User login
GI Doc Empowers Female Patients ‘To Be Themselves’
Pooja Singhal, MD, AGAF, will never forget the time a female patient came in for gastroesophageal reflux disease and dysphagia treatment, revealing that she had already gone through multiple gastroenterologists to help diagnose and treat her ailments.
“We spent a whole visit talking about it,” said Dr. Singhal, a gastroenterologist, hepatologist, and obesity medicine specialist at Oklahoma Gastro Health and Wellness in Oklahoma City. During the exam, she discovered that her middle-aged patient was wearing an adult diaper for diarrhea and leakage.
Previous GI doctors told the patient they couldn’t help her and that she had to live with these symptoms. “I was just so shocked. I told her: This is not normal. Let’s talk more about it. Let’s figure out how we can manage it,” said Dr. Singhal, who has spent her career advocating for more education about GI conditions.
There are real barriers to patients opening up and sharing their symptoms, especially if they’re female. while ensuring that the correct knowledge gets across to the public, said Dr. Singhal.
An alumna of the American Gastroenterological Association’s (AGA) Future Leaders Program, Dr. Singhal has served as the private practice course director for AGA’s Midwest Women in GI Workshop. She is a also a four-time recipient of the SCOPY award for her work in raising community awareness of colorectal cancer prevention in Oklahoma. In an interview, she discussed the critical role women GI doctors play in assisting the unique needs of female patients, and why it takes a village of doctors to treat the complexities of GI disorders.
Why did you choose GI, and more specifically, what brought about your interest in women’s GI issues?
GI is simply the best field. While I was doing my rotation in GI as a resident, I was enthralled and humbled that the field of gastroenterology offered an opportunity to prevent cancer. Colon cancer is the second leading cause of cancer related deaths, and when I realized that we could do these micro-interventions during a procedure to remove polyps that could potentially turn into cancer — or give us an opportunity to remove carcinoma in situ — that’s what really inspired me and piqued my interest in GI. As I continued to learn and explore GI more, I appreciated the opportunity the field gave us in terms of using both sides of our brains equally, the right side and the left side.
I love the diagnostic part of medicine. You have this privilege to be able to diagnose so many different diseases and perform procedures using technical skills, exploring everything from the esophagus, liver, pancreas, small bowel, and colon.
But what I really appreciate about gastroenterology is how it’s piqued my interest in women’s digestive health. How it became very close to my heart is really from my patients. I’ve learned a lot from my patients throughout the years. When I was much younger, I don’t know if I really appreciated the vulnerability it takes as a woman to go to a physician and talk about hemorrhoids and diarrhea.
One of the comments I often receive is: ‘Oh, thank God you’re a female GI. I can be myself. I can share something personal and you would understand.’
Your practice places a specific emphasis on health and wellness. Can you provide some examples of how you incorporate wellness into treatment?
I feel like wellness is very commonplace now. To me, the definition of wellness is about practicing healthy habits to attain your maximum potential, both physically and mentally — to feel the best you can. My practice specifically tries to achieve that goal by placing a strong emphasis on education and communication. We provide journals where patients can keep track of their symptoms. We encourage a lot of discussion during visits, where we talk about GI diseases and how to prevent them, or to prevent them from happening again. If you’re going to do a hemorrhoid treatment that offers hemorrhoid banding, we talk about it in detail with the patient; we don’t just do the procedure.
We have a dietitian on staff for conditions like inflammatory bowel disease, Crohn’s and ulcerative colitis, celiac disease, IBS. Some of our older patients have pelvic organ prolapse and fecal incontinence. We have a pelvic floor therapist and a urogynecologist, and we work very closely with ob-gyn teams. My practice also takes pride in communicating with primary care physicians. We’ve had patients who have had memory loss or dementia or are grieving the loss of a loved one. And we prioritize communicating and treating patients as a whole and not focusing on just their GI symptoms.
As an advocate for community education on GI disorders, where is education lacking in this field?
I think education is lacking because there is an information delivery gap. I feel the public consumes information in the form of short social media reels. The attention span is so short and any scientific information, especially around diseases, can be scary and overwhelming. Whereas I think a lot of the medical community still interacts and exchanges information in terms of journals and publications. So, we are not really trained necessarily to talk about diseases in very simple terms.
We need more advocacy efforts on Capitol Hill. AGA has been good about doing advocacy work. I had an opportunity to go to Capitol Hill a couple of times and really advocate for policy around obesity medicine coverage and procedure coverage. I was fortunate to learn so much about healthcare policy, but it also made me appreciate that there are a lot of gaps in terms of understanding common medical diseases.
You’re trained in the Orbera Intragastric balloon system for weight reduction. How does this procedure differentiate from other bariatric procedures?
Intragastric balloon is Food and Drug Administration approved for weight loss. It’s a temporary medical device, so it’s reversible. No. 2, it’s a nonsurgical intervention, so it’s usually done in an outpatient setting. We basically place a deflated gastric balloon endoscopically, similar to an upper endoscopy method. We take a pin endoscope, a deflated balloon, which is made of medical-grade material, and we inflate it with adequate fluid. The concept is when the balloon is inflated, it provides satiety. It reduces the amount of space in the stomach for food. It slows down how quickly the food is going to leave. So you feel full much of the time. And it also helps decrease a hormone called ghrelin, which is responsible for hunger. It can make a big difference when people are gaining weight and in that category of overweight before they progress to obese.
As I tell everybody, obesity is a chronic lifelong disease that is very complex and requires lifelong efforts. So, it’s truly a journey. What’s made this procedure a success is follow-up and the continued efforts of dietitians and counseling and incorporating physical exercise, because maintenance of that weight loss is also very important. Our goal is always sustained weight loss and not just short-term weight loss.
As the practice course director for the AGA’s Midwest Women in GI Workshop, can you tell me how this course came about? What does the workshop cover?
This workshop is a brainchild of AGA. This will be the third year of having these workshops. It’s been divided into regional workshops, so more people can attend. But it arose from the recognition that there is a need to have a support system, a forum where discussions on navigating career and life transitions with grace can happen, and more resources for success can be provided.
There is so much power in learning from shared experiences. And I think that was huge, to realize that we are not alone. We can celebrate our achievements together and acknowledge our challenges together, and then come together to brainstorm and innovate to solve problems and advocate for health equity.
You’ve been involved with community, non-profit organizations like the Homeless Alliance in Oklahoma City. How has this work enriched your life outside of medicine?
I feel like we sometimes get tunnel vision, talking to people in the same line of work. It was extremely important for me to broaden my horizons by learning from people outside of the medical community and from organizations like Homeless Alliance, which allowed me a platform to understand what my community needs. It’s an incredible organization that helps provide shelter not only for human beings, but also pets. The freezing temperatures over the last few months provided unique challenges like overflow in homeless shelters. I’ve learned so many things, such as how to ask for grants and how to allocate those funds. It has been absolutely enriching to me to learn about my community needs and see what an amazing difference people in the community are making.
Pooja Singhal, MD, AGAF, will never forget the time a female patient came in for gastroesophageal reflux disease and dysphagia treatment, revealing that she had already gone through multiple gastroenterologists to help diagnose and treat her ailments.
“We spent a whole visit talking about it,” said Dr. Singhal, a gastroenterologist, hepatologist, and obesity medicine specialist at Oklahoma Gastro Health and Wellness in Oklahoma City. During the exam, she discovered that her middle-aged patient was wearing an adult diaper for diarrhea and leakage.
Previous GI doctors told the patient they couldn’t help her and that she had to live with these symptoms. “I was just so shocked. I told her: This is not normal. Let’s talk more about it. Let’s figure out how we can manage it,” said Dr. Singhal, who has spent her career advocating for more education about GI conditions.
There are real barriers to patients opening up and sharing their symptoms, especially if they’re female. while ensuring that the correct knowledge gets across to the public, said Dr. Singhal.
An alumna of the American Gastroenterological Association’s (AGA) Future Leaders Program, Dr. Singhal has served as the private practice course director for AGA’s Midwest Women in GI Workshop. She is a also a four-time recipient of the SCOPY award for her work in raising community awareness of colorectal cancer prevention in Oklahoma. In an interview, she discussed the critical role women GI doctors play in assisting the unique needs of female patients, and why it takes a village of doctors to treat the complexities of GI disorders.
Why did you choose GI, and more specifically, what brought about your interest in women’s GI issues?
GI is simply the best field. While I was doing my rotation in GI as a resident, I was enthralled and humbled that the field of gastroenterology offered an opportunity to prevent cancer. Colon cancer is the second leading cause of cancer related deaths, and when I realized that we could do these micro-interventions during a procedure to remove polyps that could potentially turn into cancer — or give us an opportunity to remove carcinoma in situ — that’s what really inspired me and piqued my interest in GI. As I continued to learn and explore GI more, I appreciated the opportunity the field gave us in terms of using both sides of our brains equally, the right side and the left side.
I love the diagnostic part of medicine. You have this privilege to be able to diagnose so many different diseases and perform procedures using technical skills, exploring everything from the esophagus, liver, pancreas, small bowel, and colon.
But what I really appreciate about gastroenterology is how it’s piqued my interest in women’s digestive health. How it became very close to my heart is really from my patients. I’ve learned a lot from my patients throughout the years. When I was much younger, I don’t know if I really appreciated the vulnerability it takes as a woman to go to a physician and talk about hemorrhoids and diarrhea.
One of the comments I often receive is: ‘Oh, thank God you’re a female GI. I can be myself. I can share something personal and you would understand.’
Your practice places a specific emphasis on health and wellness. Can you provide some examples of how you incorporate wellness into treatment?
I feel like wellness is very commonplace now. To me, the definition of wellness is about practicing healthy habits to attain your maximum potential, both physically and mentally — to feel the best you can. My practice specifically tries to achieve that goal by placing a strong emphasis on education and communication. We provide journals where patients can keep track of their symptoms. We encourage a lot of discussion during visits, where we talk about GI diseases and how to prevent them, or to prevent them from happening again. If you’re going to do a hemorrhoid treatment that offers hemorrhoid banding, we talk about it in detail with the patient; we don’t just do the procedure.
We have a dietitian on staff for conditions like inflammatory bowel disease, Crohn’s and ulcerative colitis, celiac disease, IBS. Some of our older patients have pelvic organ prolapse and fecal incontinence. We have a pelvic floor therapist and a urogynecologist, and we work very closely with ob-gyn teams. My practice also takes pride in communicating with primary care physicians. We’ve had patients who have had memory loss or dementia or are grieving the loss of a loved one. And we prioritize communicating and treating patients as a whole and not focusing on just their GI symptoms.
As an advocate for community education on GI disorders, where is education lacking in this field?
I think education is lacking because there is an information delivery gap. I feel the public consumes information in the form of short social media reels. The attention span is so short and any scientific information, especially around diseases, can be scary and overwhelming. Whereas I think a lot of the medical community still interacts and exchanges information in terms of journals and publications. So, we are not really trained necessarily to talk about diseases in very simple terms.
We need more advocacy efforts on Capitol Hill. AGA has been good about doing advocacy work. I had an opportunity to go to Capitol Hill a couple of times and really advocate for policy around obesity medicine coverage and procedure coverage. I was fortunate to learn so much about healthcare policy, but it also made me appreciate that there are a lot of gaps in terms of understanding common medical diseases.
You’re trained in the Orbera Intragastric balloon system for weight reduction. How does this procedure differentiate from other bariatric procedures?
Intragastric balloon is Food and Drug Administration approved for weight loss. It’s a temporary medical device, so it’s reversible. No. 2, it’s a nonsurgical intervention, so it’s usually done in an outpatient setting. We basically place a deflated gastric balloon endoscopically, similar to an upper endoscopy method. We take a pin endoscope, a deflated balloon, which is made of medical-grade material, and we inflate it with adequate fluid. The concept is when the balloon is inflated, it provides satiety. It reduces the amount of space in the stomach for food. It slows down how quickly the food is going to leave. So you feel full much of the time. And it also helps decrease a hormone called ghrelin, which is responsible for hunger. It can make a big difference when people are gaining weight and in that category of overweight before they progress to obese.
As I tell everybody, obesity is a chronic lifelong disease that is very complex and requires lifelong efforts. So, it’s truly a journey. What’s made this procedure a success is follow-up and the continued efforts of dietitians and counseling and incorporating physical exercise, because maintenance of that weight loss is also very important. Our goal is always sustained weight loss and not just short-term weight loss.
As the practice course director for the AGA’s Midwest Women in GI Workshop, can you tell me how this course came about? What does the workshop cover?
This workshop is a brainchild of AGA. This will be the third year of having these workshops. It’s been divided into regional workshops, so more people can attend. But it arose from the recognition that there is a need to have a support system, a forum where discussions on navigating career and life transitions with grace can happen, and more resources for success can be provided.
There is so much power in learning from shared experiences. And I think that was huge, to realize that we are not alone. We can celebrate our achievements together and acknowledge our challenges together, and then come together to brainstorm and innovate to solve problems and advocate for health equity.
You’ve been involved with community, non-profit organizations like the Homeless Alliance in Oklahoma City. How has this work enriched your life outside of medicine?
I feel like we sometimes get tunnel vision, talking to people in the same line of work. It was extremely important for me to broaden my horizons by learning from people outside of the medical community and from organizations like Homeless Alliance, which allowed me a platform to understand what my community needs. It’s an incredible organization that helps provide shelter not only for human beings, but also pets. The freezing temperatures over the last few months provided unique challenges like overflow in homeless shelters. I’ve learned so many things, such as how to ask for grants and how to allocate those funds. It has been absolutely enriching to me to learn about my community needs and see what an amazing difference people in the community are making.
Pooja Singhal, MD, AGAF, will never forget the time a female patient came in for gastroesophageal reflux disease and dysphagia treatment, revealing that she had already gone through multiple gastroenterologists to help diagnose and treat her ailments.
“We spent a whole visit talking about it,” said Dr. Singhal, a gastroenterologist, hepatologist, and obesity medicine specialist at Oklahoma Gastro Health and Wellness in Oklahoma City. During the exam, she discovered that her middle-aged patient was wearing an adult diaper for diarrhea and leakage.
Previous GI doctors told the patient they couldn’t help her and that she had to live with these symptoms. “I was just so shocked. I told her: This is not normal. Let’s talk more about it. Let’s figure out how we can manage it,” said Dr. Singhal, who has spent her career advocating for more education about GI conditions.
There are real barriers to patients opening up and sharing their symptoms, especially if they’re female. while ensuring that the correct knowledge gets across to the public, said Dr. Singhal.
An alumna of the American Gastroenterological Association’s (AGA) Future Leaders Program, Dr. Singhal has served as the private practice course director for AGA’s Midwest Women in GI Workshop. She is a also a four-time recipient of the SCOPY award for her work in raising community awareness of colorectal cancer prevention in Oklahoma. In an interview, she discussed the critical role women GI doctors play in assisting the unique needs of female patients, and why it takes a village of doctors to treat the complexities of GI disorders.
Why did you choose GI, and more specifically, what brought about your interest in women’s GI issues?
GI is simply the best field. While I was doing my rotation in GI as a resident, I was enthralled and humbled that the field of gastroenterology offered an opportunity to prevent cancer. Colon cancer is the second leading cause of cancer related deaths, and when I realized that we could do these micro-interventions during a procedure to remove polyps that could potentially turn into cancer — or give us an opportunity to remove carcinoma in situ — that’s what really inspired me and piqued my interest in GI. As I continued to learn and explore GI more, I appreciated the opportunity the field gave us in terms of using both sides of our brains equally, the right side and the left side.
I love the diagnostic part of medicine. You have this privilege to be able to diagnose so many different diseases and perform procedures using technical skills, exploring everything from the esophagus, liver, pancreas, small bowel, and colon.
But what I really appreciate about gastroenterology is how it’s piqued my interest in women’s digestive health. How it became very close to my heart is really from my patients. I’ve learned a lot from my patients throughout the years. When I was much younger, I don’t know if I really appreciated the vulnerability it takes as a woman to go to a physician and talk about hemorrhoids and diarrhea.
One of the comments I often receive is: ‘Oh, thank God you’re a female GI. I can be myself. I can share something personal and you would understand.’
