surrounded by these preservatives, which are no longer only in cosmetics,” warned the dermatologist.
(Meth)acrylates
Another major allergen category is (meth)acrylates, responsible for many cases of allergic contact dermatitis. Acrylates and their derivatives are widely used in everyday items. They are low–molecular weight monomers, sensitizing on contact with the skin. Their polymerized forms include materials like Plexiglas.
“We are currently witnessing an epidemic of contact dermatitis in the general population, mainly due to nail cosmetics, such as semipermanent nail polishes and at-home false nail kits,” reported Soria. Nail cosmetics account for 97% of new sensitization cases involving (meth)acrylates. These allergens often cause severe dermatitis, prompting the European Union to mandate labeling in 2020, warning that these products are “for professional use only” and can “cause allergic reactions.”
Beyond nail cosmetics, these allergens are also found in dental products (such as trays), ECG electrodes, prosthetics, glucose sensors, surgical adhesives, and some electronic devices like earbuds and phone screens. Notably, patients sensitized to acrylates via nail kits may experience reactions during dental treatments involving acrylates.
Investigating Essential Oil Use
Essential oils, distinct from vegetable oils like almond or argan, are another known allergen. Often considered risk-free due to their “natural” label, these products are widely used topically, orally, or via inhalation for various purposes, such as treating respiratory infections or creating relaxing atmospheres. However, essential oils contain fragrant molecules like terpenes, which can become highly allergenic over time, especially after repeated exposure.
Soria emphasized the importance of asking patients about their use of essential oils, especially tea tree and lavender oils, which are commonly used but rarely mentioned by patients unless prompted.
Epoxy Resins in Recreational Use
Epoxy resins are a growing cause of contact allergies, not just in professional settings such as aeronautics and construction work but also increasingly in recreational activities. Soria highlighted the case of a 12-year-old girl hospitalized for severe facial edema after engaging in resin crafts inspired by TikTok. For 6 months, she had been creating resin objects, such as bowls and cutting boards, using vinyl gloves and a Filtering FacePiece 2 mask under adult supervision.
“The growing popularity and online availability of epoxy resins mean that allergic reactions should now be considered even in nonprofessional contexts,” warned Soria.
Clinical Approach
When dermatologists suspect allergic contact dermatitis, the first step is to treat the condition with corticosteroid creams. This is followed by a detailed patient interview to identify suspected allergens in products they’ve used.
Patch testing is then conducted to confirm the allergen. Small chambers containing potential allergens are applied to the upper back for 48 hours without removal. Results are read 2-5 days later, with some cases requiring a 7-day follow-up.
The patient’s occupation is an important factor, as certain professions, such as hairdressing, healthcare, or beauty therapy, are known to trigger allergic contact dermatitis. Similarly, certain hobbies may also play a role.
A thorough approach ensures accurate diagnosis and targeted prevention strategies.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Skin Cancer Risk Elevated Among Blood, Marrow Transplant Survivors
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA Approves Ustekinumab Biosimilar Steqeyma, the Seventh of Its Kind
The Food and Drug Administration (FDA) has approved ustekinumab-stba (Steqeyma) as a biosimilar to the interleukin-12 and -23 inhibitor ustekinumab (Stelara) for the treatment of adults with active Crohn’s disease or ulcerative colitis and for both children aged ≥ 6 years and adults with moderate to severe plaque psoriasis or active psoriatic arthritis.
This is the seventh ustekinumab biosimilar approved by the FDA. The biosimilar, developed by Celltrion, has a license entry date in February 2025 as part of the settlement and license agreement with the manufacturer of the reference biologic, Johnson & Johnson.
Ustekinumab-stba will be available in two formulations: A subcutaneous injection in two strengths — a 45 mg/0.5 mL or 90 mg/1 mL solution in a single-dose, prefilled syringe — and an intravenous infusion of a 130 mg/26 mL (5 mg/mL) solution in a single-dose vial.
“The approval of Steqeyma reflects Celltrion’s continued investment in providing treatment options to patients diagnosed with ulcerative colitis, Crohn’s disease, psoriasis, and psoriatic arthritis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, Jersey City, New Jersey, in a press release.
The FDA has previously approved the company’s adalimumab biosimilar Yuflyma and its infliximab biosimilar Zymfentra.
The full prescribing information for ustekinumab-stba is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved ustekinumab-stba (Steqeyma) as a biosimilar to the interleukin-12 and -23 inhibitor ustekinumab (Stelara) for the treatment of adults with active Crohn’s disease or ulcerative colitis and for both children aged ≥ 6 years and adults with moderate to severe plaque psoriasis or active psoriatic arthritis.
This is the seventh ustekinumab biosimilar approved by the FDA. The biosimilar, developed by Celltrion, has a license entry date in February 2025 as part of the settlement and license agreement with the manufacturer of the reference biologic, Johnson & Johnson.
Ustekinumab-stba will be available in two formulations: A subcutaneous injection in two strengths — a 45 mg/0.5 mL or 90 mg/1 mL solution in a single-dose, prefilled syringe — and an intravenous infusion of a 130 mg/26 mL (5 mg/mL) solution in a single-dose vial.
“The approval of Steqeyma reflects Celltrion’s continued investment in providing treatment options to patients diagnosed with ulcerative colitis, Crohn’s disease, psoriasis, and psoriatic arthritis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, Jersey City, New Jersey, in a press release.
The FDA has previously approved the company’s adalimumab biosimilar Yuflyma and its infliximab biosimilar Zymfentra.
The full prescribing information for ustekinumab-stba is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved ustekinumab-stba (Steqeyma) as a biosimilar to the interleukin-12 and -23 inhibitor ustekinumab (Stelara) for the treatment of adults with active Crohn’s disease or ulcerative colitis and for both children aged ≥ 6 years and adults with moderate to severe plaque psoriasis or active psoriatic arthritis.
This is the seventh ustekinumab biosimilar approved by the FDA. The biosimilar, developed by Celltrion, has a license entry date in February 2025 as part of the settlement and license agreement with the manufacturer of the reference biologic, Johnson & Johnson.
Ustekinumab-stba will be available in two formulations: A subcutaneous injection in two strengths — a 45 mg/0.5 mL or 90 mg/1 mL solution in a single-dose, prefilled syringe — and an intravenous infusion of a 130 mg/26 mL (5 mg/mL) solution in a single-dose vial.
“The approval of Steqeyma reflects Celltrion’s continued investment in providing treatment options to patients diagnosed with ulcerative colitis, Crohn’s disease, psoriasis, and psoriatic arthritis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, Jersey City, New Jersey, in a press release.
The FDA has previously approved the company’s adalimumab biosimilar Yuflyma and its infliximab biosimilar Zymfentra.
The full prescribing information for ustekinumab-stba is available here.
A version of this article first appeared on Medscape.com.
Infants Exposed to Minoxidil May Develop Hypertrichosis
OVIEDO, SPAIN – In April 2023, the Navarra Pharmacovigilance Center (NPC) became aware of a case involving an infant who developed progressive hair growth on their back, legs, and thighs (hypertrichosis) over the course of 2 months. During an interview with the family, it was revealed that the infant’s father had been using 5% topical minoxidil to treat androgenic alopecia, and he had taken a leave of absence from work to care for his child. After the medication was discontinued, the infant’s symptoms fully regressed. Specialists from the NPC presented the case at the 13th Spanish Pharmacovigilance Congress in November 2024.
A review of similar cases reported in the Spanish Pharmacovigilance System database identified six additional cases with the same characteristics, all involving infants whose caregivers were using minoxidil. Four more cases were found through the European pharmacovigilance database EudraVigilance and a review of scientific literature, bringing the total to 11 cases.
According to the Navarra Pharmacovigilance Bulletin, these cases are concerning as they highlight the exposure of vulnerable infants to a medication not indicated for their age group. Additionally, the condition can cause significant stress for the families of the affected children.
Mechanism of Transmission Unclear
In the newly identified cases, specialists suspect the drug was transmitted through direct or indirect contact. Accidental ingestion is also a possibility if the infant’s hands touched treated areas on the caregiver’s skin and were then brought to the mouth.
