User login
The Kids Are Not Alright
“...but it all started to get worse during the pandemic.”
As the patient’s† door closed, I (JS) thought about what his father had shared: his 12-year-old son had experienced a slow decline in his mental health since March 2020. There had been a gradual loss of all the things his son needed for psychological well-being: school went virtual and extracurricular activities ceased, and with them went any sense of routine, normalcy, or authentic opportunities to socialize. His feelings of isolation and depression culminated in an attempt to end his own life. My mind shifted to other patients under our care: an 8-year-old with behavioral outbursts intensifying after school-based therapy ended, a 13-year-old who became suicidal from isolation and virtual bullying. These children’s families sought emergent care because they no longer had the resources to care for their children at home. My team left each of these rooms heartbroken, unsure of exactly what to say and aware of the limitations of our current healthcare system.
Before and during the COVID-19 pandemic, many pediatric providers have had similar experiences caring for countless patients who are “boarding”—awaiting transfer to a psychiatric facility for their primary acute psychiatric issue, initially in the emergency room, often for 5 days or more,1 then ultimately admitted to a general medical floor if an appropriate psychiatric bed is still not available.2 Unfortunately, just as parents have run out of resources to care for their children’s psychiatric needs, so too is our medical system lacking in resources to provide the acute care these children need in general hospitals.
This mental health crisis began before the COVID-19 pandemic3 but has only worsened in the wake of its resulting social isolation. During the pandemic, suicide hotlines had a 1000% increase in call volumes.4 COVID-19–induced bed closures simultaneously worsened an existing critical bed shortage5,6 and led to an increase in the average length of stay (LOS) for patients boarding in the emergency department (ED).7 In the state of Massachusetts, for example, psychiatric patients awaiting inpatient beds boarded for more than 10,000 hours in January 2021—more than ever before, and up approximately 4000 hours since January 2017.6 For pediatric patients, the average wait time is now 59 hours.6 In the first 6 months of the pandemic, 39% of children presenting to EDs for mental health complaints ended up boarding, which is an astounding figure and is unfortunately 7% higher than in 2019.8 Even these staggering numbers do not capture the full range of experiences, as many statistics do not account for time spent on inpatient units by patients who do not receive a bed placement after waiting hours to several days in the ED.
Shortages of space, as well as an underfunded and understaffed mental health workforce, lead to these prolonged, often traumatic boarding periods in hospitals designed to care for acute medical, rather than acute psychiatric, conditions. Patients awaiting psychiatric placement are waiting in settings that are chaotic, inconsistent, and lacking in privacy. A patient in the throes of psychosis or suicidality needs a therapeutic milieu, not one that interrupts their daily routine,2 disconnects them from their existing support networks, and is punctuated by the incessant clangs of bedside monitors and the hubbub of code teams. These environments are not therapeutic3 for young infants with fevers, let alone for teenagers battling suicidality and eating disorders. In fact, for these reasons, we suspect that many of our patients’ inpatient ”behavioral escalations” are in fact triggered by their hospital environment, which may contribute to the 300% increase in the number of pharmacological restraints used during mental health visits in the ED over the past 10 years.9
None of us imagined when we chose to pursue pediatrics a that significant—and at times predominant—portion of our training would encompass caring for patients with acute mental health concerns. And although we did not anticipate this crisis, we have now been tasked with managing it. Throughout the day, when we are called to see our patients with primarily psychiatric pathology, we are often at war with ourselves. We weigh forming deeply meaningful relationships with these patients against the potential of unintentionally retraumatizing them or forming bonds that will be abruptly severed when patients are transferred to a psychiatric facility, which often occurs with barely a few hours’ notice. Moreover, many healthcare workers have training ill-suited to meet the needs of these patients. Just as emergency physicians can diagnose appendicitis but rely on surgeons to provide timely surgical treatment, general pediatricians identify psychiatric crises but rely on psychiatrists for ideal treatment plans. And almost daily, we are called to an “escalating” patient and arrive minutes into a stressful situation that others expect us to extinguish expeditiously. Along with nursing colleagues and the behavioral response team, we enact the treatment plan laid out by our psychiatry colleagues and wonder whether there is a better way.
We propose the following changes to create a more ideal health system (Table). We acknowledge that each health system has unique resources, challenges, and patient populations. Thus, our recommendations are not comprehensive and are largely based on experiences within our own institutions and state, but they encompass many domains that impact and are affected by child and adolescent mental healthcare in the United States, ranging from program- and hospital-level innovation to community and legislative action.
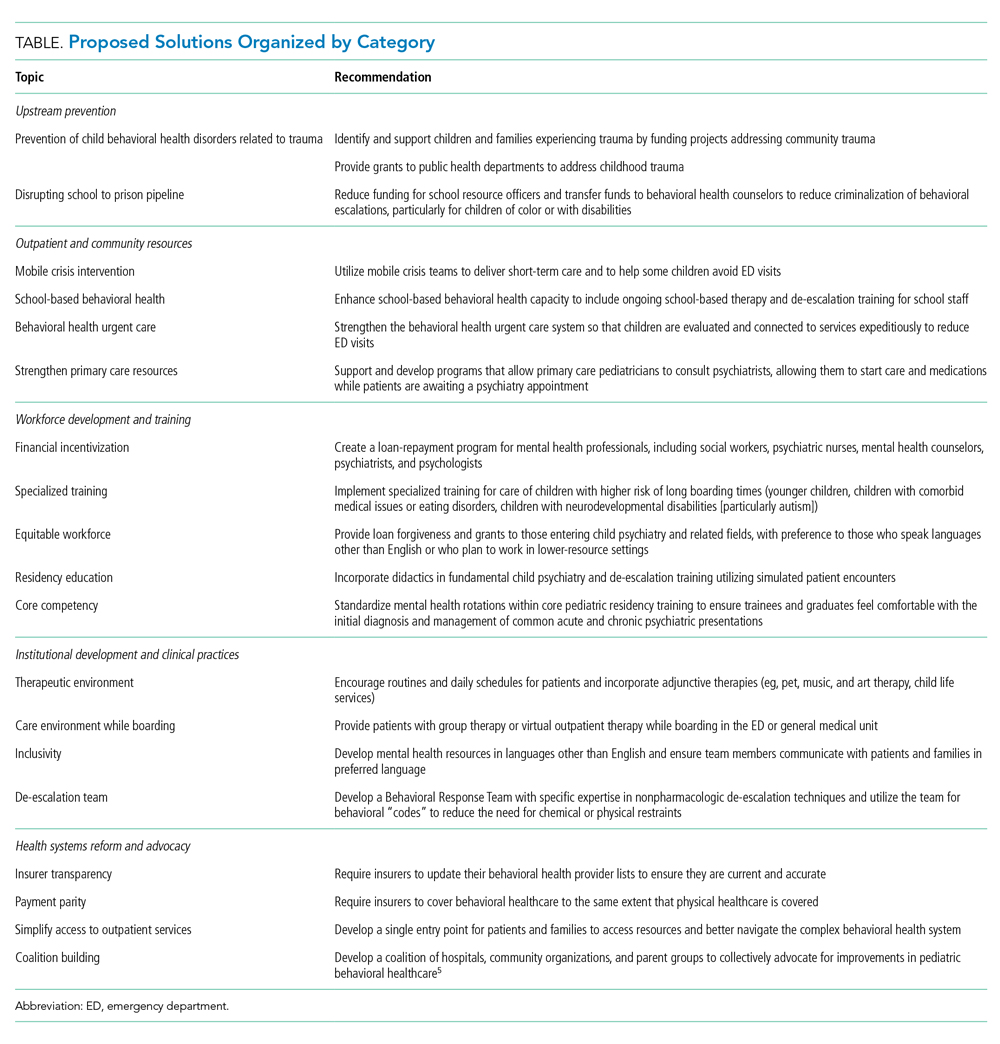
UPSTREAM PREVENTION
Like all good health system designs, we recommend prioritizing prevention. This would entail funding programs and legislation such as H.R. 3180, the RISE from Trauma Act, and H.R. 8544, the STRONG Support for Children Act of 2020 (both currently under consideration in the US House of Representatives) that support early childhood development and prevent adverse childhood experiences and trauma, averting mental health diagnoses such as depression and attention-deficit/hyperactivity disorder before they begin.10
OUTPATIENT AND COMMUNITY RESOURCES
We recognize that schools and general pediatricians have far more exposure to children at risk for mental health crises than do subspecialists. Thus, we urge an equitable increase in access to mental healthcare in the community so that patients needing assistance are screened and diagnosed earlier in their illness, allowing for secondary prevention of worsening mental health disorders. We support increased funding for programs such as the Massachusetts Child Psychiatry Access Program, which allows primary care doctors to consult psychiatrists in real time, closing the gap between a primary care visit and specialty follow-up. Telehealth services will be key to improving access for patients themselves and to allow pediatricians to consult with mental health professionals to initiate care prior to specialist availability. We envision that strengthening school-based behavioral health resources will also help prevent ED visits. Behavioral healthcare should be integrated into schools and community centers while police presence is simultaneously reduced, as there is evidence of an increased likelihood of juvenile justice involvement for children with disabilities and mental health needs.11,12
WORKFORCE DEVELOPMENT AND TRAINING
Ensuring access necessitates increasing the capacity of our psychiatric workforce by encouraging graduates to pursue mental health occupations with concrete financial incentives such as loan repayment and training grants. We thus support legislation such as H.R. 6597, the Mental Health Professionals Workforce Shortage Loan Repayment Act of 2018 (currently under consideration in the US House of Representatives). This may also improve recruitment and retention of individuals who are underrepresented in medicine, one step in helping ensure children have access to linguistically appropriate and culturally sensitive care. Residency programs and hospital systems should expand their training and education to identify and stabilize patients in mental health in extremis through culturally sensitive curricula focused on behavioral de-escalation techniques, trauma-informed care, and psychopharmacology. Our own residency program created a 2-week mental health rotation13 that includes rotating with outpatient mental health providers and our hospital’s behavioral response team, a group of trauma-informed responders for behavioral emergencies. Similar training should be available for nursing and other allied health professionals, who are often the first responders to behavioral escalations.13
INSTITUTIONAL DEVELOPMENT AND CLINICAL PRACTICES
Ideally, patients requiring higher-intensity psychiatric care would be referred to specialized pediatric behavioral health urgent care centers so their conditions can be adequately evaluated and addressed by staff trained in psychiatric management and in therapeutic environments. We believe all providers caring for children with mental health needs should be trained in basic, but core, behavioral health and de-escalation competencies, including specialized training for children with comorbid medical and neurodevelopmental diagnoses, such as autism. These centers should have specific beds for young children and those with developmental or complex care needs, and services should be available in numerous languages and levels of health literacy to allow all families to participate in their child’s care. At the same time, even nonpsychiatric EDs and inpatient units should commit resources to developing a maximally therapeutic environment, including allowing adjunctive services such as child life services, group therapy, and pet and music therapy, and create environments that support, rather than disrupt, normal routines.
HEALTH SYSTEMS REFORM AND ADVOCACY
Underpinning all the above innovations are changes to our healthcare payment system and provider networks, including the need for insurance coverage and payment parity for behavioral health, to ensure care is not only accessible but affordable. Additionally, for durable change, we need more than just education—we need coalition building and advocacy. Many organizations, including the American Academy of Pediatrics and the Children’s Hospital Association, have begun this work, which we must all continue.14 Bringing in diverse partners, including health systems, providers, educators, hospital administrators, payors, elected officials, and communities, will prioritize children’s needs and create a more ideal pediatric behavioral healthcare system.15
The COVID-19 pandemic has highlighted the dire need for comprehensive mental healthcare in the United States, a need that existed before the pandemic and will persist in a more fragile state long after it ends. Our hope is that the pandemic serves as the catalyst necessary to promote the magnitude of investments and stakeholder buy-in necessary to improve pediatric mental health and engender a radical redesign of our behavioral healthcare system. Our patients are counting on us to act. Together, we can build a system that ensures that the kids will be alright.
†Patient details have been changed for patient privacy.
Acknowledgments
The authors thank Joanna Perdomo, MD, Amara Azubuike, JD, and Josh Greenberg, JD, for reading and providing feedback on earlier versions of this work.
1. “This is a crisis”: mom whose son has boarded 33 days for psych bed calls for state action. WBUR News. Updated March 2, 2021. Accessed August 4, 2021. www.wbur.org/news/2021/02/26/mental-health-boarding-hospitals
2. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813-824. https://doi.org/10.1016/S2215-0366(20)30307-2
3. Nash KA, Zima BT, Rothenberg C, et al. Prolonged emergency department length of stay for US pediatric mental health visits (2005-2015). Pediatrics. 2021;147(5):e2020030692. https://doi.org/10.1542/peds.2020-030692
4. Cloutier RL, Marshaall R. A dangerous pandemic pair: Covid19 and adolescent mental health emergencies. Am J Emerg Med. 2021;46:776-777. https://doi.org/10.1016/j.ajem.2020.09.008
5. Schoenberg S. Lack of mental health beds means long ER waits. CommonWealth Magazine. April 15, 2021. Accessed August 5, 2021. https://commonwealthmagazine.org/health-care/lack-of-mental-health-beds-means-long-er-waits/
6. Jolicoeur L, Mullins L. Mass. physicians call on state to address ER “boarding” of patients awaiting admission. WBUR News. Updated February 3, 2021. Accessed August 5, 2021. www.wbur.org/news/2021/02/02/emergency-department-er-inpatient-beds-boarding
7. Krass P, Dalton E, Doupnik SK, Esposito J. US pediatric emergency department visits for mental health conditions during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e218533. https://doi.org/10.1001/jamanetworkopen.2021.8533
8. Impact of COVID-19 on the Massachusetts Health Care System: Interim Report. Massachusetts Health Policy Commission. April 2021. Accessed September 25, 2021. www.mass.gov/doc/impact-of-covid-19-on-the-massachusetts-health-care-system-interim-report/download
9. Foster AA, Porter JJ, Monuteaux MC, Hoffmann JA, Hudgins JD. Pharmacologic restraint use during mental health visits in pediatric emergency departments. J Pediatr. 2021;236:276-283.e2. https://doi.org/10.1016/j.jpeds.2021.03.027
10. Brown NM, Brown SN, Briggs RD, Germán M, Belamarich PF, Oyeku SO. Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad Pediatr. 2017;17(4):349-355. https://doi.org/10.1016/j.acap.2016.08.013
11. Harper K, Ryberg R, Temkin D. Black students and students with disabilities remain more likely to receive out-of-school suspensions, despite overall declines. Child Trends. April 29, 2019. Accessed August 5, 2021. www.childtrends.org/publications/black-students-disabilities-out-of-school-suspensions
12. Whitaker A, Torres-Guillén S, Morton M, et al. Cops and no counselors: how the lack of school mental health staff is harming students. American Civil Liberties Union. Accessed August 6, 2021. www.aclu.org/report/cops-and-no-counselors
13. Education. Boston Combined Residence Program. Accessed August 5, 2021. https://msbcrp.wpengine.com/program/education/
14. American Academy of Pediatrics. Interim guidance on supporting the emotional and behavioral health needs of children, adolescents, and families during the COVID-19 pandemic. Updated July 28, 2021. Accessed August 5, 2021. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-on-supporting-the-emotional-and-behavioral-health-needs-of-children-adolescents-and-families-during-the-covid-19-pandemic/
15. Advocacy. Children’s Mental Health Campaign. Accessed August 4, 2021. https://childrensmentalhealthcampaign.org/advocacy
“...but it all started to get worse during the pandemic.”
As the patient’s† door closed, I (JS) thought about what his father had shared: his 12-year-old son had experienced a slow decline in his mental health since March 2020. There had been a gradual loss of all the things his son needed for psychological well-being: school went virtual and extracurricular activities ceased, and with them went any sense of routine, normalcy, or authentic opportunities to socialize. His feelings of isolation and depression culminated in an attempt to end his own life. My mind shifted to other patients under our care: an 8-year-old with behavioral outbursts intensifying after school-based therapy ended, a 13-year-old who became suicidal from isolation and virtual bullying. These children’s families sought emergent care because they no longer had the resources to care for their children at home. My team left each of these rooms heartbroken, unsure of exactly what to say and aware of the limitations of our current healthcare system.
Before and during the COVID-19 pandemic, many pediatric providers have had similar experiences caring for countless patients who are “boarding”—awaiting transfer to a psychiatric facility for their primary acute psychiatric issue, initially in the emergency room, often for 5 days or more,1 then ultimately admitted to a general medical floor if an appropriate psychiatric bed is still not available.2 Unfortunately, just as parents have run out of resources to care for their children’s psychiatric needs, so too is our medical system lacking in resources to provide the acute care these children need in general hospitals.
This mental health crisis began before the COVID-19 pandemic3 but has only worsened in the wake of its resulting social isolation. During the pandemic, suicide hotlines had a 1000% increase in call volumes.4 COVID-19–induced bed closures simultaneously worsened an existing critical bed shortage5,6 and led to an increase in the average length of stay (LOS) for patients boarding in the emergency department (ED).7 In the state of Massachusetts, for example, psychiatric patients awaiting inpatient beds boarded for more than 10,000 hours in January 2021—more than ever before, and up approximately 4000 hours since January 2017.6 For pediatric patients, the average wait time is now 59 hours.6 In the first 6 months of the pandemic, 39% of children presenting to EDs for mental health complaints ended up boarding, which is an astounding figure and is unfortunately 7% higher than in 2019.8 Even these staggering numbers do not capture the full range of experiences, as many statistics do not account for time spent on inpatient units by patients who do not receive a bed placement after waiting hours to several days in the ED.
Shortages of space, as well as an underfunded and understaffed mental health workforce, lead to these prolonged, often traumatic boarding periods in hospitals designed to care for acute medical, rather than acute psychiatric, conditions. Patients awaiting psychiatric placement are waiting in settings that are chaotic, inconsistent, and lacking in privacy. A patient in the throes of psychosis or suicidality needs a therapeutic milieu, not one that interrupts their daily routine,2 disconnects them from their existing support networks, and is punctuated by the incessant clangs of bedside monitors and the hubbub of code teams. These environments are not therapeutic3 for young infants with fevers, let alone for teenagers battling suicidality and eating disorders. In fact, for these reasons, we suspect that many of our patients’ inpatient ”behavioral escalations” are in fact triggered by their hospital environment, which may contribute to the 300% increase in the number of pharmacological restraints used during mental health visits in the ED over the past 10 years.9
None of us imagined when we chose to pursue pediatrics a that significant—and at times predominant—portion of our training would encompass caring for patients with acute mental health concerns. And although we did not anticipate this crisis, we have now been tasked with managing it. Throughout the day, when we are called to see our patients with primarily psychiatric pathology, we are often at war with ourselves. We weigh forming deeply meaningful relationships with these patients against the potential of unintentionally retraumatizing them or forming bonds that will be abruptly severed when patients are transferred to a psychiatric facility, which often occurs with barely a few hours’ notice. Moreover, many healthcare workers have training ill-suited to meet the needs of these patients. Just as emergency physicians can diagnose appendicitis but rely on surgeons to provide timely surgical treatment, general pediatricians identify psychiatric crises but rely on psychiatrists for ideal treatment plans. And almost daily, we are called to an “escalating” patient and arrive minutes into a stressful situation that others expect us to extinguish expeditiously. Along with nursing colleagues and the behavioral response team, we enact the treatment plan laid out by our psychiatry colleagues and wonder whether there is a better way.
We propose the following changes to create a more ideal health system (Table). We acknowledge that each health system has unique resources, challenges, and patient populations. Thus, our recommendations are not comprehensive and are largely based on experiences within our own institutions and state, but they encompass many domains that impact and are affected by child and adolescent mental healthcare in the United States, ranging from program- and hospital-level innovation to community and legislative action.

UPSTREAM PREVENTION
Like all good health system designs, we recommend prioritizing prevention. This would entail funding programs and legislation such as H.R. 3180, the RISE from Trauma Act, and H.R. 8544, the STRONG Support for Children Act of 2020 (both currently under consideration in the US House of Representatives) that support early childhood development and prevent adverse childhood experiences and trauma, averting mental health diagnoses such as depression and attention-deficit/hyperactivity disorder before they begin.10
OUTPATIENT AND COMMUNITY RESOURCES
We recognize that schools and general pediatricians have far more exposure to children at risk for mental health crises than do subspecialists. Thus, we urge an equitable increase in access to mental healthcare in the community so that patients needing assistance are screened and diagnosed earlier in their illness, allowing for secondary prevention of worsening mental health disorders. We support increased funding for programs such as the Massachusetts Child Psychiatry Access Program, which allows primary care doctors to consult psychiatrists in real time, closing the gap between a primary care visit and specialty follow-up. Telehealth services will be key to improving access for patients themselves and to allow pediatricians to consult with mental health professionals to initiate care prior to specialist availability. We envision that strengthening school-based behavioral health resources will also help prevent ED visits. Behavioral healthcare should be integrated into schools and community centers while police presence is simultaneously reduced, as there is evidence of an increased likelihood of juvenile justice involvement for children with disabilities and mental health needs.11,12
WORKFORCE DEVELOPMENT AND TRAINING
Ensuring access necessitates increasing the capacity of our psychiatric workforce by encouraging graduates to pursue mental health occupations with concrete financial incentives such as loan repayment and training grants. We thus support legislation such as H.R. 6597, the Mental Health Professionals Workforce Shortage Loan Repayment Act of 2018 (currently under consideration in the US House of Representatives). This may also improve recruitment and retention of individuals who are underrepresented in medicine, one step in helping ensure children have access to linguistically appropriate and culturally sensitive care. Residency programs and hospital systems should expand their training and education to identify and stabilize patients in mental health in extremis through culturally sensitive curricula focused on behavioral de-escalation techniques, trauma-informed care, and psychopharmacology. Our own residency program created a 2-week mental health rotation13 that includes rotating with outpatient mental health providers and our hospital’s behavioral response team, a group of trauma-informed responders for behavioral emergencies. Similar training should be available for nursing and other allied health professionals, who are often the first responders to behavioral escalations.13
INSTITUTIONAL DEVELOPMENT AND CLINICAL PRACTICES
Ideally, patients requiring higher-intensity psychiatric care would be referred to specialized pediatric behavioral health urgent care centers so their conditions can be adequately evaluated and addressed by staff trained in psychiatric management and in therapeutic environments. We believe all providers caring for children with mental health needs should be trained in basic, but core, behavioral health and de-escalation competencies, including specialized training for children with comorbid medical and neurodevelopmental diagnoses, such as autism. These centers should have specific beds for young children and those with developmental or complex care needs, and services should be available in numerous languages and levels of health literacy to allow all families to participate in their child’s care. At the same time, even nonpsychiatric EDs and inpatient units should commit resources to developing a maximally therapeutic environment, including allowing adjunctive services such as child life services, group therapy, and pet and music therapy, and create environments that support, rather than disrupt, normal routines.