Your practice places a specific emphasis on health and wellness. Can you provide some examples of how you incorporate wellness into treatment?
I feel like wellness is very commonplace now. To me, the definition of wellness is about practicing healthy habits to attain your maximum potential, both physically and mentally — to feel the best you can. My practice specifically tries to achieve that goal by placing a strong emphasis on education and communication. We provide journals where patients can keep track of their symptoms. We encourage a lot of discussion during visits, where we talk about GI diseases and how to prevent them, or to prevent them from happening again. If you’re going to do a hemorrhoid treatment that offers hemorrhoid banding, we talk about it in detail with the patient; we don’t just do the procedure.
We have a dietitian on staff for conditions like inflammatory bowel disease, Crohn’s and ulcerative colitis, celiac disease, IBS. Some of our older patients have pelvic organ prolapse and fecal incontinence. We have a pelvic floor therapist and a urogynecologist, and we work very closely with ob-gyn teams. My practice also takes pride in communicating with primary care physicians. We’ve had patients who have had memory loss or dementia or are grieving the loss of a loved one. And we prioritize communicating and treating patients as a whole and not focusing on just their GI symptoms.
As an advocate for community education on GI disorders, where is education lacking in this field?
I think education is lacking because there is an information delivery gap. I feel the public consumes information in the form of short social media reels. The attention span is so short and any scientific information, especially around diseases, can be scary and overwhelming. Whereas I think a lot of the medical community still interacts and exchanges information in terms of journals and publications. So, we are not really trained necessarily to talk about diseases in very simple terms.
We need more advocacy efforts on Capitol Hill. AGA has been good about doing advocacy work. I had an opportunity to go to Capitol Hill a couple of times and really advocate for policy around obesity medicine coverage and procedure coverage. I was fortunate to learn so much about healthcare policy, but it also made me appreciate that there are a lot of gaps in terms of understanding common medical diseases.
You’re trained in the Orbera Intragastric balloon system for weight reduction. How does this procedure differentiate from other bariatric procedures?
Intragastric balloon is Food and Drug Administration approved for weight loss. It’s a temporary medical device, so it’s reversible. No. 2, it’s a nonsurgical intervention, so it’s usually done in an outpatient setting. We basically place a deflated gastric balloon endoscopically, similar to an upper endoscopy method. We take a pin endoscope, a deflated balloon, which is made of medical-grade material, and we inflate it with adequate fluid. The concept is when the balloon is inflated, it provides satiety. It reduces the amount of space in the stomach for food. It slows down how quickly the food is going to leave. So you feel full much of the time. And it also helps decrease a hormone called ghrelin, which is responsible for hunger. It can make a big difference when people are gaining weight and in that category of overweight before they progress to obese.
As I tell everybody, obesity is a chronic lifelong disease that is very complex and requires lifelong efforts. So, it’s truly a journey. What’s made this procedure a success is follow-up and the continued efforts of dietitians and counseling and incorporating physical exercise, because maintenance of that weight loss is also very important. Our goal is always sustained weight loss and not just short-term weight loss.
As the practice course director for the AGA’s Midwest Women in GI Workshop, can you tell me how this course came about? What does the workshop cover?
This workshop is a brainchild of AGA. This will be the third year of having these workshops. It’s been divided into regional workshops, so more people can attend. But it arose from the recognition that there is a need to have a support system, a forum where discussions on navigating career and life transitions with grace can happen, and more resources for success can be provided.
There is so much power in learning from shared experiences. And I think that was huge, to realize that we are not alone. We can celebrate our achievements together and acknowledge our challenges together, and then come together to brainstorm and innovate to solve problems and advocate for health equity.
You’ve been involved with community, non-profit organizations like the Homeless Alliance in Oklahoma City. How has this work enriched your life outside of medicine?
I feel like we sometimes get tunnel vision, talking to people in the same line of work. It was extremely important for me to broaden my horizons by learning from people outside of the medical community and from organizations like Homeless Alliance, which allowed me a platform to understand what my community needs. It’s an incredible organization that helps provide shelter not only for human beings, but also pets. The freezing temperatures over the last few months provided unique challenges like overflow in homeless shelters. I’ve learned so many things, such as how to ask for grants and how to allocate those funds. It has been absolutely enriching to me to learn about my community needs and see what an amazing difference people in the community are making.

Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
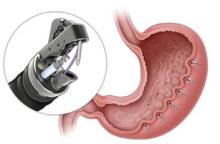
Obesity is a growing worldwide epidemic with significant implications for individual health and public health care costs. It is also associated with several medical conditions, including diabetes, cardiovascular disease, cancer, and mental health disorders.1 Comprehensive lifestyle intervention is a first-line therapy for obesity consisting of dietary and exercise interventions. Despite initial success, long-term results and durability of weight loss with lifestyle modifications are limited. 2 Bariatric surgery, including sleeve gastrectomy and gastric bypass surgery, is a more invasive approach that is highly effective in weight loss. However, these operations are not reversible, and patients may not be eligible for or may not desire surgery. Overall, bariatric surgery is widely underutilized, with < 1% of eligible patients ultimately undergoing surgery.3,4
Endoscopic bariatric therapies are increasingly popular procedures that address the need for additional treatments for obesity among individuals who have not had success with lifestyle changes and are not surgical candidates. The most common procedure is the endoscopic sleeve gastroplasty (ESG), which applies full-thickness sutures in the stomach to reduce gastric volume, delay gastric emptying, and limit food intake while keeping the fundus intact compared with sleeve gastrectomy. This procedure is typically considered in patients with body mass index (BMI) ≥ 30, who do not qualify for or do not want traditional bariatric surgery. The literature supports robust outcomes after ESG, with studies demonstrating significant and sustained total body weight loss of up to 14% to 16% at 5 years and significant improvement in ≥ 1 metabolic comorbidities in 80% of patients.5,6 ESG adverse events (AEs) include abdominal pain, nausea, and vomiting that are typically self-limited to 1 week. Rarer but more serious AEs include bleeding, perforation, or infection, and occur in 2% of cases based on large trial data.5,7
Although the weight loss benefits of ESG are well established, to date, there are limited data on the effects of endoscopic bariatric therapies like ESG on mental health conditions. Here, we describe a case of a veteran with a history of mental health disorders that prevented him from completing bariatric surgery. The patient underwent ESG and had a successful clinical course.
CASE PRESENTATION
A 59-year-old male veteran with a medical history of class III obesity (42.4 BMI), obstructive sleep apnea, hypothyroidism, hypertension, type 2 diabetes mellitus, and a large ventral hernia was referred to the MOVE! (Management of Overweight/ Obese Veterans Everywhere!) multidisciplinary high-intensity weight loss program at the US Department of Veterans Affairs (VA) West Los Angeles VA Medical Center (WLAVAMC). His psychiatric history included generalized anxiety disorder, posttraumatic stress disorder (PTSD), and panic disorder, managed by the Psychiatry Service and treated with sertraline 25 mg daily, lorazepam 0.5 mg twice daily, and hydroxyzine 20 mg nightly. He had previously implemented lifestyle changes and attended MOVE! classes and nutrition coaching for 1 year but was unsuccessful in losing weight. He had also tried liraglutide 3 mg daily for weight loss but was unable to tolerate it and reported worsening medication-related anxiety.
The patient declined further weight loss pharmacotherapy and was referred to bariatric surgery. He was scheduled for a surgical sleeve gastrectomy. However, on the day he arrived at the hospital for surgery, he developed severe anxiety and had a panic attack, and it was canceled. Due to his mental health issues, he was no longer comfortable proceeding with surgery and was left without other options for obesity treatment. The veteran was extremely disappointed because the ventral hernia caused significant quality of life impairment, limited his ability to exercise, and caused him embarrassment in public settings. The hernia could not be surgically repaired until there was significant weight loss.
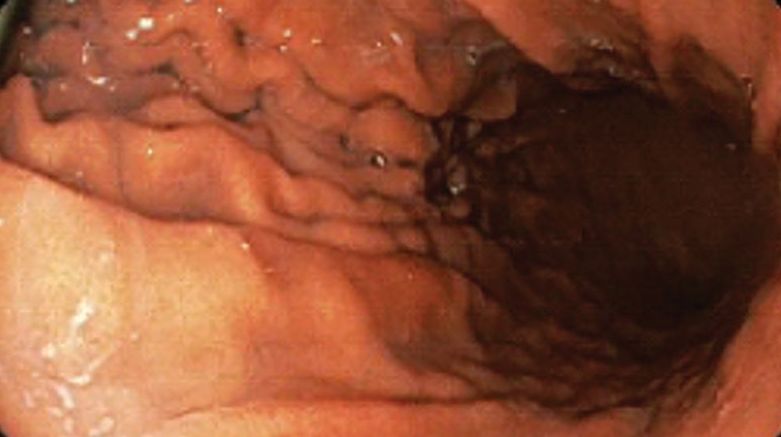
A bariatric endoscopy program within the Division of Gastroenterology was developed and implemented at the WLAVAMC in February 2023 in conjunction with MOVE! The patient was referred for consideration of an endoscopic weight loss procedure. He was determined to be a suitable candidate for ESG based on his BMI being > 40 and personal preference not to proceed with surgery to lose enough weight to qualify for hernia repair. The veteran underwent an endoscopy, which showed normal anatomy and gastric mucosa. ESG was performed in standard fashion (Figure).8 Three vertical lines were made using argon plasma coagulation from the incisura to 2 cm below the gastroesophageal junction along the anterior, posterior, and greater curvature of the stomach to mark the area for endoscopic suture placement. Starting at the incisura, 7 full-thickness sutures were placed to create a volume reduction plication, with preservation of the fundus. The patient did well postprocedure with no immediate or delayed AEs and was discharged home the same day.
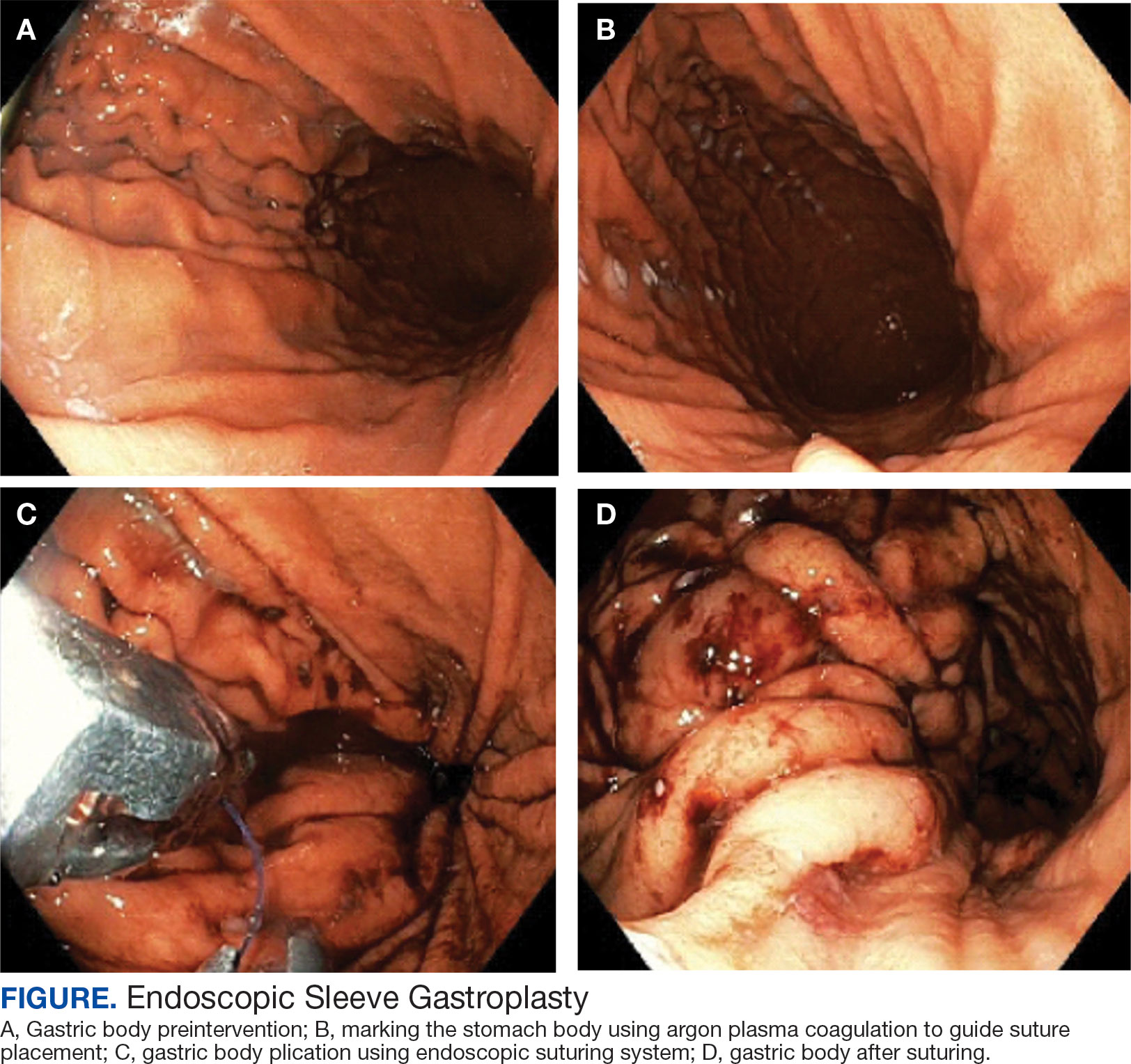
Follow-up
The veteran followed a gradual dietary advancement from a clear liquid diet to pureed and soft texture food. The patient’s weight dropped from 359 lbs preprocedure to 304 lbs 6 months postprocedure, a total body weight loss (TWBL) of 15.3%. At 12 months the veteran weighed 299 lbs (16.7% TBWL). He also had notable improvements in metabolic parameters. His systolic blood pressure decreased from ≥ 140 mm Hg to 120 to 130 mm Hg and hemoglobin A1c dropped from 7.0% to 6.3%. Remarkably, his psychiatrist noted significant improvement in his overall mental health. The veteran reported complete cessation of panic attacks since the ESG, improvements in PTSD and anxiety, and was able to discontinue lorazepam and decrease his dose of sertraline to 12.5 mg daily. He reported feeling more energetic and goal-oriented with increased clarity of thought. Perhaps the most significant outcome was that after the 55-lb weight loss at 6 months, the patient was eligible to undergo ventral hernia surgical repair, which had previously contributed to shame and social isolation. This, in turn, improved his quality of life, allowed him to start walking again, up to 8 miles daily, and to feel comfortable again going out in public settings.
DISCUSSION
Bariatric surgeries are an effective method of achieving weight loss and improving obesity-related comorbidities. However, only a small percentage of individuals with obesity are candidates for bariatric surgery. Given the dramatic increase in the prevalence of obesity, other options are needed. Specifically, within the VA, an estimated 80% of veterans are overweight or obese, but only about 500 bariatric surgeries are performed annually.9 With the need for additional weight loss therapies, VA programs are starting to offer endoscopic bariatric procedures as an alternative option. This may be a desirable choice for patients with obesity (BMI > 30), with or without associated metabolic comorbidities, who need more aggressive intervention beyond dietary and lifestyle changes and are either not interested in or not eligible for bariatric surgery or weight loss medications.
Although there is evidence that metabolic comorbidities are associated with obesity, there has been less research on obesity and mental health comorbidities such as depression and anxiety. These psychiatric conditions may even be more common among patients seeking weight loss procedures and more prominent in certain groups such as veterans, which may ultimately exclude these patients from bariatric surgery.10 Prior studies suggest that bariatric surgery can reduce the severity of depression and, to a lesser extent, anxiety symptoms at 2 years following the initial surgery; however, there is limited literature describing the impact of weight loss procedure on panic disorders.11-14 We suspect that a weight loss procedure such as ESG may have indirectly improved the veteran’s mood disorder due to the weight loss it induced, increasing the ability to exercise, quality of sleep, and participation in public settings.