The NPC explained that infants’ skin is more permeable because of the thinner stratum corneum and a higher surface area/body weight ratio, making them more susceptible to absorbing topically applied medications.
Regulatory Changes and Precautions
In light of these findings, the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee concluded that, starting in October 2024, product information for medications containing minoxidil should be updated. Specifically, new information must be added to the package insert warning about the risk for hypertrichosis in infants following accidental exposure to minoxidil.
The NPC emphasizes the importance of caregivers being aware of the risks associated with topical medications like minoxidil. Recommended precautions include thoroughly washing hands after applying the product and covering treated areas to prevent direct contact with infants’ skin.
Healthcare professionals should also be aware of this risk and consider it when diagnosing hypertrichosis in infants. Recognizing the connection can prevent unnecessary testing for the infant and alleviate stress for the family.
This story was translated from Univadis Spain using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
OVIEDO, SPAIN – In April 2023, the Navarra Pharmacovigilance Center (NPC) became aware of a case involving an infant who developed progressive hair growth on their back, legs, and thighs (hypertrichosis) over the course of 2 months. During an interview with the family, it was revealed that the infant’s father had been using 5% topical minoxidil to treat androgenic alopecia, and he had taken a leave of absence from work to care for his child. After the medication was discontinued, the infant’s symptoms fully regressed. Specialists from the NPC presented the case at the 13th Spanish Pharmacovigilance Congress in November 2024.
A review of similar cases reported in the Spanish Pharmacovigilance System database identified six additional cases with the same characteristics, all involving infants whose caregivers were using minoxidil. Four more cases were found through the European pharmacovigilance database EudraVigilance and a review of scientific literature, bringing the total to 11 cases.
According to the Navarra Pharmacovigilance Bulletin, these cases are concerning as they highlight the exposure of vulnerable infants to a medication not indicated for their age group. Additionally, the condition can cause significant stress for the families of the affected children.
Mechanism of Transmission Unclear
In the newly identified cases, specialists suspect the drug was transmitted through direct or indirect contact. Accidental ingestion is also a possibility if the infant’s hands touched treated areas on the caregiver’s skin and were then brought to the mouth.
The NPC explained that infants’ skin is more permeable because of the thinner stratum corneum and a higher surface area/body weight ratio, making them more susceptible to absorbing topically applied medications.
Regulatory Changes and Precautions
In light of these findings, the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee concluded that, starting in October 2024, product information for medications containing minoxidil should be updated. Specifically, new information must be added to the package insert warning about the risk for hypertrichosis in infants following accidental exposure to minoxidil.
The NPC emphasizes the importance of caregivers being aware of the risks associated with topical medications like minoxidil. Recommended precautions include thoroughly washing hands after applying the product and covering treated areas to prevent direct contact with infants’ skin.
Healthcare professionals should also be aware of this risk and consider it when diagnosing hypertrichosis in infants. Recognizing the connection can prevent unnecessary testing for the infant and alleviate stress for the family.
This story was translated from Univadis Spain using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
OVIEDO, SPAIN – In April 2023, the Navarra Pharmacovigilance Center (NPC) became aware of a case involving an infant who developed progressive hair growth on their back, legs, and thighs (hypertrichosis) over the course of 2 months. During an interview with the family, it was revealed that the infant’s father had been using 5% topical minoxidil to treat androgenic alopecia, and he had taken a leave of absence from work to care for his child. After the medication was discontinued, the infant’s symptoms fully regressed. Specialists from the NPC presented the case at the 13th Spanish Pharmacovigilance Congress in November 2024.
A review of similar cases reported in the Spanish Pharmacovigilance System database identified six additional cases with the same characteristics, all involving infants whose caregivers were using minoxidil. Four more cases were found through the European pharmacovigilance database EudraVigilance and a review of scientific literature, bringing the total to 11 cases.
According to the Navarra Pharmacovigilance Bulletin, these cases are concerning as they highlight the exposure of vulnerable infants to a medication not indicated for their age group. Additionally, the condition can cause significant stress for the families of the affected children.
Mechanism of Transmission Unclear
In the newly identified cases, specialists suspect the drug was transmitted through direct or indirect contact. Accidental ingestion is also a possibility if the infant’s hands touched treated areas on the caregiver’s skin and were then brought to the mouth.
The NPC explained that infants’ skin is more permeable because of the thinner stratum corneum and a higher surface area/body weight ratio, making them more susceptible to absorbing topically applied medications.
Regulatory Changes and Precautions
In light of these findings, the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee concluded that, starting in October 2024, product information for medications containing minoxidil should be updated. Specifically, new information must be added to the package insert warning about the risk for hypertrichosis in infants following accidental exposure to minoxidil.
The NPC emphasizes the importance of caregivers being aware of the risks associated with topical medications like minoxidil. Recommended precautions include thoroughly washing hands after applying the product and covering treated areas to prevent direct contact with infants’ skin.
Healthcare professionals should also be aware of this risk and consider it when diagnosing hypertrichosis in infants. Recognizing the connection can prevent unnecessary testing for the infant and alleviate stress for the family.
This story was translated from Univadis Spain using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM THE SPANISH PHARMACOVIGILANCE CONGRESS 2024
Alpha-Gal Syndrome: 5 Things to Know
Alpha-gal syndrome (AGS), a tickborne disease commonly called “red meat allergy,” is a serious, potentially life-threatening allergy to the carbohydrate alpha-gal. The alpha-gal carbohydrate is found in most mammals, though it is not in humans, apes, or old-world monkeys. People with AGS can have allergic reactions when they consume mammalian meat, dairy products, or other products derived from mammals. People often live with this disease for years before receiving a correct diagnosis, greatly impacting their quality of life. The number of suspected cases is also rising.
More than 110,000 suspected AGS cases were identified between 2010 and 2022, according to a Centers for Disease Control and Prevention (CDC) report.1 However, because the diagnosis requires a positive test and a clinical exam and some people may not get tested, as many as 450,000 people might be affected by AGS in the United States. Additionally, a CDC survey found that nearly half (42%) of US healthcare providers had never heard of AGS.2 Among those who had, less than one third (29%) knew how to diagnose the condition.
Here are 5 things clinicians need to know about AGS.
1. People can develop AGS after being bitten by a tick, primarily the lone star tick (Amblyomma americanum), in the United States.
In the United States, AGS is primarily associated with the bite of a lone star tick, but other kinds of ticks have not been ruled out. The majority of suspected AGS cases in the United States were reported in parts of Arkansas, Delaware, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Tennessee, and Virginia. The lone star tick is widely distributed with established populations in Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
While AGS is associated with tick bites, more research is needed to understand the role ticks play in starting this condition, and why certain people develop AGS. Anyone can develop AGS, but most cases have been reported in adults.
Know how to recognize the symptoms of AGS and be prepared to test, diagnose, and manage AGS, particularly in states where lone star ticks are found.
2. Tick bites are only one risk factor for developing AGS.
Many people are bitten by lone star ticks and will never develop AGS. Scientists are exploring the connection between other risk factors and developing AGS. A recent study has shown that people diagnosed with AGS may be more likely to have a family member who was also diagnosed with AGS, have another food allergy, have an allergy to stinging or biting insects, or have A or O blood types.3
Research has also shown that environmental risk factors could contribute to developing AGS,4 like living in an area with lone star ticks, remembering finding a tick on themselves, recalling multiple tick bites, living near a wooded forest, spending more time outside, or living in areas with deer, such as larger properties, wooded forests, and properties with shrubs and brush.
Ask your patient questions about other allergies and history of recent tick bites or outdoor exposure to help determine if testing for AGS is appropriate.
3. Symptoms of AGS are consistently inconsistent.
There is a spectrum of how sensitive AGS patients are to alpha-gal, and reactions are often different from person to person, which can make it difficult to diagnose. The first allergic reaction to AGS typically occurs between 1-6 months after a tick bite. Symptoms commonly appear 2-6 hours after being in contact with products containing alpha-gal, like red meat (beef, pork, lamb, venison, rabbit, or other meat from mammals), dairy, and some medications. Symptoms can range from mild to severe and include hives or itchy rash; swelling of the lips, throat, tongue, or eyelids; gastrointestinal symptoms such as nausea, vomiting, or diarrhea; heartburn or indigestion; cough, shortness of breath, or difficulty breathing; dizziness or a drop in blood pressure; or anaphylaxis.