HEALTH SYSTEMS REFORM AND ADVOCACY
Underpinning all the above innovations are changes to our healthcare payment system and provider networks, including the need for insurance coverage and payment parity for behavioral health, to ensure care is not only accessible but affordable. Additionally, for durable change, we need more than just education—we need coalition building and advocacy. Many organizations, including the American Academy of Pediatrics and the Children’s Hospital Association, have begun this work, which we must all continue.14 Bringing in diverse partners, including health systems, providers, educators, hospital administrators, payors, elected officials, and communities, will prioritize children’s needs and create a more ideal pediatric behavioral healthcare system.15
The COVID-19 pandemic has highlighted the dire need for comprehensive mental healthcare in the United States, a need that existed before the pandemic and will persist in a more fragile state long after it ends. Our hope is that the pandemic serves as the catalyst necessary to promote the magnitude of investments and stakeholder buy-in necessary to improve pediatric mental health and engender a radical redesign of our behavioral healthcare system. Our patients are counting on us to act. Together, we can build a system that ensures that the kids will be alright.
†Patient details have been changed for patient privacy.
Acknowledgments
The authors thank Joanna Perdomo, MD, Amara Azubuike, JD, and Josh Greenberg, JD, for reading and providing feedback on earlier versions of this work.
“...but it all started to get worse during the pandemic.”
As the patient’s† door closed, I (JS) thought about what his father had shared: his 12-year-old son had experienced a slow decline in his mental health since March 2020. There had been a gradual loss of all the things his son needed for psychological well-being: school went virtual and extracurricular activities ceased, and with them went any sense of routine, normalcy, or authentic opportunities to socialize. His feelings of isolation and depression culminated in an attempt to end his own life. My mind shifted to other patients under our care: an 8-year-old with behavioral outbursts intensifying after school-based therapy ended, a 13-year-old who became suicidal from isolation and virtual bullying. These children’s families sought emergent care because they no longer had the resources to care for their children at home. My team left each of these rooms heartbroken, unsure of exactly what to say and aware of the limitations of our current healthcare system.
Before and during the COVID-19 pandemic, many pediatric providers have had similar experiences caring for countless patients who are “boarding”—awaiting transfer to a psychiatric facility for their primary acute psychiatric issue, initially in the emergency room, often for 5 days or more,1 then ultimately admitted to a general medical floor if an appropriate psychiatric bed is still not available.2 Unfortunately, just as parents have run out of resources to care for their children’s psychiatric needs, so too is our medical system lacking in resources to provide the acute care these children need in general hospitals.
This mental health crisis began before the COVID-19 pandemic3 but has only worsened in the wake of its resulting social isolation. During the pandemic, suicide hotlines had a 1000% increase in call volumes.4 COVID-19–induced bed closures simultaneously worsened an existing critical bed shortage5,6 and led to an increase in the average length of stay (LOS) for patients boarding in the emergency department (ED).7 In the state of Massachusetts, for example, psychiatric patients awaiting inpatient beds boarded for more than 10,000 hours in January 2021—more than ever before, and up approximately 4000 hours since January 2017.6 For pediatric patients, the average wait time is now 59 hours.6 In the first 6 months of the pandemic, 39% of children presenting to EDs for mental health complaints ended up boarding, which is an astounding figure and is unfortunately 7% higher than in 2019.8 Even these staggering numbers do not capture the full range of experiences, as many statistics do not account for time spent on inpatient units by patients who do not receive a bed placement after waiting hours to several days in the ED.
Shortages of space, as well as an underfunded and understaffed mental health workforce, lead to these prolonged, often traumatic boarding periods in hospitals designed to care for acute medical, rather than acute psychiatric, conditions. Patients awaiting psychiatric placement are waiting in settings that are chaotic, inconsistent, and lacking in privacy. A patient in the throes of psychosis or suicidality needs a therapeutic milieu, not one that interrupts their daily routine,2 disconnects them from their existing support networks, and is punctuated by the incessant clangs of bedside monitors and the hubbub of code teams. These environments are not therapeutic3 for young infants with fevers, let alone for teenagers battling suicidality and eating disorders. In fact, for these reasons, we suspect that many of our patients’ inpatient ”behavioral escalations” are in fact triggered by their hospital environment, which may contribute to the 300% increase in the number of pharmacological restraints used during mental health visits in the ED over the past 10 years.9
None of us imagined when we chose to pursue pediatrics a that significant—and at times predominant—portion of our training would encompass caring for patients with acute mental health concerns. And although we did not anticipate this crisis, we have now been tasked with managing it. Throughout the day, when we are called to see our patients with primarily psychiatric pathology, we are often at war with ourselves. We weigh forming deeply meaningful relationships with these patients against the potential of unintentionally retraumatizing them or forming bonds that will be abruptly severed when patients are transferred to a psychiatric facility, which often occurs with barely a few hours’ notice. Moreover, many healthcare workers have training ill-suited to meet the needs of these patients. Just as emergency physicians can diagnose appendicitis but rely on surgeons to provide timely surgical treatment, general pediatricians identify psychiatric crises but rely on psychiatrists for ideal treatment plans. And almost daily, we are called to an “escalating” patient and arrive minutes into a stressful situation that others expect us to extinguish expeditiously. Along with nursing colleagues and the behavioral response team, we enact the treatment plan laid out by our psychiatry colleagues and wonder whether there is a better way.
We propose the following changes to create a more ideal health system (Table). We acknowledge that each health system has unique resources, challenges, and patient populations. Thus, our recommendations are not comprehensive and are largely based on experiences within our own institutions and state, but they encompass many domains that impact and are affected by child and adolescent mental healthcare in the United States, ranging from program- and hospital-level innovation to community and legislative action.

UPSTREAM PREVENTION
Like all good health system designs, we recommend prioritizing prevention. This would entail funding programs and legislation such as H.R. 3180, the RISE from Trauma Act, and H.R. 8544, the STRONG Support for Children Act of 2020 (both currently under consideration in the US House of Representatives) that support early childhood development and prevent adverse childhood experiences and trauma, averting mental health diagnoses such as depression and attention-deficit/hyperactivity disorder before they begin.10
OUTPATIENT AND COMMUNITY RESOURCES
We recognize that schools and general pediatricians have far more exposure to children at risk for mental health crises than do subspecialists. Thus, we urge an equitable increase in access to mental healthcare in the community so that patients needing assistance are screened and diagnosed earlier in their illness, allowing for secondary prevention of worsening mental health disorders. We support increased funding for programs such as the Massachusetts Child Psychiatry Access Program, which allows primary care doctors to consult psychiatrists in real time, closing the gap between a primary care visit and specialty follow-up. Telehealth services will be key to improving access for patients themselves and to allow pediatricians to consult with mental health professionals to initiate care prior to specialist availability. We envision that strengthening school-based behavioral health resources will also help prevent ED visits. Behavioral healthcare should be integrated into schools and community centers while police presence is simultaneously reduced, as there is evidence of an increased likelihood of juvenile justice involvement for children with disabilities and mental health needs.11,12
WORKFORCE DEVELOPMENT AND TRAINING
Ensuring access necessitates increasing the capacity of our psychiatric workforce by encouraging graduates to pursue mental health occupations with concrete financial incentives such as loan repayment and training grants. We thus support legislation such as H.R. 6597, the Mental Health Professionals Workforce Shortage Loan Repayment Act of 2018 (currently under consideration in the US House of Representatives). This may also improve recruitment and retention of individuals who are underrepresented in medicine, one step in helping ensure children have access to linguistically appropriate and culturally sensitive care. Residency programs and hospital systems should expand their training and education to identify and stabilize patients in mental health in extremis through culturally sensitive curricula focused on behavioral de-escalation techniques, trauma-informed care, and psychopharmacology. Our own residency program created a 2-week mental health rotation13 that includes rotating with outpatient mental health providers and our hospital’s behavioral response team, a group of trauma-informed responders for behavioral emergencies. Similar training should be available for nursing and other allied health professionals, who are often the first responders to behavioral escalations.13
INSTITUTIONAL DEVELOPMENT AND CLINICAL PRACTICES
Ideally, patients requiring higher-intensity psychiatric care would be referred to specialized pediatric behavioral health urgent care centers so their conditions can be adequately evaluated and addressed by staff trained in psychiatric management and in therapeutic environments. We believe all providers caring for children with mental health needs should be trained in basic, but core, behavioral health and de-escalation competencies, including specialized training for children with comorbid medical and neurodevelopmental diagnoses, such as autism. These centers should have specific beds for young children and those with developmental or complex care needs, and services should be available in numerous languages and levels of health literacy to allow all families to participate in their child’s care. At the same time, even nonpsychiatric EDs and inpatient units should commit resources to developing a maximally therapeutic environment, including allowing adjunctive services such as child life services, group therapy, and pet and music therapy, and create environments that support, rather than disrupt, normal routines.
HEALTH SYSTEMS REFORM AND ADVOCACY
Underpinning all the above innovations are changes to our healthcare payment system and provider networks, including the need for insurance coverage and payment parity for behavioral health, to ensure care is not only accessible but affordable. Additionally, for durable change, we need more than just education—we need coalition building and advocacy. Many organizations, including the American Academy of Pediatrics and the Children’s Hospital Association, have begun this work, which we must all continue.14 Bringing in diverse partners, including health systems, providers, educators, hospital administrators, payors, elected officials, and communities, will prioritize children’s needs and create a more ideal pediatric behavioral healthcare system.15
The COVID-19 pandemic has highlighted the dire need for comprehensive mental healthcare in the United States, a need that existed before the pandemic and will persist in a more fragile state long after it ends. Our hope is that the pandemic serves as the catalyst necessary to promote the magnitude of investments and stakeholder buy-in necessary to improve pediatric mental health and engender a radical redesign of our behavioral healthcare system. Our patients are counting on us to act. Together, we can build a system that ensures that the kids will be alright.
†Patient details have been changed for patient privacy.
Acknowledgments
The authors thank Joanna Perdomo, MD, Amara Azubuike, JD, and Josh Greenberg, JD, for reading and providing feedback on earlier versions of this work.
1. “This is a crisis”: mom whose son has boarded 33 days for psych bed calls for state action. WBUR News. Updated March 2, 2021. Accessed August 4, 2021. www.wbur.org/news/2021/02/26/mental-health-boarding-hospitals
2. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813-824. https://doi.org/10.1016/S2215-0366(20)30307-2
3. Nash KA, Zima BT, Rothenberg C, et al. Prolonged emergency department length of stay for US pediatric mental health visits (2005-2015). Pediatrics. 2021;147(5):e2020030692. https://doi.org/10.1542/peds.2020-030692
4. Cloutier RL, Marshaall R. A dangerous pandemic pair: Covid19 and adolescent mental health emergencies. Am J Emerg Med. 2021;46:776-777. https://doi.org/10.1016/j.ajem.2020.09.008
5. Schoenberg S. Lack of mental health beds means long ER waits. CommonWealth Magazine. April 15, 2021. Accessed August 5, 2021. https://commonwealthmagazine.org/health-care/lack-of-mental-health-beds-means-long-er-waits/
6. Jolicoeur L, Mullins L. Mass. physicians call on state to address ER “boarding” of patients awaiting admission. WBUR News. Updated February 3, 2021. Accessed August 5, 2021. www.wbur.org/news/2021/02/02/emergency-department-er-inpatient-beds-boarding
7. Krass P, Dalton E, Doupnik SK, Esposito J. US pediatric emergency department visits for mental health conditions during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e218533. https://doi.org/10.1001/jamanetworkopen.2021.8533
8. Impact of COVID-19 on the Massachusetts Health Care System: Interim Report. Massachusetts Health Policy Commission. April 2021. Accessed September 25, 2021. www.mass.gov/doc/impact-of-covid-19-on-the-massachusetts-health-care-system-interim-report/download
9. Foster AA, Porter JJ, Monuteaux MC, Hoffmann JA, Hudgins JD. Pharmacologic restraint use during mental health visits in pediatric emergency departments. J Pediatr. 2021;236:276-283.e2. https://doi.org/10.1016/j.jpeds.2021.03.027
10. Brown NM, Brown SN, Briggs RD, Germán M, Belamarich PF, Oyeku SO. Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad Pediatr. 2017;17(4):349-355. https://doi.org/10.1016/j.acap.2016.08.013
11. Harper K, Ryberg R, Temkin D. Black students and students with disabilities remain more likely to receive out-of-school suspensions, despite overall declines. Child Trends. April 29, 2019. Accessed August 5, 2021. www.childtrends.org/publications/black-students-disabilities-out-of-school-suspensions
12. Whitaker A, Torres-Guillén S, Morton M, et al. Cops and no counselors: how the lack of school mental health staff is harming students. American Civil Liberties Union. Accessed August 6, 2021. www.aclu.org/report/cops-and-no-counselors
13. Education. Boston Combined Residence Program. Accessed August 5, 2021. https://msbcrp.wpengine.com/program/education/
14. American Academy of Pediatrics. Interim guidance on supporting the emotional and behavioral health needs of children, adolescents, and families during the COVID-19 pandemic. Updated July 28, 2021. Accessed August 5, 2021. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-on-supporting-the-emotional-and-behavioral-health-needs-of-children-adolescents-and-families-during-the-covid-19-pandemic/
15. Advocacy. Children’s Mental Health Campaign. Accessed August 4, 2021. https://childrensmentalhealthcampaign.org/advocacy
1. “This is a crisis”: mom whose son has boarded 33 days for psych bed calls for state action. WBUR News. Updated March 2, 2021. Accessed August 4, 2021. www.wbur.org/news/2021/02/26/mental-health-boarding-hospitals
2. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813-824. https://doi.org/10.1016/S2215-0366(20)30307-2
3. Nash KA, Zima BT, Rothenberg C, et al. Prolonged emergency department length of stay for US pediatric mental health visits (2005-2015). Pediatrics. 2021;147(5):e2020030692. https://doi.org/10.1542/peds.2020-030692
4. Cloutier RL, Marshaall R. A dangerous pandemic pair: Covid19 and adolescent mental health emergencies. Am J Emerg Med. 2021;46:776-777. https://doi.org/10.1016/j.ajem.2020.09.008
5. Schoenberg S. Lack of mental health beds means long ER waits. CommonWealth Magazine. April 15, 2021. Accessed August 5, 2021. https://commonwealthmagazine.org/health-care/lack-of-mental-health-beds-means-long-er-waits/
6. Jolicoeur L, Mullins L. Mass. physicians call on state to address ER “boarding” of patients awaiting admission. WBUR News. Updated February 3, 2021. Accessed August 5, 2021. www.wbur.org/news/2021/02/02/emergency-department-er-inpatient-beds-boarding
7. Krass P, Dalton E, Doupnik SK, Esposito J. US pediatric emergency department visits for mental health conditions during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e218533. https://doi.org/10.1001/jamanetworkopen.2021.8533
8. Impact of COVID-19 on the Massachusetts Health Care System: Interim Report. Massachusetts Health Policy Commission. April 2021. Accessed September 25, 2021. www.mass.gov/doc/impact-of-covid-19-on-the-massachusetts-health-care-system-interim-report/download
9. Foster AA, Porter JJ, Monuteaux MC, Hoffmann JA, Hudgins JD. Pharmacologic restraint use during mental health visits in pediatric emergency departments. J Pediatr. 2021;236:276-283.e2. https://doi.org/10.1016/j.jpeds.2021.03.027
10. Brown NM, Brown SN, Briggs RD, Germán M, Belamarich PF, Oyeku SO. Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad Pediatr. 2017;17(4):349-355. https://doi.org/10.1016/j.acap.2016.08.013
11. Harper K, Ryberg R, Temkin D. Black students and students with disabilities remain more likely to receive out-of-school suspensions, despite overall declines. Child Trends. April 29, 2019. Accessed August 5, 2021. www.childtrends.org/publications/black-students-disabilities-out-of-school-suspensions
12. Whitaker A, Torres-Guillén S, Morton M, et al. Cops and no counselors: how the lack of school mental health staff is harming students. American Civil Liberties Union. Accessed August 6, 2021. www.aclu.org/report/cops-and-no-counselors
13. Education. Boston Combined Residence Program. Accessed August 5, 2021. https://msbcrp.wpengine.com/program/education/
14. American Academy of Pediatrics. Interim guidance on supporting the emotional and behavioral health needs of children, adolescents, and families during the COVID-19 pandemic. Updated July 28, 2021. Accessed August 5, 2021. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-on-supporting-the-emotional-and-behavioral-health-needs-of-children-adolescents-and-families-during-the-covid-19-pandemic/
15. Advocacy. Children’s Mental Health Campaign. Accessed August 4, 2021. https://childrensmentalhealthcampaign.org/advocacy
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Discontinuing Urate-Lowering Therapy on Admission
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: 2020 American Society of Addiction Medicine Clinical Practice Guideline on Alcohol Withdrawal Management
Alcohol is the most common substance implicated in hospitalizations for substance use disorders,1 and as a result, hospitalists commonly diagnose and manage alcohol withdrawal syndrome (AWS) in the inpatient medical setting. The 2020 guidelines of the American Society of Addiction Medicine (ASAM) provide updated recommendations for the diagnosis, monitoring, and treatment of patients hospitalized with AWS, which we have condensed to emphasize key changes from the last update2 and clarify ongoing areas of uncertainty.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnosis
Recommendation 1. All inpatients who have used alcohol recently or regularly should be risk-stratified for AWS, regardless of whether or not they have suggestive symptoms (recommendations I.3, I.4, I.5, II.10). The Alcohol Use Disorders Identification Test-(Piccinelli) Consumption (AUDIT-PC) identifies patients at risk for AWS, and the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) identifies those at risk for severe or complicated AWS, which includes seizures and alcohol withdrawal delirium (formerly delirium tremens). The guideline emphasizes use of these tools rather than simply initiating Clinical Institute Withdrawal Assessment for Alcohol, revised (CIWA-Ar) monitoring on all such patients to diagnose AWS, as CIWA-Ar was developed for monitoring response to treatment, not diagnosis (recommendation I.6).
Treating Mild/Moderate or Uncomplicated AWS
Recommendation 2. Because of their proven track record of reducing the incidence of seizure and alcohol withdrawal delirium, benzodiazepines remain the recommended first-line therapy (recommendations V.13, V.16). Symptom-triggered administration of benzodiazepines (via CIWA-Ar) is recommended over fixed-dose administration because the former is associated with shorter length of stay and lower cumulative benzodiazepine administration3,4 (recommendation V.23). Patients with mild AWS who are at low risk for severe or complicated withdrawal should be monitored for up to 36 hours for the development of worsening symptoms (recommendation V.1). For patients with high CIWA-Ar scores or who are at increased risk for severe or complicated AWS, frequent administration of moderate to high doses of a long-acting benzodiazepine early in AWS treatment (a practice called frontloading) is recommended to quickly control symptoms and prevent clinical worsening. This approach has been shown to reduce the incidence of seizures and alcohol withdrawal delirium (recommendations V.14, V.19, V.24).
Carbamazepine or gabapentin may be used in mild or moderate AWS if benzodiazepines are contraindicated; however, neither agent is recommended as first-line therapy because a clear reduction in seizure and withdrawal delirium has not been established (recommendation V.16). Alpha-2 agonists (eg, clonidine, dexmedetomidine) may be used to treat persistent autonomic hyperactivity or anxiety when these are not adequately controlled by benzodiazepines alone (recommendation V.36).
Treating Severe or Complicated AWS
Recommendation 3. The guideline defines severe AWS as withdrawal with severe signs and symptoms, and complicated AWS as withdrawal accompanied by seizures or delirium (Appendix Table5). The development of complications warrants prompt treatment. Patients who experience seizure should receive a fast-acting benzodiazepine (eg, intravenous [IV] diazepam or lorazepam) (recommendation VI.4). Patients with withdrawal delirium should receive a benzodiazepine (preferably parenterally) dosed to achieve light sedation. Clinicians should be prepared for the possibility that large doses may be required and to monitor patients for oversedation and respiratory depression (recommendations VI.13, VI.17). Antipsychotics may be used as adjuncts when withdrawal delirium or other symptoms, such as hallucinosis, are not adequately controlled by benzodiazepines alone, but should not be used as monotherapy (recommendation VI.20). The guideline emphasizes that alpha-2 agonists should not be used to treat withdrawal delirium (recommendation VI.21), but they may be used as adjuncts for resistant alcohol withdrawal in the intensive care unit (ICU) (recommendations VI.27, VI.29). Phenobarbital is an acceptable alternative to benzodiazepines for severe withdrawal (recommendation V.17); however, the guideline recommends that clinicians should be experienced in its use.
Treating Wernicke Encephalopathy
Recommendation 4. Thiamine should be administered to prevent Wernicke encephalopathy (WE), with parenteral formulations recommended in patients with malnutrition, severe/complicated withdrawal, or requiring ICU-level care (recommendations V.7, V.8). In particular, all patients admitted to an ICU for AWS should receive thiamine, as diagnosis of WE is often difficult in this population. Although there is no consensus on the required dose of thiamine to treat WE, 100 mg IV or intramuscularly (IM) daily for 3 to 5 days is commonly administered (recommendation V.7). Because of a lack of evidence of harm, thiamine may be given before, after, or concurrently with glucose or dextrose (recommendation V.7). The guideline does not make a specific recommendation regarding how to risk-stratify patients for WE.
Treating Underlying Alcohol Use Disorder
Recommendation 5. Hospitalization for AWS is an important opportunity to engage patients in treatment for alcohol use disorder (AUD), including pharmacotherapy and connection with outpatient providers (recommendation V.12). The guideline emphasizes that treatment for AUD should be initiated concomitantly with AWS management whenever possible but does not make recommendations regarding specific pharmacotherapies.
CRITIQUE
This guideline was authored by a committee of emergency medicine physicians, psychiatrists, and internists using the Department of Veterans Affairs/Department of Defense guidelines and the RAND/UCLA appropriateness method to combine the scientific literature with expert opinion. The result is a series of recommendations for physicians, physician assistants, nurse practitioners, and pharmacists that are not rated by strength; an assessment of the quality of the supporting evidence is available in an appendix. Four of the nine guideline committee members reported significant financial relationships with industry and other entities relevant to these guidelines.
Despite concern about oversedation from phenobarbital raised in small case series,6 observational studies comparing phenobarbital with benzodiazepines suggest phenobarbital has similar efficacy for treating AWS and that oversedation is rare.7-9 Large randomized controlled trials in this area are lacking; however, at least one small randomized controlled trial10 among patients with AWS presenting to emergency departments supports the safety and efficacy of phenobarbital when used in combination with benzodiazepines. Given the growing body of evidence supporting the safety of phenobarbital, we believe a stronger recommendation for use in patients presenting with alcohol withdrawal delirium or treatment-resistant alcohol withdrawal is warranted. The guidelines also suggest that only “experienced clinicians” use phenobarbital for AWS, which may suppress appropriate use. Nationally, phenobarbital use for AWS remains low.11Finally, although the guideline recommends initiation of treatment for AUD, specific recommendations for pharmacotherapy are not provided. Three medications currently have approval from the US Food and Drug Administration for treatment of AUD: acamprosate, naltrexone, and disulfiram. Large randomized controlled trials support the safety and efficacy of acamprosate and naltrexone, with or without counselling, in the treatment of AUD,12 and disulfiram may be appropriate for selected highly motivated patients. We believe more specific recommendations to assist in choosing among these options would be useful.
AREAS IN NEED OF FUTURE STUDY
More data are needed on the safety and efficacy of phenobarbital in patients with AWS, as well as comparative effectiveness against benzodiazepines. Recruitment is ongoing for a single clinical trial comparing the effect of phenobarbital and lorazepam on length of stay among patients in the ICU with AWS (NCT04156464); to date, no randomized trials of phenobarbital have been conducted in medical inpatients with AWS. In addition, gaps in the literature exist regarding benzodiazepine selection, and head-to-head comparisons of symptom-triggered usage of different benzodiazepines are lacking.