This case highlights a veteran who did not tolerate weight loss medication and had severe anxiety and PTSD that prevented him from going through with bariatric surgery. He then underwent an endoscopic weight loss procedure. The ESG helped him successfully achieve significant weight loss, increase his physical activity, reduce his anxiety and panic disorder, and overall, significantly improve his quality of life. More than 1 year after the procedure, the patient has sustained improvements in his psychiatric and emotional health along with durable weight loss, maintaining > 15% of his total weight lost. Additional studies are needed to further understand the prevalence and long-term outcomes of mental health comorbidities, as well as weight loss outcomes in this group of patients who undergo endoscopic bariatric procedures.
CONCLUSIONS
We describe a case of a veteran with severe obesity and significant psychiatric comorbidities that prevented him from undergoing bariatric surgery, who underwent an ESG. This procedure led to significant weight loss, improvement of metabolic parameters, reduction in anxiety and PTSD, and enhancement of his quality of life. This case emphasizes the unique advantages of ESG and supports the expansion of endoscopic bariatric programs in the VA.
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319-326. doi:10.1016/j.numecd.2006.07.005
- Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404-412. doi:10.1016/j.soard.2017.11.012
- Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150- 163. doi:10.1001/jama.2015.18118
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al.. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441-451. doi:10.1016/S0140-6736(22)01280-6
- Matteo MV, Bove V, Ciasca G, et al. Success predictors of endoscopic sleeve gastroplasty. Obes Surg. 2024;34(5):1496-1504. doi:10.1007/s11695-024-07109-4
- Maselli DB, Hoff AC, Kucera A, et al. Endoscopic sleeve gastroplasty in class III obesity: efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc. 2023;15(6):469-479. doi:10.4253/wjge.v15.i6.469
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159-2164. doi:10.1007/s00464-017-5869-2
- Maggard-Gibbons M, Shekelle PG, Girgis MD, et al. Endoscopic Bariatric Interventions versus lifestyle interventions or surgery for weight loss in patients with obesity: a systematic review and meta-analysis. Department of Veterans Affairs (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK587943/
- Maggard Gibbons MA, Maher AM, Dawes AJ, et al. Psychological clearance for bariatric surgery: a systematic review. VA-ESP project #05-2262014.
- van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552-560. doi:10.1381/0960892053723484
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348-358. doi:10.1016/j.biopsych.2006.03.040
- Aylward L, Lilly C, Konsor M, et al. How soon do depression and anxiety symptoms improve after bariatric surgery?. Healthcare (Basel). 2023;11(6):862. doi:10.3390/healthcare11060862
- Law S, Dong S, Zhou F, Zheng D, Wang C, Dong Z. Bariatric surgery and mental health outcomes: an umbrella review. Front Endocrinol (Lausanne). 2023;14:1283621. doi:10.3389/fendo.2023.1283621
Obesity is a growing worldwide epidemic with significant implications for individual health and public health care costs. It is also associated with several medical conditions, including diabetes, cardiovascular disease, cancer, and mental health disorders.1 Comprehensive lifestyle intervention is a first-line therapy for obesity consisting of dietary and exercise interventions. Despite initial success, long-term results and durability of weight loss with lifestyle modifications are limited. 2 Bariatric surgery, including sleeve gastrectomy and gastric bypass surgery, is a more invasive approach that is highly effective in weight loss. However, these operations are not reversible, and patients may not be eligible for or may not desire surgery. Overall, bariatric surgery is widely underutilized, with < 1% of eligible patients ultimately undergoing surgery.3,4
Endoscopic bariatric therapies are increasingly popular procedures that address the need for additional treatments for obesity among individuals who have not had success with lifestyle changes and are not surgical candidates. The most common procedure is the endoscopic sleeve gastroplasty (ESG), which applies full-thickness sutures in the stomach to reduce gastric volume, delay gastric emptying, and limit food intake while keeping the fundus intact compared with sleeve gastrectomy. This procedure is typically considered in patients with body mass index (BMI) ≥ 30, who do not qualify for or do not want traditional bariatric surgery. The literature supports robust outcomes after ESG, with studies demonstrating significant and sustained total body weight loss of up to 14% to 16% at 5 years and significant improvement in ≥ 1 metabolic comorbidities in 80% of patients.5,6 ESG adverse events (AEs) include abdominal pain, nausea, and vomiting that are typically self-limited to 1 week. Rarer but more serious AEs include bleeding, perforation, or infection, and occur in 2% of cases based on large trial data.5,7
Although the weight loss benefits of ESG are well established, to date, there are limited data on the effects of endoscopic bariatric therapies like ESG on mental health conditions. Here, we describe a case of a veteran with a history of mental health disorders that prevented him from completing bariatric surgery. The patient underwent ESG and had a successful clinical course.
CASE PRESENTATION
A 59-year-old male veteran with a medical history of class III obesity (42.4 BMI), obstructive sleep apnea, hypothyroidism, hypertension, type 2 diabetes mellitus, and a large ventral hernia was referred to the MOVE! (Management of Overweight/ Obese Veterans Everywhere!) multidisciplinary high-intensity weight loss program at the US Department of Veterans Affairs (VA) West Los Angeles VA Medical Center (WLAVAMC). His psychiatric history included generalized anxiety disorder, posttraumatic stress disorder (PTSD), and panic disorder, managed by the Psychiatry Service and treated with sertraline 25 mg daily, lorazepam 0.5 mg twice daily, and hydroxyzine 20 mg nightly. He had previously implemented lifestyle changes and attended MOVE! classes and nutrition coaching for 1 year but was unsuccessful in losing weight. He had also tried liraglutide 3 mg daily for weight loss but was unable to tolerate it and reported worsening medication-related anxiety.
The patient declined further weight loss pharmacotherapy and was referred to bariatric surgery. He was scheduled for a surgical sleeve gastrectomy. However, on the day he arrived at the hospital for surgery, he developed severe anxiety and had a panic attack, and it was canceled. Due to his mental health issues, he was no longer comfortable proceeding with surgery and was left without other options for obesity treatment. The veteran was extremely disappointed because the ventral hernia caused significant quality of life impairment, limited his ability to exercise, and caused him embarrassment in public settings. The hernia could not be surgically repaired until there was significant weight loss.
A bariatric endoscopy program within the Division of Gastroenterology was developed and implemented at the WLAVAMC in February 2023 in conjunction with MOVE! The patient was referred for consideration of an endoscopic weight loss procedure. He was determined to be a suitable candidate for ESG based on his BMI being > 40 and personal preference not to proceed with surgery to lose enough weight to qualify for hernia repair. The veteran underwent an endoscopy, which showed normal anatomy and gastric mucosa. ESG was performed in standard fashion (Figure).8 Three vertical lines were made using argon plasma coagulation from the incisura to 2 cm below the gastroesophageal junction along the anterior, posterior, and greater curvature of the stomach to mark the area for endoscopic suture placement. Starting at the incisura, 7 full-thickness sutures were placed to create a volume reduction plication, with preservation of the fundus. The patient did well postprocedure with no immediate or delayed AEs and was discharged home the same day.

Follow-up
The veteran followed a gradual dietary advancement from a clear liquid diet to pureed and soft texture food. The patient’s weight dropped from 359 lbs preprocedure to 304 lbs 6 months postprocedure, a total body weight loss (TWBL) of 15.3%. At 12 months the veteran weighed 299 lbs (16.7% TBWL). He also had notable improvements in metabolic parameters. His systolic blood pressure decreased from ≥ 140 mm Hg to 120 to 130 mm Hg and hemoglobin A1c dropped from 7.0% to 6.3%. Remarkably, his psychiatrist noted significant improvement in his overall mental health. The veteran reported complete cessation of panic attacks since the ESG, improvements in PTSD and anxiety, and was able to discontinue lorazepam and decrease his dose of sertraline to 12.5 mg daily. He reported feeling more energetic and goal-oriented with increased clarity of thought. Perhaps the most significant outcome was that after the 55-lb weight loss at 6 months, the patient was eligible to undergo ventral hernia surgical repair, which had previously contributed to shame and social isolation. This, in turn, improved his quality of life, allowed him to start walking again, up to 8 miles daily, and to feel comfortable again going out in public settings.
DISCUSSION
Bariatric surgeries are an effective method of achieving weight loss and improving obesity-related comorbidities. However, only a small percentage of individuals with obesity are candidates for bariatric surgery. Given the dramatic increase in the prevalence of obesity, other options are needed. Specifically, within the VA, an estimated 80% of veterans are overweight or obese, but only about 500 bariatric surgeries are performed annually.9 With the need for additional weight loss therapies, VA programs are starting to offer endoscopic bariatric procedures as an alternative option. This may be a desirable choice for patients with obesity (BMI > 30), with or without associated metabolic comorbidities, who need more aggressive intervention beyond dietary and lifestyle changes and are either not interested in or not eligible for bariatric surgery or weight loss medications.
Although there is evidence that metabolic comorbidities are associated with obesity, there has been less research on obesity and mental health comorbidities such as depression and anxiety. These psychiatric conditions may even be more common among patients seeking weight loss procedures and more prominent in certain groups such as veterans, which may ultimately exclude these patients from bariatric surgery.10 Prior studies suggest that bariatric surgery can reduce the severity of depression and, to a lesser extent, anxiety symptoms at 2 years following the initial surgery; however, there is limited literature describing the impact of weight loss procedure on panic disorders.11-14 We suspect that a weight loss procedure such as ESG may have indirectly improved the veteran’s mood disorder due to the weight loss it induced, increasing the ability to exercise, quality of sleep, and participation in public settings.
This case highlights a veteran who did not tolerate weight loss medication and had severe anxiety and PTSD that prevented him from going through with bariatric surgery. He then underwent an endoscopic weight loss procedure. The ESG helped him successfully achieve significant weight loss, increase his physical activity, reduce his anxiety and panic disorder, and overall, significantly improve his quality of life. More than 1 year after the procedure, the patient has sustained improvements in his psychiatric and emotional health along with durable weight loss, maintaining > 15% of his total weight lost. Additional studies are needed to further understand the prevalence and long-term outcomes of mental health comorbidities, as well as weight loss outcomes in this group of patients who undergo endoscopic bariatric procedures.
CONCLUSIONS
We describe a case of a veteran with severe obesity and significant psychiatric comorbidities that prevented him from undergoing bariatric surgery, who underwent an ESG. This procedure led to significant weight loss, improvement of metabolic parameters, reduction in anxiety and PTSD, and enhancement of his quality of life. This case emphasizes the unique advantages of ESG and supports the expansion of endoscopic bariatric programs in the VA.
Obesity is a growing worldwide epidemic with significant implications for individual health and public health care costs. It is also associated with several medical conditions, including diabetes, cardiovascular disease, cancer, and mental health disorders.1 Comprehensive lifestyle intervention is a first-line therapy for obesity consisting of dietary and exercise interventions. Despite initial success, long-term results and durability of weight loss with lifestyle modifications are limited. 2 Bariatric surgery, including sleeve gastrectomy and gastric bypass surgery, is a more invasive approach that is highly effective in weight loss. However, these operations are not reversible, and patients may not be eligible for or may not desire surgery. Overall, bariatric surgery is widely underutilized, with < 1% of eligible patients ultimately undergoing surgery.3,4
Endoscopic bariatric therapies are increasingly popular procedures that address the need for additional treatments for obesity among individuals who have not had success with lifestyle changes and are not surgical candidates. The most common procedure is the endoscopic sleeve gastroplasty (ESG), which applies full-thickness sutures in the stomach to reduce gastric volume, delay gastric emptying, and limit food intake while keeping the fundus intact compared with sleeve gastrectomy. This procedure is typically considered in patients with body mass index (BMI) ≥ 30, who do not qualify for or do not want traditional bariatric surgery. The literature supports robust outcomes after ESG, with studies demonstrating significant and sustained total body weight loss of up to 14% to 16% at 5 years and significant improvement in ≥ 1 metabolic comorbidities in 80% of patients.5,6 ESG adverse events (AEs) include abdominal pain, nausea, and vomiting that are typically self-limited to 1 week. Rarer but more serious AEs include bleeding, perforation, or infection, and occur in 2% of cases based on large trial data.5,7
Although the weight loss benefits of ESG are well established, to date, there are limited data on the effects of endoscopic bariatric therapies like ESG on mental health conditions. Here, we describe a case of a veteran with a history of mental health disorders that prevented him from completing bariatric surgery. The patient underwent ESG and had a successful clinical course.
CASE PRESENTATION
A 59-year-old male veteran with a medical history of class III obesity (42.4 BMI), obstructive sleep apnea, hypothyroidism, hypertension, type 2 diabetes mellitus, and a large ventral hernia was referred to the MOVE! (Management of Overweight/ Obese Veterans Everywhere!) multidisciplinary high-intensity weight loss program at the US Department of Veterans Affairs (VA) West Los Angeles VA Medical Center (WLAVAMC). His psychiatric history included generalized anxiety disorder, posttraumatic stress disorder (PTSD), and panic disorder, managed by the Psychiatry Service and treated with sertraline 25 mg daily, lorazepam 0.5 mg twice daily, and hydroxyzine 20 mg nightly. He had previously implemented lifestyle changes and attended MOVE! classes and nutrition coaching for 1 year but was unsuccessful in losing weight. He had also tried liraglutide 3 mg daily for weight loss but was unable to tolerate it and reported worsening medication-related anxiety.
The patient declined further weight loss pharmacotherapy and was referred to bariatric surgery. He was scheduled for a surgical sleeve gastrectomy. However, on the day he arrived at the hospital for surgery, he developed severe anxiety and had a panic attack, and it was canceled. Due to his mental health issues, he was no longer comfortable proceeding with surgery and was left without other options for obesity treatment. The veteran was extremely disappointed because the ventral hernia caused significant quality of life impairment, limited his ability to exercise, and caused him embarrassment in public settings. The hernia could not be surgically repaired until there was significant weight loss.
A bariatric endoscopy program within the Division of Gastroenterology was developed and implemented at the WLAVAMC in February 2023 in conjunction with MOVE! The patient was referred for consideration of an endoscopic weight loss procedure. He was determined to be a suitable candidate for ESG based on his BMI being > 40 and personal preference not to proceed with surgery to lose enough weight to qualify for hernia repair. The veteran underwent an endoscopy, which showed normal anatomy and gastric mucosa. ESG was performed in standard fashion (Figure).8 Three vertical lines were made using argon plasma coagulation from the incisura to 2 cm below the gastroesophageal junction along the anterior, posterior, and greater curvature of the stomach to mark the area for endoscopic suture placement. Starting at the incisura, 7 full-thickness sutures were placed to create a volume reduction plication, with preservation of the fundus. The patient did well postprocedure with no immediate or delayed AEs and was discharged home the same day.

Follow-up
The veteran followed a gradual dietary advancement from a clear liquid diet to pureed and soft texture food. The patient’s weight dropped from 359 lbs preprocedure to 304 lbs 6 months postprocedure, a total body weight loss (TWBL) of 15.3%. At 12 months the veteran weighed 299 lbs (16.7% TBWL). He also had notable improvements in metabolic parameters. His systolic blood pressure decreased from ≥ 140 mm Hg to 120 to 130 mm Hg and hemoglobin A1c dropped from 7.0% to 6.3%. Remarkably, his psychiatrist noted significant improvement in his overall mental health. The veteran reported complete cessation of panic attacks since the ESG, improvements in PTSD and anxiety, and was able to discontinue lorazepam and decrease his dose of sertraline to 12.5 mg daily. He reported feeling more energetic and goal-oriented with increased clarity of thought. Perhaps the most significant outcome was that after the 55-lb weight loss at 6 months, the patient was eligible to undergo ventral hernia surgical repair, which had previously contributed to shame and social isolation. This, in turn, improved his quality of life, allowed him to start walking again, up to 8 miles daily, and to feel comfortable again going out in public settings.