Consider AGS if a patient reports waking up in the middle of the night with allergic symptoms after eating alpha-gal containing products for dinner, if allergic reactions are delayed, or if a patient has anaphylaxis of unknown cause, adult-onset allergy, or allergic symptoms and reports a recent tick bite.
4. Diagnosing AGS requires a combination of a blood test and a physical exam.
Diagnosing AGS requires a detailed patient history, physical exam, and a blood test to detect specific immunoglobulin E (IgE) antibodies specific to alpha-gal (alpha-gal sIgE). Tests for alpha-gal sIgE antibodies are available at several large commercial laboratories and some academic institutions. Skin tests to identify reactions to allergens like pork or beef may also be used to inform AGS diagnosis. However, a positive alpha-gal sIgE test or skin test does not mean a person has AGS. Many people, particularly those who live in regions with lone star ticks, have positive alpha-gal specific IgE tests without having AGS.
Consider the test results along with your patient’s symptoms and risk factors.
5. There is no treatment for AGS, but people can take prevention steps and AGS can be managed.
People can protect themselves and their family from AGS by preventing tick bites. Encourage your patients to use an Environmental Protection Agency–registered insect repellent outdoors, wear permethrin-treated clothing, and conduct thorough tick checks after outdoor activities.
Once a person is no longer exposed to alpha-gal containing products, they should no longer experience symptoms. People with AGS should also proactively prevent tick bites. Tick bites can trigger or reactivate AGS.
For patients who have AGS, help manage their symptoms and identify alpha-gal containing products to avoid.
Dr. Kersh is Chief of the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, and disclosed no relevant conflicts of interest.
CDC resources:
About Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Testing and Diagnosis for Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Resources | Alpha-gal Syndrome | CDC
References
Thompson JM et al. MMWR Morb Mortal Wkly Rep. 2023;72:815-820.
Carpenter A et al. MMWR Morb Mortal Wkly Rep. 2023;72:809-814. Taylor ML et al. Ann Allergy, Asthma & Immunol. 2024 Jun;132(6):759.e2-764.e2. Kersh GJ et al. Ann Allergy, Asthma & Immunol. 2023 Apr;130(4):472-478.
Alpha-gal syndrome (AGS), a tickborne disease commonly called “red meat allergy,” is a serious, potentially life-threatening allergy to the carbohydrate alpha-gal. The alpha-gal carbohydrate is found in most mammals, though it is not in humans, apes, or old-world monkeys. People with AGS can have allergic reactions when they consume mammalian meat, dairy products, or other products derived from mammals. People often live with this disease for years before receiving a correct diagnosis, greatly impacting their quality of life. The number of suspected cases is also rising.
More than 110,000 suspected AGS cases were identified between 2010 and 2022, according to a Centers for Disease Control and Prevention (CDC) report.1 However, because the diagnosis requires a positive test and a clinical exam and some people may not get tested, as many as 450,000 people might be affected by AGS in the United States. Additionally, a CDC survey found that nearly half (42%) of US healthcare providers had never heard of AGS.2 Among those who had, less than one third (29%) knew how to diagnose the condition.
Here are 5 things clinicians need to know about AGS.
1. People can develop AGS after being bitten by a tick, primarily the lone star tick (Amblyomma americanum), in the United States.
In the United States, AGS is primarily associated with the bite of a lone star tick, but other kinds of ticks have not been ruled out. The majority of suspected AGS cases in the United States were reported in parts of Arkansas, Delaware, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Tennessee, and Virginia. The lone star tick is widely distributed with established populations in Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
While AGS is associated with tick bites, more research is needed to understand the role ticks play in starting this condition, and why certain people develop AGS. Anyone can develop AGS, but most cases have been reported in adults.
Know how to recognize the symptoms of AGS and be prepared to test, diagnose, and manage AGS, particularly in states where lone star ticks are found.
2. Tick bites are only one risk factor for developing AGS.
Many people are bitten by lone star ticks and will never develop AGS. Scientists are exploring the connection between other risk factors and developing AGS. A recent study has shown that people diagnosed with AGS may be more likely to have a family member who was also diagnosed with AGS, have another food allergy, have an allergy to stinging or biting insects, or have A or O blood types.3
Research has also shown that environmental risk factors could contribute to developing AGS,4 like living in an area with lone star ticks, remembering finding a tick on themselves, recalling multiple tick bites, living near a wooded forest, spending more time outside, or living in areas with deer, such as larger properties, wooded forests, and properties with shrubs and brush.
Ask your patient questions about other allergies and history of recent tick bites or outdoor exposure to help determine if testing for AGS is appropriate.
3. Symptoms of AGS are consistently inconsistent.
There is a spectrum of how sensitive AGS patients are to alpha-gal, and reactions are often different from person to person, which can make it difficult to diagnose. The first allergic reaction to AGS typically occurs between 1-6 months after a tick bite. Symptoms commonly appear 2-6 hours after being in contact with products containing alpha-gal, like red meat (beef, pork, lamb, venison, rabbit, or other meat from mammals), dairy, and some medications. Symptoms can range from mild to severe and include hives or itchy rash; swelling of the lips, throat, tongue, or eyelids; gastrointestinal symptoms such as nausea, vomiting, or diarrhea; heartburn or indigestion; cough, shortness of breath, or difficulty breathing; dizziness or a drop in blood pressure; or anaphylaxis.
Consider AGS if a patient reports waking up in the middle of the night with allergic symptoms after eating alpha-gal containing products for dinner, if allergic reactions are delayed, or if a patient has anaphylaxis of unknown cause, adult-onset allergy, or allergic symptoms and reports a recent tick bite.
4. Diagnosing AGS requires a combination of a blood test and a physical exam.
Diagnosing AGS requires a detailed patient history, physical exam, and a blood test to detect specific immunoglobulin E (IgE) antibodies specific to alpha-gal (alpha-gal sIgE). Tests for alpha-gal sIgE antibodies are available at several large commercial laboratories and some academic institutions. Skin tests to identify reactions to allergens like pork or beef may also be used to inform AGS diagnosis. However, a positive alpha-gal sIgE test or skin test does not mean a person has AGS. Many people, particularly those who live in regions with lone star ticks, have positive alpha-gal specific IgE tests without having AGS.
Consider the test results along with your patient’s symptoms and risk factors.
5. There is no treatment for AGS, but people can take prevention steps and AGS can be managed.
People can protect themselves and their family from AGS by preventing tick bites. Encourage your patients to use an Environmental Protection Agency–registered insect repellent outdoors, wear permethrin-treated clothing, and conduct thorough tick checks after outdoor activities.
Once a person is no longer exposed to alpha-gal containing products, they should no longer experience symptoms. People with AGS should also proactively prevent tick bites. Tick bites can trigger or reactivate AGS.
For patients who have AGS, help manage their symptoms and identify alpha-gal containing products to avoid.
Dr. Kersh is Chief of the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, and disclosed no relevant conflicts of interest.
CDC resources:
About Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Testing and Diagnosis for Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Resources | Alpha-gal Syndrome | CDC
References
Thompson JM et al. MMWR Morb Mortal Wkly Rep. 2023;72:815-820.
Carpenter A et al. MMWR Morb Mortal Wkly Rep. 2023;72:809-814. Taylor ML et al. Ann Allergy, Asthma & Immunol. 2024 Jun;132(6):759.e2-764.e2. Kersh GJ et al. Ann Allergy, Asthma & Immunol. 2023 Apr;130(4):472-478.
Alpha-gal syndrome (AGS), a tickborne disease commonly called “red meat allergy,” is a serious, potentially life-threatening allergy to the carbohydrate alpha-gal. The alpha-gal carbohydrate is found in most mammals, though it is not in humans, apes, or old-world monkeys. People with AGS can have allergic reactions when they consume mammalian meat, dairy products, or other products derived from mammals. People often live with this disease for years before receiving a correct diagnosis, greatly impacting their quality of life. The number of suspected cases is also rising.