1. Heslin KC, Elixhauser A, Steiner CA. Hospitalizations involving mental and substance use disorders among adults, 2012. HCUP Statistical Brief #191. June 2015. Accessed November 17, 2021. www.hcup-us.ahrq.gov/reports/statbriefs/sb191-Hospitalization-Mental-Substance-Use-Disorders-2012.pdf
2. Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405-1412. https://doi.org/10.1001/archinte.164.13.1405
3. Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519-523.
4. Daeppen J-B, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. https://doi.org/10.1001/archinte.162.10.1117
5. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. J Addict Med. 2020;14(3S suppl):1-72. https://doi.org/10.1097/ADM.0000000000000668
6. Oks M, Cleven KL, Healy L, et al. The safety and utility of phenobarbital use for the treatment of severe alcohol withdrawal syndrome in the medical intensive care unit. J Intensive Care Med. 2020;35(9):844-850. https://doi.org/10.1177/0885066618783947
7. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357. https://doi.org/10.1111/j.1360-0443.1989.tb00737.x
8. Ibarra Jr F. Single dose phenobarbital in addition to symptom-triggered lorazepam in alcohol withdrawal. Am J Emerg Med. 2020;38(2):178-181. https://doi.org/10.1016/j.ajem.2019.01.053
9. Nisavic M, Nejad SH, Isenberg BM, et al. Use of phenobarbital in alcohol withdrawal management–a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics. 2019;60(5):458-467. https://doi.org/10.1016/j.psym.2019.02.002
10. Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med. 2013;44(3):592-598.e2. https://doi.org/10.1016/j.jemermed.2012.07.056
11. Gupta N, Emerman CL. Trends in the management of inpatients with alcohol withdrawal syndrome. Addict Disord Their Treat. 2021;20(1):29-32. https://doi.org/10.1097/ADT.0000000000000203
12. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017. https://doi.org/10.1001/jama.295.17.2003
Alcohol is the most common substance implicated in hospitalizations for substance use disorders,1 and as a result, hospitalists commonly diagnose and manage alcohol withdrawal syndrome (AWS) in the inpatient medical setting. The 2020 guidelines of the American Society of Addiction Medicine (ASAM) provide updated recommendations for the diagnosis, monitoring, and treatment of patients hospitalized with AWS, which we have condensed to emphasize key changes from the last update2 and clarify ongoing areas of uncertainty.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnosis
Recommendation 1. All inpatients who have used alcohol recently or regularly should be risk-stratified for AWS, regardless of whether or not they have suggestive symptoms (recommendations I.3, I.4, I.5, II.10). The Alcohol Use Disorders Identification Test-(Piccinelli) Consumption (AUDIT-PC) identifies patients at risk for AWS, and the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) identifies those at risk for severe or complicated AWS, which includes seizures and alcohol withdrawal delirium (formerly delirium tremens). The guideline emphasizes use of these tools rather than simply initiating Clinical Institute Withdrawal Assessment for Alcohol, revised (CIWA-Ar) monitoring on all such patients to diagnose AWS, as CIWA-Ar was developed for monitoring response to treatment, not diagnosis (recommendation I.6).
Treating Mild/Moderate or Uncomplicated AWS
Recommendation 2. Because of their proven track record of reducing the incidence of seizure and alcohol withdrawal delirium, benzodiazepines remain the recommended first-line therapy (recommendations V.13, V.16). Symptom-triggered administration of benzodiazepines (via CIWA-Ar) is recommended over fixed-dose administration because the former is associated with shorter length of stay and lower cumulative benzodiazepine administration3,4 (recommendation V.23). Patients with mild AWS who are at low risk for severe or complicated withdrawal should be monitored for up to 36 hours for the development of worsening symptoms (recommendation V.1). For patients with high CIWA-Ar scores or who are at increased risk for severe or complicated AWS, frequent administration of moderate to high doses of a long-acting benzodiazepine early in AWS treatment (a practice called frontloading) is recommended to quickly control symptoms and prevent clinical worsening. This approach has been shown to reduce the incidence of seizures and alcohol withdrawal delirium (recommendations V.14, V.19, V.24).
Carbamazepine or gabapentin may be used in mild or moderate AWS if benzodiazepines are contraindicated; however, neither agent is recommended as first-line therapy because a clear reduction in seizure and withdrawal delirium has not been established (recommendation V.16). Alpha-2 agonists (eg, clonidine, dexmedetomidine) may be used to treat persistent autonomic hyperactivity or anxiety when these are not adequately controlled by benzodiazepines alone (recommendation V.36).
Treating Severe or Complicated AWS
Recommendation 3. The guideline defines severe AWS as withdrawal with severe signs and symptoms, and complicated AWS as withdrawal accompanied by seizures or delirium (Appendix Table5). The development of complications warrants prompt treatment. Patients who experience seizure should receive a fast-acting benzodiazepine (eg, intravenous [IV] diazepam or lorazepam) (recommendation VI.4). Patients with withdrawal delirium should receive a benzodiazepine (preferably parenterally) dosed to achieve light sedation. Clinicians should be prepared for the possibility that large doses may be required and to monitor patients for oversedation and respiratory depression (recommendations VI.13, VI.17). Antipsychotics may be used as adjuncts when withdrawal delirium or other symptoms, such as hallucinosis, are not adequately controlled by benzodiazepines alone, but should not be used as monotherapy (recommendation VI.20). The guideline emphasizes that alpha-2 agonists should not be used to treat withdrawal delirium (recommendation VI.21), but they may be used as adjuncts for resistant alcohol withdrawal in the intensive care unit (ICU) (recommendations VI.27, VI.29). Phenobarbital is an acceptable alternative to benzodiazepines for severe withdrawal (recommendation V.17); however, the guideline recommends that clinicians should be experienced in its use.
Treating Wernicke Encephalopathy
Recommendation 4. Thiamine should be administered to prevent Wernicke encephalopathy (WE), with parenteral formulations recommended in patients with malnutrition, severe/complicated withdrawal, or requiring ICU-level care (recommendations V.7, V.8). In particular, all patients admitted to an ICU for AWS should receive thiamine, as diagnosis of WE is often difficult in this population. Although there is no consensus on the required dose of thiamine to treat WE, 100 mg IV or intramuscularly (IM) daily for 3 to 5 days is commonly administered (recommendation V.7). Because of a lack of evidence of harm, thiamine may be given before, after, or concurrently with glucose or dextrose (recommendation V.7). The guideline does not make a specific recommendation regarding how to risk-stratify patients for WE.
Treating Underlying Alcohol Use Disorder
Recommendation 5. Hospitalization for AWS is an important opportunity to engage patients in treatment for alcohol use disorder (AUD), including pharmacotherapy and connection with outpatient providers (recommendation V.12). The guideline emphasizes that treatment for AUD should be initiated concomitantly with AWS management whenever possible but does not make recommendations regarding specific pharmacotherapies.
CRITIQUE
This guideline was authored by a committee of emergency medicine physicians, psychiatrists, and internists using the Department of Veterans Affairs/Department of Defense guidelines and the RAND/UCLA appropriateness method to combine the scientific literature with expert opinion. The result is a series of recommendations for physicians, physician assistants, nurse practitioners, and pharmacists that are not rated by strength; an assessment of the quality of the supporting evidence is available in an appendix. Four of the nine guideline committee members reported significant financial relationships with industry and other entities relevant to these guidelines.
Despite concern about oversedation from phenobarbital raised in small case series,6 observational studies comparing phenobarbital with benzodiazepines suggest phenobarbital has similar efficacy for treating AWS and that oversedation is rare.7-9 Large randomized controlled trials in this area are lacking; however, at least one small randomized controlled trial10 among patients with AWS presenting to emergency departments supports the safety and efficacy of phenobarbital when used in combination with benzodiazepines. Given the growing body of evidence supporting the safety of phenobarbital, we believe a stronger recommendation for use in patients presenting with alcohol withdrawal delirium or treatment-resistant alcohol withdrawal is warranted. The guidelines also suggest that only “experienced clinicians” use phenobarbital for AWS, which may suppress appropriate use. Nationally, phenobarbital use for AWS remains low.11Finally, although the guideline recommends initiation of treatment for AUD, specific recommendations for pharmacotherapy are not provided. Three medications currently have approval from the US Food and Drug Administration for treatment of AUD: acamprosate, naltrexone, and disulfiram. Large randomized controlled trials support the safety and efficacy of acamprosate and naltrexone, with or without counselling, in the treatment of AUD,12 and disulfiram may be appropriate for selected highly motivated patients. We believe more specific recommendations to assist in choosing among these options would be useful.
AREAS IN NEED OF FUTURE STUDY
More data are needed on the safety and efficacy of phenobarbital in patients with AWS, as well as comparative effectiveness against benzodiazepines. Recruitment is ongoing for a single clinical trial comparing the effect of phenobarbital and lorazepam on length of stay among patients in the ICU with AWS (NCT04156464); to date, no randomized trials of phenobarbital have been conducted in medical inpatients with AWS. In addition, gaps in the literature exist regarding benzodiazepine selection, and head-to-head comparisons of symptom-triggered usage of different benzodiazepines are lacking.
Alcohol is the most common substance implicated in hospitalizations for substance use disorders,1 and as a result, hospitalists commonly diagnose and manage alcohol withdrawal syndrome (AWS) in the inpatient medical setting. The 2020 guidelines of the American Society of Addiction Medicine (ASAM) provide updated recommendations for the diagnosis, monitoring, and treatment of patients hospitalized with AWS, which we have condensed to emphasize key changes from the last update2 and clarify ongoing areas of uncertainty.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnosis
Recommendation 1. All inpatients who have used alcohol recently or regularly should be risk-stratified for AWS, regardless of whether or not they have suggestive symptoms (recommendations I.3, I.4, I.5, II.10). The Alcohol Use Disorders Identification Test-(Piccinelli) Consumption (AUDIT-PC) identifies patients at risk for AWS, and the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) identifies those at risk for severe or complicated AWS, which includes seizures and alcohol withdrawal delirium (formerly delirium tremens). The guideline emphasizes use of these tools rather than simply initiating Clinical Institute Withdrawal Assessment for Alcohol, revised (CIWA-Ar) monitoring on all such patients to diagnose AWS, as CIWA-Ar was developed for monitoring response to treatment, not diagnosis (recommendation I.6).
Treating Mild/Moderate or Uncomplicated AWS
Recommendation 2. Because of their proven track record of reducing the incidence of seizure and alcohol withdrawal delirium, benzodiazepines remain the recommended first-line therapy (recommendations V.13, V.16). Symptom-triggered administration of benzodiazepines (via CIWA-Ar) is recommended over fixed-dose administration because the former is associated with shorter length of stay and lower cumulative benzodiazepine administration3,4 (recommendation V.23). Patients with mild AWS who are at low risk for severe or complicated withdrawal should be monitored for up to 36 hours for the development of worsening symptoms (recommendation V.1). For patients with high CIWA-Ar scores or who are at increased risk for severe or complicated AWS, frequent administration of moderate to high doses of a long-acting benzodiazepine early in AWS treatment (a practice called frontloading) is recommended to quickly control symptoms and prevent clinical worsening. This approach has been shown to reduce the incidence of seizures and alcohol withdrawal delirium (recommendations V.14, V.19, V.24).
Carbamazepine or gabapentin may be used in mild or moderate AWS if benzodiazepines are contraindicated; however, neither agent is recommended as first-line therapy because a clear reduction in seizure and withdrawal delirium has not been established (recommendation V.16). Alpha-2 agonists (eg, clonidine, dexmedetomidine) may be used to treat persistent autonomic hyperactivity or anxiety when these are not adequately controlled by benzodiazepines alone (recommendation V.36).
Treating Severe or Complicated AWS
Recommendation 3. The guideline defines severe AWS as withdrawal with severe signs and symptoms, and complicated AWS as withdrawal accompanied by seizures or delirium (Appendix Table5). The development of complications warrants prompt treatment. Patients who experience seizure should receive a fast-acting benzodiazepine (eg, intravenous [IV] diazepam or lorazepam) (recommendation VI.4). Patients with withdrawal delirium should receive a benzodiazepine (preferably parenterally) dosed to achieve light sedation. Clinicians should be prepared for the possibility that large doses may be required and to monitor patients for oversedation and respiratory depression (recommendations VI.13, VI.17). Antipsychotics may be used as adjuncts when withdrawal delirium or other symptoms, such as hallucinosis, are not adequately controlled by benzodiazepines alone, but should not be used as monotherapy (recommendation VI.20). The guideline emphasizes that alpha-2 agonists should not be used to treat withdrawal delirium (recommendation VI.21), but they may be used as adjuncts for resistant alcohol withdrawal in the intensive care unit (ICU) (recommendations VI.27, VI.29). Phenobarbital is an acceptable alternative to benzodiazepines for severe withdrawal (recommendation V.17); however, the guideline recommends that clinicians should be experienced in its use.
Treating Wernicke Encephalopathy
Recommendation 4. Thiamine should be administered to prevent Wernicke encephalopathy (WE), with parenteral formulations recommended in patients with malnutrition, severe/complicated withdrawal, or requiring ICU-level care (recommendations V.7, V.8). In particular, all patients admitted to an ICU for AWS should receive thiamine, as diagnosis of WE is often difficult in this population. Although there is no consensus on the required dose of thiamine to treat WE, 100 mg IV or intramuscularly (IM) daily for 3 to 5 days is commonly administered (recommendation V.7). Because of a lack of evidence of harm, thiamine may be given before, after, or concurrently with glucose or dextrose (recommendation V.7). The guideline does not make a specific recommendation regarding how to risk-stratify patients for WE.
Treating Underlying Alcohol Use Disorder
Recommendation 5. Hospitalization for AWS is an important opportunity to engage patients in treatment for alcohol use disorder (AUD), including pharmacotherapy and connection with outpatient providers (recommendation V.12). The guideline emphasizes that treatment for AUD should be initiated concomitantly with AWS management whenever possible but does not make recommendations regarding specific pharmacotherapies.
CRITIQUE
This guideline was authored by a committee of emergency medicine physicians, psychiatrists, and internists using the Department of Veterans Affairs/Department of Defense guidelines and the RAND/UCLA appropriateness method to combine the scientific literature with expert opinion. The result is a series of recommendations for physicians, physician assistants, nurse practitioners, and pharmacists that are not rated by strength; an assessment of the quality of the supporting evidence is available in an appendix. Four of the nine guideline committee members reported significant financial relationships with industry and other entities relevant to these guidelines.
Despite concern about oversedation from phenobarbital raised in small case series,6 observational studies comparing phenobarbital with benzodiazepines suggest phenobarbital has similar efficacy for treating AWS and that oversedation is rare.7-9 Large randomized controlled trials in this area are lacking; however, at least one small randomized controlled trial10 among patients with AWS presenting to emergency departments supports the safety and efficacy of phenobarbital when used in combination with benzodiazepines. Given the growing body of evidence supporting the safety of phenobarbital, we believe a stronger recommendation for use in patients presenting with alcohol withdrawal delirium or treatment-resistant alcohol withdrawal is warranted. The guidelines also suggest that only “experienced clinicians” use phenobarbital for AWS, which may suppress appropriate use. Nationally, phenobarbital use for AWS remains low.11Finally, although the guideline recommends initiation of treatment for AUD, specific recommendations for pharmacotherapy are not provided. Three medications currently have approval from the US Food and Drug Administration for treatment of AUD: acamprosate, naltrexone, and disulfiram. Large randomized controlled trials support the safety and efficacy of acamprosate and naltrexone, with or without counselling, in the treatment of AUD,12 and disulfiram may be appropriate for selected highly motivated patients. We believe more specific recommendations to assist in choosing among these options would be useful.
AREAS IN NEED OF FUTURE STUDY
More data are needed on the safety and efficacy of phenobarbital in patients with AWS, as well as comparative effectiveness against benzodiazepines. Recruitment is ongoing for a single clinical trial comparing the effect of phenobarbital and lorazepam on length of stay among patients in the ICU with AWS (NCT04156464); to date, no randomized trials of phenobarbital have been conducted in medical inpatients with AWS. In addition, gaps in the literature exist regarding benzodiazepine selection, and head-to-head comparisons of symptom-triggered usage of different benzodiazepines are lacking.
1. Heslin KC, Elixhauser A, Steiner CA. Hospitalizations involving mental and substance use disorders among adults, 2012. HCUP Statistical Brief #191. June 2015. Accessed November 17, 2021. www.hcup-us.ahrq.gov/reports/statbriefs/sb191-Hospitalization-Mental-Substance-Use-Disorders-2012.pdf
2. Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405-1412. https://doi.org/10.1001/archinte.164.13.1405
3. Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519-523.
4. Daeppen J-B, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. https://doi.org/10.1001/archinte.162.10.1117
5. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. J Addict Med. 2020;14(3S suppl):1-72. https://doi.org/10.1097/ADM.0000000000000668
6. Oks M, Cleven KL, Healy L, et al. The safety and utility of phenobarbital use for the treatment of severe alcohol withdrawal syndrome in the medical intensive care unit. J Intensive Care Med. 2020;35(9):844-850. https://doi.org/10.1177/0885066618783947
7. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357. https://doi.org/10.1111/j.1360-0443.1989.tb00737.x
8. Ibarra Jr F. Single dose phenobarbital in addition to symptom-triggered lorazepam in alcohol withdrawal. Am J Emerg Med. 2020;38(2):178-181. https://doi.org/10.1016/j.ajem.2019.01.053
9. Nisavic M, Nejad SH, Isenberg BM, et al. Use of phenobarbital in alcohol withdrawal management–a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics. 2019;60(5):458-467. https://doi.org/10.1016/j.psym.2019.02.002
10. Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med. 2013;44(3):592-598.e2. https://doi.org/10.1016/j.jemermed.2012.07.056
11. Gupta N, Emerman CL. Trends in the management of inpatients with alcohol withdrawal syndrome. Addict Disord Their Treat. 2021;20(1):29-32. https://doi.org/10.1097/ADT.0000000000000203
12. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017. https://doi.org/10.1001/jama.295.17.2003
1. Heslin KC, Elixhauser A, Steiner CA. Hospitalizations involving mental and substance use disorders among adults, 2012. HCUP Statistical Brief #191. June 2015. Accessed November 17, 2021. www.hcup-us.ahrq.gov/reports/statbriefs/sb191-Hospitalization-Mental-Substance-Use-Disorders-2012.pdf
2. Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405-1412. https://doi.org/10.1001/archinte.164.13.1405
3. Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519-523.
4. Daeppen J-B, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. https://doi.org/10.1001/archinte.162.10.1117
5. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. J Addict Med. 2020;14(3S suppl):1-72. https://doi.org/10.1097/ADM.0000000000000668
6. Oks M, Cleven KL, Healy L, et al. The safety and utility of phenobarbital use for the treatment of severe alcohol withdrawal syndrome in the medical intensive care unit. J Intensive Care Med. 2020;35(9):844-850. https://doi.org/10.1177/0885066618783947
7. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357. https://doi.org/10.1111/j.1360-0443.1989.tb00737.x
8. Ibarra Jr F. Single dose phenobarbital in addition to symptom-triggered lorazepam in alcohol withdrawal. Am J Emerg Med. 2020;38(2):178-181. https://doi.org/10.1016/j.ajem.2019.01.053
9. Nisavic M, Nejad SH, Isenberg BM, et al. Use of phenobarbital in alcohol withdrawal management–a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics. 2019;60(5):458-467. https://doi.org/10.1016/j.psym.2019.02.002
10. Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med. 2013;44(3):592-598.e2. https://doi.org/10.1016/j.jemermed.2012.07.056
11. Gupta N, Emerman CL. Trends in the management of inpatients with alcohol withdrawal syndrome. Addict Disord Their Treat. 2021;20(1):29-32. https://doi.org/10.1097/ADT.0000000000000203
12. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017. https://doi.org/10.1001/jama.295.17.2003
© 2021 Society of Hospital Medicine
The VA My Life My Story Project: Keeping Medical Students and Veterans Socially Connected While Physically Distanced
Narrative competence is the ability to acquire, interpret, and act on the stories of others.1 Developing this skill through guided medical storytelling can improve health care practitioners’ (HCPs) sense of empathy and satisfaction with their work.2 Narrative medicine experiences for medical students can foster a deeper understanding of their patients beyond illness-associated identities.3
Within narrative medicine, the “life story” is a specific technique that allows patients to share experiences through open-ended interviews that are entered into the health record.4,5 By sharing life stories, patients control a narrative encompassing more than their illness and can reinforce a sense of purpose in their lives.6 The US Department of Veterans Affairs (VA) My Life My Story (MLMS) program gives veterans the opportunity to share their narrative with staff and volunteer interviewers. MLMS is well received by veterans, has durable positive effects for HCPs who read the stories, and has been used as a tool to teach patient-centered care to medical trainees.7-9
We created a narrative medicine curriculum at the San Francisco VA Medical Center (SFVAMC) in which medical students interviewed veterans for the MLMS program. Medical students initially collected life stories through in-person conversation. During the COVID-19 pandemic, physical distancing regulations limited direct patient interaction for students and prompted a switch to phone and video interviews. This shift paralleled the widespread adoption of telehealth, which will persist beyond the pandemic and require teachers and learners to develop competency in forming personal connections with patients through videoconferencing.10,11
There are no published studies describing how to guide medical students (or other historians) in generating life stories without in-person patient contact. This article details the design of a medical student curriculum incorporating MLMS and the transition to remote interaction between instructors, students, and veterans during the early COVID-19 pandemic.
MLMS Program Origins
The MLMS project began at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin, in 2013 with staff and volunteer interviewers and has expanded to more than 60 VA facilities.7 In January 2020, we initiated a narrative medicine curriculum incorporating MLMS at the SFVAMC as a required component of a third-year internal medicine clerkship for medical students at the University of California San Francisco (UCSF). Fifty-four medical students in 10 cohorts participated in the curriculum in 2020. The primary program objectives were for medical students to develop skills for eliciting and recording a life story and to appreciate the impact of this activity on a veteran’s experience of receiving health care. Secondary objectives were for students to understand the mission of the VA health care system and veteran demographics.
The first cohort of 6 UCSF medical students participated in MLMS during their 8-week VA clerkship. Students attended a 1-hour small group session to introduce the program and build narrative medicine skills. Preparation for this session involved listening to 2 podcast episodes introducing the VA health care system and MLMS.12,13 The session began with a short interactive discussion of veteran demographics with an emphasis on addressing assumptions students might have about the veteran population. Students were taught strategies for engaging in open-ended conversations without emphasizing illness. Each student practiced collecting a life story with a simulated patient portrayed by an instructor and received feedback from classmates and instructors.
Over the following weeks, students selected a hospitalized veteran, typically a patient they were caring for, introduced MLMS, and obtained verbal consent to participate. They conducted a 60- to 90-minute interview, wrote and organized the life story, read it to the veteran, and solicited edits. Once a final version was generated, the student provided the veteran with printed copies and offered to place the story in the Computerized Patient Record System (CPRS).