DISCUSSION
Bariatric surgeries are an effective method of achieving weight loss and improving obesity-related comorbidities. However, only a small percentage of individuals with obesity are candidates for bariatric surgery. Given the dramatic increase in the prevalence of obesity, other options are needed. Specifically, within the VA, an estimated 80% of veterans are overweight or obese, but only about 500 bariatric surgeries are performed annually.9 With the need for additional weight loss therapies, VA programs are starting to offer endoscopic bariatric procedures as an alternative option. This may be a desirable choice for patients with obesity (BMI > 30), with or without associated metabolic comorbidities, who need more aggressive intervention beyond dietary and lifestyle changes and are either not interested in or not eligible for bariatric surgery or weight loss medications.
Although there is evidence that metabolic comorbidities are associated with obesity, there has been less research on obesity and mental health comorbidities such as depression and anxiety. These psychiatric conditions may even be more common among patients seeking weight loss procedures and more prominent in certain groups such as veterans, which may ultimately exclude these patients from bariatric surgery.10 Prior studies suggest that bariatric surgery can reduce the severity of depression and, to a lesser extent, anxiety symptoms at 2 years following the initial surgery; however, there is limited literature describing the impact of weight loss procedure on panic disorders.11-14 We suspect that a weight loss procedure such as ESG may have indirectly improved the veteran’s mood disorder due to the weight loss it induced, increasing the ability to exercise, quality of sleep, and participation in public settings.
This case highlights a veteran who did not tolerate weight loss medication and had severe anxiety and PTSD that prevented him from going through with bariatric surgery. He then underwent an endoscopic weight loss procedure. The ESG helped him successfully achieve significant weight loss, increase his physical activity, reduce his anxiety and panic disorder, and overall, significantly improve his quality of life. More than 1 year after the procedure, the patient has sustained improvements in his psychiatric and emotional health along with durable weight loss, maintaining > 15% of his total weight lost. Additional studies are needed to further understand the prevalence and long-term outcomes of mental health comorbidities, as well as weight loss outcomes in this group of patients who undergo endoscopic bariatric procedures.
CONCLUSIONS
We describe a case of a veteran with severe obesity and significant psychiatric comorbidities that prevented him from undergoing bariatric surgery, who underwent an ESG. This procedure led to significant weight loss, improvement of metabolic parameters, reduction in anxiety and PTSD, and enhancement of his quality of life. This case emphasizes the unique advantages of ESG and supports the expansion of endoscopic bariatric programs in the VA.
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319-326. doi:10.1016/j.numecd.2006.07.005
- Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404-412. doi:10.1016/j.soard.2017.11.012
- Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150- 163. doi:10.1001/jama.2015.18118
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al.. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441-451. doi:10.1016/S0140-6736(22)01280-6
- Matteo MV, Bove V, Ciasca G, et al. Success predictors of endoscopic sleeve gastroplasty. Obes Surg. 2024;34(5):1496-1504. doi:10.1007/s11695-024-07109-4
- Maselli DB, Hoff AC, Kucera A, et al. Endoscopic sleeve gastroplasty in class III obesity: efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc. 2023;15(6):469-479. doi:10.4253/wjge.v15.i6.469
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159-2164. doi:10.1007/s00464-017-5869-2
- Maggard-Gibbons M, Shekelle PG, Girgis MD, et al. Endoscopic Bariatric Interventions versus lifestyle interventions or surgery for weight loss in patients with obesity: a systematic review and meta-analysis. Department of Veterans Affairs (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK587943/
- Maggard Gibbons MA, Maher AM, Dawes AJ, et al. Psychological clearance for bariatric surgery: a systematic review. VA-ESP project #05-2262014.
- van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552-560. doi:10.1381/0960892053723484
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348-358. doi:10.1016/j.biopsych.2006.03.040
- Aylward L, Lilly C, Konsor M, et al. How soon do depression and anxiety symptoms improve after bariatric surgery?. Healthcare (Basel). 2023;11(6):862. doi:10.3390/healthcare11060862
- Law S, Dong S, Zhou F, Zheng D, Wang C, Dong Z. Bariatric surgery and mental health outcomes: an umbrella review. Front Endocrinol (Lausanne). 2023;14:1283621. doi:10.3389/fendo.2023.1283621
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319-326. doi:10.1016/j.numecd.2006.07.005
- Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404-412. doi:10.1016/j.soard.2017.11.012
- Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150- 163. doi:10.1001/jama.2015.18118
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al.. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441-451. doi:10.1016/S0140-6736(22)01280-6
- Matteo MV, Bove V, Ciasca G, et al. Success predictors of endoscopic sleeve gastroplasty. Obes Surg. 2024;34(5):1496-1504. doi:10.1007/s11695-024-07109-4
- Maselli DB, Hoff AC, Kucera A, et al. Endoscopic sleeve gastroplasty in class III obesity: efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc. 2023;15(6):469-479. doi:10.4253/wjge.v15.i6.469
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159-2164. doi:10.1007/s00464-017-5869-2
- Maggard-Gibbons M, Shekelle PG, Girgis MD, et al. Endoscopic Bariatric Interventions versus lifestyle interventions or surgery for weight loss in patients with obesity: a systematic review and meta-analysis. Department of Veterans Affairs (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK587943/
- Maggard Gibbons MA, Maher AM, Dawes AJ, et al. Psychological clearance for bariatric surgery: a systematic review. VA-ESP project #05-2262014.
- van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552-560. doi:10.1381/0960892053723484
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348-358. doi:10.1016/j.biopsych.2006.03.040
- Aylward L, Lilly C, Konsor M, et al. How soon do depression and anxiety symptoms improve after bariatric surgery?. Healthcare (Basel). 2023;11(6):862. doi:10.3390/healthcare11060862
- Law S, Dong S, Zhou F, Zheng D, Wang C, Dong Z. Bariatric surgery and mental health outcomes: an umbrella review. Front Endocrinol (Lausanne). 2023;14:1283621. doi:10.3389/fendo.2023.1283621
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Duodenal Mucosal Resurfacing Curbs Weight Gain Post-GLP-1
, initial results of the open-label, multistage REMAIN-1 trial showed.
In addition, “the procedure was well tolerated, with only minor, transient TEAEs [treatment-emergent adverse events] consistent with routine upper endoscopy,” said Shailendra Singh, MD, of West Virginia University in Morgantown, West Virginia, who presented the findings at The Obesity Society’s Obesity Week 2025 meeting in Atlanta.
DMR uses hydrothermal ablation to treat the duodenal mucosa, which may be dysfunctional in both obesity and impaired glucose tolerance. A previous pooled clinical trial analysis of more than 100 patients with type 2 diabetes demonstrated that DMR helped patients maintain body weight loss up to 48 weeks post-procedure.
Metabolic therapeutics company Fractyl Health, Burlington, Massachusetts, developed the procedure, called Revita, and is sponsoring the current study. The trial’s aim is to determine the effect of DMR on weight-loss maintenance in patients with ≥ 15% total body weight loss using a GLP-1 RA in both an open-label arm and a prospective, randomized, double-blind, sham-controlled multicenter arm.
‘Encouraging Preliminary Findings’
The open-label arm included 15 DMR-treated participants (mean age, 49 years, 87% female ), all of whom had taken tirzepatide for a minimum of 5 months and a maximum of 3 years prior to DMR and had lost at least 15% of their total body weight.
Participants had a mean pre-GLP-1 RA weight of 104.8 kg and a mean weight prior to DMR of 79.4 kg, for a mean total body weight loss from the start of GLP-1 RA of 23.8%. Weight loss was heterogeneous and reflective of the real-world patient population taking GLP-1 medications, according to the poster presentation.
Participants discontinued their GLP-1 medication, underwent the DMR procedure, and were followed for 3 months. A total of 12 of 13 patients maintained or lost weight at that point, with 6 of 13 losing additional weight.
Specifically, participants experienced a median of 0.46% weight change (approximately 1 lb) compared with the 5%-6% weight regain (10-15 lb) observed after GLP-1 discontinuation in the literature.
The procedure was well tolerated, with most patients experiencing no TEAEs and none experiencing an event greater than grade 1. Grade 1 events occurred in three patients; 23% were transient in nature, lasting 2-5 days, and were similar to those typically seen with a routine upper endoscopy.
“These encouraging preliminary findings suggest that DMR may safely achieve durable weight maintenance for patients who wish to discontinue GLP-1 RA therapy,” the study authors stated.
Randomization is anticipated in early 2026, with 6-month topline data and a potential premarket approval filing expected in the second half of 2026.
A version of this article appeared on Medscape.com.
, initial results of the open-label, multistage REMAIN-1 trial showed.
In addition, “the procedure was well tolerated, with only minor, transient TEAEs [treatment-emergent adverse events] consistent with routine upper endoscopy,” said Shailendra Singh, MD, of West Virginia University in Morgantown, West Virginia, who presented the findings at The Obesity Society’s Obesity Week 2025 meeting in Atlanta.
DMR uses hydrothermal ablation to treat the duodenal mucosa, which may be dysfunctional in both obesity and impaired glucose tolerance. A previous pooled clinical trial analysis of more than 100 patients with type 2 diabetes demonstrated that DMR helped patients maintain body weight loss up to 48 weeks post-procedure.
Metabolic therapeutics company Fractyl Health, Burlington, Massachusetts, developed the procedure, called Revita, and is sponsoring the current study. The trial’s aim is to determine the effect of DMR on weight-loss maintenance in patients with ≥ 15% total body weight loss using a GLP-1 RA in both an open-label arm and a prospective, randomized, double-blind, sham-controlled multicenter arm.
‘Encouraging Preliminary Findings’
The open-label arm included 15 DMR-treated participants (mean age, 49 years, 87% female ), all of whom had taken tirzepatide for a minimum of 5 months and a maximum of 3 years prior to DMR and had lost at least 15% of their total body weight.
Participants had a mean pre-GLP-1 RA weight of 104.8 kg and a mean weight prior to DMR of 79.4 kg, for a mean total body weight loss from the start of GLP-1 RA of 23.8%. Weight loss was heterogeneous and reflective of the real-world patient population taking GLP-1 medications, according to the poster presentation.
Participants discontinued their GLP-1 medication, underwent the DMR procedure, and were followed for 3 months. A total of 12 of 13 patients maintained or lost weight at that point, with 6 of 13 losing additional weight.
Specifically, participants experienced a median of 0.46% weight change (approximately 1 lb) compared with the 5%-6% weight regain (10-15 lb) observed after GLP-1 discontinuation in the literature.
The procedure was well tolerated, with most patients experiencing no TEAEs and none experiencing an event greater than grade 1. Grade 1 events occurred in three patients; 23% were transient in nature, lasting 2-5 days, and were similar to those typically seen with a routine upper endoscopy.
“These encouraging preliminary findings suggest that DMR may safely achieve durable weight maintenance for patients who wish to discontinue GLP-1 RA therapy,” the study authors stated.
Randomization is anticipated in early 2026, with 6-month topline data and a potential premarket approval filing expected in the second half of 2026.
A version of this article appeared on Medscape.com.
, initial results of the open-label, multistage REMAIN-1 trial showed.
In addition, “the procedure was well tolerated, with only minor, transient TEAEs [treatment-emergent adverse events] consistent with routine upper endoscopy,” said Shailendra Singh, MD, of West Virginia University in Morgantown, West Virginia, who presented the findings at The Obesity Society’s Obesity Week 2025 meeting in Atlanta.
DMR uses hydrothermal ablation to treat the duodenal mucosa, which may be dysfunctional in both obesity and impaired glucose tolerance. A previous pooled clinical trial analysis of more than 100 patients with type 2 diabetes demonstrated that DMR helped patients maintain body weight loss up to 48 weeks post-procedure.
Metabolic therapeutics company Fractyl Health, Burlington, Massachusetts, developed the procedure, called Revita, and is sponsoring the current study. The trial’s aim is to determine the effect of DMR on weight-loss maintenance in patients with ≥ 15% total body weight loss using a GLP-1 RA in both an open-label arm and a prospective, randomized, double-blind, sham-controlled multicenter arm.
‘Encouraging Preliminary Findings’
The open-label arm included 15 DMR-treated participants (mean age, 49 years, 87% female ), all of whom had taken tirzepatide for a minimum of 5 months and a maximum of 3 years prior to DMR and had lost at least 15% of their total body weight.
Participants had a mean pre-GLP-1 RA weight of 104.8 kg and a mean weight prior to DMR of 79.4 kg, for a mean total body weight loss from the start of GLP-1 RA of 23.8%. Weight loss was heterogeneous and reflective of the real-world patient population taking GLP-1 medications, according to the poster presentation.
Participants discontinued their GLP-1 medication, underwent the DMR procedure, and were followed for 3 months. A total of 12 of 13 patients maintained or lost weight at that point, with 6 of 13 losing additional weight.
Specifically, participants experienced a median of 0.46% weight change (approximately 1 lb) compared with the 5%-6% weight regain (10-15 lb) observed after GLP-1 discontinuation in the literature.
The procedure was well tolerated, with most patients experiencing no TEAEs and none experiencing an event greater than grade 1. Grade 1 events occurred in three patients; 23% were transient in nature, lasting 2-5 days, and were similar to those typically seen with a routine upper endoscopy.
“These encouraging preliminary findings suggest that DMR may safely achieve durable weight maintenance for patients who wish to discontinue GLP-1 RA therapy,” the study authors stated.
Randomization is anticipated in early 2026, with 6-month topline data and a potential premarket approval filing expected in the second half of 2026.
A version of this article appeared on Medscape.com.
New Drug Eases Side Effects of Weight-Loss Meds
A new drug currently known as NG101 reduced nausea and vomiting in patients with obesity using GLP-1s by 40% and 67%, respectively, based on data from a phase 2 trial presented at the Obesity Society’s Obesity Week 2025 in Atlanta.
Previous research published in JAMA Network Open showed a nearly 65% discontinuation rate for three GLP-1s (liraglutide, semaglutide, or tirzepatide) among adults with overweight or obesity and without type 2 diabetes. Gastrointestinal (GI) side effects topped the list of reasons for dropping the medications.
Given the impact of nausea and vomiting on discontinuation, there is an unmet need for therapies to manage GI symptoms, said Kimberley Cummings, PhD, of Neurogastrx, Inc., in her presentation.
In the new study, Cummings and colleagues randomly assigned 90 adults aged 18-55 years with overweight or obesity (defined as a BMI ranging from 22.0 to 35.0) to receive a single subcutaneous dose of semaglutide (0.5 mg) plus 5 days of NG101 at 20 mg twice daily, or a placebo.
NG101 is a peripherally acting D2 antagonist designed to reduce nausea and vomiting associated with GLP-1 use, Cummings said. NG101 targets the nausea center of the brain but is peripherally restricted to prevent central nervous system side effects, she explained.
Compared with placebo, NG101 significantly reduced the incidence of nausea and vomiting by 40% and 67%, respectively. Use of NG101 also was associated with a significant reduction in the duration of nausea and vomiting; GI events lasting longer than 1 day were reported in 22% and 51% of the NG101 patients and placebo patients, respectively.
In addition, participants who received NG101 reported a 70% decrease in nausea severity from baseline.
Overall, patients in the NG101 group also reported significantly fewer adverse events than those in the placebo group (74 vs 135), suggesting an improved safety profile when semaglutide is administered in conjunction with NG101, the researchers noted. No serious adverse events related to the study drug were reported in either group.
The findings were limited by several factors including the relatively small sample size. Additional research is needed with other GLP-1 agonists in larger populations with longer follow-up periods, Cummings said. However, the results suggest that NG101 was safe and effectively improved side effects associated with GLP-1 agonists.
“We know there are receptors for GLP-1 in the area postrema (nausea center of the brain), and that NG101 works on this area to reduce nausea and vomiting, so the study findings were not unexpected,” said Jim O’Mara, president and CEO of Neurogastrx, in an interview.
The study was a single-dose study designed to show proof of concept, and future studies would involve treating patients going through the recommended titration schedule for their GLP-1s, O’Mara said. However, NG101 offers an opportunity to keep more patients on GLP-1 therapy and help them reach their long-term therapeutic goals, he said.
Decrease Side Effects for Weight-Loss Success
“GI side effects are often the rate-limiting step in implementing an effective medication that patients want to take but may not be able to tolerate,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for Weight and Diabetes Management, Burlington, Ontario, Canada, in an interview. “If we can decrease side effects, these medications could improve patients’ lives,” said Wharton, who was not involved in the study.