More than 110,000 suspected AGS cases were identified between 2010 and 2022, according to a Centers for Disease Control and Prevention (CDC) report.1 However, because the diagnosis requires a positive test and a clinical exam and some people may not get tested, as many as 450,000 people might be affected by AGS in the United States. Additionally, a CDC survey found that nearly half (42%) of US healthcare providers had never heard of AGS.2 Among those who had, less than one third (29%) knew how to diagnose the condition.
Here are 5 things clinicians need to know about AGS.
1. People can develop AGS after being bitten by a tick, primarily the lone star tick (Amblyomma americanum), in the United States.
In the United States, AGS is primarily associated with the bite of a lone star tick, but other kinds of ticks have not been ruled out. The majority of suspected AGS cases in the United States were reported in parts of Arkansas, Delaware, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Tennessee, and Virginia. The lone star tick is widely distributed with established populations in Alabama, Arkansas, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, and West Virginia.
While AGS is associated with tick bites, more research is needed to understand the role ticks play in starting this condition, and why certain people develop AGS. Anyone can develop AGS, but most cases have been reported in adults.
Know how to recognize the symptoms of AGS and be prepared to test, diagnose, and manage AGS, particularly in states where lone star ticks are found.
2. Tick bites are only one risk factor for developing AGS.
Many people are bitten by lone star ticks and will never develop AGS. Scientists are exploring the connection between other risk factors and developing AGS. A recent study has shown that people diagnosed with AGS may be more likely to have a family member who was also diagnosed with AGS, have another food allergy, have an allergy to stinging or biting insects, or have A or O blood types.3
Research has also shown that environmental risk factors could contribute to developing AGS,4 like living in an area with lone star ticks, remembering finding a tick on themselves, recalling multiple tick bites, living near a wooded forest, spending more time outside, or living in areas with deer, such as larger properties, wooded forests, and properties with shrubs and brush.
Ask your patient questions about other allergies and history of recent tick bites or outdoor exposure to help determine if testing for AGS is appropriate.
3. Symptoms of AGS are consistently inconsistent.
There is a spectrum of how sensitive AGS patients are to alpha-gal, and reactions are often different from person to person, which can make it difficult to diagnose. The first allergic reaction to AGS typically occurs between 1-6 months after a tick bite. Symptoms commonly appear 2-6 hours after being in contact with products containing alpha-gal, like red meat (beef, pork, lamb, venison, rabbit, or other meat from mammals), dairy, and some medications. Symptoms can range from mild to severe and include hives or itchy rash; swelling of the lips, throat, tongue, or eyelids; gastrointestinal symptoms such as nausea, vomiting, or diarrhea; heartburn or indigestion; cough, shortness of breath, or difficulty breathing; dizziness or a drop in blood pressure; or anaphylaxis.
Consider AGS if a patient reports waking up in the middle of the night with allergic symptoms after eating alpha-gal containing products for dinner, if allergic reactions are delayed, or if a patient has anaphylaxis of unknown cause, adult-onset allergy, or allergic symptoms and reports a recent tick bite.
4. Diagnosing AGS requires a combination of a blood test and a physical exam.
Diagnosing AGS requires a detailed patient history, physical exam, and a blood test to detect specific immunoglobulin E (IgE) antibodies specific to alpha-gal (alpha-gal sIgE). Tests for alpha-gal sIgE antibodies are available at several large commercial laboratories and some academic institutions. Skin tests to identify reactions to allergens like pork or beef may also be used to inform AGS diagnosis. However, a positive alpha-gal sIgE test or skin test does not mean a person has AGS. Many people, particularly those who live in regions with lone star ticks, have positive alpha-gal specific IgE tests without having AGS.
Consider the test results along with your patient’s symptoms and risk factors.
5. There is no treatment for AGS, but people can take prevention steps and AGS can be managed.
People can protect themselves and their family from AGS by preventing tick bites. Encourage your patients to use an Environmental Protection Agency–registered insect repellent outdoors, wear permethrin-treated clothing, and conduct thorough tick checks after outdoor activities.
Once a person is no longer exposed to alpha-gal containing products, they should no longer experience symptoms. People with AGS should also proactively prevent tick bites. Tick bites can trigger or reactivate AGS.
For patients who have AGS, help manage their symptoms and identify alpha-gal containing products to avoid.
Dr. Kersh is Chief of the Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, and disclosed no relevant conflicts of interest.
CDC resources:
About Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Testing and Diagnosis for Alpha-gal Syndrome | Alpha-gal Syndrome | CDC
Clinical Resources | Alpha-gal Syndrome | CDC
References
Thompson JM et al. MMWR Morb Mortal Wkly Rep. 2023;72:815-820.
Carpenter A et al. MMWR Morb Mortal Wkly Rep. 2023;72:809-814. Taylor ML et al. Ann Allergy, Asthma & Immunol. 2024 Jun;132(6):759.e2-764.e2. Kersh GJ et al. Ann Allergy, Asthma & Immunol. 2023 Apr;130(4):472-478.
Understanding of Hidradenitis Suppurativa Pathophysiology Advancing
NEW YORK, NY — , according to two investigators intimately involved in much of the recent progress.
“Success is being achieved by targeting multiple inflammatory axes in HS, and therapeutics are evolving rapidly,” reported James G. Krueger, MD, PhD, head of the Laboratory of Investigative Dermatology, Rockefeller University, New York, NY.
The activity of targeted anti-inflammatory therapies — bimekizumab just joined adalimumab and secukinumab as a third approved biologic for HS — is not news, but the degree to which inflammation is upregulated systemically, not just at areas of skin involvement, has changed the conceptualization of HS.
HS Is a Systemic Inflammatory Disease
Relative to psoriasis, for which there are many parallels, “HS is hugely more inflammatory in the systemic circulation,” Krueger said at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024. Yet, HS is also more complex involving additional pathways that appear to include dysbiosis. The concept of follicular occlusion, once a common explanation for HS, has been left far behind.
“Unlike psoriasis, which we can treat really well by inhibiting a single pathway target, HS is just not that simple,” Krueger said. Although largely an inflammatory process, the cascade of inflammatory factors for specific manifestations, such as tunnels, means that optimal therapy in one case might have little benefit in another.
The relatively new evidence that HS activity is not confined to lesional skin might be the most important recent step toward new strategies to target disease. These studies were performed by Kristina Navrazhina, MD, PhD, now a resident in dermatology at the Icahn School of Medicine at Mount Sinai, New York. She received her PhD while studying HS activity in non-lesional skin. Her work has led her to conclude that the best chance for better outcomes in HS is early diagnosis and treatment. Although this is generally true of any pathology, the changes in the HS phenotype once fistulae form includes a poor response to conventional therapies.
In fact, based on her work in evaluating HS activity in non-lesional skin, Navrazhina has shown that “many patients with modest lesions already have advanced disease.” Consistent with the premise that HS is a deeply systemic inflammatory process, nodules, considered an early manifestation, turn out to be “the tip of the iceberg.”
Non-Lesional HS Skin Is Inflamed
When she has employed RNA sequencing based on tape strip sampling from completely normal skin away from nodules, interleukin (IL)-17 and a broad array of other inflammatory markers were found to be upregulated. When she performed ultrasound to look for disease activity under the normal skin, she has often found tunnels already formed. Doppler ultrasound showed some of these tunnels were actively draining.
This might provide a partial explanation for why therapies are not always effective even when clinical signs of disease are modest.
“Are we missing the opportunity for intervening?” Navrazhina asked, noting that early intervention has been limited traditionally by extremely long diagnostic delays. Citing the literature, Navrazhina said the average delay is 7 years for HS versus 1 year for psoriasis. Patients often cycle through 3 or 4 providers before the diagnosis is made, she said.
Awakening first-line clinicians to the signs and symptoms of HS, whether in the emergency room or primary care, is a critical message because of the incrementally difficult task to control disease once fistulae have formed.