Near the end of their rotation, students attended a 1-hour small group session in which they shared reflections on the experience of collecting a life story, the impact of veterans’ life experiences on their health and illness, and moments when students confronted their own stereotypes and implicit biases. Students then reviewed narrative medicine skills that are generalizable to all patient interactions.
COVID-19-Related Adaptation
In March 2020, shortly after the second student cohort began, medical students were removed from the clinical setting in response to the COVID-19 pandemic. The 8-week clerkship was converted to a 3-week remote learning rotation. The MLMS experience was preserved by converting small group sessions to videoconferences and expanding the pool of eligible patients to include veterans who students had met on prior rotations, current inpatients, and outpatients from VA primary care clinics. Students contacted veterans after an instructor had introduced MLMS to the veteran and confirmed that the veteran was interested in participating.
Students in the second and third cohorts completed a telephone-based iteration of MLMS in which interviews and life story reviews were conducted over the telephone and printed copies mailed to the veteran. For the fourth, fifth, and sixth cohorts, MLMS was transitioned to a video-based program with inpatients. Instructors collaborated with a volunteer group supplying tablet devices to inpatients to make video calls to their families during the pandemic.14 Clerkship students coordinated with that volunteer group to interview veterans and review their stories through the tablet devices.
From July to December 2020 medical students returned to 4-week on-site clinical rotations at the SFVAMC. The program returned to the original format for cohorts 7 to 10, with students attending in-person small group sessions and conducting in-person interviews with inpatients.
Curriculum Evaluation
Students completed surveys in the week after the curriculum concluded. Survey completion was voluntary, anonymous, and had no bearing on their evaluation or grade (pass/fail only). Likert scale questions (1, strongly disagree; 5, strongly agree) were used to assess the program (eAppendix 1). One-way analysis of variance testing was used to compare means stratified by method of interview (in person, telephone, or video). Surveys also included free-response questions asking students to highlight aspects of the program they valued or would change; responses were summarized by theme. This program evaluation was deemed exempt from review by the UCSF Human Research Protection Program Institutional Review Board.
Sixty-two veteran stories were collected by 54 participating students (one student was unable to complete an interview, while several students completed multiple interviews). Fifty-four (87%) veterans requested their stories be entered into the medical record.
All 54 students completed the survey. Students reported that the MLMS curriculum helped them develop new skills for eliciting and recording a life story (mean [SD] 4.5 [0.7]). Most students strongly agreed that MLMS helped them understand how sharing a life story can impact a veteran’s experience of receiving health care, with a mean (SD) score of 4.8 (0.4). After completing MLMS, students also reported a better understanding of the mission of the VA and veteran demographics with a mean (SD) score of 4.4 (0.7) and 4.3 (0.7), respectively. Stratification of survey responses by method of interview (in person, telephone, or video) revealed no statistically significant differences in evaluations (Table 1).
Fifty-two (96%) students provided responses to free-response survey questions. Students reported that they valued shifting the focus of an interview from medical history to rapport-building and patient engagement, having protected time to focus on the humanistic aspect of doctoring, and redefining healing as a process that occurs in the greater context of a patient’s life. One student reported, “We talk so much about seeing the person instead of the disease, but this is the first time that I really felt like I had the opportunity to wholeheartedly commit myself to that. It was an incredible opportunity and something I wish all medical trainees would have the chance to do.” Another student, after participating in the video version of the project, reported, “I found so much comfort in the time that I just sat and listened to another person’s story firsthand. Not only did this opportunity remind me of why I wanted to work in medicine, but also why I wanted to work with and for other people.” Thirty-three (61%) students provided constructive feedback in response to a free-response question soliciting suggestions for improvement, which guided iterative programmatic changes. For example, 3 students who completed the telephone iteration of MLMS felt that patient engagement suffered due to the lack of nonverbal cues and body language that can enhance the bond between storyteller and interviewer. This prompted a switch to video interviews beginning with the fourth cohort.
The second small group session provided space for students to reflect on their experience. During this session, students frequently referenced the unique connections they developed with veterans. Several students described feeling refreshed by these connections and that MLMS helped them recall their original commitment to become physicians. Students also discovered that the events veterans included in their stories often echoed current societal issues. For example, as social unrest and protests related to racial injustice occurred in the summer of 2020, veterans’ life stories more frequently incorporated examples of prejudice or inequities in the justice system. As the use of force by police moved to the forefront of political discourse, life stories more often included veterans’ experiences working as military and nonmilitary law enforcement. In identifying these common themes, students reported a greater appreciation of the impact of society on patients’ overall health and well-being.
Stories were recorded as CPRS notes titled “My Story,” and completion of a note generated a “My Story” alert on the CPRS landing page at the SFVAMC (eAppendix 2). Physicians and nurses who have discovered the notes reported that patient care has been enhanced by the contextualization provided by a life story. HCPs now frequently contact MLMS instructors inquiring whether students are available to collect life stories for their patients. One physician wrote, “I learned so much from what you documented—much more than I could appreciate in my clinic visits with him. His voice comes shining through. Thank you for highlighting the humanism of medicine in the medical record.” Another physician noted, “The story captured his voice so well. I reread it over the weekend after I got the news that he died, and it helped me celebrate his life. Please tell your students how much their work means to patients, families, and the providers who care for them.”
Discussion
Previous research has demonstrated that a narrative medicine curriculum can help medicine clerkship students develop narrative competence through patient storytelling with a focus on a patient’s illness narrative.15 The VA MLMS program extends the patient narrative beyond health care–related experiences and encompasses their broader life story. This article adds to the MLMS and narrative medicine literature by demonstrating that the efficacy of teaching patient-centered care to medical trainees through direct interviews can be maintained in remote formats.9 The article also provides guidance for MLMS programs that wish to conduct remote veteran interviews.
The widespread adoption of telemedicine will require trainees to develop communication skills to establish therapeutic relationships with patients both face-to-face and through videoconferencing. In order to promote this important skill across varying levels of physical distancing, narrative medicine programs should be adaptable to a virtual learning environment. As we redesigned MLMS for the remote setting, we learned several key lessons that can guide similar curricular and programmatic innovations at other institutions. For example, videoconferencing created stronger connections between the students and veterans than telephone calls. However, tablet-based video interviews also introduced many technological challenges and required on-site personnel (nurses and volunteers) to connect students, veterans, and technology. Solutions for technology and communication challenges related to the basic personnel and infrastructure needed to start and maintain a remote MLMS program are outlined in Table 2.
We are now using this experience to guide the expansion of life story curricula to other affiliated clerkship sites and other medical student rotations. We also are expanding the interviewer pool beyond medical students to VA staff and volunteers, some of whom may be restricted from direct patient contact in the future but who could participate through the remote protocols that we developed.
Limitations
Limitations of this study include the participation of trainees from a single institution and a lack of assessment of the impact of MLMS on veterans. Future research could assess whether life story skills and practices are maintained after the medicine clerkship. In addition, future studies could examine veterans’ perspectives through interviews with qualitative analysis to learn how MLMS affected their experience of receiving health care.
Conclusions
This is the first report of a remote-capable life story curriculum for medical students. Shifting to a virtual MLMS curriculum requires protocols and people to link interviewers, veterans, and technology. Training for in-person interactions while being prepared for remote interviewing is essential to ensure that the MLMS experience remains available to interviewers and veterans who otherwise may never have the chance to connect. The restrictions and isolation of the COVID-19 pandemic will fade, but using MLMS to virtually connect patients, providers, and students will remain an important capability and opportunity as health care shifts to more virtual interaction.
Acknowledgments
The authors thank Emma Levine, MD, for her assistance coordinating video interviews; Thor Ringler, MS, MFA, for his assistance with manuscript review; and the veterans of the San Francisco VA Health Care System for sharing their stories.
1. Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
2. Milota MM, van Thiel GJMW, van Delden JJM. Narrative medicine as a medical education tool: a systematic review. Med Teach. 2019;41(7):802-810. doi:10.1080/0142159X.2019.1584274
3. Garrison D, Lyness JM, Frank JB, Epstein RM. Qualitative analysis of medical student impressions of a narrative exercise in the third-year psychiatry clerkship. Acad Med. 2011;86(1):85-89. doi:10.1097/ACM.0b013e3181ff7a63
4. Divinsky M. Stories for life: introduction to narrative medicine. Can Fam Physician. 2007;53(2):203-211.
5. McAdams DP, McLean KC. Narrative identity. Curr Dir Psychol Sci. 2013;22(3):233-238. doi:10.1177 /0963721413475622
6. Fitchett G, Emanuel L, Handzo G, Boyken L, Wilkie DJ. Care of the human spirit and the role of dignity therapy: a systematic review of dignity therapy research. BMC Palliat Care. 2015;14:8. Published 2015 Mar 21. doi:10.1186/s12904-015-0007-1
7. Ringler T, Ahearn EP, Wise M, Lee ER, Krahn D. Using life stories to connect veterans and providers. Fed Pract. 2015;32(6):8-14.
8. Roberts TJ, Ringler T, Krahn D, Ahearn E. The My Life, My Story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
9. Nathan S, Fiore LL, Saunders S, et al. My Life, My Story: Teaching patient centered care competencies for older adults through life story work [published online ahead of print, 2019 Sep 9] [published correction appears in Gerontol Geriatr Educ. 2019 Oct 15;:1]. Gerontol Geriatr Educ. 2019;1-14. doi:10.1080/02701960.2019.1665038
10. Dorsey ER, Topol EJ. Telemedicine 2020 and the next decade. Lancet. 2020;395(10227):859. doi:10.1016/S0140-6736(20)30424-4
11. Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020 [published correction appears in MMWR Morb Mortal Wkly Rep. 2020 Nov 13;69(45):1711]. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595-1599. Published 2020 Oct 30. doi:10.15585/mmwr.mm6943a3
12. Caputo LV. Across the Street. The VA philosophy: with Dr. Goldberg. July 14, 2019. Accessed November 5, 2021. https://soundcloud.com/user-911014559/the-va-philosophy-with-dr-goldberg-1
13. Sable-Smith B. Storytelling helps hospital staff discover the person within the patient. NPR. Published June 8, 2019. Accessed November 5, 2021. https://www.npr.org/sections/health-shots/2019/06/08/729351842/storytelling-helps-hospital-staff-discover-the-person-within-the-patient
14. Ganeshan S, Hsiang E, Peng T, et al. Enabling patient communication for hospitalised patients during and beyond the COVID-19 pandemic. BMJ Innov. 2021;7(2):316-320. doi:10.1136/bmjinnov-2020-000636
15. Chretien KC, Swenson R, Yoon B, et al. Tell me your story: a pilot narrative medicine curriculum during the medicine clerkship. J Gen Intern Med. 2015;30(7):1025-1028. doi:10.1007/s11606-015-3211-z
Narrative competence is the ability to acquire, interpret, and act on the stories of others.1 Developing this skill through guided medical storytelling can improve health care practitioners’ (HCPs) sense of empathy and satisfaction with their work.2 Narrative medicine experiences for medical students can foster a deeper understanding of their patients beyond illness-associated identities.3
Within narrative medicine, the “life story” is a specific technique that allows patients to share experiences through open-ended interviews that are entered into the health record.4,5 By sharing life stories, patients control a narrative encompassing more than their illness and can reinforce a sense of purpose in their lives.6 The US Department of Veterans Affairs (VA) My Life My Story (MLMS) program gives veterans the opportunity to share their narrative with staff and volunteer interviewers. MLMS is well received by veterans, has durable positive effects for HCPs who read the stories, and has been used as a tool to teach patient-centered care to medical trainees.7-9
We created a narrative medicine curriculum at the San Francisco VA Medical Center (SFVAMC) in which medical students interviewed veterans for the MLMS program. Medical students initially collected life stories through in-person conversation. During the COVID-19 pandemic, physical distancing regulations limited direct patient interaction for students and prompted a switch to phone and video interviews. This shift paralleled the widespread adoption of telehealth, which will persist beyond the pandemic and require teachers and learners to develop competency in forming personal connections with patients through videoconferencing.10,11
There are no published studies describing how to guide medical students (or other historians) in generating life stories without in-person patient contact. This article details the design of a medical student curriculum incorporating MLMS and the transition to remote interaction between instructors, students, and veterans during the early COVID-19 pandemic.
MLMS Program Origins
The MLMS project began at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin, in 2013 with staff and volunteer interviewers and has expanded to more than 60 VA facilities.7 In January 2020, we initiated a narrative medicine curriculum incorporating MLMS at the SFVAMC as a required component of a third-year internal medicine clerkship for medical students at the University of California San Francisco (UCSF). Fifty-four medical students in 10 cohorts participated in the curriculum in 2020. The primary program objectives were for medical students to develop skills for eliciting and recording a life story and to appreciate the impact of this activity on a veteran’s experience of receiving health care. Secondary objectives were for students to understand the mission of the VA health care system and veteran demographics.
The first cohort of 6 UCSF medical students participated in MLMS during their 8-week VA clerkship. Students attended a 1-hour small group session to introduce the program and build narrative medicine skills. Preparation for this session involved listening to 2 podcast episodes introducing the VA health care system and MLMS.12,13 The session began with a short interactive discussion of veteran demographics with an emphasis on addressing assumptions students might have about the veteran population. Students were taught strategies for engaging in open-ended conversations without emphasizing illness. Each student practiced collecting a life story with a simulated patient portrayed by an instructor and received feedback from classmates and instructors.
Over the following weeks, students selected a hospitalized veteran, typically a patient they were caring for, introduced MLMS, and obtained verbal consent to participate. They conducted a 60- to 90-minute interview, wrote and organized the life story, read it to the veteran, and solicited edits. Once a final version was generated, the student provided the veteran with printed copies and offered to place the story in the Computerized Patient Record System (CPRS).
Near the end of their rotation, students attended a 1-hour small group session in which they shared reflections on the experience of collecting a life story, the impact of veterans’ life experiences on their health and illness, and moments when students confronted their own stereotypes and implicit biases. Students then reviewed narrative medicine skills that are generalizable to all patient interactions.
COVID-19-Related Adaptation
In March 2020, shortly after the second student cohort began, medical students were removed from the clinical setting in response to the COVID-19 pandemic. The 8-week clerkship was converted to a 3-week remote learning rotation. The MLMS experience was preserved by converting small group sessions to videoconferences and expanding the pool of eligible patients to include veterans who students had met on prior rotations, current inpatients, and outpatients from VA primary care clinics. Students contacted veterans after an instructor had introduced MLMS to the veteran and confirmed that the veteran was interested in participating.
Students in the second and third cohorts completed a telephone-based iteration of MLMS in which interviews and life story reviews were conducted over the telephone and printed copies mailed to the veteran. For the fourth, fifth, and sixth cohorts, MLMS was transitioned to a video-based program with inpatients. Instructors collaborated with a volunteer group supplying tablet devices to inpatients to make video calls to their families during the pandemic.14 Clerkship students coordinated with that volunteer group to interview veterans and review their stories through the tablet devices.
From July to December 2020 medical students returned to 4-week on-site clinical rotations at the SFVAMC. The program returned to the original format for cohorts 7 to 10, with students attending in-person small group sessions and conducting in-person interviews with inpatients.
Curriculum Evaluation
Students completed surveys in the week after the curriculum concluded. Survey completion was voluntary, anonymous, and had no bearing on their evaluation or grade (pass/fail only). Likert scale questions (1, strongly disagree; 5, strongly agree) were used to assess the program (eAppendix 1). One-way analysis of variance testing was used to compare means stratified by method of interview (in person, telephone, or video). Surveys also included free-response questions asking students to highlight aspects of the program they valued or would change; responses were summarized by theme. This program evaluation was deemed exempt from review by the UCSF Human Research Protection Program Institutional Review Board.
Sixty-two veteran stories were collected by 54 participating students (one student was unable to complete an interview, while several students completed multiple interviews). Fifty-four (87%) veterans requested their stories be entered into the medical record.
All 54 students completed the survey. Students reported that the MLMS curriculum helped them develop new skills for eliciting and recording a life story (mean [SD] 4.5 [0.7]). Most students strongly agreed that MLMS helped them understand how sharing a life story can impact a veteran’s experience of receiving health care, with a mean (SD) score of 4.8 (0.4). After completing MLMS, students also reported a better understanding of the mission of the VA and veteran demographics with a mean (SD) score of 4.4 (0.7) and 4.3 (0.7), respectively. Stratification of survey responses by method of interview (in person, telephone, or video) revealed no statistically significant differences in evaluations (Table 1).
Fifty-two (96%) students provided responses to free-response survey questions. Students reported that they valued shifting the focus of an interview from medical history to rapport-building and patient engagement, having protected time to focus on the humanistic aspect of doctoring, and redefining healing as a process that occurs in the greater context of a patient’s life. One student reported, “We talk so much about seeing the person instead of the disease, but this is the first time that I really felt like I had the opportunity to wholeheartedly commit myself to that. It was an incredible opportunity and something I wish all medical trainees would have the chance to do.” Another student, after participating in the video version of the project, reported, “I found so much comfort in the time that I just sat and listened to another person’s story firsthand. Not only did this opportunity remind me of why I wanted to work in medicine, but also why I wanted to work with and for other people.” Thirty-three (61%) students provided constructive feedback in response to a free-response question soliciting suggestions for improvement, which guided iterative programmatic changes. For example, 3 students who completed the telephone iteration of MLMS felt that patient engagement suffered due to the lack of nonverbal cues and body language that can enhance the bond between storyteller and interviewer. This prompted a switch to video interviews beginning with the fourth cohort.
The second small group session provided space for students to reflect on their experience. During this session, students frequently referenced the unique connections they developed with veterans. Several students described feeling refreshed by these connections and that MLMS helped them recall their original commitment to become physicians. Students also discovered that the events veterans included in their stories often echoed current societal issues. For example, as social unrest and protests related to racial injustice occurred in the summer of 2020, veterans’ life stories more frequently incorporated examples of prejudice or inequities in the justice system. As the use of force by police moved to the forefront of political discourse, life stories more often included veterans’ experiences working as military and nonmilitary law enforcement. In identifying these common themes, students reported a greater appreciation of the impact of society on patients’ overall health and well-being.
Stories were recorded as CPRS notes titled “My Story,” and completion of a note generated a “My Story” alert on the CPRS landing page at the SFVAMC (eAppendix 2). Physicians and nurses who have discovered the notes reported that patient care has been enhanced by the contextualization provided by a life story. HCPs now frequently contact MLMS instructors inquiring whether students are available to collect life stories for their patients. One physician wrote, “I learned so much from what you documented—much more than I could appreciate in my clinic visits with him. His voice comes shining through. Thank you for highlighting the humanism of medicine in the medical record.” Another physician noted, “The story captured his voice so well. I reread it over the weekend after I got the news that he died, and it helped me celebrate his life. Please tell your students how much their work means to patients, families, and the providers who care for them.”
Discussion
Previous research has demonstrated that a narrative medicine curriculum can help medicine clerkship students develop narrative competence through patient storytelling with a focus on a patient’s illness narrative.15 The VA MLMS program extends the patient narrative beyond health care–related experiences and encompasses their broader life story. This article adds to the MLMS and narrative medicine literature by demonstrating that the efficacy of teaching patient-centered care to medical trainees through direct interviews can be maintained in remote formats.9 The article also provides guidance for MLMS programs that wish to conduct remote veteran interviews.
The widespread adoption of telemedicine will require trainees to develop communication skills to establish therapeutic relationships with patients both face-to-face and through videoconferencing. In order to promote this important skill across varying levels of physical distancing, narrative medicine programs should be adaptable to a virtual learning environment. As we redesigned MLMS for the remote setting, we learned several key lessons that can guide similar curricular and programmatic innovations at other institutions. For example, videoconferencing created stronger connections between the students and veterans than telephone calls. However, tablet-based video interviews also introduced many technological challenges and required on-site personnel (nurses and volunteers) to connect students, veterans, and technology. Solutions for technology and communication challenges related to the basic personnel and infrastructure needed to start and maintain a remote MLMS program are outlined in Table 2.
We are now using this experience to guide the expansion of life story curricula to other affiliated clerkship sites and other medical student rotations. We also are expanding the interviewer pool beyond medical students to VA staff and volunteers, some of whom may be restricted from direct patient contact in the future but who could participate through the remote protocols that we developed.
Limitations
Limitations of this study include the participation of trainees from a single institution and a lack of assessment of the impact of MLMS on veterans. Future research could assess whether life story skills and practices are maintained after the medicine clerkship. In addition, future studies could examine veterans’ perspectives through interviews with qualitative analysis to learn how MLMS affected their experience of receiving health care.
Conclusions
This is the first report of a remote-capable life story curriculum for medical students. Shifting to a virtual MLMS curriculum requires protocols and people to link interviewers, veterans, and technology. Training for in-person interactions while being prepared for remote interviewing is essential to ensure that the MLMS experience remains available to interviewers and veterans who otherwise may never have the chance to connect. The restrictions and isolation of the COVID-19 pandemic will fade, but using MLMS to virtually connect patients, providers, and students will remain an important capability and opportunity as health care shifts to more virtual interaction.
Acknowledgments
The authors thank Emma Levine, MD, for her assistance coordinating video interviews; Thor Ringler, MS, MFA, for his assistance with manuscript review; and the veterans of the San Francisco VA Health Care System for sharing their stories.
Narrative competence is the ability to acquire, interpret, and act on the stories of others.1 Developing this skill through guided medical storytelling can improve health care practitioners’ (HCPs) sense of empathy and satisfaction with their work.2 Narrative medicine experiences for medical students can foster a deeper understanding of their patients beyond illness-associated identities.3
Within narrative medicine, the “life story” is a specific technique that allows patients to share experiences through open-ended interviews that are entered into the health record.4,5 By sharing life stories, patients control a narrative encompassing more than their illness and can reinforce a sense of purpose in their lives.6 The US Department of Veterans Affairs (VA) My Life My Story (MLMS) program gives veterans the opportunity to share their narrative with staff and volunteer interviewers. MLMS is well received by veterans, has durable positive effects for HCPs who read the stories, and has been used as a tool to teach patient-centered care to medical trainees.7-9
We created a narrative medicine curriculum at the San Francisco VA Medical Center (SFVAMC) in which medical students interviewed veterans for the MLMS program. Medical students initially collected life stories through in-person conversation. During the COVID-19 pandemic, physical distancing regulations limited direct patient interaction for students and prompted a switch to phone and video interviews. This shift paralleled the widespread adoption of telehealth, which will persist beyond the pandemic and require teachers and learners to develop competency in forming personal connections with patients through videoconferencing.10,11
There are no published studies describing how to guide medical students (or other historians) in generating life stories without in-person patient contact. This article details the design of a medical student curriculum incorporating MLMS and the transition to remote interaction between instructors, students, and veterans during the early COVID-19 pandemic.
MLMS Program Origins
The MLMS project began at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin, in 2013 with staff and volunteer interviewers and has expanded to more than 60 VA facilities.7 In January 2020, we initiated a narrative medicine curriculum incorporating MLMS at the SFVAMC as a required component of a third-year internal medicine clerkship for medical students at the University of California San Francisco (UCSF). Fifty-four medical students in 10 cohorts participated in the curriculum in 2020. The primary program objectives were for medical students to develop skills for eliciting and recording a life story and to appreciate the impact of this activity on a veteran’s experience of receiving health care. Secondary objectives were for students to understand the mission of the VA health care system and veteran demographics.