The improvement after a single dose of NG101 in patients receiving a single dose of semaglutide was impressive and in keeping with the mechanism of the drug action, said Wharton. “I was not surprised by the result but pleased that this single dose was shown to reduce the overall incidence of nausea and vomiting, the duration of nausea, the severity of nausea as rated by the study participants compared to placebo,” he said.
Ultimately, the clinical implications for NG101 are improved patient tolerance for GLP-1s and the ability to titrate and stay on them long term, incurring greater cardiometabolic benefit, Wharton told this news organization.
The current trial was limited to GLP1-1s on the market; newer medications may have fewer side effects, Wharton noted. “In clinical practice, patients often decrease the medication or titrate slower, and this could be the comparator,” he added.
The study was funded by Neurogastrx.
Wharton disclosed serving as a consultant for Neurogastrx but not as an investigator on the current study. He also reported having disclosed research on various GLP-1 medications.
A version of this article first appeared on Medscape.com.
A new drug currently known as NG101 reduced nausea and vomiting in patients with obesity using GLP-1s by 40% and 67%, respectively, based on data from a phase 2 trial presented at the Obesity Society’s Obesity Week 2025 in Atlanta.
Previous research published in JAMA Network Open showed a nearly 65% discontinuation rate for three GLP-1s (liraglutide, semaglutide, or tirzepatide) among adults with overweight or obesity and without type 2 diabetes. Gastrointestinal (GI) side effects topped the list of reasons for dropping the medications.
Given the impact of nausea and vomiting on discontinuation, there is an unmet need for therapies to manage GI symptoms, said Kimberley Cummings, PhD, of Neurogastrx, Inc., in her presentation.
In the new study, Cummings and colleagues randomly assigned 90 adults aged 18-55 years with overweight or obesity (defined as a BMI ranging from 22.0 to 35.0) to receive a single subcutaneous dose of semaglutide (0.5 mg) plus 5 days of NG101 at 20 mg twice daily, or a placebo.
NG101 is a peripherally acting D2 antagonist designed to reduce nausea and vomiting associated with GLP-1 use, Cummings said. NG101 targets the nausea center of the brain but is peripherally restricted to prevent central nervous system side effects, she explained.
Compared with placebo, NG101 significantly reduced the incidence of nausea and vomiting by 40% and 67%, respectively. Use of NG101 also was associated with a significant reduction in the duration of nausea and vomiting; GI events lasting longer than 1 day were reported in 22% and 51% of the NG101 patients and placebo patients, respectively.
In addition, participants who received NG101 reported a 70% decrease in nausea severity from baseline.
Overall, patients in the NG101 group also reported significantly fewer adverse events than those in the placebo group (74 vs 135), suggesting an improved safety profile when semaglutide is administered in conjunction with NG101, the researchers noted. No serious adverse events related to the study drug were reported in either group.
The findings were limited by several factors including the relatively small sample size. Additional research is needed with other GLP-1 agonists in larger populations with longer follow-up periods, Cummings said. However, the results suggest that NG101 was safe and effectively improved side effects associated with GLP-1 agonists.
“We know there are receptors for GLP-1 in the area postrema (nausea center of the brain), and that NG101 works on this area to reduce nausea and vomiting, so the study findings were not unexpected,” said Jim O’Mara, president and CEO of Neurogastrx, in an interview.
The study was a single-dose study designed to show proof of concept, and future studies would involve treating patients going through the recommended titration schedule for their GLP-1s, O’Mara said. However, NG101 offers an opportunity to keep more patients on GLP-1 therapy and help them reach their long-term therapeutic goals, he said.
Decrease Side Effects for Weight-Loss Success
“GI side effects are often the rate-limiting step in implementing an effective medication that patients want to take but may not be able to tolerate,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for Weight and Diabetes Management, Burlington, Ontario, Canada, in an interview. “If we can decrease side effects, these medications could improve patients’ lives,” said Wharton, who was not involved in the study.
The improvement after a single dose of NG101 in patients receiving a single dose of semaglutide was impressive and in keeping with the mechanism of the drug action, said Wharton. “I was not surprised by the result but pleased that this single dose was shown to reduce the overall incidence of nausea and vomiting, the duration of nausea, the severity of nausea as rated by the study participants compared to placebo,” he said.
Ultimately, the clinical implications for NG101 are improved patient tolerance for GLP-1s and the ability to titrate and stay on them long term, incurring greater cardiometabolic benefit, Wharton told this news organization.
The current trial was limited to GLP1-1s on the market; newer medications may have fewer side effects, Wharton noted. “In clinical practice, patients often decrease the medication or titrate slower, and this could be the comparator,” he added.
The study was funded by Neurogastrx.
Wharton disclosed serving as a consultant for Neurogastrx but not as an investigator on the current study. He also reported having disclosed research on various GLP-1 medications.
A version of this article first appeared on Medscape.com.
A new drug currently known as NG101 reduced nausea and vomiting in patients with obesity using GLP-1s by 40% and 67%, respectively, based on data from a phase 2 trial presented at the Obesity Society’s Obesity Week 2025 in Atlanta.
Previous research published in JAMA Network Open showed a nearly 65% discontinuation rate for three GLP-1s (liraglutide, semaglutide, or tirzepatide) among adults with overweight or obesity and without type 2 diabetes. Gastrointestinal (GI) side effects topped the list of reasons for dropping the medications.
Given the impact of nausea and vomiting on discontinuation, there is an unmet need for therapies to manage GI symptoms, said Kimberley Cummings, PhD, of Neurogastrx, Inc., in her presentation.
In the new study, Cummings and colleagues randomly assigned 90 adults aged 18-55 years with overweight or obesity (defined as a BMI ranging from 22.0 to 35.0) to receive a single subcutaneous dose of semaglutide (0.5 mg) plus 5 days of NG101 at 20 mg twice daily, or a placebo.
NG101 is a peripherally acting D2 antagonist designed to reduce nausea and vomiting associated with GLP-1 use, Cummings said. NG101 targets the nausea center of the brain but is peripherally restricted to prevent central nervous system side effects, she explained.
Compared with placebo, NG101 significantly reduced the incidence of nausea and vomiting by 40% and 67%, respectively. Use of NG101 also was associated with a significant reduction in the duration of nausea and vomiting; GI events lasting longer than 1 day were reported in 22% and 51% of the NG101 patients and placebo patients, respectively.
In addition, participants who received NG101 reported a 70% decrease in nausea severity from baseline.
Overall, patients in the NG101 group also reported significantly fewer adverse events than those in the placebo group (74 vs 135), suggesting an improved safety profile when semaglutide is administered in conjunction with NG101, the researchers noted. No serious adverse events related to the study drug were reported in either group.
The findings were limited by several factors including the relatively small sample size. Additional research is needed with other GLP-1 agonists in larger populations with longer follow-up periods, Cummings said. However, the results suggest that NG101 was safe and effectively improved side effects associated with GLP-1 agonists.
“We know there are receptors for GLP-1 in the area postrema (nausea center of the brain), and that NG101 works on this area to reduce nausea and vomiting, so the study findings were not unexpected,” said Jim O’Mara, president and CEO of Neurogastrx, in an interview.
The study was a single-dose study designed to show proof of concept, and future studies would involve treating patients going through the recommended titration schedule for their GLP-1s, O’Mara said. However, NG101 offers an opportunity to keep more patients on GLP-1 therapy and help them reach their long-term therapeutic goals, he said.
Decrease Side Effects for Weight-Loss Success
“GI side effects are often the rate-limiting step in implementing an effective medication that patients want to take but may not be able to tolerate,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for Weight and Diabetes Management, Burlington, Ontario, Canada, in an interview. “If we can decrease side effects, these medications could improve patients’ lives,” said Wharton, who was not involved in the study.
The improvement after a single dose of NG101 in patients receiving a single dose of semaglutide was impressive and in keeping with the mechanism of the drug action, said Wharton. “I was not surprised by the result but pleased that this single dose was shown to reduce the overall incidence of nausea and vomiting, the duration of nausea, the severity of nausea as rated by the study participants compared to placebo,” he said.
Ultimately, the clinical implications for NG101 are improved patient tolerance for GLP-1s and the ability to titrate and stay on them long term, incurring greater cardiometabolic benefit, Wharton told this news organization.
The current trial was limited to GLP1-1s on the market; newer medications may have fewer side effects, Wharton noted. “In clinical practice, patients often decrease the medication or titrate slower, and this could be the comparator,” he added.
The study was funded by Neurogastrx.
Wharton disclosed serving as a consultant for Neurogastrx but not as an investigator on the current study. He also reported having disclosed research on various GLP-1 medications.
A version of this article first appeared on Medscape.com.
FROM OBESITY WEEK 2025
Approach to Weight Management in GI Practice
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Introduction
The majority of patients in the United States are now overweight or obese, and as gastroenterologists we treat a number of conditions that are caused or worsened by obesity.1 Cirrhosis related to metabolic associated fatty liver disease (MAFLD) is now a leading indication for liver transplantation in the US2 and obesity is a clear risk factor for all major malignancies of the GI tract, including esophageal, gastric cardia, pancreatic, liver, gallbladder, colon, and rectum.3 Obesity is associated with dysbiosis and impacts barrier function: increasing permeability, abnormal gut bacterial translocation, and inflammation.4 It is more common than malnutrition in our patients with inflammatory bowel disease (IBD), where it impacts response to biologic drugs, increases the technical difficulty of surgeries, such as IPAA, and is associated with worse surgical outcomes.5 Furthermore, patients with obesity may be less likely to undergo preventative cancer screenings and are at increased risk related to sedation for endoscopic procedures.6 With over 40% of Americans suffering from obesity, and increasingly effective treatments available,
Understanding the Mechanisms of Obesity
There are complex orexigenic and anorexigenic brain pathways in the hypothalamus which control global energy balance.7 Obesity results when energy intake exceeds energy expenditure. While overeating and a sedentary lifestyle are commonly blamed, there are a number of elements that contribute, including genetics, medical conditions, medications, psychosocial factors, and environmental components. For example, sleep loss contributes to weight gain by several mechanisms including increasing ghrelin and decreasing leptin levels, thereby increasing hunger and appetite, as well as by decreasing insulin sensitivity and increasing cortisol. Subjects exposed to sleep deprivation in research settings take in 550 kcal more the following day.8 Medications used commonly in GI practice including corticosteroids, antihistamines, propranolol, and amitriptyline, are obesogenic9 and cannabis can impact hypothalamic pathways to stimulate hunger.10
When patients diet or exercise to lose weight, as we have traditionally advised, there are strong hormonal changes and metabolic adaptations that occur to preserve the defended fat mass or “set point.” Loss of adipose tissue results in decreased production of leptin, a hormone that stimulates satiety pathways and inhibits orexigenic pathways, greatly increasing hunger and cravings. Increases in ghrelin production by the stomach decreases perceptions of fullness. With weight loss, energy requirements decrease, and muscles become more efficient, meaning fewer kcal are needed to maintain bodily processes.11 Eventually a plateau is reached, while motivation to diet and restraint around food wane, and hedonistic (reward) pathways are activated. These powerful factors result in the regain of lost weight within one year in the majority of patients.
Implementing Weight Management into GI Practice
Given the stigma and bias around obesity, patients often feel shame and vulnerability around the condition. It is important to have empathy in your approach, asking permission to discuss weight and using patient-first language (e.g. “patient with obesity” not “obese patient”). While BMI is predictive of health outcomes, it does not measure body fat percentage and may be misleading, such as in muscular individuals. Other measures of adiposity including waist circumference and body composition testing, such as with DEXA, may provide additional data. A BMI of 30 or above defines obesity, though newer definitions incorporate related symptoms, organ disfunction, and metabolic abnormalities into the term “clinical obesity.”12 Asian patients experience metabolic complications at a lower BMI, and therefore the definition of obese is 27.5kg/m2 in this population.
Begin by taking a weight history. Has this been a lifelong struggle or is there a particular life circumstance, such as working a third shift or recent pregnancy which precipitated weight gain? Patients should be asked about binge eating or eating late into the evening or waking at night to eat, as these disordered eating behaviors are managed with specific medications and behavioral therapies. Inquire about sleep duration and quality and refer for a sleep study if there is suspicion for obstructive sleep apnea. Other weight-related comorbidities including hyperlipidemia, type 2 diabetes mellitus (T2DM), and MAFLD should be considered and merit a more aggressive approach, as does more severe obesity (class III, BMI ≥40). Questions about marijuana and alcohol use as well as review of the medication list for obesogenic medications can provide further insight into modifiable contributing factors.
Pillars of Weight Management
The internet is awash with trendy diet recommendations, and widespread misconceptions about obesity management are even ingrained into how physicians approach the disease. It is critical to remember that this is not a consequence of bad choices or lack of self-control. Exercise alone is insufficient to result in significant weight loss.13 Furthermore, whether it is through low fat, low carb, or intermittent fasting, weight loss will occur with calorie deficit.14 Evidence-based diet and lifestyle recommendations to lay the groundwork for success should be discussed at each visit (see Table 1). The Mediterranean diet is recommended for weight loss as well as for several GI disorders (i.e., MAFLD and IBD) and is the optimal eating strategy for cardiovascular health.15 Patients should be advised to engage in 150 minutes of moderate exercise per week, such as brisk walking, and should incorporate resistance training to build muscle and maintain bone density.
Anti-obesity Medications
There are a number of medications, either FDA approved or used off label, for treatment of obesity (see Table 2).16 All are indicated for patients with a BMI of ≥ 30 kg/m2 or for those with a BMI between 27-29 kg/m2 with weight-related comorbidities and should be used in combination with diet and lifestyle interventions. None are approved or safe in pregnancy. Mechanisms of action vary by type and include decreased appetite, increased energy expenditure, improved insulin sensitivity, and interfere with absorption.
The newest and most effective anti-obesity medications (AOM), the glucagon-like peptide-1 receptor agonists (GLP-1 RA) are derived from gut hormones secreted in the distal small bowel and colon in response to a meal, which function to delay gastric emptying, increase insulin release from the pancreas, and reduce hepatic gluconeogenesis. Central nervous system effects are not yet entirely understood, but function to decrease appetite and increase satiety. Initially developed for treatment of T2DM, observed weight reduction in patients treated with GLP-1 RA led to clinical trials for treatment of obesity. Semaglutide treatment resulted in weight reduction of 16.9% of total body weight (TBW), and one third of subjects lost ≥ 20% of TBW.17 Tirzepatide combines GLP-1 RA and a gastric inhibitory polypeptide (GIP) receptor agonist, which also has an incretin effect and functions to slow gastric emptying. In the pivotal SURMOUNT trial, approximately 58% of patients achieved ≥20% loss of TBW18 with 15mg weekly dosing of tirzepatide. This class of drugs is a logical choice in patients with T2DM and obesity. Long-term treatment appears necessary, as patients typically regain two-thirds of lost weight within a year after GLP-1 RA are stopped.
Based on tumors observed in rodents, GLP-1 RA are contraindicated in patients with a personal or family history of multiple endocrine neoplasia type 2 (MEN II) or medullary thyroid cancer. These tumors have not been observed in humans treated with GLP-1 RA. They should be used with caution in patients with history of pancreatitis, gastroparesis, or diabetic retinopathy, though a recent systematic review and meta-analysis suggests showed little to no increased risk for biliary events from GLP-1 RA.19 Side effects are most commonly gastrointestinal in nature (nausea, reflux, constipation or diarrhea) and are typically most severe with initiation of the drug and with dose escalation. Side effects can be mitigated by initiating these drugs at lowest doses and gradually titrating up (every four weeks) based on effectiveness and tolerability. Antisecretory, antiemetic, and laxative medications can also be used to help manage GLP-1 RA related side effects.
There is no reason to escalate to highest doses if patients are experiencing weight loss and reduction in food cravings at lower doses. Both semaglutide and tirzepatide are administered subcutaneously every seven days. Once patients have reached goal weight, they can either continue maintenance therapy at that same dose/interval, or if motivated to do so, may gradually reduce the weekly dose in a stepwise approach to determine the minimally effective dose to maintain weight loss. There are not yet published maintenance studies to guide this process. Currently the price of GLP-1 RA and inconsistent insurance coverage make them inaccessible to many patients. The manufacturers of both semaglutide and tirzepatide offer direct to consumer pricing and home delivery.