Krueger made the same appeal. For the neutrophilic inflammation that characterizes nodules, targeted therapies are often effective, but he agreed that available therapies are generally far less so once tunnels form.
Role Seen for Bacteria in HS Pathogenesis
One reason might be an interaction between anaerobic bacteria and the keratinocytes that form the tunnel walls, according to Krueger. Although HS is not typically considered an infectious disease, he reported that the interaction of these bacteria with keratinocytes is associated with expression of approximately 1000 inflammatory gene products. The process of tunnel formation is traced to how factors recruited by upregulated inflammation, such as chemokines, coordinate.
He described recent work pursing novel strategies such as highly targeted antibiotics or inhibitors of complement factor C5a, which has been proposed as a biomarker for HS, to intervene in preventing or reversing HS tunnels.
While this work progresses, one of the most Important unmet needs in HS is an accepted measure of clinically meaningful improvement in advanced disease, particularly the impact of therapy on HS tunnels, according to Krueger.
“There is no measure of tunnel activity that the FDA accepts in evaluating drugs,” he noted, which will be essential for approving therapies that offer this benefit.
A phase 3 trials program for one of the promising drugs, sonelokimab, was announced early in 2024. A nanobody that targets IL-17A/A, IL-17A/F, and IL-17F/F, the small size of this molecule permits exceptional tissue penetration while the broad anti-IL-17 activity has a high degree of theoretical potential in late-stage HS, according to Krueger.
There are numerous pieces of the HS puzzle that are still missing, but both Krueger and Navrazhina are enthusiastic about new targets and opportunities for disease control that are stemming from a better understanding of the underlying pathophysiology. Not least, both indicated that testing for inflammatory phenotypes will allow for individualized therapeutic choices with a maximum likelihood of response, particularly if earlier diagnosis permits earlier treatment.
“Due to the heterogeneity of HS, it is hard to know who will respond to which treatment or which treatment should be started first,” Navrazhina said. She thinks that early measures of the inflammatory profile in nodules or even non-lesional skin might provide that guidance.
Both Krueger and Navrazhina reported no financial relationships relevant to this work.
A version of this article appeared on Medscape.com.
NEW YORK, NY — , according to two investigators intimately involved in much of the recent progress.
“Success is being achieved by targeting multiple inflammatory axes in HS, and therapeutics are evolving rapidly,” reported James G. Krueger, MD, PhD, head of the Laboratory of Investigative Dermatology, Rockefeller University, New York, NY.
The activity of targeted anti-inflammatory therapies — bimekizumab just joined adalimumab and secukinumab as a third approved biologic for HS — is not news, but the degree to which inflammation is upregulated systemically, not just at areas of skin involvement, has changed the conceptualization of HS.
HS Is a Systemic Inflammatory Disease
Relative to psoriasis, for which there are many parallels, “HS is hugely more inflammatory in the systemic circulation,” Krueger said at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024. Yet, HS is also more complex involving additional pathways that appear to include dysbiosis. The concept of follicular occlusion, once a common explanation for HS, has been left far behind.
“Unlike psoriasis, which we can treat really well by inhibiting a single pathway target, HS is just not that simple,” Krueger said. Although largely an inflammatory process, the cascade of inflammatory factors for specific manifestations, such as tunnels, means that optimal therapy in one case might have little benefit in another.
The relatively new evidence that HS activity is not confined to lesional skin might be the most important recent step toward new strategies to target disease. These studies were performed by Kristina Navrazhina, MD, PhD, now a resident in dermatology at the Icahn School of Medicine at Mount Sinai, New York. She received her PhD while studying HS activity in non-lesional skin. Her work has led her to conclude that the best chance for better outcomes in HS is early diagnosis and treatment. Although this is generally true of any pathology, the changes in the HS phenotype once fistulae form includes a poor response to conventional therapies.
In fact, based on her work in evaluating HS activity in non-lesional skin, Navrazhina has shown that “many patients with modest lesions already have advanced disease.” Consistent with the premise that HS is a deeply systemic inflammatory process, nodules, considered an early manifestation, turn out to be “the tip of the iceberg.”
Non-Lesional HS Skin Is Inflamed
When she has employed RNA sequencing based on tape strip sampling from completely normal skin away from nodules, interleukin (IL)-17 and a broad array of other inflammatory markers were found to be upregulated. When she performed ultrasound to look for disease activity under the normal skin, she has often found tunnels already formed. Doppler ultrasound showed some of these tunnels were actively draining.
This might provide a partial explanation for why therapies are not always effective even when clinical signs of disease are modest.
“Are we missing the opportunity for intervening?” Navrazhina asked, noting that early intervention has been limited traditionally by extremely long diagnostic delays. Citing the literature, Navrazhina said the average delay is 7 years for HS versus 1 year for psoriasis. Patients often cycle through 3 or 4 providers before the diagnosis is made, she said.
Awakening first-line clinicians to the signs and symptoms of HS, whether in the emergency room or primary care, is a critical message because of the incrementally difficult task to control disease once fistulae have formed.
Krueger made the same appeal. For the neutrophilic inflammation that characterizes nodules, targeted therapies are often effective, but he agreed that available therapies are generally far less so once tunnels form.
Role Seen for Bacteria in HS Pathogenesis
One reason might be an interaction between anaerobic bacteria and the keratinocytes that form the tunnel walls, according to Krueger. Although HS is not typically considered an infectious disease, he reported that the interaction of these bacteria with keratinocytes is associated with expression of approximately 1000 inflammatory gene products. The process of tunnel formation is traced to how factors recruited by upregulated inflammation, such as chemokines, coordinate.
He described recent work pursing novel strategies such as highly targeted antibiotics or inhibitors of complement factor C5a, which has been proposed as a biomarker for HS, to intervene in preventing or reversing HS tunnels.
While this work progresses, one of the most Important unmet needs in HS is an accepted measure of clinically meaningful improvement in advanced disease, particularly the impact of therapy on HS tunnels, according to Krueger.
“There is no measure of tunnel activity that the FDA accepts in evaluating drugs,” he noted, which will be essential for approving therapies that offer this benefit.
A phase 3 trials program for one of the promising drugs, sonelokimab, was announced early in 2024. A nanobody that targets IL-17A/A, IL-17A/F, and IL-17F/F, the small size of this molecule permits exceptional tissue penetration while the broad anti-IL-17 activity has a high degree of theoretical potential in late-stage HS, according to Krueger.
There are numerous pieces of the HS puzzle that are still missing, but both Krueger and Navrazhina are enthusiastic about new targets and opportunities for disease control that are stemming from a better understanding of the underlying pathophysiology. Not least, both indicated that testing for inflammatory phenotypes will allow for individualized therapeutic choices with a maximum likelihood of response, particularly if earlier diagnosis permits earlier treatment.
“Due to the heterogeneity of HS, it is hard to know who will respond to which treatment or which treatment should be started first,” Navrazhina said. She thinks that early measures of the inflammatory profile in nodules or even non-lesional skin might provide that guidance.
Both Krueger and Navrazhina reported no financial relationships relevant to this work.
A version of this article appeared on Medscape.com.
NEW YORK, NY — , according to two investigators intimately involved in much of the recent progress.
“Success is being achieved by targeting multiple inflammatory axes in HS, and therapeutics are evolving rapidly,” reported James G. Krueger, MD, PhD, head of the Laboratory of Investigative Dermatology, Rockefeller University, New York, NY.
The activity of targeted anti-inflammatory therapies — bimekizumab just joined adalimumab and secukinumab as a third approved biologic for HS — is not news, but the degree to which inflammation is upregulated systemically, not just at areas of skin involvement, has changed the conceptualization of HS.
HS Is a Systemic Inflammatory Disease
Relative to psoriasis, for which there are many parallels, “HS is hugely more inflammatory in the systemic circulation,” Krueger said at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024. Yet, HS is also more complex involving additional pathways that appear to include dysbiosis. The concept of follicular occlusion, once a common explanation for HS, has been left far behind.