The first cohort of 6 UCSF medical students participated in MLMS during their 8-week VA clerkship. Students attended a 1-hour small group session to introduce the program and build narrative medicine skills. Preparation for this session involved listening to 2 podcast episodes introducing the VA health care system and MLMS.12,13 The session began with a short interactive discussion of veteran demographics with an emphasis on addressing assumptions students might have about the veteran population. Students were taught strategies for engaging in open-ended conversations without emphasizing illness. Each student practiced collecting a life story with a simulated patient portrayed by an instructor and received feedback from classmates and instructors.
Over the following weeks, students selected a hospitalized veteran, typically a patient they were caring for, introduced MLMS, and obtained verbal consent to participate. They conducted a 60- to 90-minute interview, wrote and organized the life story, read it to the veteran, and solicited edits. Once a final version was generated, the student provided the veteran with printed copies and offered to place the story in the Computerized Patient Record System (CPRS).
Near the end of their rotation, students attended a 1-hour small group session in which they shared reflections on the experience of collecting a life story, the impact of veterans’ life experiences on their health and illness, and moments when students confronted their own stereotypes and implicit biases. Students then reviewed narrative medicine skills that are generalizable to all patient interactions.
COVID-19-Related Adaptation
In March 2020, shortly after the second student cohort began, medical students were removed from the clinical setting in response to the COVID-19 pandemic. The 8-week clerkship was converted to a 3-week remote learning rotation. The MLMS experience was preserved by converting small group sessions to videoconferences and expanding the pool of eligible patients to include veterans who students had met on prior rotations, current inpatients, and outpatients from VA primary care clinics. Students contacted veterans after an instructor had introduced MLMS to the veteran and confirmed that the veteran was interested in participating.
Students in the second and third cohorts completed a telephone-based iteration of MLMS in which interviews and life story reviews were conducted over the telephone and printed copies mailed to the veteran. For the fourth, fifth, and sixth cohorts, MLMS was transitioned to a video-based program with inpatients. Instructors collaborated with a volunteer group supplying tablet devices to inpatients to make video calls to their families during the pandemic.14 Clerkship students coordinated with that volunteer group to interview veterans and review their stories through the tablet devices.
From July to December 2020 medical students returned to 4-week on-site clinical rotations at the SFVAMC. The program returned to the original format for cohorts 7 to 10, with students attending in-person small group sessions and conducting in-person interviews with inpatients.
Curriculum Evaluation
Students completed surveys in the week after the curriculum concluded. Survey completion was voluntary, anonymous, and had no bearing on their evaluation or grade (pass/fail only). Likert scale questions (1, strongly disagree; 5, strongly agree) were used to assess the program (eAppendix 1). One-way analysis of variance testing was used to compare means stratified by method of interview (in person, telephone, or video). Surveys also included free-response questions asking students to highlight aspects of the program they valued or would change; responses were summarized by theme. This program evaluation was deemed exempt from review by the UCSF Human Research Protection Program Institutional Review Board.
Sixty-two veteran stories were collected by 54 participating students (one student was unable to complete an interview, while several students completed multiple interviews). Fifty-four (87%) veterans requested their stories be entered into the medical record.
All 54 students completed the survey. Students reported that the MLMS curriculum helped them develop new skills for eliciting and recording a life story (mean [SD] 4.5 [0.7]). Most students strongly agreed that MLMS helped them understand how sharing a life story can impact a veteran’s experience of receiving health care, with a mean (SD) score of 4.8 (0.4). After completing MLMS, students also reported a better understanding of the mission of the VA and veteran demographics with a mean (SD) score of 4.4 (0.7) and 4.3 (0.7), respectively. Stratification of survey responses by method of interview (in person, telephone, or video) revealed no statistically significant differences in evaluations (Table 1).
Fifty-two (96%) students provided responses to free-response survey questions. Students reported that they valued shifting the focus of an interview from medical history to rapport-building and patient engagement, having protected time to focus on the humanistic aspect of doctoring, and redefining healing as a process that occurs in the greater context of a patient’s life. One student reported, “We talk so much about seeing the person instead of the disease, but this is the first time that I really felt like I had the opportunity to wholeheartedly commit myself to that. It was an incredible opportunity and something I wish all medical trainees would have the chance to do.” Another student, after participating in the video version of the project, reported, “I found so much comfort in the time that I just sat and listened to another person’s story firsthand. Not only did this opportunity remind me of why I wanted to work in medicine, but also why I wanted to work with and for other people.” Thirty-three (61%) students provided constructive feedback in response to a free-response question soliciting suggestions for improvement, which guided iterative programmatic changes. For example, 3 students who completed the telephone iteration of MLMS felt that patient engagement suffered due to the lack of nonverbal cues and body language that can enhance the bond between storyteller and interviewer. This prompted a switch to video interviews beginning with the fourth cohort.
The second small group session provided space for students to reflect on their experience. During this session, students frequently referenced the unique connections they developed with veterans. Several students described feeling refreshed by these connections and that MLMS helped them recall their original commitment to become physicians. Students also discovered that the events veterans included in their stories often echoed current societal issues. For example, as social unrest and protests related to racial injustice occurred in the summer of 2020, veterans’ life stories more frequently incorporated examples of prejudice or inequities in the justice system. As the use of force by police moved to the forefront of political discourse, life stories more often included veterans’ experiences working as military and nonmilitary law enforcement. In identifying these common themes, students reported a greater appreciation of the impact of society on patients’ overall health and well-being.
Stories were recorded as CPRS notes titled “My Story,” and completion of a note generated a “My Story” alert on the CPRS landing page at the SFVAMC (eAppendix 2). Physicians and nurses who have discovered the notes reported that patient care has been enhanced by the contextualization provided by a life story. HCPs now frequently contact MLMS instructors inquiring whether students are available to collect life stories for their patients. One physician wrote, “I learned so much from what you documented—much more than I could appreciate in my clinic visits with him. His voice comes shining through. Thank you for highlighting the humanism of medicine in the medical record.” Another physician noted, “The story captured his voice so well. I reread it over the weekend after I got the news that he died, and it helped me celebrate his life. Please tell your students how much their work means to patients, families, and the providers who care for them.”
Discussion
Previous research has demonstrated that a narrative medicine curriculum can help medicine clerkship students develop narrative competence through patient storytelling with a focus on a patient’s illness narrative.15 The VA MLMS program extends the patient narrative beyond health care–related experiences and encompasses their broader life story. This article adds to the MLMS and narrative medicine literature by demonstrating that the efficacy of teaching patient-centered care to medical trainees through direct interviews can be maintained in remote formats.9 The article also provides guidance for MLMS programs that wish to conduct remote veteran interviews.
The widespread adoption of telemedicine will require trainees to develop communication skills to establish therapeutic relationships with patients both face-to-face and through videoconferencing. In order to promote this important skill across varying levels of physical distancing, narrative medicine programs should be adaptable to a virtual learning environment. As we redesigned MLMS for the remote setting, we learned several key lessons that can guide similar curricular and programmatic innovations at other institutions. For example, videoconferencing created stronger connections between the students and veterans than telephone calls. However, tablet-based video interviews also introduced many technological challenges and required on-site personnel (nurses and volunteers) to connect students, veterans, and technology. Solutions for technology and communication challenges related to the basic personnel and infrastructure needed to start and maintain a remote MLMS program are outlined in Table 2.
We are now using this experience to guide the expansion of life story curricula to other affiliated clerkship sites and other medical student rotations. We also are expanding the interviewer pool beyond medical students to VA staff and volunteers, some of whom may be restricted from direct patient contact in the future but who could participate through the remote protocols that we developed.
Limitations
Limitations of this study include the participation of trainees from a single institution and a lack of assessment of the impact of MLMS on veterans. Future research could assess whether life story skills and practices are maintained after the medicine clerkship. In addition, future studies could examine veterans’ perspectives through interviews with qualitative analysis to learn how MLMS affected their experience of receiving health care.
Conclusions
This is the first report of a remote-capable life story curriculum for medical students. Shifting to a virtual MLMS curriculum requires protocols and people to link interviewers, veterans, and technology. Training for in-person interactions while being prepared for remote interviewing is essential to ensure that the MLMS experience remains available to interviewers and veterans who otherwise may never have the chance to connect. The restrictions and isolation of the COVID-19 pandemic will fade, but using MLMS to virtually connect patients, providers, and students will remain an important capability and opportunity as health care shifts to more virtual interaction.
Acknowledgments
The authors thank Emma Levine, MD, for her assistance coordinating video interviews; Thor Ringler, MS, MFA, for his assistance with manuscript review; and the veterans of the San Francisco VA Health Care System for sharing their stories.
1. Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
2. Milota MM, van Thiel GJMW, van Delden JJM. Narrative medicine as a medical education tool: a systematic review. Med Teach. 2019;41(7):802-810. doi:10.1080/0142159X.2019.1584274
3. Garrison D, Lyness JM, Frank JB, Epstein RM. Qualitative analysis of medical student impressions of a narrative exercise in the third-year psychiatry clerkship. Acad Med. 2011;86(1):85-89. doi:10.1097/ACM.0b013e3181ff7a63
4. Divinsky M. Stories for life: introduction to narrative medicine. Can Fam Physician. 2007;53(2):203-211.
5. McAdams DP, McLean KC. Narrative identity. Curr Dir Psychol Sci. 2013;22(3):233-238. doi:10.1177 /0963721413475622
6. Fitchett G, Emanuel L, Handzo G, Boyken L, Wilkie DJ. Care of the human spirit and the role of dignity therapy: a systematic review of dignity therapy research. BMC Palliat Care. 2015;14:8. Published 2015 Mar 21. doi:10.1186/s12904-015-0007-1
7. Ringler T, Ahearn EP, Wise M, Lee ER, Krahn D. Using life stories to connect veterans and providers. Fed Pract. 2015;32(6):8-14.
8. Roberts TJ, Ringler T, Krahn D, Ahearn E. The My Life, My Story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
9. Nathan S, Fiore LL, Saunders S, et al. My Life, My Story: Teaching patient centered care competencies for older adults through life story work [published online ahead of print, 2019 Sep 9] [published correction appears in Gerontol Geriatr Educ. 2019 Oct 15;:1]. Gerontol Geriatr Educ. 2019;1-14. doi:10.1080/02701960.2019.1665038
10. Dorsey ER, Topol EJ. Telemedicine 2020 and the next decade. Lancet. 2020;395(10227):859. doi:10.1016/S0140-6736(20)30424-4
11. Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020 [published correction appears in MMWR Morb Mortal Wkly Rep. 2020 Nov 13;69(45):1711]. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595-1599. Published 2020 Oct 30. doi:10.15585/mmwr.mm6943a3
12. Caputo LV. Across the Street. The VA philosophy: with Dr. Goldberg. July 14, 2019. Accessed November 5, 2021. https://soundcloud.com/user-911014559/the-va-philosophy-with-dr-goldberg-1
13. Sable-Smith B. Storytelling helps hospital staff discover the person within the patient. NPR. Published June 8, 2019. Accessed November 5, 2021. https://www.npr.org/sections/health-shots/2019/06/08/729351842/storytelling-helps-hospital-staff-discover-the-person-within-the-patient
14. Ganeshan S, Hsiang E, Peng T, et al. Enabling patient communication for hospitalised patients during and beyond the COVID-19 pandemic. BMJ Innov. 2021;7(2):316-320. doi:10.1136/bmjinnov-2020-000636
15. Chretien KC, Swenson R, Yoon B, et al. Tell me your story: a pilot narrative medicine curriculum during the medicine clerkship. J Gen Intern Med. 2015;30(7):1025-1028. doi:10.1007/s11606-015-3211-z
1. Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
2. Milota MM, van Thiel GJMW, van Delden JJM. Narrative medicine as a medical education tool: a systematic review. Med Teach. 2019;41(7):802-810. doi:10.1080/0142159X.2019.1584274
3. Garrison D, Lyness JM, Frank JB, Epstein RM. Qualitative analysis of medical student impressions of a narrative exercise in the third-year psychiatry clerkship. Acad Med. 2011;86(1):85-89. doi:10.1097/ACM.0b013e3181ff7a63
4. Divinsky M. Stories for life: introduction to narrative medicine. Can Fam Physician. 2007;53(2):203-211.
5. McAdams DP, McLean KC. Narrative identity. Curr Dir Psychol Sci. 2013;22(3):233-238. doi:10.1177 /0963721413475622
6. Fitchett G, Emanuel L, Handzo G, Boyken L, Wilkie DJ. Care of the human spirit and the role of dignity therapy: a systematic review of dignity therapy research. BMC Palliat Care. 2015;14:8. Published 2015 Mar 21. doi:10.1186/s12904-015-0007-1
7. Ringler T, Ahearn EP, Wise M, Lee ER, Krahn D. Using life stories to connect veterans and providers. Fed Pract. 2015;32(6):8-14.
8. Roberts TJ, Ringler T, Krahn D, Ahearn E. The My Life, My Story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
9. Nathan S, Fiore LL, Saunders S, et al. My Life, My Story: Teaching patient centered care competencies for older adults through life story work [published online ahead of print, 2019 Sep 9] [published correction appears in Gerontol Geriatr Educ. 2019 Oct 15;:1]. Gerontol Geriatr Educ. 2019;1-14. doi:10.1080/02701960.2019.1665038
10. Dorsey ER, Topol EJ. Telemedicine 2020 and the next decade. Lancet. 2020;395(10227):859. doi:10.1016/S0140-6736(20)30424-4
11. Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020 [published correction appears in MMWR Morb Mortal Wkly Rep. 2020 Nov 13;69(45):1711]. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595-1599. Published 2020 Oct 30. doi:10.15585/mmwr.mm6943a3
12. Caputo LV. Across the Street. The VA philosophy: with Dr. Goldberg. July 14, 2019. Accessed November 5, 2021. https://soundcloud.com/user-911014559/the-va-philosophy-with-dr-goldberg-1
13. Sable-Smith B. Storytelling helps hospital staff discover the person within the patient. NPR. Published June 8, 2019. Accessed November 5, 2021. https://www.npr.org/sections/health-shots/2019/06/08/729351842/storytelling-helps-hospital-staff-discover-the-person-within-the-patient
14. Ganeshan S, Hsiang E, Peng T, et al. Enabling patient communication for hospitalised patients during and beyond the COVID-19 pandemic. BMJ Innov. 2021;7(2):316-320. doi:10.1136/bmjinnov-2020-000636
15. Chretien KC, Swenson R, Yoon B, et al. Tell me your story: a pilot narrative medicine curriculum during the medicine clerkship. J Gen Intern Med. 2015;30(7):1025-1028. doi:10.1007/s11606-015-3211-z
Assessing Outcomes Between Risperidone Microspheres and Paliperidone Palmitate Long-Acting Injectable Antipsychotics Among Veterans
Medication nonadherence is common with oral antipsychotic formulations, resulting in relapse, increased morbidity, and more frequent psychiatric hospitalization.1-7 Psychiatric hospitalization and illness decompensation is costly to health care systems and leads to reduced quality of life for veterans and families.6,7 Long-acting injectable antipsychotics (LAIAs) were developed to enhance antipsychotic adherence and improve patient outcomes, including reduced psychiatric hospitalization.8-12
Little outcomes data exist comparing LAIAs, including biweekly risperidone microspheres and monthly paliperidone palmitate.10-13 Risperidone microspheres require a 3-week oral crossover and are administered every 2 weeks, whereas paliperidone palmitate does not require an oral crossover and is administered every 4 weeks. The paliperidone palmitate loading regimen replaces an oral crossover.
The primary objective of this study was to compare the number of psychiatric hospitalizations between veterans administered risperidone microspheres and those on paliperidone palmitate pre- and post-LAIA initiation. Secondary objectives were to assess rehospitalization rates between patients taking risperidone microspheres and paliperidone palmitate, reduction in pre- and posthospitalization rates with LAIAs, and medication adherence.
Methods
This observational study with a retrospective cohort design was conducted at the Veterans Affairs Loma Linda Healthcare System (VALLHS) in California. We examined veterans who were initiated on LAIAs risperidone microspheres or paliperidone palmitate from January 01, 2016 through December 31, 2018. Veterans who were aged ≥ 18 years and received ≥ 2 injections of either risperidone microspheres or paliperidone palmitate during the study period were included. Veterans were excluded if they had received < 2 doses of either LAIA, received the LAIA outside of the review period, were nonadherent to risperidone crossover if they received risperidone microspheres, or transferred their care to another facility. At VALLHS, LAIA injections are administered by a nurse, and veterans must travel to the facility to receive the injections.
Extracted patient chart elements included participant demographics; diagnoses; comorbid alcohol, nicotine, opioid, or other substance use; duration on LAIA; psychiatric hospitalizations pre- and postinitiation of the LAIA; medication adherence; and medication discontinuation based on clinician documentation and clinic orders (Table 1).
Nonadherence to LAIA was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. This time frame was based on pharmacokinetic information listed in the products’ package inserts.14,15 Nonadherence to oral risperidone crossover with risperidone microspheres was defined as ≤ 80% of days covered.
Data Analysis
Patient demographics were analyzed using descriptive statistics and experimental comparisons between the risperidone microspheres and paliperidone palmitate groups to assess baseline differences between groups. Psychiatric hospitalizations pre- and post-LAIA were analyzed with parallel group (between veterans–independent groups) and pre-post (within veterans–dependent groups) designs. Index hospitalizations were examined for a period equivalent to the length of time veterans were on the LAIA. Psychiatric rehospitalization rates were analyzed for patients who had index hospitalizations and were rehospitalized for any period when they were receiving the LAIA. Incidences of pre- and post-LAIA hospitalizations were calculated in 100 person-years.
Parallel-group analysis was analyzed using the χ2 and Mann-Whitney U tests. Pre-post analyses were analyzed using the Wilcoxon rank sum test. P was set at < .05 for statistical significance.
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18
The researchers established reference ranges for risperidone and paliperidone plasma concentrations that represented expected variability within a population and were derived from population pharmacokinetic models.18 Gopal and colleagues conducted a post hoc comparison between paliperidone palmitate and oral risperidone during initiation of long-acting injectable risperidone in patients with acute schizophrenia.19 The researchers found that during the first month after initiating long-acting injectable risperidone, paliperidone palmitate without oral supplementation had similar efficacy and safety to oral risperidone among these patients.19
LAIAs can create a steadier drug plasma concentration compared with oral antipsychotics and do not need to be taken daily. These agents improve adherence by reducing the frequency of medication administrations.20-24 Assessing nonadherence is easier with LAIAs by counting missed injections compared with oral antipsychotics that require calculation of percentage of days covered.25
The results in our study are somewhat unexpected in part because of the close relationship between risperidone and paliperidone. Risperidone is converted to paliperidone (9-OH-risperidone) via hepatic cytochrome P450 2D6. Although the molecules do not have identical pharmacologic profiles, it is accepted that they are similar enough that risperidone can establish oral tolerability when transitioning therapy to paliperidone palmitate and vice versa.24 Although the active moiety in risperidone microspheres and paliperidone palmitate is similar, the dosing interval for risperidone microspheres is 2 weeks compared with 4 weeks with paliperidone palmitate. One potential explanation as to why veterans started on risperidone microspheres experienced better outcomes is because they had twice as many office visits with the health care team. Facility procedures dictate veterans receive the LAIA at an on-site clinic. During the visits, a licensed vocational nurse administers the injection and monitors the patient for 15 to 30 minutes afterward.
Despite new LAIAs coming to market, high-quality data examining potential differences in treatment outcomes among agents are limited. This is problematic for clinicians who want to optimize care by understanding how administration schedules or other aspects of LAIA use could modify treatment outcomes. Our results suggest that an advantage might exist in selecting an agent with a more frequent administration schedule, at least initially. This could allow for close monitoring and regular therapeutic contact, which could improve short-term outcomes. This conclusion is supported by meta-analyses, randomized controlled trials, and conceptual articles conducted by Wehring and colleagues, Berwaerts and colleagues, and Parellada and colleagues, respectively, who examined patients on different LAIAs and contact with health care professionals as part of their research.26-28 These researchers concluded that patients who had regular contact with a health care professional had better outcomes when initiated on a LAIA.26-28
Limitations
There are several limitations in this study. Retrospective and observational methods introduce risks of bias and confounding variables. Sample size might have limited statistical power to detect differences. Veterans might have had undocumented pre- or posthospitalizations at other institutions, which was not accounted for and lack of rehospitalization is not conclusive of a positive outcome. Institutions could improve on our study and help to fill gaps in comparative data by conducting larger analyses over longer periods and including more LAIA agents.
Conclusions
Although veterans that were administered risperidone microspheres had a shorter treatment duration, they were less likely to be rehospitalized, had a fewer mean number of post-LAIA hospitalizations, and had a larger difference in incidence in 100 person-years compared with veterans on paliperidone palmitate. Nonadherence and discontinuation rates were comparable between risperidone microspheres and paliperidone palmitate. Future studies could aim to further clarify differences in outcomes among agents or administration schedules.
1. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
2. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi:10.1056/NEJMoa051688
3. Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59(5):500-506. doi:10.1176/ps.2008.59.5.500
4. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152(6):856-561. doi:10.1176/ajp.152.6.856
5. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi:10.1186/1471-244X-8-32
6. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. doi:10.1176/appi.ps.55.8.886
7. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-699. doi:10.1176/appi.ajp.161.4.692
8. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655. doi:10.1185/03007995.2014.915211
9. Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S-53S. doi:10.1177/1077558703256724
10. Duncan EJ, Woolson SL, Hamer RM. Treatment compliance in veterans administration schizophrenia spectrum patients treated with risperidone long-acting injectable. Int Clin Psychopharmacol. 2012;27(5):283-290. doi:10.1097/YIC.0b013e328354b534
11. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
12. Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis Veterans Affairs Health Care System. J Clin Psychopharmacol. 2017;37(5):605-608. doi:10.1097/JCP.0000000000000755
13. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
14. Risperdal Consta. Package insert. Janssen Pharmaceutical; 2007.
15. Invega Sustenna. Package insert. Janssen Pharmaceutical; 2009.
16. Limosin F, Belhadi D, Comet D, et al. Comparison of paliperidone palmitate and risperidone long-acting injection in schizophrenic patients: results from a multicenter retrospective cohort study in France. J Clin Psychopharmacol. 2018;38(1):19-26. doi:10.1097/JCP.0000000000000827
17. Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706
18. Korell J, Green B, Remmerie B, Vermeulen A. Determination of plasma concentration reference ranges for risperidone and paliperidone. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):589-595. doi:10.1002/psp4.12217
19. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33.
20. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
21. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
22. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. doi:10.4088/JCP.13m08838
23. Rezansoff SN, Moniruzzaman A, Fazel S, Procyshyn R, Somers JM. Adherence to antipsychotic medication among homeless adults in Vancouver, Canada: a 15-year retrospective cohort study. Soc Psychiatry Psychiatr Epidemiol. 2016;51(12):1623-1632. doi:10.1007/s00127-016-1259-7
24. Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Ment Health. 2015;18(2):36-39. doi:10.1136/eb-2015-102086
25. Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 (suppl 16):3-9.