Bariatric Surgery
In patients with higher BMI (≥35kg/m2) or those with BMI ≥30kg/m2 and obesity-related metabolic disease and the desire to avoid lifelong medications or who fail or are intolerant of AOM, bariatric options should be considered.20 Sleeve gastrectomy has become the most performed surgery for treatment of obesity. It is a restrictive procedure, removing 80% of the stomach, but a drop in circulating levels of ghrelin afterwards also leads to decreased feelings of hunger. It results in weight loss of 25-30% TBW loss. It is not a good choice for patients who suffer from severe GERD, as this typically worsens afterwards; furthermore, de novo Barrett’s has been observed in nearly 6% of patients who undergo sleeve gastrectomy.21
Roux-en-Y gastric bypass is a restrictive and malabsorptive procedure, resulting in 30-35% TBW loss. It has beneficial and immediate metabolic effects, including increased release of endogenous GLP-1, which leads to improvements in weight-related T2DM. The newer single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S) starts with a sleeve gastrectomy, making a smaller tube-shaped stomach. The duodenum is divided just after the stomach and then a loop of ileum is brought up and connected to the stomach (see Figure 1). This procedure is highly effective, with patients losing 75-95% of excess body weight and is becoming a preferred option for patients with greater BMI (≥50kg/m2). It is also an option for patients who have already had a sleeve gastrectomy and are seeking further weight loss. Because there is only one anastomosis, perioperative complications, such as anastomotic leaks, are reduced. The risk of micronutrient deficiencies is present with all malabsorptive procedures, and these patients must supplement with multivitamins, iron, vitamin D, and calcium.
Endoscopic Therapies
Endoscopic bariatric and metabolic therapies (EBMTs) have been increasingly studied and utilized, and this less invasive option may be more appropriate for or attractive to many patients. Intragastric balloons, which reduce meal volume and delay gastric emptying, can be used short term only (six months) resulting in loss of about 6.9% of total body weight (TBW) greater than lifestyle modification (LM) alone, and may be considered in limited situations, such as need for pre-operative weight loss to reduce risks in very obese individuals.22
Endoscopic gastric remodeling (EGR), also known as endoscopic sleeve gastrectomy (ESG), is a purely restrictive procedure in which the stomach is cinched to resize and reshape using an endoscopic suturing device (see Figure 2).23 It is an option for patients with class 1 or 2 obesity, with data from a randomized controlled trial in this population demonstrating mean percentage of TBW loss of 13.6% at 52 weeks compared to 0.8% in those treated with LM alone.24 A recent meta-analysis of 21 observational studies, including patients with higher BMIs (32.5 to 49.9 kg/m2) showed pooled average weight loss of 17.3% TBW at 12 months with EGR.22 This procedure has potential advantages of fewer complications, quicker recovery, and much less new-onset GERD compared to laparoscopic sleeve gastrectomy. Furthermore, it may be utilized in combination with AOMs to achieve optimum weight loss and metabolic outcomes.25,26 Potential adverse events include abdominal pain, nausea and vomiting (which may be severe), as well as rare instances of intra/extra luminal bleeding or abdominal abscess requiring drainage.22
Recent joint American/European Gastrointestinal Endoscopy guidelines suggest the use of EBMTs plus lifestyle modification in patients with a BMI of ≥ 30 kg/m2, or with a BMI of 27.0-29.9 kg/m2 with at least 1 obesity-related comorbidity.22 Small bowel interventions including duodenal-jejunal bypass liner and duodenal mucosal resurfacing are being investigated for patients with obesity and type 2 diabetes but not yet commercially available.
Conclusion
Given the overlap of obesity with many GI disorders, it is entirely appropriate for gastroenterologists to consider it worthy of aggressive treatment, particularly in patients with MAFLD and other serious weight related comorbidities. With a compassionate and empathetic approach, and a number of highly effective medical, endoscopic, and surgical therapies now available, weight management has the potential to be extremely rewarding when implemented in GI practice.
Dr. Kelly is based in the Department of Medicine, Division of Gastroenterology, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston, Massachusetts. She serves on the clinical advisory board for OpenBiome (unpaid) and has served on an advisory board for Eli Lilly and Company.
References
1. Hales CM, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief 2020 Feb:(360):1–8.
2. Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016 Dec. doi: 10.1016/j.jhep.2016.07.033.
3. Lauby-Secretan B, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016 Aug. doi: 10.1056/NEJMsr1606602.
4. Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015 Nov-Dec. doi: 10.1097/MCG.0000000000000356.
5. Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb. doi: 10.1038/nrgastro.2016.181.
6. Sundararaman L, Goudra B. Sedation for GI Endoscopy in the Morbidly Obese: Challenges and Possible Solutions. J Clin Med. 2024 Aug. doi: 10.3390/jcm13164635.
7. Bombassaro B, et al. The hypothalamus as the central regulator of energy balance and its impact on current and future obesity treatments. Arch Endocrinol Metab. 2024 Nov. doi: 10.20945/2359-4292-2024-0082.
8. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011 Jul. doi: 10.1097/MCO.0b013e3283479109.
9. Desalermos A, et al. Effect of Obesogenic Medications on Weight-Loss Outcomes in a Behavioral Weight-Management Program. Obesity (Silver Spring). 2019 May. doi: 10.1002/oby.22444.
10. Lord MN, Noble EE. Hypothalamic cannabinoid signaling: Consequences for eating behavior. Pharmacol Res Perspect. 2024 Oct. doi: 10.1002/prp2.1251.
11. Farhana A, Rehman A. Metabolic Consequences of Weight Reduction. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572145/.
12. Rubino F, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025 Mar. doi: 10.1016/S2213-8587(24)00316-4.
13. Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017 Aug. doi: 10.2337/ds17-0013.
14. Chaput JP, et al. Widespread misconceptions about obesity. Can Fam Physician. 2014 Nov. PMID: 25392431.
15. Muscogiuri G, et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr Obes Rep. 2022 Dec. doi: 10.1007/s13679-022-00481-1.
16. Gudzune KA, Kushner RF. Medications for Obesity: A Review. JAMA. 2024 Aug. doi: 10.1001/jama.2024.10816.
17. Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Feb. doi: 10.1056/NEJMoa2032183.
18. Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jun. doi: 10.1056/NEJMoa2206038.
19. Chiang CH, et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology. 2025 Nov. doi: 10.1053/j.gastro.2025.06.003.
20. Aderinto N, et al. Recent advances in bariatric surgery: a narrative review of weight loss procedures. Ann Med Surg (Lond). 2023 Nov. doi: 10.1097/MS9.0000000000001472.
21. Chandan S, et al. Risk of De Novo Barrett’s Esophagus Post Sleeve Gastrectomy: A Systematic Review and Meta-Analysis of Studies With Long-Term Follow-Up. Clin Gastroenterol Hepatol. 2025 Jan. doi: 10.1016/j.cgh.2024.06.041.
22. Jirapinyo P, et al. American Society for Gastrointestinal Endoscopy-European Society of Gastrointestinal Endoscopy guideline on primary endoscopic bariatric and metabolic therapies for adults with obesity. Gastrointest Endosc. 2024 Jun. doi: 10.1016/j.gie.2023.12.004.
23. Nduma BN, et al. Endoscopic Gastric Sleeve: A Review of Literature. Cureus. 2023 Mar. doi: 10.7759/cureus.36353.
24. Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022 Aug. doi: 10.1016/S0140-6736(22)01280-6.
25. Gala K, et al. Outcomes of concomitant antiobesity medication use with endoscopic sleeve gastroplasty in clinical US settings. Obes Pillars. 2024 May. doi: 10.1016/j.obpill.2024.100112.
26. Chung CS, et al. Endoscopic sleeve gastroplasty combined with anti-obesity medication for better control of weight and diabetes. Clin Endosc. 2025 May. doi: 10.5946/ce.2024.274.
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Deconditioning among hospitalized older adults contributes to significant decline in posthospitalization functional ability, physical performance, and physical activity.1-10 Previous hospital-to-home interventions have targeted improving function and physical activity, including recent programs leveraging home telehealth as a feasible and potentially effective mode of delivering in-home exercise and rehabilitation.11-14 However, pilot interventions have shown mixed effectiveness.11,12,14 This study expands on a previously published intervention describing a pilot home telehealth program for veterans posthospital discharge that demonstrated significant 6-month improvement in physical activity as well as trends in physical function improvement, including among those with cognitive impairment.15 Factors that contribute to improved outcomes are the focus of the present study.
Key factors underlying the complexity of hospital-to-home transitions include hospitalization elements (ie, reason for admission and length of stay), associated posthospital syndromes (ie, postdischarge falls, medication changes, cognitive impairment, and pain), and postdischarge health care application (ie, physical therapy and hospital readmission).16-18 These factors may be associated with postdischarge functional ability, physical performance, and physical activity, but their direct influence on intervention outcomes is unclear (Figure 1).5,7,9,16-20 The objective of this study was to examine the influence of hospitalization, posthospital syndrome, and postdischarge health care application factors on outcomes of a US Department of Veterans Affairs (VA) Video Connect (VVC) intervention to enhance function and physical activity in older adults posthospital discharge.
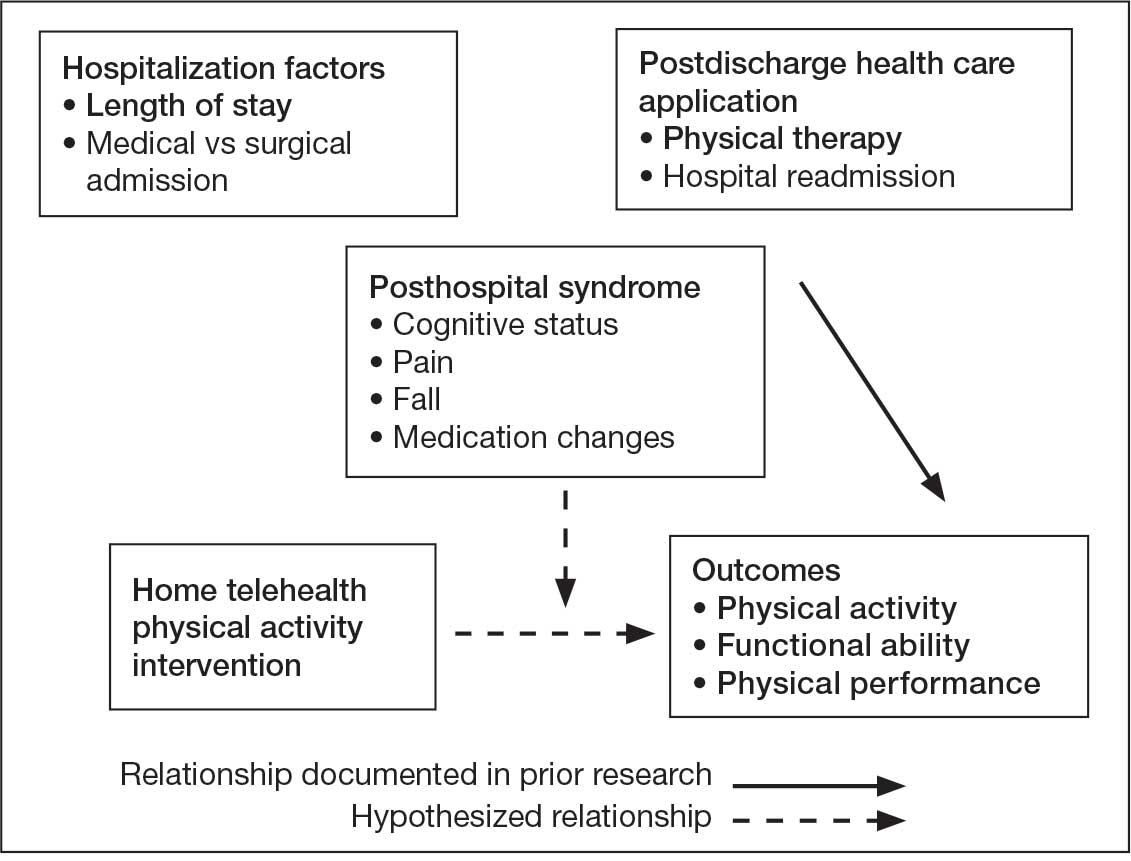
health care application factors on physical activity, functional ability, and
physical performance intervention outcomes.
Methods
The previous analysis reported on patient characteristics, program feasibility, and preliminary outcomes.13,15 The current study reports on relationships between hospitalization, posthospital syndrome, and postdischarge health care application factors and change in key outcomes, namely postdischarge self-reported functional ability, physical performance, and physical activity from baseline to endpoint.
Participants provided written informed consent. The protocol and consent forms were approved by the VA Ann Arbor Healthcare System (VAAAHS) Research and Development Committee, and the project was registered on clinicaltrials.gov (NCT04045054).
Intervention
The pilot program targeted older adults following recent hospital discharge from VAAAHS. Participants were eligible if they were aged ≥ 50 years, had been discharged following an inpatient stay in the past 1 to 2 weeks, evaluated by physical therapy during hospitalization with stated rehabilitation goals on discharge, and followed by a VAAAHS primary care physician. Participants were either recruited during hospital admission or shortly after discharge.13
An experienced physical activity trainer (PAT) supported the progression of participants’ rehabilitation goals via a home exercise program and coached the patient and caregiver to optimize functional ability, physical performance, and physical activity. The PAT was a nonlicensed research assistant with extensive experience in applying standard physical activity enhancement protocols (eg, increased walking) to older adults with comorbidities. Participation in the program lasted about 6 months. Initiation of the PAT program was delayed if the patient was already receiving postdischarge home-based or outpatient physical therapy. The PAT contacted the patient weekly via VVC for the first 6 weeks, then monthly for a total of 6 months. Each contact included information on optimal walking form, injury prevention, program progression, and ways to incorporate sit-to-stand transitions, nonsitting behavior, and walking into daily routines. The initial VVC contact lasted about 60 minutes and subsequent sessions lasted about 30 minutes.13
Demographic characteristics were self-reported by participants and included age, sex, race, years of education, and marital status. Clinical characteristics were obtained from each participant’s electronic health record (EHR), including copay status, index hospitalization length of stay, admission diagnosis, and postsurgery status (postsurgery vs nonpostsurgery). Intervention adherence was tracked as the number of PAT sessions attended.
Posthospital Syndrome Factors
Participant falls (categorized as those who reported a fall vs those who did not) and medication changes (number of changes reported, including new medication, discontinued medication, dose changes, medication changes, or changes in medication schedule) were reported by participants or caregivers during each VVC contact. Participants completed the Montreal Cognitive Assessment (MoCA) at baseline, and were dichotomized into 2 groups: no cognitive impairment (MoCA score ≥ 26) and mild to moderate cognitive impairment (MoCA score 10-25).13,21
Participants rated how much pain interfered with their normal daily activities since the previous VVC session on a 5-point Likert scale (1, not at all; to 5, extremely).22 Similar to prior research, participants were placed into 2 groups based on their mean pain interference score (individuals with scores from 1.0 to 2.0 in 1 group, and individuals with > 2.0 in another).23-25 Participants were separated into a no or mild pain interference group and a moderate to severe pain interference group. Hospital readmissions (VA and non-VA) and postdischarge physical therapy outcomes were obtained from the participant’s EHR, including primary care visits.
Outcomes
Outcomes were collected at baseline (posthospital discharge) and 6 months postenrollment.