“Unlike psoriasis, which we can treat really well by inhibiting a single pathway target, HS is just not that simple,” Krueger said. Although largely an inflammatory process, the cascade of inflammatory factors for specific manifestations, such as tunnels, means that optimal therapy in one case might have little benefit in another.
The relatively new evidence that HS activity is not confined to lesional skin might be the most important recent step toward new strategies to target disease. These studies were performed by Kristina Navrazhina, MD, PhD, now a resident in dermatology at the Icahn School of Medicine at Mount Sinai, New York. She received her PhD while studying HS activity in non-lesional skin. Her work has led her to conclude that the best chance for better outcomes in HS is early diagnosis and treatment. Although this is generally true of any pathology, the changes in the HS phenotype once fistulae form includes a poor response to conventional therapies.
In fact, based on her work in evaluating HS activity in non-lesional skin, Navrazhina has shown that “many patients with modest lesions already have advanced disease.” Consistent with the premise that HS is a deeply systemic inflammatory process, nodules, considered an early manifestation, turn out to be “the tip of the iceberg.”
Non-Lesional HS Skin Is Inflamed
When she has employed RNA sequencing based on tape strip sampling from completely normal skin away from nodules, interleukin (IL)-17 and a broad array of other inflammatory markers were found to be upregulated. When she performed ultrasound to look for disease activity under the normal skin, she has often found tunnels already formed. Doppler ultrasound showed some of these tunnels were actively draining.
This might provide a partial explanation for why therapies are not always effective even when clinical signs of disease are modest.
“Are we missing the opportunity for intervening?” Navrazhina asked, noting that early intervention has been limited traditionally by extremely long diagnostic delays. Citing the literature, Navrazhina said the average delay is 7 years for HS versus 1 year for psoriasis. Patients often cycle through 3 or 4 providers before the diagnosis is made, she said.
Awakening first-line clinicians to the signs and symptoms of HS, whether in the emergency room or primary care, is a critical message because of the incrementally difficult task to control disease once fistulae have formed.
Krueger made the same appeal. For the neutrophilic inflammation that characterizes nodules, targeted therapies are often effective, but he agreed that available therapies are generally far less so once tunnels form.
Role Seen for Bacteria in HS Pathogenesis
One reason might be an interaction between anaerobic bacteria and the keratinocytes that form the tunnel walls, according to Krueger. Although HS is not typically considered an infectious disease, he reported that the interaction of these bacteria with keratinocytes is associated with expression of approximately 1000 inflammatory gene products. The process of tunnel formation is traced to how factors recruited by upregulated inflammation, such as chemokines, coordinate.
He described recent work pursing novel strategies such as highly targeted antibiotics or inhibitors of complement factor C5a, which has been proposed as a biomarker for HS, to intervene in preventing or reversing HS tunnels.
While this work progresses, one of the most Important unmet needs in HS is an accepted measure of clinically meaningful improvement in advanced disease, particularly the impact of therapy on HS tunnels, according to Krueger.
“There is no measure of tunnel activity that the FDA accepts in evaluating drugs,” he noted, which will be essential for approving therapies that offer this benefit.
A phase 3 trials program for one of the promising drugs, sonelokimab, was announced early in 2024. A nanobody that targets IL-17A/A, IL-17A/F, and IL-17F/F, the small size of this molecule permits exceptional tissue penetration while the broad anti-IL-17 activity has a high degree of theoretical potential in late-stage HS, according to Krueger.
There are numerous pieces of the HS puzzle that are still missing, but both Krueger and Navrazhina are enthusiastic about new targets and opportunities for disease control that are stemming from a better understanding of the underlying pathophysiology. Not least, both indicated that testing for inflammatory phenotypes will allow for individualized therapeutic choices with a maximum likelihood of response, particularly if earlier diagnosis permits earlier treatment.
“Due to the heterogeneity of HS, it is hard to know who will respond to which treatment or which treatment should be started first,” Navrazhina said. She thinks that early measures of the inflammatory profile in nodules or even non-lesional skin might provide that guidance.
Both Krueger and Navrazhina reported no financial relationships relevant to this work.
A version of this article appeared on Medscape.com.
Topical Tapinarof Approved for Treating Atopic Dermatitis, Ages 2 and Up
An aryl hydrocarbon receptor agonist, tapinarof cream, 1% was first approved in May 2022 for the topical treatment of plaque psoriasis in adults.
According to a press release from the manufacturer, Organon — which markets tapinarof cream, 1%, under the brand name VTAMA — the new indication for AD is based on results from the ADORING pivotal studies. In ADORING 1, the proportion of patients in the tapinarof cream, 1% treatment group who achieved a score of clear (0) or almost clear (1) and a minimum 2-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD was 45.4%, compared with 13.9% of patients who received vehicle alone. ADORING 2 yielded similar results (46.4% vs 18.0%, respectively; P < .0001 for both associations).
Secondary endpoints measured at week 8 also significantly favored the treatment group over the vehicle group, including the Eczema Area and Severity Index score improvement of at least 75% from baseline and achievement of a ≥ 4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline.
The most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection (12%), folliculitis (9%), lower respiratory tract infection (5%), headache (4%), asthma (2%), vomiting (2%), ear infection (2%), pain in extremity (2%), and abdominal pain (1%), according to the release.
Among 728 patients in the ADORING studies who enrolled in an open-label 48-week extension trial (ADORING 3), 378 entered with or achieved complete disease clearance and discontinued treatment. In this subset of patients, the mean duration of the first treatment-free interval was approximately 80 consecutive days, according to the release.
A version of this article first appeared on Medscape.com.
An aryl hydrocarbon receptor agonist, tapinarof cream, 1% was first approved in May 2022 for the topical treatment of plaque psoriasis in adults.
According to a press release from the manufacturer, Organon — which markets tapinarof cream, 1%, under the brand name VTAMA — the new indication for AD is based on results from the ADORING pivotal studies. In ADORING 1, the proportion of patients in the tapinarof cream, 1% treatment group who achieved a score of clear (0) or almost clear (1) and a minimum 2-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD was 45.4%, compared with 13.9% of patients who received vehicle alone. ADORING 2 yielded similar results (46.4% vs 18.0%, respectively; P < .0001 for both associations).
Secondary endpoints measured at week 8 also significantly favored the treatment group over the vehicle group, including the Eczema Area and Severity Index score improvement of at least 75% from baseline and achievement of a ≥ 4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline.
The most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection (12%), folliculitis (9%), lower respiratory tract infection (5%), headache (4%), asthma (2%), vomiting (2%), ear infection (2%), pain in extremity (2%), and abdominal pain (1%), according to the release.
Among 728 patients in the ADORING studies who enrolled in an open-label 48-week extension trial (ADORING 3), 378 entered with or achieved complete disease clearance and discontinued treatment. In this subset of patients, the mean duration of the first treatment-free interval was approximately 80 consecutive days, according to the release.
A version of this article first appeared on Medscape.com.
An aryl hydrocarbon receptor agonist, tapinarof cream, 1% was first approved in May 2022 for the topical treatment of plaque psoriasis in adults.
According to a press release from the manufacturer, Organon — which markets tapinarof cream, 1%, under the brand name VTAMA — the new indication for AD is based on results from the ADORING pivotal studies. In ADORING 1, the proportion of patients in the tapinarof cream, 1% treatment group who achieved a score of clear (0) or almost clear (1) and a minimum 2-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD was 45.4%, compared with 13.9% of patients who received vehicle alone. ADORING 2 yielded similar results (46.4% vs 18.0%, respectively; P < .0001 for both associations).
Secondary endpoints measured at week 8 also significantly favored the treatment group over the vehicle group, including the Eczema Area and Severity Index score improvement of at least 75% from baseline and achievement of a ≥ 4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline.
The most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection (12%), folliculitis (9%), lower respiratory tract infection (5%), headache (4%), asthma (2%), vomiting (2%), ear infection (2%), pain in extremity (2%), and abdominal pain (1%), according to the release.
Among 728 patients in the ADORING studies who enrolled in an open-label 48-week extension trial (ADORING 3), 378 entered with or achieved complete disease clearance and discontinued treatment. In this subset of patients, the mean duration of the first treatment-free interval was approximately 80 consecutive days, according to the release.