26. Wehring HJ, Thedford S, Koola M, Kelly DL. Patient and health care provider perspectives on long acting injectable antipsychotics in schizophrenia and the introduction of olanzapine long-acting injection. J Cent Nerv Syst Dis. 2011;2011(3):107-123. doi:10.4137/JCNSD.S4091
27. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. doi:10.1001/jamapsychiatry.2015.0241
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi:10.1007/s40263-016-0350-7
Medication nonadherence is common with oral antipsychotic formulations, resulting in relapse, increased morbidity, and more frequent psychiatric hospitalization.1-7 Psychiatric hospitalization and illness decompensation is costly to health care systems and leads to reduced quality of life for veterans and families.6,7 Long-acting injectable antipsychotics (LAIAs) were developed to enhance antipsychotic adherence and improve patient outcomes, including reduced psychiatric hospitalization.8-12
Little outcomes data exist comparing LAIAs, including biweekly risperidone microspheres and monthly paliperidone palmitate.10-13 Risperidone microspheres require a 3-week oral crossover and are administered every 2 weeks, whereas paliperidone palmitate does not require an oral crossover and is administered every 4 weeks. The paliperidone palmitate loading regimen replaces an oral crossover.
The primary objective of this study was to compare the number of psychiatric hospitalizations between veterans administered risperidone microspheres and those on paliperidone palmitate pre- and post-LAIA initiation. Secondary objectives were to assess rehospitalization rates between patients taking risperidone microspheres and paliperidone palmitate, reduction in pre- and posthospitalization rates with LAIAs, and medication adherence.
Methods
This observational study with a retrospective cohort design was conducted at the Veterans Affairs Loma Linda Healthcare System (VALLHS) in California. We examined veterans who were initiated on LAIAs risperidone microspheres or paliperidone palmitate from January 01, 2016 through December 31, 2018. Veterans who were aged ≥ 18 years and received ≥ 2 injections of either risperidone microspheres or paliperidone palmitate during the study period were included. Veterans were excluded if they had received < 2 doses of either LAIA, received the LAIA outside of the review period, were nonadherent to risperidone crossover if they received risperidone microspheres, or transferred their care to another facility. At VALLHS, LAIA injections are administered by a nurse, and veterans must travel to the facility to receive the injections.
Extracted patient chart elements included participant demographics; diagnoses; comorbid alcohol, nicotine, opioid, or other substance use; duration on LAIA; psychiatric hospitalizations pre- and postinitiation of the LAIA; medication adherence; and medication discontinuation based on clinician documentation and clinic orders (Table 1).
Nonadherence to LAIA was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. This time frame was based on pharmacokinetic information listed in the products’ package inserts.14,15 Nonadherence to oral risperidone crossover with risperidone microspheres was defined as ≤ 80% of days covered.
Data Analysis
Patient demographics were analyzed using descriptive statistics and experimental comparisons between the risperidone microspheres and paliperidone palmitate groups to assess baseline differences between groups. Psychiatric hospitalizations pre- and post-LAIA were analyzed with parallel group (between veterans–independent groups) and pre-post (within veterans–dependent groups) designs. Index hospitalizations were examined for a period equivalent to the length of time veterans were on the LAIA. Psychiatric rehospitalization rates were analyzed for patients who had index hospitalizations and were rehospitalized for any period when they were receiving the LAIA. Incidences of pre- and post-LAIA hospitalizations were calculated in 100 person-years.
Parallel-group analysis was analyzed using the χ2 and Mann-Whitney U tests. Pre-post analyses were analyzed using the Wilcoxon rank sum test. P was set at < .05 for statistical significance.
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18
The researchers established reference ranges for risperidone and paliperidone plasma concentrations that represented expected variability within a population and were derived from population pharmacokinetic models.18 Gopal and colleagues conducted a post hoc comparison between paliperidone palmitate and oral risperidone during initiation of long-acting injectable risperidone in patients with acute schizophrenia.19 The researchers found that during the first month after initiating long-acting injectable risperidone, paliperidone palmitate without oral supplementation had similar efficacy and safety to oral risperidone among these patients.19
LAIAs can create a steadier drug plasma concentration compared with oral antipsychotics and do not need to be taken daily. These agents improve adherence by reducing the frequency of medication administrations.20-24 Assessing nonadherence is easier with LAIAs by counting missed injections compared with oral antipsychotics that require calculation of percentage of days covered.25
The results in our study are somewhat unexpected in part because of the close relationship between risperidone and paliperidone. Risperidone is converted to paliperidone (9-OH-risperidone) via hepatic cytochrome P450 2D6. Although the molecules do not have identical pharmacologic profiles, it is accepted that they are similar enough that risperidone can establish oral tolerability when transitioning therapy to paliperidone palmitate and vice versa.24 Although the active moiety in risperidone microspheres and paliperidone palmitate is similar, the dosing interval for risperidone microspheres is 2 weeks compared with 4 weeks with paliperidone palmitate. One potential explanation as to why veterans started on risperidone microspheres experienced better outcomes is because they had twice as many office visits with the health care team. Facility procedures dictate veterans receive the LAIA at an on-site clinic. During the visits, a licensed vocational nurse administers the injection and monitors the patient for 15 to 30 minutes afterward.
Despite new LAIAs coming to market, high-quality data examining potential differences in treatment outcomes among agents are limited. This is problematic for clinicians who want to optimize care by understanding how administration schedules or other aspects of LAIA use could modify treatment outcomes. Our results suggest that an advantage might exist in selecting an agent with a more frequent administration schedule, at least initially. This could allow for close monitoring and regular therapeutic contact, which could improve short-term outcomes. This conclusion is supported by meta-analyses, randomized controlled trials, and conceptual articles conducted by Wehring and colleagues, Berwaerts and colleagues, and Parellada and colleagues, respectively, who examined patients on different LAIAs and contact with health care professionals as part of their research.26-28 These researchers concluded that patients who had regular contact with a health care professional had better outcomes when initiated on a LAIA.26-28
Limitations
There are several limitations in this study. Retrospective and observational methods introduce risks of bias and confounding variables. Sample size might have limited statistical power to detect differences. Veterans might have had undocumented pre- or posthospitalizations at other institutions, which was not accounted for and lack of rehospitalization is not conclusive of a positive outcome. Institutions could improve on our study and help to fill gaps in comparative data by conducting larger analyses over longer periods and including more LAIA agents.
Conclusions
Although veterans that were administered risperidone microspheres had a shorter treatment duration, they were less likely to be rehospitalized, had a fewer mean number of post-LAIA hospitalizations, and had a larger difference in incidence in 100 person-years compared with veterans on paliperidone palmitate. Nonadherence and discontinuation rates were comparable between risperidone microspheres and paliperidone palmitate. Future studies could aim to further clarify differences in outcomes among agents or administration schedules.
Medication nonadherence is common with oral antipsychotic formulations, resulting in relapse, increased morbidity, and more frequent psychiatric hospitalization.1-7 Psychiatric hospitalization and illness decompensation is costly to health care systems and leads to reduced quality of life for veterans and families.6,7 Long-acting injectable antipsychotics (LAIAs) were developed to enhance antipsychotic adherence and improve patient outcomes, including reduced psychiatric hospitalization.8-12
Little outcomes data exist comparing LAIAs, including biweekly risperidone microspheres and monthly paliperidone palmitate.10-13 Risperidone microspheres require a 3-week oral crossover and are administered every 2 weeks, whereas paliperidone palmitate does not require an oral crossover and is administered every 4 weeks. The paliperidone palmitate loading regimen replaces an oral crossover.
The primary objective of this study was to compare the number of psychiatric hospitalizations between veterans administered risperidone microspheres and those on paliperidone palmitate pre- and post-LAIA initiation. Secondary objectives were to assess rehospitalization rates between patients taking risperidone microspheres and paliperidone palmitate, reduction in pre- and posthospitalization rates with LAIAs, and medication adherence.
Methods
This observational study with a retrospective cohort design was conducted at the Veterans Affairs Loma Linda Healthcare System (VALLHS) in California. We examined veterans who were initiated on LAIAs risperidone microspheres or paliperidone palmitate from January 01, 2016 through December 31, 2018. Veterans who were aged ≥ 18 years and received ≥ 2 injections of either risperidone microspheres or paliperidone palmitate during the study period were included. Veterans were excluded if they had received < 2 doses of either LAIA, received the LAIA outside of the review period, were nonadherent to risperidone crossover if they received risperidone microspheres, or transferred their care to another facility. At VALLHS, LAIA injections are administered by a nurse, and veterans must travel to the facility to receive the injections.
Extracted patient chart elements included participant demographics; diagnoses; comorbid alcohol, nicotine, opioid, or other substance use; duration on LAIA; psychiatric hospitalizations pre- and postinitiation of the LAIA; medication adherence; and medication discontinuation based on clinician documentation and clinic orders (Table 1).
Nonadherence to LAIA was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. This time frame was based on pharmacokinetic information listed in the products’ package inserts.14,15 Nonadherence to oral risperidone crossover with risperidone microspheres was defined as ≤ 80% of days covered.
Data Analysis
Patient demographics were analyzed using descriptive statistics and experimental comparisons between the risperidone microspheres and paliperidone palmitate groups to assess baseline differences between groups. Psychiatric hospitalizations pre- and post-LAIA were analyzed with parallel group (between veterans–independent groups) and pre-post (within veterans–dependent groups) designs. Index hospitalizations were examined for a period equivalent to the length of time veterans were on the LAIA. Psychiatric rehospitalization rates were analyzed for patients who had index hospitalizations and were rehospitalized for any period when they were receiving the LAIA. Incidences of pre- and post-LAIA hospitalizations were calculated in 100 person-years.
Parallel-group analysis was analyzed using the χ2 and Mann-Whitney U tests. Pre-post analyses were analyzed using the Wilcoxon rank sum test. P was set at < .05 for statistical significance.
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18
The researchers established reference ranges for risperidone and paliperidone plasma concentrations that represented expected variability within a population and were derived from population pharmacokinetic models.18 Gopal and colleagues conducted a post hoc comparison between paliperidone palmitate and oral risperidone during initiation of long-acting injectable risperidone in patients with acute schizophrenia.19 The researchers found that during the first month after initiating long-acting injectable risperidone, paliperidone palmitate without oral supplementation had similar efficacy and safety to oral risperidone among these patients.19
LAIAs can create a steadier drug plasma concentration compared with oral antipsychotics and do not need to be taken daily. These agents improve adherence by reducing the frequency of medication administrations.20-24 Assessing nonadherence is easier with LAIAs by counting missed injections compared with oral antipsychotics that require calculation of percentage of days covered.25
The results in our study are somewhat unexpected in part because of the close relationship between risperidone and paliperidone. Risperidone is converted to paliperidone (9-OH-risperidone) via hepatic cytochrome P450 2D6. Although the molecules do not have identical pharmacologic profiles, it is accepted that they are similar enough that risperidone can establish oral tolerability when transitioning therapy to paliperidone palmitate and vice versa.24 Although the active moiety in risperidone microspheres and paliperidone palmitate is similar, the dosing interval for risperidone microspheres is 2 weeks compared with 4 weeks with paliperidone palmitate. One potential explanation as to why veterans started on risperidone microspheres experienced better outcomes is because they had twice as many office visits with the health care team. Facility procedures dictate veterans receive the LAIA at an on-site clinic. During the visits, a licensed vocational nurse administers the injection and monitors the patient for 15 to 30 minutes afterward.
Despite new LAIAs coming to market, high-quality data examining potential differences in treatment outcomes among agents are limited. This is problematic for clinicians who want to optimize care by understanding how administration schedules or other aspects of LAIA use could modify treatment outcomes. Our results suggest that an advantage might exist in selecting an agent with a more frequent administration schedule, at least initially. This could allow for close monitoring and regular therapeutic contact, which could improve short-term outcomes. This conclusion is supported by meta-analyses, randomized controlled trials, and conceptual articles conducted by Wehring and colleagues, Berwaerts and colleagues, and Parellada and colleagues, respectively, who examined patients on different LAIAs and contact with health care professionals as part of their research.26-28 These researchers concluded that patients who had regular contact with a health care professional had better outcomes when initiated on a LAIA.26-28
Limitations
There are several limitations in this study. Retrospective and observational methods introduce risks of bias and confounding variables. Sample size might have limited statistical power to detect differences. Veterans might have had undocumented pre- or posthospitalizations at other institutions, which was not accounted for and lack of rehospitalization is not conclusive of a positive outcome. Institutions could improve on our study and help to fill gaps in comparative data by conducting larger analyses over longer periods and including more LAIA agents.
Conclusions
Although veterans that were administered risperidone microspheres had a shorter treatment duration, they were less likely to be rehospitalized, had a fewer mean number of post-LAIA hospitalizations, and had a larger difference in incidence in 100 person-years compared with veterans on paliperidone palmitate. Nonadherence and discontinuation rates were comparable between risperidone microspheres and paliperidone palmitate. Future studies could aim to further clarify differences in outcomes among agents or administration schedules.
1. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
2. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi:10.1056/NEJMoa051688
3. Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59(5):500-506. doi:10.1176/ps.2008.59.5.500
4. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152(6):856-561. doi:10.1176/ajp.152.6.856
5. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi:10.1186/1471-244X-8-32
6. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. doi:10.1176/appi.ps.55.8.886
7. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-699. doi:10.1176/appi.ajp.161.4.692
8. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655. doi:10.1185/03007995.2014.915211
9. Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S-53S. doi:10.1177/1077558703256724
10. Duncan EJ, Woolson SL, Hamer RM. Treatment compliance in veterans administration schizophrenia spectrum patients treated with risperidone long-acting injectable. Int Clin Psychopharmacol. 2012;27(5):283-290. doi:10.1097/YIC.0b013e328354b534
11. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
12. Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis Veterans Affairs Health Care System. J Clin Psychopharmacol. 2017;37(5):605-608. doi:10.1097/JCP.0000000000000755
13. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
14. Risperdal Consta. Package insert. Janssen Pharmaceutical; 2007.
15. Invega Sustenna. Package insert. Janssen Pharmaceutical; 2009.
16. Limosin F, Belhadi D, Comet D, et al. Comparison of paliperidone palmitate and risperidone long-acting injection in schizophrenic patients: results from a multicenter retrospective cohort study in France. J Clin Psychopharmacol. 2018;38(1):19-26. doi:10.1097/JCP.0000000000000827
17. Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706
18. Korell J, Green B, Remmerie B, Vermeulen A. Determination of plasma concentration reference ranges for risperidone and paliperidone. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):589-595. doi:10.1002/psp4.12217
19. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33.
20. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
21. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
22. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. doi:10.4088/JCP.13m08838
23. Rezansoff SN, Moniruzzaman A, Fazel S, Procyshyn R, Somers JM. Adherence to antipsychotic medication among homeless adults in Vancouver, Canada: a 15-year retrospective cohort study. Soc Psychiatry Psychiatr Epidemiol. 2016;51(12):1623-1632. doi:10.1007/s00127-016-1259-7
24. Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Ment Health. 2015;18(2):36-39. doi:10.1136/eb-2015-102086
25. Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 (suppl 16):3-9.
26. Wehring HJ, Thedford S, Koola M, Kelly DL. Patient and health care provider perspectives on long acting injectable antipsychotics in schizophrenia and the introduction of olanzapine long-acting injection. J Cent Nerv Syst Dis. 2011;2011(3):107-123. doi:10.4137/JCNSD.S4091
27. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. doi:10.1001/jamapsychiatry.2015.0241
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi:10.1007/s40263-016-0350-7
1. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
2. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi:10.1056/NEJMoa051688
3. Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59(5):500-506. doi:10.1176/ps.2008.59.5.500
4. Haywood TW, Kravitz HM, Grossman LS, Cavanaugh JL Jr, Davis JM, Lewis DA. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152(6):856-561. doi:10.1176/ajp.152.6.856
5. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8:32. doi:10.1186/1471-244X-8-32
6. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. doi:10.1176/appi.ps.55.8.886
7. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692-699. doi:10.1176/appi.ajp.161.4.692
8. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655. doi:10.1185/03007995.2014.915211
9. Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S-53S. doi:10.1177/1077558703256724
10. Duncan EJ, Woolson SL, Hamer RM. Treatment compliance in veterans administration schizophrenia spectrum patients treated with risperidone long-acting injectable. Int Clin Psychopharmacol. 2012;27(5):283-290. doi:10.1097/YIC.0b013e328354b534
11. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
12. Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis Veterans Affairs Health Care System. J Clin Psychopharmacol. 2017;37(5):605-608. doi:10.1097/JCP.0000000000000755
13. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
14. Risperdal Consta. Package insert. Janssen Pharmaceutical; 2007.
15. Invega Sustenna. Package insert. Janssen Pharmaceutical; 2009.
16. Limosin F, Belhadi D, Comet D, et al. Comparison of paliperidone palmitate and risperidone long-acting injection in schizophrenic patients: results from a multicenter retrospective cohort study in France. J Clin Psychopharmacol. 2018;38(1):19-26. doi:10.1097/JCP.0000000000000827
17. Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706
18. Korell J, Green B, Remmerie B, Vermeulen A. Determination of plasma concentration reference ranges for risperidone and paliperidone. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):589-595. doi:10.1002/psp4.12217
19. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33.
20. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. doi:10.18553/jmcp.2015.21.9.754
21. Romstadt N, Wonson E. Outcomes comparison of long-acting injectable antipsychotic initiation in treatment-naïve veterans in the inpatient versus outpatient setting. Ment Health Clin. 2018;8(1):24-27. doi:10.9740/mhc.2018.01.024
22. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. doi:10.4088/JCP.13m08838
23. Rezansoff SN, Moniruzzaman A, Fazel S, Procyshyn R, Somers JM. Adherence to antipsychotic medication among homeless adults in Vancouver, Canada: a 15-year retrospective cohort study. Soc Psychiatry Psychiatr Epidemiol. 2016;51(12):1623-1632. doi:10.1007/s00127-016-1259-7
24. Castillo EG, Stroup TS. Effectiveness of long-acting injectable antipsychotics: a clinical perspective. Evid Based Ment Health. 2015;18(2):36-39. doi:10.1136/eb-2015-102086
25. Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 (suppl 16):3-9.
26. Wehring HJ, Thedford S, Koola M, Kelly DL. Patient and health care provider perspectives on long acting injectable antipsychotics in schizophrenia and the introduction of olanzapine long-acting injection. J Cent Nerv Syst Dis. 2011;2011(3):107-123. doi:10.4137/JCNSD.S4091
27. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. doi:10.1001/jamapsychiatry.2015.0241
28. Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi:10.1007/s40263-016-0350-7
The Angel of Death in Clarksburg
Readers of this column may recall that since I have been the Editor-in-Chief of Federal Practitioner, my December editorial focuses on the best and worst of the year in federal medicine. In 2021, these evaluative terms fail to capture the sadness and global devastation that mark this grim epoch of the continuing pandemic, increasing climate disasters, rising political tensions, and racial violence. Thus, this year my editorial is framed in terms of the philosophical or theological categories of good and evil as the only concepts that can even begin to express the horrendous events that occurred in West Virginia.
On June 28, 2018, then US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Executive-in-Charge, Carolyn Clancy, MD, contacted Inspector General Michael Missal to alert him that “there may be an ‘Angel of Death’ in Clarksburg [West Virginia].”1 Two years later Reta Mays, a 46-year-old VA nursing assistant, entered a guilty plea in federal court to the deaths of 7 vulnerable veterans. The legal charges were second-degree murder and 1 count of assault with intent to commit murder by injecting insulin. The victims were all patients on Ward 3 at the Louis A. Johnson VA, Medical Center in Clarksburg, where Mays worked the night shift from 2015 to 2018.2 Mays was sentenced in May of this year to 7 consecutive life terms for each of the veterans whose lives she cruelly ended and an additional 240 months for the eighth patient who survived her murder attempt.3
The term angel of death has religious roots in Judaism, although not strictly in the Hebrew scriptures. Neither the Jewish nor Christian Bible identifies a specific figure who is the angel of death. The idea first appears in Rabbinic literature and Jewish tradition.4 The angel God sends as a messenger of death is known as malakh ha-mavet in Hebrew. The revered Jewish physician and philosopher Moses Maimonides taught in his Guide for the Perplexed the angel of death is synonymous with the devil, and the evil inclination that dwells in the mind of all human beings.5In modern times, the concept of an angel of death has come to designate a serial killer who is a health care professional (HCP). A group of forensic scientists, HCPs, and attorneys, including former VA Under Secretary for Health Dr. Kenneth Kizer, published a study of HCPs who had been prosecuted or convicted of serial murder. Nurses constituted the largest group of offenders (60%) with nursing aides like Mays responsible for 18% of murders, and physicians 12%. The review found that though health care serial killers are rare, they operate in nations across the Western world, in many different states in this country, and in almost all health care settings, including previous VA angels of death.6Nursing aides who are not supposed to have access to medications—a major problem in Mays’ case—nor permitted to administer them more often resort to noncontrolled substances to kill their victims.1 Mays chose insulin as her murder weapon as did 13% of serial killers. Just as insulin may be difficult to detect in toxicology, so Mays and others like her committed their crimes on the night shift when they were less likely to be discovered.6
Many of us feel compelled to seek a rational motivation for why healers would mutate into killers: If we can find a reason for this heinous behavior it somehow helps us feel the world is more intelligible and controllable. Unfortunately, despite intensive forensic investigations of multiple angels of death, there is little definitive understanding of the motives of these murders.6 Mays disclosed more than most. As part of a plea bargain, she provided investigators with 2 rationales for her killing: She wanted to ease the patients’ suffering. Such claims of being an angel of mercy are common among HCP serial murders, which the patterns of the killings generally disprove. The patients Mays lethally injected, while mostly old and ill, were all expected to recover and leave the hospital. The Inspector General report uncovered a cautionary detail that has at least indirect bearing on the nursing assistant’s contention that she “wanted to let the patient’s die gently”: Contrary to VHA requirements, the facility had no functioning palliative care team. This finding in no way excuses or even explains Mays’ actions; it does, however, reinforce the essential value of palliative care expertise in an aging veteran population with many life-limiting conditions.7
Mays’ second motivation seems more plausible, based on her life narrative and the literature on HCP serial killers. Mays disclosed to investigators that she “had a lot of stress and chaos in [her] personal and professional life and these actions gave [her] a sense of control.”1 Her prior use of excessive force when employed at a prison as well as forensic science indicating that feelings of wielding power over life and death often drive health care murders, suggest this may have been a factor in Mays’ horrific conduct.8
It seems blasphemous to associate the word good in the same pages with this terrible evil. Nothing can compensate or justify the betrayal of the sacred oath of an HCP and the public trust of a VHA employee. Yet that very impossibility carries with it an obligation to ask, as did the author of an article about a recent Canadian nurse serial killer, “What can we learn from the [Mays] story?”9
Mays could never have taken the lives of 8 patients without clinical and administrative lapses and shortcuts at all levels of the health care system. Indeed, the 100-plus page Inspector General report makes 15 recommendations for the VHA, the Veterans Integrated Service Network, and the facility, encompassing areas of personnel hiring and performance evaluation, medication management and security, reporting and responding to unexplained events, quality and safety programs oversight, leaders’ responses, corrective actions, and even computer systems data analysis.
I want to suggest 2 ethical additions to this list addressed to all of us as VHA staff and especially to those of us who are HCPs. From the perspective of virtue ethics, Reta Mays is a tragedy about complacency and compromise in everyday work that the pandemic has made even more frequent and challenging to avoid and resist. This is what the Roman Virgil means in the epigraph that the road down to hell is easy and the road back very difficult.