Self-Reported Functional Ability. This measure is provided by participants or caregivers and measured by the Katz Index of Independence in Activities of Daily Living (ADL), Lawton and Brody Instrumental ADL Scale (IADL), Nagi Disability Model, and Rosow-Breslau Scale. The Katz ADL assesses the ability to complete 6 self-care activities and awards 1 point for independence and 0 if the individual is dependent (total score range, 0-6).26 The Lawton and Brody IADL measures an individual’s independence in 8 instrumental ADLs; it awards 1 point for independence and 0 if the individual is dependent (total score range, 0-8).27 The Nagi Disability Model evaluates an individual’s difficulty performing 5 tasks (total score range, 0-5) and tallies the number of items with a response other than “no difficulty at all” (higher total score indicates greater difficulty). 28 The Rosow-Breslau Scale is a 3-item measure of mobility disability; individual responses are 0 (no help) and 1 (requires help or unable); higher total score (range, 0-3) indicates greater disability.29
Physical Performance. Measured using the Short Physical Performance Battery (SPPB), which evaluates standing balance, sit to stand, and walking performance. Scores range from 0 to 4 on the balance, gait speed, and chair stand tests, for a total composite score between 0 and 12 (higher score indicates better performance).30
Physical Activity. Measured using actigraphy, namely a physical activity monitor adherent to the thigh (activ-PAL3TM, PAL Technologies Ltd., Glasgow, UK).31 Participants were instructed to wear the activPal for ≥ 1 week. Participants with a minimum of 5 days of wear were included in this analysis.
Data Analyses
Analyses were performed using SPSS software version 29.0.32 Continuous variables were summarized using mean (SD) or median and IQR using the weighted average method; categorical variables were summarized using frequencies and percentages. Baseline scores on outcome variables were compared by categorical hospitalization, posthospital syndrome, and postdischarge health care application factor variables using Mann-Whitney U tests. The differences between outcome variables from baseline to endpoint were then calculated to produce change scores. Relationships between the number of PAT sessions attended and baseline outcomes and outcome change scores were estimated using Spearman correlations. Relationships between categorical factors (hospitalization, posthospital syndrome, and postdischarge health care application) and outcome variable change scores (which were normally distributed) were examined using Mann-Whitney U tests. Relationships with continuous hospitalization (length of stay) and posthospital syndrome factors (medication changes) were estimated using Spearman correlations. Effect sizes (ES) were estimated with Cohen d; small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Missing data were handled using pairwise deletion.33 Therefore, sample sizes were reported for each analysis. For all statistical tests, P < .05 was considered significant.
Results
Twenty-four individuals completed the pilot intervention.15 Mean (SD) age was 73.6 (8.1) years (range, 64-93 years) and participants were predominantly White males (Table 1). Eight participants had a high school education only and 13 had more than a high school education. Diagnoses at admission included 9 patients with orthopedic/musculoskeletal conditions (6 were for joint replacement), 6 patients with vascular/pulmonary conditions, and 4 with gastrointestinal/renal/urological conditions. Of the 11 postsurgery participants, 7 were orthopedic, 4 were gastrointestinal, and 1 was peripheral vascular.
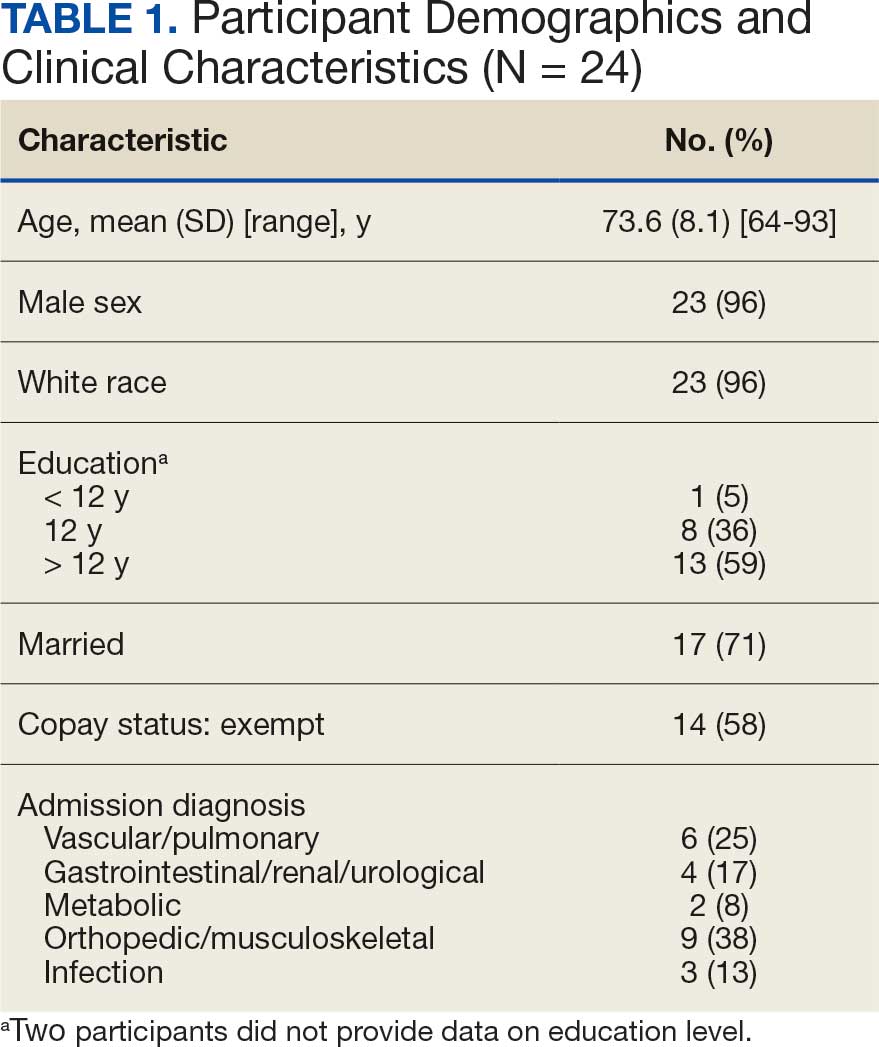
Baseline outcome scores did not differ significantly between groups, except individuals with moderate to severe pain interference reported a significantly lower IADL score (median [IQR] 4 [2-7]) than individuals with mild or moderate pain interference (median [IQR] 8 [7-8]; P = .02) (Table 2). The mean (SD) number of PAT sessions attended was 9.3 (3.7) (range, 3-19). There were no significant relationships between number of sessions attended and any baseline outcome variables or outcome change scores.
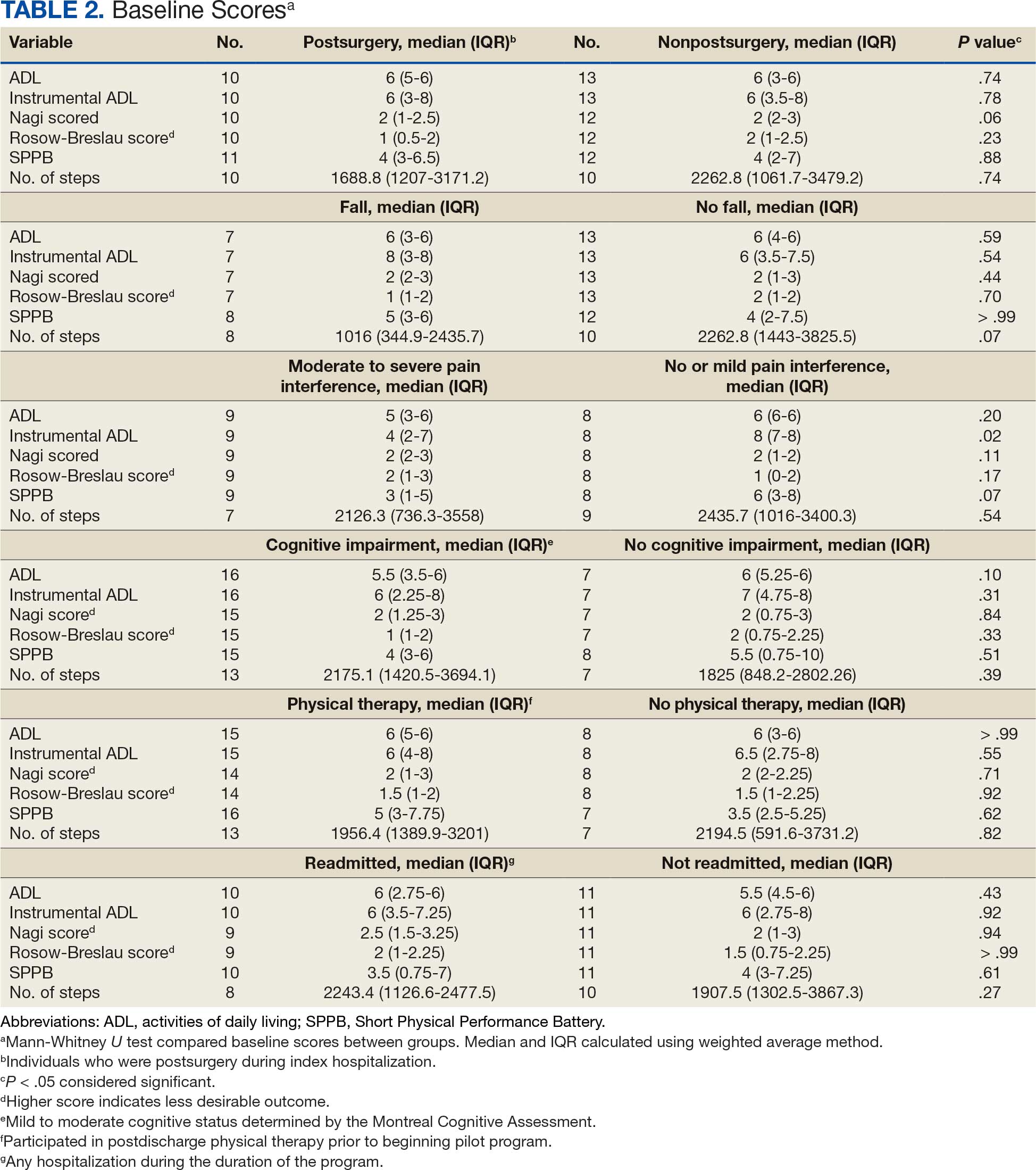
Hospitalization Factors
Participants who were postsurgery tended to have greater improvement than individuals who were nonpostsurgery in ADLs (median [IQR] 0 [0-1.5]; ES, 0.6; P = .10) and SPPB (median [IQR] 2 [1.5-9]; ES, 0.9; P = .07), but the improvements were not statistically significant (Table 3). Mean (SD) length of stay of the index hospitalization was 6.7 (6.1) days. Longer length of stay was significantly correlated with an increase in Nagi score (ρ, 0.45; 95% CI, 0.01-0.75). There were no other significant or trending relationships between length of stay and outcome variables.
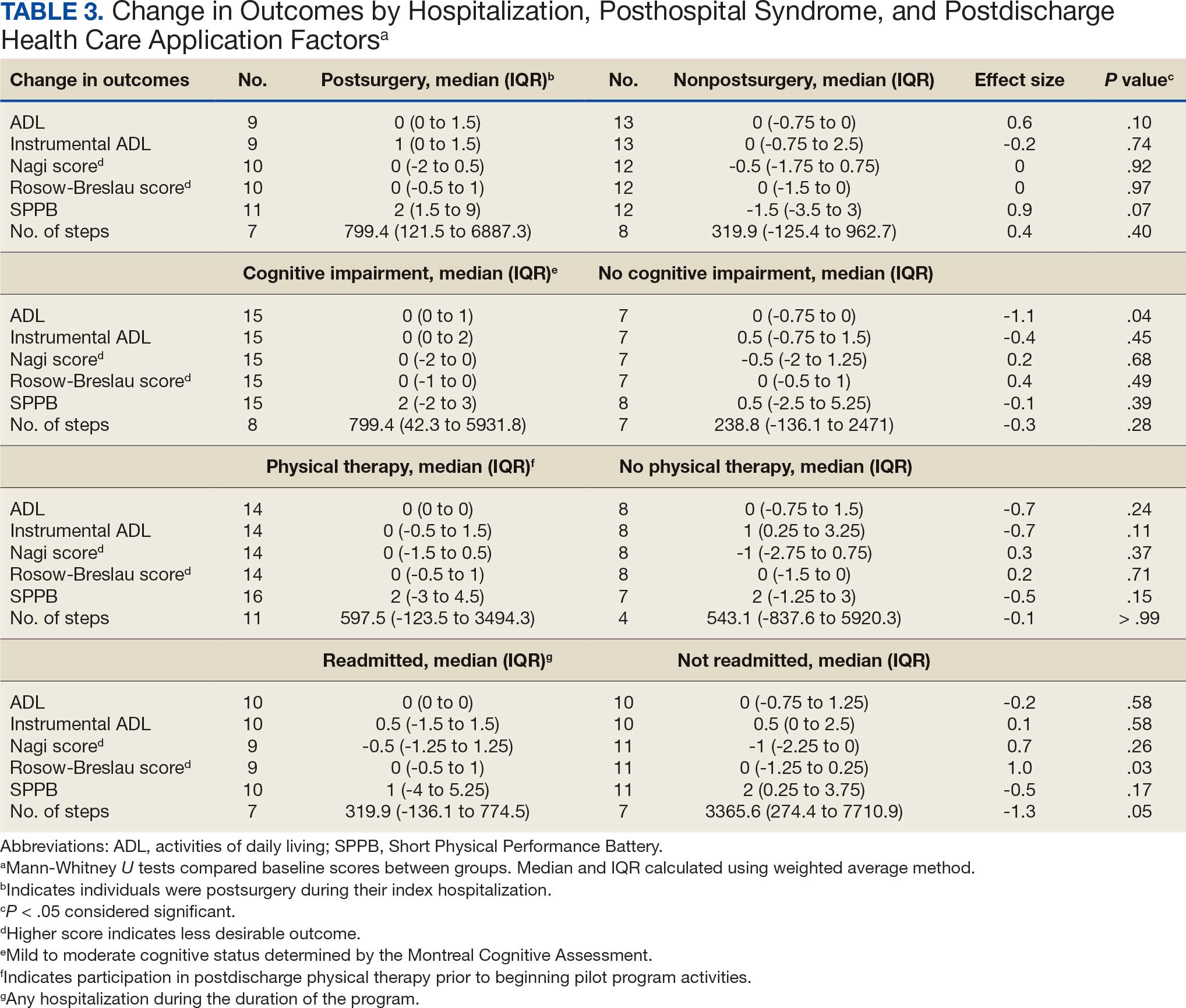
Posthospital Syndrome Factors
The 16 participants with mild to moderate cognitive impairment had less improvement in ADLs (median [IQR] 0 [0-1]) than the 8 participants with no impairment (median [IQR] 0 [-0.75 to 0]; ES, -1.1; P = .04). Change in outcome variables from baseline to endpoint did not significantly differ between the 8 patients who reported a fall compared with the 13 who did not, nor were any trends observed. Change in outcome variables from baseline to endpoint also did not significantly differ between the 8 participants who reported no or mild pain interference compared with the 10 patients with moderate to severe pain interference, nor were any trends observed. Mean (SD) number of medication changes was 2.5 (1.6). Higher number of medication changes was significantly correlated with a decrease in Rosow-Breslau score (ρ, -0.47; 95% CI, -0.76 to -0.02). There were no other significant or trending relationships between number of medication changes and outcome variables.
Postdischarge Health Care Application Factors
The 16 participants who attended posthospital physical therapy trended towards less improvement in IADLs (median [IQR] 0 [-0.5 to 1.5]; ES, -0.7; P = .11) and SPPB (median [IQR] 2 [-3.0 to 4.5]; ES, -0.5; P = .15) than the 8 patients with no postdischarge physical therapy. Eleven participants were readmitted, while 13 had no readmissions in their medical records between baseline and endpoint. Participants with ≥ 1 readmission experienced a greater increase in Rosow-Breslau score (median [IQR] 0 [-0.5 to 1.0]) than those not readmitted (median [IQR] 0 [-1.25 to 0.25]; ES, 1.0; P = .03). Borderline greater improvement in number of steps was found in those not readmitted (median [IQR] 3365.6 [274.4-7710.9]) compared with those readmitted (median [IQR] 319.9 [-136.1 to 774.5]; ES, -1.3; P = .05). Patients who were readmitted also tended to have lower and not statistically significant improvements in SPPB (median [IQR] 1 [-4.0 to 5.3]) compared with those not readmitted (median [IQR] 2 [0.3-3.8]; ES, -0.5; P = .17) (Table 3).