A version of this article first appeared on Medscape.com.
A 16-Year-Old Hispanic Male with a History of Hyperlipidemia Reports a Pruritic Rash on His Neck and Chest
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Case Report
A 16-year-old Hispanic male with a history of hyperlipidemia presents to his pediatrician's office for a routine well-child check-up. He reports a pruritic rash on his neck and chest that has been present for the past 1.5 weeks. The rash is itchy, and although a cream from Mexico initially helped, it has not been effective recently. The patient mentions that he has increased his gym workouts and has been training for basketball. He has a history of obesity but has lost almost 100 pounds in the last 6 months. Most recently, he has stopped consuming carbohydrates and has been fasting in the mornings.
There is no history of eczema or psoriasis, either in the patient or his family.
Physical Examination
The patient weighs 147 pounds, a significant decrease from his previous weight of 270 pounds 6 months ago. Other vital signs are within normal limits.
On physical examination, the patient presents with net-like, pink, scaly plaques on his neck, with no other rashes on the body (see Pictures 1 and 2).
Skin Cancer Screening: Biopsy-Free Technology Advancing
NEW YORK CITY — now in routine use at his own institution.
For skin cancer screening, existing and coming technologies represent “the future of dermatology,” but “we can and should be [already] trying to incorporate these into routine practice,” said Jonathan Ungar, MD, assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York City.
Technologies such as electrical impedance spectroscopy (EIS), optical coherence tomography (OCT), and reflectance confocal microscopy (RCM) have immediate utility for improving skin cancer detection with fewer biopsies, but this is just the beginning, according to Ungar, who is also medical director of the Kimberly and Eric J. Waldman Melanoma and Skin Cancer Center at Mount Sinai, New York City.
“There is going to be a day when we are not cutting to make a diagnosis,” he said during a presentation at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024.
Four Noninvasive Tools Are in Routine Use
Each of these technologies, along with total body photography (TBP), is currently in use at Mount Sinai as well as other tertiary centers to improve diagnostic accuracy at the same time they reduce invasive tests. The initial excitement about these technologies was based on their potential to avoid biopsy in cosmetically sensitive areas, but Ungar suggested that wider application is being driven by better rates of detection, less morbidity, and improved patient satisfaction.
Patients are happy to avoid invasive procedures whenever they can, Ungar noted. In addition to concern about pain or discomfort and a small but measurable risk for infection, patients face a wound that requires healing and the potential for an enduring scar whether the histology is positive for a malignancy.
While none of the four technologies Ungar outlined typically provide a yes or no answer regarding the presence of a malignancy, they do improve diagnostic accuracy with a lower rate of biopsy.
Each Noninvasive Tool Reduces Biopsy Rates
In the case of EIS, for example, the impedance of a painless and harmless electrical current directed into the skin with a handheld probe differentiates normal from abnormal skin through an EIS algorithm. Ungar said it does not require training. A result negative for an abnormality has about a 90% predictive value, and it means that a biopsy can be avoided if there are no other reasons for suspicion.
With a price estimated in the thousands of dollars, the device and software are “not so expensive,” particularly when the tool results in fewer biopsies, Ungar noted.
OCT has a similar profile. Again, used as an adjunct to other types of evaluations, including a history and visual inspection, this helps in modulating suspicion of malignancy. In published studies, OCT has proven superior to dermatoscopy for cancer detection. Citing a 14-study meta-analysis, Ungar said that the sensitivity of OCT for melanoma exceeds, and the specificity approaches, 90%. For basal cell cancers, it is even better.
RCM involves directing a laser into the skin to detect abnormal cells that reflect light. It enables visualization of the skin by layers to the papillary dermis in a detail that is comparable with histology, according to Ungar. Imaging performed with the device used at Mount Sinai (VivaScope 1500, Caliber Imaging & Diagnostics) is reimbursed by Medicare.
Once comfortable with the technology, scanning and interpretation take slightly more time than that required of EIS or OCT, but, like the others, it is painless and helpful for determining whether further evaluation is needed, according to Ungar.
“It is extremely useful in reducing the number of biopsies,” whether melanoma or basal cell malignancies, he said.
Total Body Photograph Helps With Serial Screens
While not specifically a diagnostic tool, TBP can also play a role in reducing biopsies through its highly efficient ability to document the evolution of lesions over time.
As its name implies, essentially the entire body surface is captured by multiple cameras mounted in a circle around the patient. Unlike sequential photos that require far more time to take and store and are challenging to organize and retrieve, the device used at Mount Sinai (Vectra Wb180 1360, Canfield Scientific) can complete the photos in about 2 minutes.
Software for organizing and storing the photos, to which dermatoscope images of individual lesions can be attached if helpful, results in efficient retrieval of photos at sequential visits for evaluating change in any specific lesion.
“It is very easy to use,” according to Ungar, who noted that although the underlying idea is not, the technology of taking, storing, and retrieving photographs has been “perhaps perfected” with this approach.
Noninvasive Screening Training Is Appropriate
Year after year, dermatology residents undergo intensive instruction to master the traditional methods of skin examination with the naked eye and the help of a dermatoscope, but Ungar considers the noninvasive tools to be another step forward. They lower miss rates while reducing the need for histopathology.
Adding these new technologies to routine patient care resonates for many experts, even if the protocols of when to use with the tool are not well established.
Angela J. Lamb, MD, an associate professor of dermatology at Mount Sinai, who has been following the work of Ungar with interest, sees merit in his argument. Not surprisingly, she thinks that any approach shown to boost skin cancer detection is something that deserves attention, but she thinks the effort to safely eliminate biopsies with a low likelihood of a positive finding cannot be ignored.
“Patients want to avoid biopsies when they can,” Lamb told this news organization, and she does not think this is limited to biopsies on the face or other cosmetically sensitive areas.
As a result, she said that she does see the rationale for incorporating the newer technologies into routine care and called this an “important” effort to improve the patient experience as well as reduce missed lesions.
Ungar reported financial relationships with AbbVie, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Janssen Pharmaceuticals, Menlo Therapeutics, Mitsubishi Tanabe Pharma America, and UCB. Lamb reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — now in routine use at his own institution.
For skin cancer screening, existing and coming technologies represent “the future of dermatology,” but “we can and should be [already] trying to incorporate these into routine practice,” said Jonathan Ungar, MD, assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York City.
Technologies such as electrical impedance spectroscopy (EIS), optical coherence tomography (OCT), and reflectance confocal microscopy (RCM) have immediate utility for improving skin cancer detection with fewer biopsies, but this is just the beginning, according to Ungar, who is also medical director of the Kimberly and Eric J. Waldman Melanoma and Skin Cancer Center at Mount Sinai, New York City.
“There is going to be a day when we are not cutting to make a diagnosis,” he said during a presentation at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024.
Four Noninvasive Tools Are in Routine Use
Each of these technologies, along with total body photography (TBP), is currently in use at Mount Sinai as well as other tertiary centers to improve diagnostic accuracy at the same time they reduce invasive tests. The initial excitement about these technologies was based on their potential to avoid biopsy in cosmetically sensitive areas, but Ungar suggested that wider application is being driven by better rates of detection, less morbidity, and improved patient satisfaction.
Patients are happy to avoid invasive procedures whenever they can, Ungar noted. In addition to concern about pain or discomfort and a small but measurable risk for infection, patients face a wound that requires healing and the potential for an enduring scar whether the histology is positive for a malignancy.
While none of the four technologies Ungar outlined typically provide a yes or no answer regarding the presence of a malignancy, they do improve diagnostic accuracy with a lower rate of biopsy.
Each Noninvasive Tool Reduces Biopsy Rates
In the case of EIS, for example, the impedance of a painless and harmless electrical current directed into the skin with a handheld probe differentiates normal from abnormal skin through an EIS algorithm. Ungar said it does not require training. A result negative for an abnormality has about a 90% predictive value, and it means that a biopsy can be avoided if there are no other reasons for suspicion.