I propose the need for discernment in trying to listen to our moral intuitions that tell us something is amiss and diligence in adhering to best practices even when we are fearful, exhausted, demoralized, or apathetic. These 2 habits of commitment to veterans, one of compassion and the other of competence, can help us follow the good inclinations of our hearts and together with system changes can bar the doors of our hospitals to the visits of future angels of death. This dedication is the least we owe to the families of the patients at Clarksburg whose loved ones never came home and whose questions likely can never be fully answered.
1. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration: care and oversight deficiencies related to multiple homicides at the Louis A. Johnson VA Medical Center in Clarksburg, West Virginia. Healthcare Inspection Report #20-035993-140. Published May 11 2021. Accessed November 22, 2021. https://www.va.gov/oig/pubs/VAOIG-20-03593-140.pdf
2. Kennedy M, Schwartz M. Former VA medical worker pleads guilty to murdering 7 patients in West Virginia. Published July 14, 2020. Accessed November 22, 2021. https://www.npr.org/2020/07/14/890776010/former-va-medical-worker-charged-with-7-murders-in-west-virginia
3. US Department of Justice, US Attorney’s Office Northern District of West Virginia. Former VA hospital nursing assistant sentenced to seven consecutive life sentences for murdering seven veterans and assault with intent to commit murder of an eighth [press release]. Published May 11, 2021. Accessed November 22, 2021. https://www.justice.gov/usao-ndwv/pr/former-va-hospital-nursing-assistant-sentenced-seven-consecutive-life-sentences.
4. Jacobs L. The Jewish Religion: A Companion. 1st ed. Oxford University Press;1995:116.
5. Maimonides. Guide for the Perplexed. Frielander M, trans. Routledge and Kegan Paul Ltd; 1904:pt 3, chap 22.
6. Yorker BC, Kizer KW, Lampe P, Forrest AR, Lannan JM, Russell DA. Serial murder by healthcare professionals. J Forensic Sci. 2006;51(6):1362-1371. doi:10.1111/j.1556-4029.2006.00273.x
7. VHA Directive 1139. Palliative care consult teams (PCCT) and VISN leads. Published June 14, 2017.
8. Rourke S, Ward T. Healthcare serial killers: patterns and policies. Published August 14, 2017. Accessed November 22, 2021. https://www.medscape.com/viewarticle/884136
9. Frank C. Health care serial murder: what can we learn from the Wettlaufer story? Can Fam Physician. 2020;66(10):719-722.
Readers of this column may recall that since I have been the Editor-in-Chief of Federal Practitioner, my December editorial focuses on the best and worst of the year in federal medicine. In 2021, these evaluative terms fail to capture the sadness and global devastation that mark this grim epoch of the continuing pandemic, increasing climate disasters, rising political tensions, and racial violence. Thus, this year my editorial is framed in terms of the philosophical or theological categories of good and evil as the only concepts that can even begin to express the horrendous events that occurred in West Virginia.
On June 28, 2018, then US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Executive-in-Charge, Carolyn Clancy, MD, contacted Inspector General Michael Missal to alert him that “there may be an ‘Angel of Death’ in Clarksburg [West Virginia].”1 Two years later Reta Mays, a 46-year-old VA nursing assistant, entered a guilty plea in federal court to the deaths of 7 vulnerable veterans. The legal charges were second-degree murder and 1 count of assault with intent to commit murder by injecting insulin. The victims were all patients on Ward 3 at the Louis A. Johnson VA, Medical Center in Clarksburg, where Mays worked the night shift from 2015 to 2018.2 Mays was sentenced in May of this year to 7 consecutive life terms for each of the veterans whose lives she cruelly ended and an additional 240 months for the eighth patient who survived her murder attempt.3
The term angel of death has religious roots in Judaism, although not strictly in the Hebrew scriptures. Neither the Jewish nor Christian Bible identifies a specific figure who is the angel of death. The idea first appears in Rabbinic literature and Jewish tradition.4 The angel God sends as a messenger of death is known as malakh ha-mavet in Hebrew. The revered Jewish physician and philosopher Moses Maimonides taught in his Guide for the Perplexed the angel of death is synonymous with the devil, and the evil inclination that dwells in the mind of all human beings.5In modern times, the concept of an angel of death has come to designate a serial killer who is a health care professional (HCP). A group of forensic scientists, HCPs, and attorneys, including former VA Under Secretary for Health Dr. Kenneth Kizer, published a study of HCPs who had been prosecuted or convicted of serial murder. Nurses constituted the largest group of offenders (60%) with nursing aides like Mays responsible for 18% of murders, and physicians 12%. The review found that though health care serial killers are rare, they operate in nations across the Western world, in many different states in this country, and in almost all health care settings, including previous VA angels of death.6Nursing aides who are not supposed to have access to medications—a major problem in Mays’ case—nor permitted to administer them more often resort to noncontrolled substances to kill their victims.1 Mays chose insulin as her murder weapon as did 13% of serial killers. Just as insulin may be difficult to detect in toxicology, so Mays and others like her committed their crimes on the night shift when they were less likely to be discovered.6
Many of us feel compelled to seek a rational motivation for why healers would mutate into killers: If we can find a reason for this heinous behavior it somehow helps us feel the world is more intelligible and controllable. Unfortunately, despite intensive forensic investigations of multiple angels of death, there is little definitive understanding of the motives of these murders.6 Mays disclosed more than most. As part of a plea bargain, she provided investigators with 2 rationales for her killing: She wanted to ease the patients’ suffering. Such claims of being an angel of mercy are common among HCP serial murders, which the patterns of the killings generally disprove. The patients Mays lethally injected, while mostly old and ill, were all expected to recover and leave the hospital. The Inspector General report uncovered a cautionary detail that has at least indirect bearing on the nursing assistant’s contention that she “wanted to let the patient’s die gently”: Contrary to VHA requirements, the facility had no functioning palliative care team. This finding in no way excuses or even explains Mays’ actions; it does, however, reinforce the essential value of palliative care expertise in an aging veteran population with many life-limiting conditions.7
Mays’ second motivation seems more plausible, based on her life narrative and the literature on HCP serial killers. Mays disclosed to investigators that she “had a lot of stress and chaos in [her] personal and professional life and these actions gave [her] a sense of control.”1 Her prior use of excessive force when employed at a prison as well as forensic science indicating that feelings of wielding power over life and death often drive health care murders, suggest this may have been a factor in Mays’ horrific conduct.8
It seems blasphemous to associate the word good in the same pages with this terrible evil. Nothing can compensate or justify the betrayal of the sacred oath of an HCP and the public trust of a VHA employee. Yet that very impossibility carries with it an obligation to ask, as did the author of an article about a recent Canadian nurse serial killer, “What can we learn from the [Mays] story?”9
Mays could never have taken the lives of 8 patients without clinical and administrative lapses and shortcuts at all levels of the health care system. Indeed, the 100-plus page Inspector General report makes 15 recommendations for the VHA, the Veterans Integrated Service Network, and the facility, encompassing areas of personnel hiring and performance evaluation, medication management and security, reporting and responding to unexplained events, quality and safety programs oversight, leaders’ responses, corrective actions, and even computer systems data analysis.
I want to suggest 2 ethical additions to this list addressed to all of us as VHA staff and especially to those of us who are HCPs. From the perspective of virtue ethics, Reta Mays is a tragedy about complacency and compromise in everyday work that the pandemic has made even more frequent and challenging to avoid and resist. This is what the Roman Virgil means in the epigraph that the road down to hell is easy and the road back very difficult.
I propose the need for discernment in trying to listen to our moral intuitions that tell us something is amiss and diligence in adhering to best practices even when we are fearful, exhausted, demoralized, or apathetic. These 2 habits of commitment to veterans, one of compassion and the other of competence, can help us follow the good inclinations of our hearts and together with system changes can bar the doors of our hospitals to the visits of future angels of death. This dedication is the least we owe to the families of the patients at Clarksburg whose loved ones never came home and whose questions likely can never be fully answered.
Readers of this column may recall that since I have been the Editor-in-Chief of Federal Practitioner, my December editorial focuses on the best and worst of the year in federal medicine. In 2021, these evaluative terms fail to capture the sadness and global devastation that mark this grim epoch of the continuing pandemic, increasing climate disasters, rising political tensions, and racial violence. Thus, this year my editorial is framed in terms of the philosophical or theological categories of good and evil as the only concepts that can even begin to express the horrendous events that occurred in West Virginia.
On June 28, 2018, then US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Executive-in-Charge, Carolyn Clancy, MD, contacted Inspector General Michael Missal to alert him that “there may be an ‘Angel of Death’ in Clarksburg [West Virginia].”1 Two years later Reta Mays, a 46-year-old VA nursing assistant, entered a guilty plea in federal court to the deaths of 7 vulnerable veterans. The legal charges were second-degree murder and 1 count of assault with intent to commit murder by injecting insulin. The victims were all patients on Ward 3 at the Louis A. Johnson VA, Medical Center in Clarksburg, where Mays worked the night shift from 2015 to 2018.2 Mays was sentenced in May of this year to 7 consecutive life terms for each of the veterans whose lives she cruelly ended and an additional 240 months for the eighth patient who survived her murder attempt.3
The term angel of death has religious roots in Judaism, although not strictly in the Hebrew scriptures. Neither the Jewish nor Christian Bible identifies a specific figure who is the angel of death. The idea first appears in Rabbinic literature and Jewish tradition.4 The angel God sends as a messenger of death is known as malakh ha-mavet in Hebrew. The revered Jewish physician and philosopher Moses Maimonides taught in his Guide for the Perplexed the angel of death is synonymous with the devil, and the evil inclination that dwells in the mind of all human beings.5In modern times, the concept of an angel of death has come to designate a serial killer who is a health care professional (HCP). A group of forensic scientists, HCPs, and attorneys, including former VA Under Secretary for Health Dr. Kenneth Kizer, published a study of HCPs who had been prosecuted or convicted of serial murder. Nurses constituted the largest group of offenders (60%) with nursing aides like Mays responsible for 18% of murders, and physicians 12%. The review found that though health care serial killers are rare, they operate in nations across the Western world, in many different states in this country, and in almost all health care settings, including previous VA angels of death.6Nursing aides who are not supposed to have access to medications—a major problem in Mays’ case—nor permitted to administer them more often resort to noncontrolled substances to kill their victims.1 Mays chose insulin as her murder weapon as did 13% of serial killers. Just as insulin may be difficult to detect in toxicology, so Mays and others like her committed their crimes on the night shift when they were less likely to be discovered.6
Many of us feel compelled to seek a rational motivation for why healers would mutate into killers: If we can find a reason for this heinous behavior it somehow helps us feel the world is more intelligible and controllable. Unfortunately, despite intensive forensic investigations of multiple angels of death, there is little definitive understanding of the motives of these murders.6 Mays disclosed more than most. As part of a plea bargain, she provided investigators with 2 rationales for her killing: She wanted to ease the patients’ suffering. Such claims of being an angel of mercy are common among HCP serial murders, which the patterns of the killings generally disprove. The patients Mays lethally injected, while mostly old and ill, were all expected to recover and leave the hospital. The Inspector General report uncovered a cautionary detail that has at least indirect bearing on the nursing assistant’s contention that she “wanted to let the patient’s die gently”: Contrary to VHA requirements, the facility had no functioning palliative care team. This finding in no way excuses or even explains Mays’ actions; it does, however, reinforce the essential value of palliative care expertise in an aging veteran population with many life-limiting conditions.7
Mays’ second motivation seems more plausible, based on her life narrative and the literature on HCP serial killers. Mays disclosed to investigators that she “had a lot of stress and chaos in [her] personal and professional life and these actions gave [her] a sense of control.”1 Her prior use of excessive force when employed at a prison as well as forensic science indicating that feelings of wielding power over life and death often drive health care murders, suggest this may have been a factor in Mays’ horrific conduct.8
It seems blasphemous to associate the word good in the same pages with this terrible evil. Nothing can compensate or justify the betrayal of the sacred oath of an HCP and the public trust of a VHA employee. Yet that very impossibility carries with it an obligation to ask, as did the author of an article about a recent Canadian nurse serial killer, “What can we learn from the [Mays] story?”9
Mays could never have taken the lives of 8 patients without clinical and administrative lapses and shortcuts at all levels of the health care system. Indeed, the 100-plus page Inspector General report makes 15 recommendations for the VHA, the Veterans Integrated Service Network, and the facility, encompassing areas of personnel hiring and performance evaluation, medication management and security, reporting and responding to unexplained events, quality and safety programs oversight, leaders’ responses, corrective actions, and even computer systems data analysis.
I want to suggest 2 ethical additions to this list addressed to all of us as VHA staff and especially to those of us who are HCPs. From the perspective of virtue ethics, Reta Mays is a tragedy about complacency and compromise in everyday work that the pandemic has made even more frequent and challenging to avoid and resist. This is what the Roman Virgil means in the epigraph that the road down to hell is easy and the road back very difficult.
I propose the need for discernment in trying to listen to our moral intuitions that tell us something is amiss and diligence in adhering to best practices even when we are fearful, exhausted, demoralized, or apathetic. These 2 habits of commitment to veterans, one of compassion and the other of competence, can help us follow the good inclinations of our hearts and together with system changes can bar the doors of our hospitals to the visits of future angels of death. This dedication is the least we owe to the families of the patients at Clarksburg whose loved ones never came home and whose questions likely can never be fully answered.
1. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration: care and oversight deficiencies related to multiple homicides at the Louis A. Johnson VA Medical Center in Clarksburg, West Virginia. Healthcare Inspection Report #20-035993-140. Published May 11 2021. Accessed November 22, 2021. https://www.va.gov/oig/pubs/VAOIG-20-03593-140.pdf
2. Kennedy M, Schwartz M. Former VA medical worker pleads guilty to murdering 7 patients in West Virginia. Published July 14, 2020. Accessed November 22, 2021. https://www.npr.org/2020/07/14/890776010/former-va-medical-worker-charged-with-7-murders-in-west-virginia
3. US Department of Justice, US Attorney’s Office Northern District of West Virginia. Former VA hospital nursing assistant sentenced to seven consecutive life sentences for murdering seven veterans and assault with intent to commit murder of an eighth [press release]. Published May 11, 2021. Accessed November 22, 2021. https://www.justice.gov/usao-ndwv/pr/former-va-hospital-nursing-assistant-sentenced-seven-consecutive-life-sentences.
4. Jacobs L. The Jewish Religion: A Companion. 1st ed. Oxford University Press;1995:116.
5. Maimonides. Guide for the Perplexed. Frielander M, trans. Routledge and Kegan Paul Ltd; 1904:pt 3, chap 22.
6. Yorker BC, Kizer KW, Lampe P, Forrest AR, Lannan JM, Russell DA. Serial murder by healthcare professionals. J Forensic Sci. 2006;51(6):1362-1371. doi:10.1111/j.1556-4029.2006.00273.x
7. VHA Directive 1139. Palliative care consult teams (PCCT) and VISN leads. Published June 14, 2017.
8. Rourke S, Ward T. Healthcare serial killers: patterns and policies. Published August 14, 2017. Accessed November 22, 2021. https://www.medscape.com/viewarticle/884136
9. Frank C. Health care serial murder: what can we learn from the Wettlaufer story? Can Fam Physician. 2020;66(10):719-722.
1. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration: care and oversight deficiencies related to multiple homicides at the Louis A. Johnson VA Medical Center in Clarksburg, West Virginia. Healthcare Inspection Report #20-035993-140. Published May 11 2021. Accessed November 22, 2021. https://www.va.gov/oig/pubs/VAOIG-20-03593-140.pdf
2. Kennedy M, Schwartz M. Former VA medical worker pleads guilty to murdering 7 patients in West Virginia. Published July 14, 2020. Accessed November 22, 2021. https://www.npr.org/2020/07/14/890776010/former-va-medical-worker-charged-with-7-murders-in-west-virginia
3. US Department of Justice, US Attorney’s Office Northern District of West Virginia. Former VA hospital nursing assistant sentenced to seven consecutive life sentences for murdering seven veterans and assault with intent to commit murder of an eighth [press release]. Published May 11, 2021. Accessed November 22, 2021. https://www.justice.gov/usao-ndwv/pr/former-va-hospital-nursing-assistant-sentenced-seven-consecutive-life-sentences.
4. Jacobs L. The Jewish Religion: A Companion. 1st ed. Oxford University Press;1995:116.
5. Maimonides. Guide for the Perplexed. Frielander M, trans. Routledge and Kegan Paul Ltd; 1904:pt 3, chap 22.
6. Yorker BC, Kizer KW, Lampe P, Forrest AR, Lannan JM, Russell DA. Serial murder by healthcare professionals. J Forensic Sci. 2006;51(6):1362-1371. doi:10.1111/j.1556-4029.2006.00273.x
7. VHA Directive 1139. Palliative care consult teams (PCCT) and VISN leads. Published June 14, 2017.
8. Rourke S, Ward T. Healthcare serial killers: patterns and policies. Published August 14, 2017. Accessed November 22, 2021. https://www.medscape.com/viewarticle/884136
9. Frank C. Health care serial murder: what can we learn from the Wettlaufer story? Can Fam Physician. 2020;66(10):719-722.
Evaluation of Intermittent Energy Restriction and Continuous Energy Restriction on Weight Loss and Blood Pressure Control in Overweight and Obese Patients With Hypertension
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
Study Overview
Objective. To compare the effects of intermittent energy restriction (IER) with those of continuous energy restriction (CER) on blood pressure control and weight loss in overweight and obese patients with hypertension during a 6-month period.
Design. Randomized controlled trial.
Settings and participants. The trial was conducted at the Affiliated Hospital of Jiaxing University from June 1, 2020, to April 30, 2021. Chinese adults were recruited using advertisements and flyers posted in the hospital and local communities. Prior to participation in study activities, all participants gave informed consent prior to recruitment and were provided compensation in the form of a $38 voucher at 3 and 6 months for their time for participating in the study.
The main inclusion criteria were patients between the ages of 18 and 70 years, hypertension, and body mass index (BMI) ranging from 24 to 40 kg/m2. The exclusion criteria were systolic blood pressure (SBP) ≥ 180 mmHg or diastolic blood pressure (DBP) ≥ 120 mmHg, type 1 or 2 diabetes with a history of severe hypoglycemic episodes, pregnancy or breastfeeding, usage of glucagon-like peptide 1 receptor agonists, weight loss > 5 kg within the past 3 months or previous weight loss surgery, and inability to adhere to the dietary protocol.
Of the 294 participants screened for eligibility, 205 were randomized in a 1:1 ratio to the IER group (n = 102) or the CER group (n = 103), stratified by sex and BMI (as overweight or obese). All participants were required to have a stable medication regimen and weight in the 3 months prior to enrollment and not to use weight-loss drugs or vitamin supplements for the duration of the study. Researchers and participants were not blinded to the study group assignment.
Interventions. Participants randomly assigned to the IER group followed a 5:2 eating pattern: a very-low-energy diet of 500-600 kcal for 2 days of the week along with their usual diet for the other 5 days. The 2 days of calorie restriction could be consecutive or nonconsecutive, with a minimum of 0.8 g supplemental protein per kg of body weight per day, in accordance with the 2016 Dietary Guidelines for Chinese Residents. The CER group was advised to consume 1000 kcal/day for women and 1200 kcal/day for men on a 7-day energy restriction. That is, they were prescribed a daily 25% restriction based on the general principles of a Mediterranean-type diet (30% fat, 45-50% carbohydrate, and 20-25% protein).
Both groups received dietary education from a qualified dietitian and were recommended to maintain their current daily activity levels throughout the trial. Written dietary information brochures with portion advice and sample meal plans were provided to improve compliance in each group. All participants received a digital cooking scale to weigh foods to ensure accuracy of intake and were required to keep a food diary while following the recommended recipe on 2 days/week during calorie restriction to help with adherence. No food was provided. All participants were followed up by regular outpatient visits to both cardiologists and dietitians once a month. Diet checklists, activity schedules, and weight were reviewed to assess compliance with dietary advice at each visit.
Of note, participants were encouraged to measure and record their BP twice daily, and if 2 consecutive BP readings were < 110/70 mmHg and/or accompanied by hypotensive episodes with symptoms (dizziness, nausea, headache, and fatigue), they were asked to contact the investigators directly. Antihypertensive medication changes were then made in consultation with cardiologists. In addition, a medication management protocol (ie, doses of antidiabetic medications, including insulin and sulfonylurea) was designed to avoid hypoglycemia. Medication could be reduced in the CER group based on the basal dose at the endocrinologist’s discretion. In the IER group, insulin and sulfonylureas were discontinued on calorie restriction days only, and long-acting insulin was discontinued the night before the IER day. Insulin was not to be resumed until a full day’s caloric intake was achieved.
Measures and analysis. The primary outcomes of this study were changes in BP and weight (measured using an automatic digital sphygmomanometer and an electronic scale), and the secondary outcomes were changes in body composition (assessed by dual-energy x-ray absorptiometry scanning), as well as glycosylated hemoglobin A1c (HbA1c) levels and blood lipids after 6 months. All outcome measures were recorded at baseline and at each monthly visit. Incidence rates of hypoglycemia were based on blood glucose (defined as blood glucose < 70 mg/dL) and/or symptomatic hypoglycemia (symptoms of sweating, paleness, dizziness, and confusion). Two cardiologists who were blind to the patients’ diet condition measured and recorded all pertinent clinical parameters and adjudicated serious adverse events.
Data were compared using independent-samples t-tests or the Mann–Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorial variables as appropriate. Repeated-measures ANOVA via a linear mixed model was employed to test the effects of diet, time, and their interaction. In subgroup analyses, differential effects of the intervention on the primary outcomes were evaluated with respect to patients’ level of education, domicile, and sex based on the statistical significance of the interaction term for the subgroup of interest in the multivariate model. Analyses were performed based on completers and on an intention-to-treat principle.
Main results. Among the 205 randomized participants, 118 were women and 87 were men; mean (SD) age was 50.5 (8.8) years; mean (SD) BMI was 28.7 (2.6); mean (SD) SBP was 143 (10) mmHg; and mean (SD) DBP was 91 (9) mmHg. At the end of the 6-month intervention, 173 (84.4%) completed the study (IER group: n = 88; CER group: n = 85). Both groups had similar dropout rates at 6 months (IER group: 14 participants [13.7%]; CER group: 18 participants [17.5%]; P = .83) and were well matched for baseline characteristics except for triglyceride levels.
In the completers analysis, both groups experienced significant reductions in weight (mean [SEM]), but there was no difference between treatment groups (−7.2 [0.6] kg in the IER group vs −7.1 [0.6] kg in the CER group; diet by time P = .72). Similarly, the change in SBP and DBP achieved was statistically significant over time, but there was also no difference between the dietary interventions (−8 [0.7] mmHg in the IER group vs −8 [0.6] mmHg in the CER group, diet by time P = .68; −6 [0.6] mmHg in the IER group vs −6 [0.5] mmHg in the CER group, diet by time P = .53]. Subgroup analyses of the association of the intervention with weight, SBP and DBP by sex, education, and domicile showed no significant between-group differences.
All measures of body composition decreased significantly at 6 months with both groups experiencing comparable reductions in total fat mass (−5.5 [0.6] kg in the IER group vs −4.8 [0.5] kg in the CER group, diet by time P = .08) and android fat mass (−1.1 [0.2] kg in the IER group vs −0.8 [0.2] kg in the CER group, diet by time P = .16). Of note, participants in the CER group lost significantly more total fat-free mass than did participants in the IER group (mean [SEM], −2.3 [0.2] kg vs −1.7 [0.2] kg; P = .03], and there was a trend toward a greater change in total fat mass in the IER group (P = .08). The secondary outcome of mean (SEM) HbA1c (−0.2% [0.1%]) and blood lipid levels (triglyceride level, −1.0 [0.3] mmol/L; total cholesterol level, −0.9 [0.2] mmol/L; low-density lipoprotein cholesterol level, −0.9 [0.2 mmol/L; high-density lipoprotein cholesterol level, 0.7 [0.3] mmol/L] improved with weight loss (P < .05), with no differences between groups (diet by time P > .05).
The intention-to-treat analysis demonstrated that IER and CER are equally effective for weight loss and blood pressure control: both groups experienced significant reductions in weight, SBP, and DBP, but with no difference between treatment groups – mean (SEM) weight change with IER was −7.0 (0.6) kg vs −6.8 (0.6) kg with CER; the mean (SEM) SBP with IER was −7 (0.7) mmHg vs −7 (0.6) mmHg with CER; and the mean (SEM) DBP with IER was −6 (0.5) mmHg vs −5 (0.5) mmHg with CER, (diet by time P = .62, .39, and .41, respectively). There were favorable improvements in
Conclusion. A 2-day severe energy restriction with 5 days of habitual eating compared to 7 days of CER provides an acceptable alternative for BP control and weight loss in overweight and obese individuals with hypertension after 6 months. IER may offer a useful alternative strategy for this population, who find continuous weight-loss diets too difficult to maintain.
Commentary
Globally, obesity represents a major health challenge as it substantially increases the risk of diseases such as hypertension, type 2 diabetes, and coronary heart disease.1 Lifestyle modifications, including weight loss and increased physical activity, are recommended in major guidelines as a first-step intervention in the treatment of hypertensive patients.2 However, lifestyle and behavioral interventions aimed at reducing calorie intake through low-calorie dieting is challenging as it is dependent on individual motivation and adherence to a strict, continuous protocol. Further, CER strategies have limited effectiveness because complex and persistent hormonal, metabolic, and neurochemical adaptations defend against weight loss and promote weight regain.3-4 IER has drawn attention in the popular media as an alternative to CER due to its feasibility and even potential for higher rates of compliance.5
This study adds to the literature as it is the first randomized controlled trial (to the knowledge of the authors at the time of publication) to explore 2 forms of energy restriction – CER and IER – and their impact on weight loss, BP, body composition, HbA1c, and blood lipid levels in overweight and obese patients with high blood pressure. Results from this study showed that IER is as effective as, but not superior to, CER (in terms of the outcomes measures assessed). Specifically, findings highlighted that the 5:2 diet is an effective strategy and noninferior to that of daily calorie restriction for BP and weight control. In addition, both weight loss and BP reduction were greater in a subgroup of obese compared with overweight participants, which indicates that obese populations may benefit more from energy restriction. As the authors highlight, this study both aligns with and expands on current related literature.
This study has both strengths and limitations, especially with regard to the design and data analysis strategy. A key strength is the randomized controlled trial design which enables increased internal validity and decreases several sources of bias, including selection bias and confounding. In addition, it was also designed as a pragmatic trial, with the protocol reflecting efforts to replicate the real-world environment by not supplying meal replacements or food. Notably, only 9 patients could not comply with the protocol, indicating that acceptability of the diet protocol was high. However, as this was only a 6-month long study, further studies are needed to determine whether a 5:2 diet is sustainable (and effective) in the long-term compared with CER, which the authors highlight. The study was also adequately powered to detect clinically meaningful differences in weight loss and SBP, and appropriate analyses were performed on both the basis of completers and on an intention-to-treat principle. However, further studies are needed that are adequately powered to also detect clinically meaningful differences in the other measures, ie, body composition, HbA1c, and blood lipid levels. Importantly, generalizability of findings from this study is limited as the study population comprises only Chinese adults, predominately middle-aged, overweight, and had mildly to moderately elevated SBP and DBP, and excluded diabetic patients. Thus, findings are not necessarily applicable to individuals with highly elevated blood pressure or poorly controlled diabetes.
Applications for Clinical Practice
Results of this study demonstrated that IER is an effective alternative diet strategy for weight loss and blood pressure control in overweight and obese patients with hypertension and is comparable to CER. This is relevant for clinical practice as IER may be easier to maintain in this population compared to continuous weight-loss diets. Importantly, both types of calorie restriction require clinical oversight as medication changes and periodic monitoring of hypotensive and hypoglycemic episodes are needed. Clinicians should consider what is feasible and sustainable for their patients when recommending intermittent energy restriction.
Financial disclosures: None.
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi:10.1038/s41574-019-0176-8
2. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global hypertension practice guidelines. J Hypertens. 2020;38(6):982-1004. doi:10.1097/HJH.0000000000002453
3. Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413-423. doi:10.1007/s13679-016-0237-4
4. Sainsbury A, Wood RE, Seimon RV, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction. Obes Rev. 2018;19 Suppl 1:47–60. doi:10.1111/obr.12787
5. Davis CS, Clarke RE, Coulter SN, et al. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292-299. doi:10.1038/ejcn.2015.195
Preoperative Code Status Discussion in Older Adults: Are We Doing Enough?
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
Study Overview
Objective. The objective of this study was to evaluate orders and documentation describing perioperative management of code status in adults.
Design. A retrospective case series of all adult inpatients admitted to hospitals at 1 academic health system in the US.
Setting and participants. This retrospective case series was conducted at 5 hospitals within the University of Pennsylvania Health System. Cases included all adult inpatients admitted to hospitals between March 2017 and September 2018 who had a Do-Not-Resuscitate (DNR) order placed in their medical record during admission and subsequently underwent a surgical procedure that required anesthesia care.
Main outcome measures. Medical records of included cases were manually reviewed by the authors to verify whether a DNR order was in place at the time surgical intervention was discussed with a patient. Clinical notes and DNR orders of eligible cases were reviewed to identify documentation and outcome of goals of care discussions that were conducted within 48 hours prior to the surgical procedure. Collected data included patient demographics (age, sex, race); case characteristics (American Society of Anesthesiologists [ASA] physical status score, anesthesia type [general vs others such as regional], emergency status [emergent vs elective surgery], procedures by service [surgical including hip fracture repair, gastrostomy or jejunostomy, or exploratory laparotomy vs medical including endoscopy, bronchoscopy, or transesophageal echocardiogram]); and hospital policy for perioperative management of DNR orders (written policy encouraging discussion vs written policy plus additional initiatives, including procedure-specific DNR form). The primary outcome was the presence of a preoperative order or note documenting code status discussion or change. Data were analyzed using χ2 and Fisher exact tests and the threshold for statistical significance was P < .05.
Main results. Of the 27 665 inpatient procedures identified across 5 hospitals, 444 (1.6%) cases met the inclusion criteria. Patients from these cases aged 75 (SD 13) years (95% CI, 72-77 years); 247 (56%, 95% CI, 55%-57%) were women; and 300 (68%, 95% CI, 65%-71%) were White. A total of 426 patients (96%, 95% CI, 90%-100%) had an ASA physical status score of 3 or higher and 237 (53%, 95% CI, 51%-56%) received general anesthesia. The most common procedures performed were endoscopy (148 [33%]), hip fracture repair (43 [10%]), and gastrostomy or jejunostomy (28 [6%]). Reevaluation of code status was documented in 126 cases (28%, 95% CI, 25%-31%); code status orders were changed in 20 of 126 cases (16%, 95% CI, 7%-24%); and a note was filed without a corresponding order for 106 of 126 cases (84%, 95% CI, 75%-95%). In the majority of cases (109 of 126 [87%], 95% CI, 78%-95%) in which documented discussion occurred, DNR orders were suspended. Of 126 cases in which a discussion was documented, participants of these discussions included surgeons 10% of the time (13 cases, 95% CI, 8%-13%), members of the anesthesia team 51% of the time (64 cases, 95% CI, 49%-53%), and medicine or palliative care clinicians 39% of the time (49 cases, 95% CI, 37%-41%).
The rate of documented preoperative code status discussion was higher in patients with higher ASA physical status score (35% in patients with an ASA physical status score ≥ 4 [55 of 155] vs 25% in those with an ASA physical status score ≤ 3 [71 of 289]; P = .02). The rates of documented preoperative code status discussion were similar by anesthesia type (29% for general anesthesia [69 of 237 cases] vs 28% [57 of 207 cases] for other modalities; P = .70). The hospitals involved in this study all had a written policy encouraging rediscussion of code status before surgery. However, only 1 hospital reported added measures (eg, provision of a procedure-specific DNR form) to increase documentation of preoperative code status discussions. In this specific hospital, documentation of preoperative code status discussions was higher compared to other hospitals (67% [37 of 55 cases] vs 23% [89 of 389 cases]; P < .01).
Conclusion. In a retrospective case series conducted at 5 hospitals within 1 academic health system in the US, fewer than 1 in 5 patients with preexisting DNR orders had a documented discussion of code status prior to undergoing surgery. Additional strategies including the development of institutional protocols that facilitate perioperative management of advance directives, identification of local champions, and patient education, should be explored as means to improve preoperative code status reevaulation per guideline recommendations.
Commentary
It is not unusual that patients with a DNR order may require and undergo surgical interventions to treat reversible conditions, prevent progression of underlying disease, or mitigate distressing symptoms such as pain. For instance, intubation, mechanical ventilation, and administration of vasoactive drugs are resuscitative measures that may be needed to safely anesthetize and sedate a patient. As such, the American College of Surgeons1 has provided a statement on advance directives by patients with an existing DNR order to guide management. Specifically, the statement indicates that the best approach for these patients is a policy of “required reconsideration” of the existing DNR order. Required reconsideration means that “the patient or designated surrogate and the physicians who will be responsible for the patient’s care should, when possible, discuss the new intraoperative and perioperative risks associated with the surgical procedure, the patient’s treatment goals, and an approach for potentially life-threatening problems consistent with the patient’s values and preferences.” Moreover, the required reconsideration discussion needs to occur as early as it is practical once a decision is made to have surgery because the discussion “may result in the patient agreeing to suspend the DNR order during surgery and the perioperative period, retaining the original DNR order, or modifying the DNR order.” Given that surgical patients with DNR orders have significant comorbidities, many sustain postoperative complications, and nearly 1 in 4 die within 30 days of surgery, preoperative advance care planning (ACP) and code status discussions are particularly essential to delivering high quality surgical care.2
In the current study, Hadler et al3 conducted a retrospective analysis to evaluate orders and documentation describing perioperative management of code status in patients with existing DNR order at an academic health system in the US. The authors reported that fewer than 20% of patients with existing DNR orders had a documented discussion of code status prior to undergoing surgery. These findings add to the notion that compliance with such guidance on required reconsideration discussion is suboptimal in perioperative care in the US.4,5 A recently published study focused on patients aged more than 60 years undergoing high-risk oncologic or vascular surgeries similarly showed that the frequency of ACP discussions or advance directive documentations among older patients was low.6 This growing body of evidence is highly clinically relevant in that preoperative discussion on code status is highly relevant to the care of older adults, a population group that accounts for the majority of surgeries and is most vulnerable to poor surgical outcomes. Additionally, it highlights a disconnect between the shared recognition by surgeons and patients that ACP discussion is important in perioperative care and its low implementation rates.
Unsurprisingly, Hadler et al3 reported that added measures such as the provision of a procedure-specific DNR form led to an increase in the documentation of preoperative code status discussions in 1 of the hospitals studied. The authors suggested that strategies such as the development of institutional protocols aimed to facilitate perioperative advance directive discussions, identify local champions, and educate patients may be ways to improve preoperative code status reevaulation. The idea that institutional value and culture are key factors impacting surgeon behavior and may influence the practice of ACP discussion is not new. Thus, creative and adaptable strategies, resources, and trainings that are required by medical institutions and hospitals to support preoperative ACP discussions with patients undergoing surgeries need to be identified, validated, and implemented to optimize perioperative care in vulnerable patients.
Applications for Clinical Practice
The findings from the current study indicate that less than 20% of patients with preexisting DNR orders have a documented discussion of code status prior to undergoing surgery. Physicians and health care institutions need to identify barriers to, and implement strategies that, facilitate and optimize preoperative ACP discussions in order to provide patient-centered care in vulnerable surgical patients.
Financial disclosures: None.
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
1. American College of Surgeons Board of Regents. Statement on Advance Directives by Patients: “Do Not Resuscitate” in the Operating Room. American College of Surgeons. January 3, 2014. Accessed November 6, 2021. https://www.facs.org/about-acs/statements/19-advance-directives
2. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. doi:10.1001/archsurg.2011.69
3. Hadler RA, Fatuzzo M, Sahota G, Neuman MD. Perioperative Management of Do-Not-Resuscitate Orders at a Large Academic Health System. JAMA Surg. 2021;e214135. doi:10.1001/jamasurg.2021.4135
4. Coopmans VC, Gries CA. CRNA awareness and experience with perioperative DNR orders. AANA J. 2000;68(3):247-256.
5. Urman RD, Lilley EJ, Changala M, Lindvall C, Hepner DL, Bader AM. A Pilot Study to Evaluate Compliance with Guidelines for Preprocedural Reconsideration of Code Status Limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
6. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
FFR-Guided or Angiography-Guided Nonculprit Lesion PCI in Patients With STEMI Without Cardiogenic Shock
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
Association Between Physiotherapy Outcome Measures and the Functional Independence Measure: A Retrospective Analysis
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)
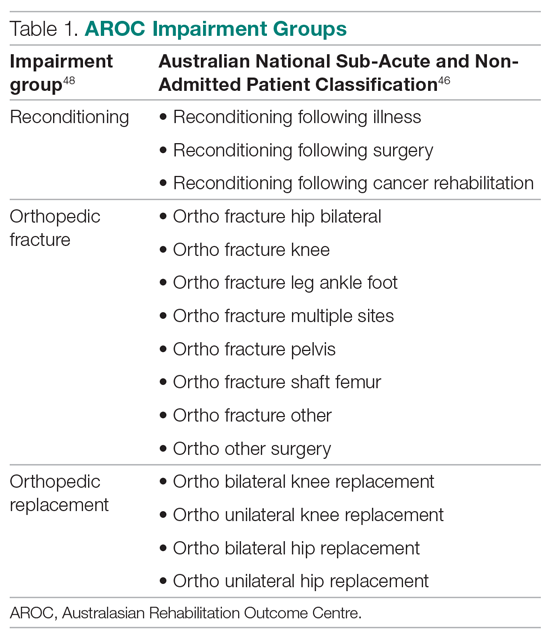
Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.
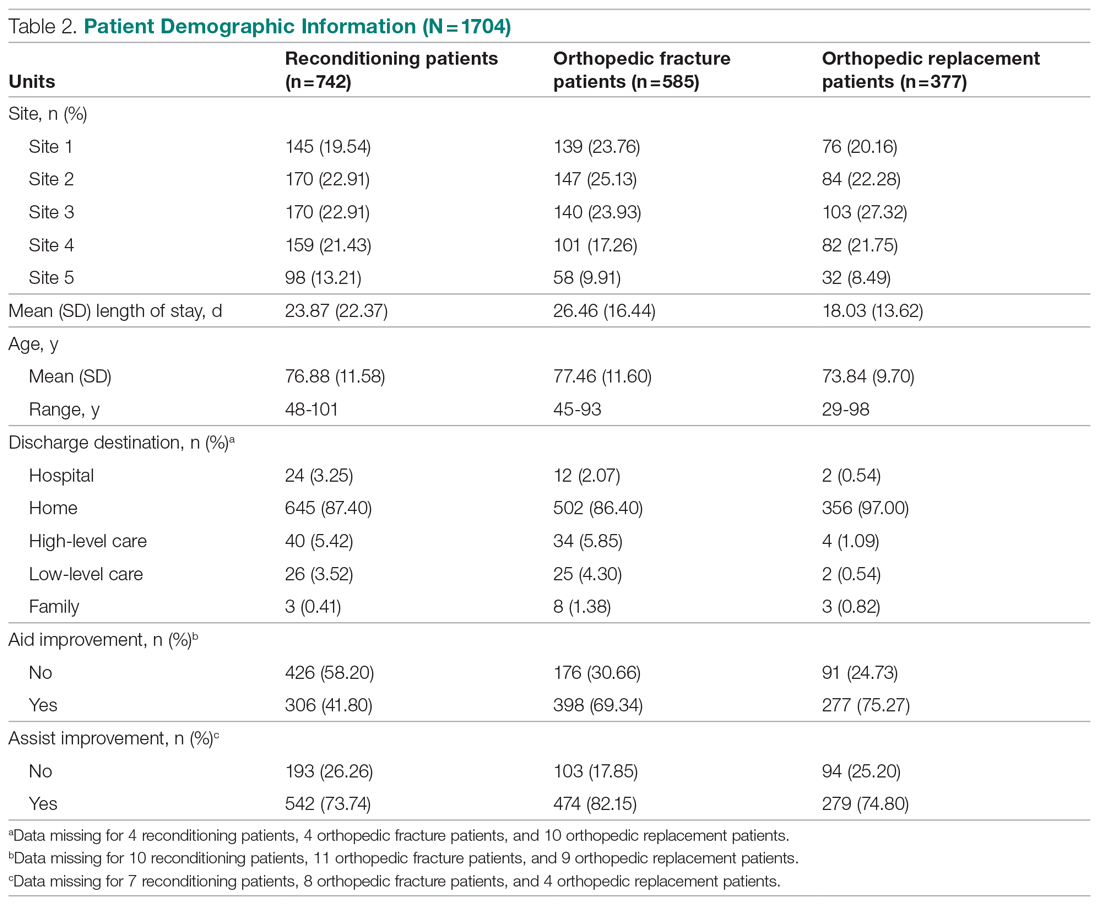
Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).
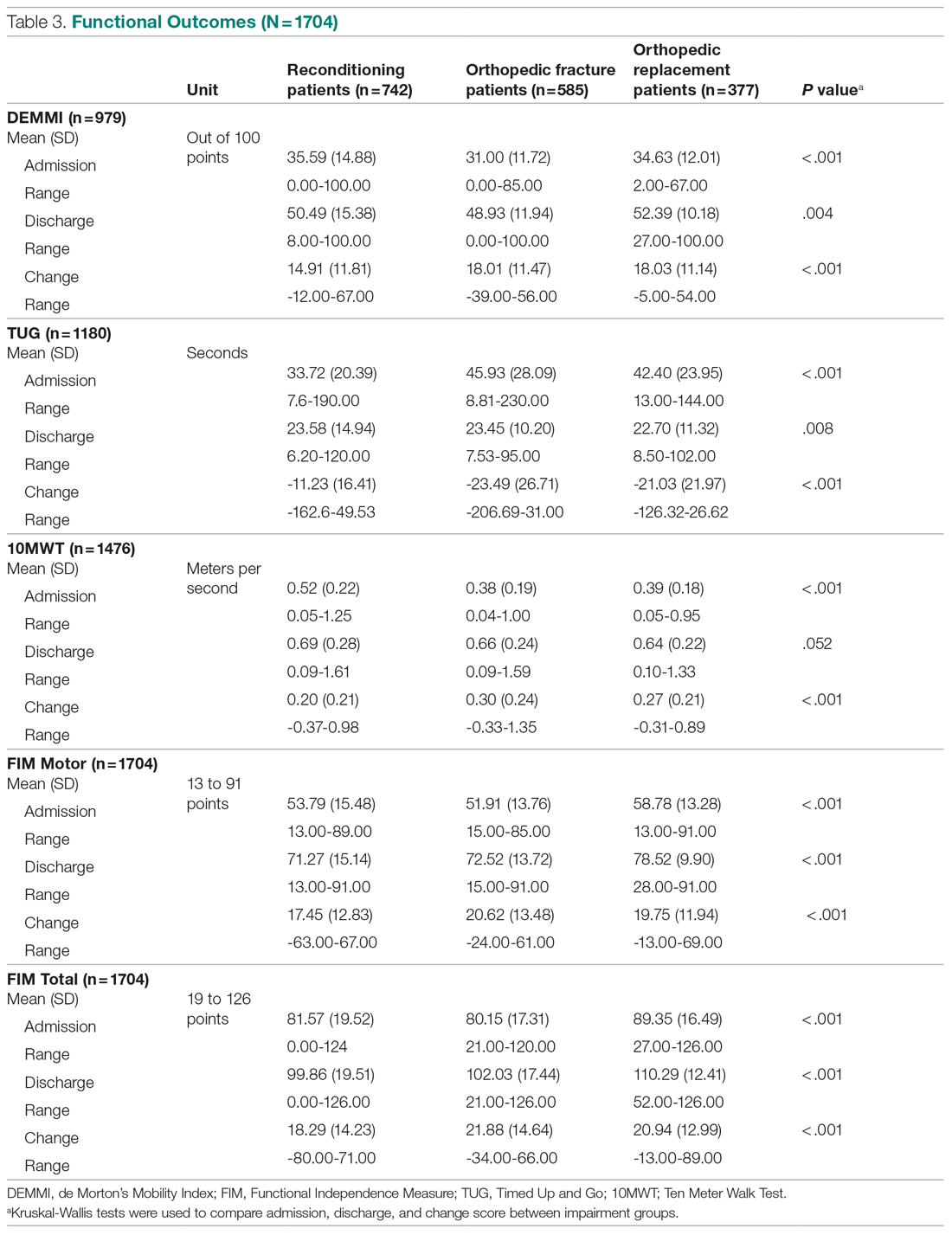
Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).
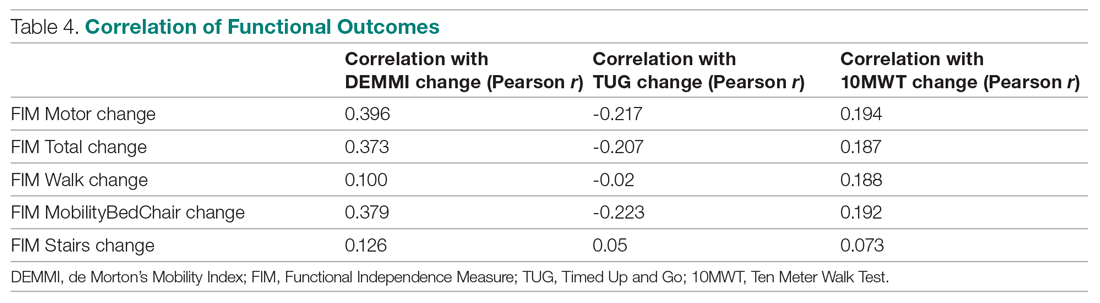
Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; maren.jones@health.nsw.gov.au.
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)

Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.

Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).

Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).

Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; maren.jones@health.nsw.gov.au.
Financial disclosures: None.
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)

Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.

Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).

Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).

Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; maren.jones@health.nsw.gov.au.
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.