With a price estimated in the thousands of dollars, the device and software are “not so expensive,” particularly when the tool results in fewer biopsies, Ungar noted.
OCT has a similar profile. Again, used as an adjunct to other types of evaluations, including a history and visual inspection, this helps in modulating suspicion of malignancy. In published studies, OCT has proven superior to dermatoscopy for cancer detection. Citing a 14-study meta-analysis, Ungar said that the sensitivity of OCT for melanoma exceeds, and the specificity approaches, 90%. For basal cell cancers, it is even better.
RCM involves directing a laser into the skin to detect abnormal cells that reflect light. It enables visualization of the skin by layers to the papillary dermis in a detail that is comparable with histology, according to Ungar. Imaging performed with the device used at Mount Sinai (VivaScope 1500, Caliber Imaging & Diagnostics) is reimbursed by Medicare.
Once comfortable with the technology, scanning and interpretation take slightly more time than that required of EIS or OCT, but, like the others, it is painless and helpful for determining whether further evaluation is needed, according to Ungar.
“It is extremely useful in reducing the number of biopsies,” whether melanoma or basal cell malignancies, he said.
Total Body Photograph Helps With Serial Screens
While not specifically a diagnostic tool, TBP can also play a role in reducing biopsies through its highly efficient ability to document the evolution of lesions over time.
As its name implies, essentially the entire body surface is captured by multiple cameras mounted in a circle around the patient. Unlike sequential photos that require far more time to take and store and are challenging to organize and retrieve, the device used at Mount Sinai (Vectra Wb180 1360, Canfield Scientific) can complete the photos in about 2 minutes.
Software for organizing and storing the photos, to which dermatoscope images of individual lesions can be attached if helpful, results in efficient retrieval of photos at sequential visits for evaluating change in any specific lesion.
“It is very easy to use,” according to Ungar, who noted that although the underlying idea is not, the technology of taking, storing, and retrieving photographs has been “perhaps perfected” with this approach.
Noninvasive Screening Training Is Appropriate
Year after year, dermatology residents undergo intensive instruction to master the traditional methods of skin examination with the naked eye and the help of a dermatoscope, but Ungar considers the noninvasive tools to be another step forward. They lower miss rates while reducing the need for histopathology.
Adding these new technologies to routine patient care resonates for many experts, even if the protocols of when to use with the tool are not well established.
Angela J. Lamb, MD, an associate professor of dermatology at Mount Sinai, who has been following the work of Ungar with interest, sees merit in his argument. Not surprisingly, she thinks that any approach shown to boost skin cancer detection is something that deserves attention, but she thinks the effort to safely eliminate biopsies with a low likelihood of a positive finding cannot be ignored.
“Patients want to avoid biopsies when they can,” Lamb told this news organization, and she does not think this is limited to biopsies on the face or other cosmetically sensitive areas.
As a result, she said that she does see the rationale for incorporating the newer technologies into routine care and called this an “important” effort to improve the patient experience as well as reduce missed lesions.
Ungar reported financial relationships with AbbVie, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Janssen Pharmaceuticals, Menlo Therapeutics, Mitsubishi Tanabe Pharma America, and UCB. Lamb reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — now in routine use at his own institution.
For skin cancer screening, existing and coming technologies represent “the future of dermatology,” but “we can and should be [already] trying to incorporate these into routine practice,” said Jonathan Ungar, MD, assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York City.
Technologies such as electrical impedance spectroscopy (EIS), optical coherence tomography (OCT), and reflectance confocal microscopy (RCM) have immediate utility for improving skin cancer detection with fewer biopsies, but this is just the beginning, according to Ungar, who is also medical director of the Kimberly and Eric J. Waldman Melanoma and Skin Cancer Center at Mount Sinai, New York City.
“There is going to be a day when we are not cutting to make a diagnosis,” he said during a presentation at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024.
Four Noninvasive Tools Are in Routine Use
Each of these technologies, along with total body photography (TBP), is currently in use at Mount Sinai as well as other tertiary centers to improve diagnostic accuracy at the same time they reduce invasive tests. The initial excitement about these technologies was based on their potential to avoid biopsy in cosmetically sensitive areas, but Ungar suggested that wider application is being driven by better rates of detection, less morbidity, and improved patient satisfaction.
Patients are happy to avoid invasive procedures whenever they can, Ungar noted. In addition to concern about pain or discomfort and a small but measurable risk for infection, patients face a wound that requires healing and the potential for an enduring scar whether the histology is positive for a malignancy.
While none of the four technologies Ungar outlined typically provide a yes or no answer regarding the presence of a malignancy, they do improve diagnostic accuracy with a lower rate of biopsy.
Each Noninvasive Tool Reduces Biopsy Rates
In the case of EIS, for example, the impedance of a painless and harmless electrical current directed into the skin with a handheld probe differentiates normal from abnormal skin through an EIS algorithm. Ungar said it does not require training. A result negative for an abnormality has about a 90% predictive value, and it means that a biopsy can be avoided if there are no other reasons for suspicion.
With a price estimated in the thousands of dollars, the device and software are “not so expensive,” particularly when the tool results in fewer biopsies, Ungar noted.
OCT has a similar profile. Again, used as an adjunct to other types of evaluations, including a history and visual inspection, this helps in modulating suspicion of malignancy. In published studies, OCT has proven superior to dermatoscopy for cancer detection. Citing a 14-study meta-analysis, Ungar said that the sensitivity of OCT for melanoma exceeds, and the specificity approaches, 90%. For basal cell cancers, it is even better.
RCM involves directing a laser into the skin to detect abnormal cells that reflect light. It enables visualization of the skin by layers to the papillary dermis in a detail that is comparable with histology, according to Ungar. Imaging performed with the device used at Mount Sinai (VivaScope 1500, Caliber Imaging & Diagnostics) is reimbursed by Medicare.
Once comfortable with the technology, scanning and interpretation take slightly more time than that required of EIS or OCT, but, like the others, it is painless and helpful for determining whether further evaluation is needed, according to Ungar.
“It is extremely useful in reducing the number of biopsies,” whether melanoma or basal cell malignancies, he said.
Total Body Photograph Helps With Serial Screens
While not specifically a diagnostic tool, TBP can also play a role in reducing biopsies through its highly efficient ability to document the evolution of lesions over time.
As its name implies, essentially the entire body surface is captured by multiple cameras mounted in a circle around the patient. Unlike sequential photos that require far more time to take and store and are challenging to organize and retrieve, the device used at Mount Sinai (Vectra Wb180 1360, Canfield Scientific) can complete the photos in about 2 minutes.
Software for organizing and storing the photos, to which dermatoscope images of individual lesions can be attached if helpful, results in efficient retrieval of photos at sequential visits for evaluating change in any specific lesion.
“It is very easy to use,” according to Ungar, who noted that although the underlying idea is not, the technology of taking, storing, and retrieving photographs has been “perhaps perfected” with this approach.
Noninvasive Screening Training Is Appropriate
Year after year, dermatology residents undergo intensive instruction to master the traditional methods of skin examination with the naked eye and the help of a dermatoscope, but Ungar considers the noninvasive tools to be another step forward. They lower miss rates while reducing the need for histopathology.
Adding these new technologies to routine patient care resonates for many experts, even if the protocols of when to use with the tool are not well established.
Angela J. Lamb, MD, an associate professor of dermatology at Mount Sinai, who has been following the work of Ungar with interest, sees merit in his argument. Not surprisingly, she thinks that any approach shown to boost skin cancer detection is something that deserves attention, but she thinks the effort to safely eliminate biopsies with a low likelihood of a positive finding cannot be ignored.
“Patients want to avoid biopsies when they can,” Lamb told this news organization, and she does not think this is limited to biopsies on the face or other cosmetically sensitive areas.
As a result, she said that she does see the rationale for incorporating the newer technologies into routine care and called this an “important” effort to improve the patient experience as well as reduce missed lesions.
Ungar reported financial relationships with AbbVie, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Janssen Pharmaceuticals, Menlo Therapeutics, Mitsubishi Tanabe Pharma America, and UCB. Lamb reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM MSWS 2024