Discussion
This study examined the association between hospitalization, posthospital syndrome, and postdischarge health care use in patients undergoing a VVC-based intervention following hospital discharge. Participants who had no or mild cognitive impairment, no readmissions, higher medication changes, and a shorter hospital length of stay tended to experience lower disability, including in mobility and ADLs. This suggests individuals who are less clinically complex may be more likely to benefit from this type of virtual rehabilitation program. These findings are consistent with clinical experiences; home-based programs to improve physical activity posthospital discharge can be challenging for those who were medically ill (and did not undergo a specific surgical procedure), cognitively impaired, and become acutely ill and trigger hospital readmission. 15 For example, the sample in this study had higher rates of falls, pain, and readmissions compared to previous research.2,3,34-39
The importance of posthospital syndrome in the context of recovery of function and health at home following hospitalization is well documented.16-18 The potential impact of posthospital syndrome on physical activity-focused interventions is less understood. In our analysis, participants with mild or moderate cognitive impairment tended to become more dependent in their ADLs, while those with no cognitive impairment tended to become more independent in their ADLs. This functional decline over time is perhaps expected in persons with cognitive impairment, but the significant difference with a large ES warrants further consideration on how to tailor interventions to better promote functional recovery in these individuals.40,41 While some cognitive decline may not be preventable, this finding supports the need to promote healthy cognitive aging, identify declines in cognition, and work to mitigate additional decline. Programs specifically designed to promote function and physical activity in older adults with cognitive impairment are needed, especially during care transitions.41-43
While participants reported that falls and pain interference did not have a significant impact on change in outcomes between baseline and endpoint, these areas need further investigation. Falls and pain have been associated with function and physical activity in older adults.42-46 Pain is common, yet underappreciated during older adult hospital-to-home transitions.11,12,45,46 There is a need for more comprehensive assessment of pain (including pain intensity) and qualitative research.
Hospitalization and postdischarge health care application factors may have a significant impact on home-telehealth physical activity intervention success. Individuals who were postsurgery tended to have greater improvements in ADLs and physical performance. Most postsurgery participants had joint replacement surgery. Postsurgery status may not be modifiable, but it is important to note expected differences in recovery between medical and surgical admissions and the need to tailor care based on admission diagnosis. Those with a longer length of hospital stay may be considered at higher risk of suboptimal outcomes postdischarge, which indicates an opportunity for targeting resources and support, in addition to efforts of reducing length of stay where possible.47
Readmissions were significantly related to a change in Rosow-Breslau mobility disability score. This may indicate the detrimental impact a readmission can have on increasing mobility and physical activity postdischarge, or the potential of this pilot program to impact readmissions by increasing mobility and physical activity, contrary to prior physical exercise interventions.5,7,9,48 With 5% to 79% of readmissions considered preventable, continued efforts and program dissemination and implementation to address preventable readmissions are warranted.49 Individuals with postdischarge physical therapy (prior to beginning the pilot program) tended to demonstrate less improvement in disability and physical performance. This relationship needs further investigation; the 2 groups did not appear to have significant differences at baseline, albeit with a small sample size. It is possible they experienced initial improvements with postdischarge physical therapy and plateaued or had little further reserve to improve upon entering the VVC program.
Strengths and Limitations
This pilot program provided evaluative data on the use of VVC to enhance function and physical activity in older adults posthospital discharge. It included individual (eg, fall, pain, cognitive impairment) and health service (eg, readmission, physical therapy) level factors as predictors of function and physical activity posthospitalization.5,7,9,15-19
The results of this pilot project stem from a small sample lacking diversity in terms of race, ethnicity, and sex. There was some variation in baseline and endpoints between participants, and when hospitalization, posthospital syndrome, and postdischarge health care application factors were collected. The majority of participants were recruited within a month postdischarge, and the program lasted about 6 months. Data collection was attempted at regular PAT contacts, but there was some variation in when visits occurred based on participant availability and preference. Some participants had missing data, which was handled using pairwise deletion.33 Larger studies are needed to confirm the findings of this study, particularly the trends that did not reach statistical significance. Home health services other than physical therapy (eg, nursing, occupational therapy) were not fully accounted for and should be considered in future research.
Conclusions
In patients undergoing a 6-month pilot VVC-based physical activity intervention posthospital discharge, improvements in mobility and disability were most likely in those who had no cognitive impairment and were not readmitted. Larger sample and qualitative investigations are necessary to optimize outcomes for patients who meet these clinical profiles.
- Liebzeit D, Bratzke L, Boltz M, Purvis S, King B. Getting back to normal: a grounded theory study of function in post-hospitalized older adults. Gerontologist. 2020;60:704-714. doi:10.1093/geront/gnz057
- Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83-91. doi:10.1016/s0167-4943(02)00061-4
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi:10.1371/journal.pone.0026951
- Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049-1054. doi:10.1093/gerona/58.11.m1049
- Huang HT, Chang CM, Liu LF, Lin HS, Chen CH. Trajectories and predictors of functional decline of hospitalised older patients. J Clin Nurs. 2013;22:1322-1331. doi:10.1111/jocn.12055
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171- 2179. doi:10.1111/j.1532-5415.2008.02023.x
- Helvik AS, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57:305-310. doi:10.1016/j.archger.2013.05.008
- Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol Biol Sci Med Sci. 2015;70:381-386. doi:10.1093/gerona/glu168
- Chen CC, Wang C, Huang GH. Functional trajectory 6 months posthospitalization: a cohort study of older hospitalized patients in Taiwan. Nurs Res. 2008;57:93-100. doi:10.1097/01.NNR.0000313485.18670.e2
- Kleinpell RM, Fletcher K, Jennings BM. Reducing functional decline in hospitalized elderly. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008. Accessed September 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK2629/
- Liebzeit D, Rutkowski R, Arbaje AI, Fields B, Werner NE. A scoping review of interventions for older adults transitioning from hospital to home. J Am Geriatr Soc. 2021;69:2950-2962. doi:10.1111/jgs.17323
- Hladkowicz E, Dumitrascu F, Auais M, et al. Evaluations of postoperative transitions in care for older adults: a scoping review. BMC Geriatr. 2022;22:329. doi:10.1186/s12877-022-02989-6
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi:10.1186/s12877-021-02454-w
- Dawson R, Oliveira JS, Kwok WS, et al. Exercise interventions delivered through telehealth to improve physical functioning for older adults with frailty, cognitive, or mobility disability: a systematic review and meta-analysis. Telemed J E Health. 2024;30:940-950. doi:10.1089/tmj.2023.0177
- Liebzeit D, Phillips KK, Hogikyan RV, Cigolle CT, Alexander NB. A pilot home-telehealth program to enhance functional ability, physical performance, and physical activity in older adult veterans post-hospital discharge. Res Gerontol Nurs. 2024;17:271-279. doi:10.3928/19404921-20241105-01
- Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100-102. doi:10.1056/NEJMp1212324
- Caraballo C, Dharmarajan K, Krumholz HM. Post hospital syndrome: is the stress of hospitalization causing harm? Rev Esp Cardiol (Engl Ed). 2019;72:896-898. doi:10.1016/j.rec.2019.04.010
- Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179:38- 45. doi:10.1001/jamainternmed.2018.5100
- Dutzi I, Schwenk M, Kirchner M, Jooss E, Bauer JM, Hauer K. Influence of cognitive impairment on rehabilitation received and its mediating effect on functional recovery. J Alzheimers Dis. 2021;84:745-756. doi:10.3233/JAD-210620
- Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence ofcognitiveimpairment. JAmMedDirAssoc. 2015;16:215-220. doi:10.1016/j.jamda.2014.09.009
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. doi:10.1111/j.1532-5415.2005.53221.x
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
- White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014;62:2350-2356. doi:10.1111/jgs.13135
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134. doi:10.1016/s0304-3959(00)00355-9
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368. doi:10.1016/j.pain.2004.04.017
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi:10.1001/jama.1963.03060120024016
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
- Alexander NB, Guire KE, Thelen DG, et al. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48:1408-1413. doi:10.1111/j.1532-5415.2000.tb02630.x
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556-559. doi:10.1093/geronj/21.4.556
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. doi:10.1093/geronj/49.2.m85
- Chan CS, Slaughter SE, Jones CA, Ickert C, Wagg AS. Measuring activity performance of older adults using the activPAL: a rapid review. Healthcare (Basel). 2017;5:94. doi:10.3390/healthcare5040094
- IBM SPSS software. IBM Corp; 2019. Accessed September 3, 2025. https://www.ibm.com/spss
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402-406. doi:10.4097/kjae.2013.64.5.402
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287-2295. doi:10.1056/NEJMsa1101942
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63:548-552. doi:10.1111/jgs.13317
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. doi:10.1056/NEJMsa0803563
- Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277-282. doi:10.1002/jhm.2152
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. J Am Geriatr Soc. 1994;42:269- 274. doi:10.1111/j.1532-5415.1994.tb01750.x
- Hoffman GJ, Liu H, Alexander NB, Tinetti M, Braun TM, Min LC. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276. doi:10.1001/jamanetworkopen.2019.4276
- Gill DP, Hubbard RA, Koepsell TD, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9:S63-S71. doi:10.1016/j.jalz.2012.10.010
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi:10.1159/000154929
- Patti A, Zangla D, Sahin FN, et al. Physical exercise and prevention of falls. Effects of a Pilates training method compared with a general physical activity program. Medicine (Baltimore). 2021;100:e25289. doi:10.1097/MD.0000000000025289
- Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. 2022;22:203. doi:10.1186/s12877-022-02879-x
- Ek S, Rizzuto D, Xu W, Calderón-Larrañaga A, Welmer AK. Predictors for functional decline after an injurious fall: a population-based cohort study. Aging Clin Exp Res. 2021;33:2183-2190. doi:10.1007/s40520-020-01747-1
- Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. 2022;16:736688. doi:10.3389/fnhum.2022.736688
- Ritchie CS, Patel K, Boscardin J, et al. Impact of persistent pain on function, cognition, and well-being of older adults. J Am Geriatr Soc. 2023;71:26-35. doi:10.1111/jgs.18125
- Han TS, Murray P, Robin J, et al. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Health Care. 2022;34:mzab160. doi:10.1093/intqhc/ mzab160
- Lærum-Onsager E, Molin M, Olsen CF, et al. Effect of nutritional and physical exercise intervention on hospital readmission for patients aged 65 or older: a systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2021;18:62. doi:10.1186/s12966-021-01123-w
- Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-E402. doi:10.1503/cmaj.101860
Deconditioning among hospitalized older adults contributes to significant decline in posthospitalization functional ability, physical performance, and physical activity.1-10 Previous hospital-to-home interventions have targeted improving function and physical activity, including recent programs leveraging home telehealth as a feasible and potentially effective mode of delivering in-home exercise and rehabilitation.11-14 However, pilot interventions have shown mixed effectiveness.11,12,14 This study expands on a previously published intervention describing a pilot home telehealth program for veterans posthospital discharge that demonstrated significant 6-month improvement in physical activity as well as trends in physical function improvement, including among those with cognitive impairment.15 Factors that contribute to improved outcomes are the focus of the present study.
Key factors underlying the complexity of hospital-to-home transitions include hospitalization elements (ie, reason for admission and length of stay), associated posthospital syndromes (ie, postdischarge falls, medication changes, cognitive impairment, and pain), and postdischarge health care application (ie, physical therapy and hospital readmission).16-18 These factors may be associated with postdischarge functional ability, physical performance, and physical activity, but their direct influence on intervention outcomes is unclear (Figure 1).5,7,9,16-20 The objective of this study was to examine the influence of hospitalization, posthospital syndrome, and postdischarge health care application factors on outcomes of a US Department of Veterans Affairs (VA) Video Connect (VVC) intervention to enhance function and physical activity in older adults posthospital discharge.

health care application factors on physical activity, functional ability, and
physical performance intervention outcomes.
Methods
The previous analysis reported on patient characteristics, program feasibility, and preliminary outcomes.13,15 The current study reports on relationships between hospitalization, posthospital syndrome, and postdischarge health care application factors and change in key outcomes, namely postdischarge self-reported functional ability, physical performance, and physical activity from baseline to endpoint.
Participants provided written informed consent. The protocol and consent forms were approved by the VA Ann Arbor Healthcare System (VAAAHS) Research and Development Committee, and the project was registered on clinicaltrials.gov (NCT04045054).
Intervention
The pilot program targeted older adults following recent hospital discharge from VAAAHS. Participants were eligible if they were aged ≥ 50 years, had been discharged following an inpatient stay in the past 1 to 2 weeks, evaluated by physical therapy during hospitalization with stated rehabilitation goals on discharge, and followed by a VAAAHS primary care physician. Participants were either recruited during hospital admission or shortly after discharge.13
An experienced physical activity trainer (PAT) supported the progression of participants’ rehabilitation goals via a home exercise program and coached the patient and caregiver to optimize functional ability, physical performance, and physical activity. The PAT was a nonlicensed research assistant with extensive experience in applying standard physical activity enhancement protocols (eg, increased walking) to older adults with comorbidities. Participation in the program lasted about 6 months. Initiation of the PAT program was delayed if the patient was already receiving postdischarge home-based or outpatient physical therapy. The PAT contacted the patient weekly via VVC for the first 6 weeks, then monthly for a total of 6 months. Each contact included information on optimal walking form, injury prevention, program progression, and ways to incorporate sit-to-stand transitions, nonsitting behavior, and walking into daily routines. The initial VVC contact lasted about 60 minutes and subsequent sessions lasted about 30 minutes.13
Demographic characteristics were self-reported by participants and included age, sex, race, years of education, and marital status. Clinical characteristics were obtained from each participant’s electronic health record (EHR), including copay status, index hospitalization length of stay, admission diagnosis, and postsurgery status (postsurgery vs nonpostsurgery). Intervention adherence was tracked as the number of PAT sessions attended.
Posthospital Syndrome Factors
Participant falls (categorized as those who reported a fall vs those who did not) and medication changes (number of changes reported, including new medication, discontinued medication, dose changes, medication changes, or changes in medication schedule) were reported by participants or caregivers during each VVC contact. Participants completed the Montreal Cognitive Assessment (MoCA) at baseline, and were dichotomized into 2 groups: no cognitive impairment (MoCA score ≥ 26) and mild to moderate cognitive impairment (MoCA score 10-25).13,21
Participants rated how much pain interfered with their normal daily activities since the previous VVC session on a 5-point Likert scale (1, not at all; to 5, extremely).22 Similar to prior research, participants were placed into 2 groups based on their mean pain interference score (individuals with scores from 1.0 to 2.0 in 1 group, and individuals with > 2.0 in another).23-25 Participants were separated into a no or mild pain interference group and a moderate to severe pain interference group. Hospital readmissions (VA and non-VA) and postdischarge physical therapy outcomes were obtained from the participant’s EHR, including primary care visits.
Outcomes
Outcomes were collected at baseline (posthospital discharge) and 6 months postenrollment.
Self-Reported Functional Ability. This measure is provided by participants or caregivers and measured by the Katz Index of Independence in Activities of Daily Living (ADL), Lawton and Brody Instrumental ADL Scale (IADL), Nagi Disability Model, and Rosow-Breslau Scale. The Katz ADL assesses the ability to complete 6 self-care activities and awards 1 point for independence and 0 if the individual is dependent (total score range, 0-6).26 The Lawton and Brody IADL measures an individual’s independence in 8 instrumental ADLs; it awards 1 point for independence and 0 if the individual is dependent (total score range, 0-8).27 The Nagi Disability Model evaluates an individual’s difficulty performing 5 tasks (total score range, 0-5) and tallies the number of items with a response other than “no difficulty at all” (higher total score indicates greater difficulty). 28 The Rosow-Breslau Scale is a 3-item measure of mobility disability; individual responses are 0 (no help) and 1 (requires help or unable); higher total score (range, 0-3) indicates greater disability.29
Physical Performance. Measured using the Short Physical Performance Battery (SPPB), which evaluates standing balance, sit to stand, and walking performance. Scores range from 0 to 4 on the balance, gait speed, and chair stand tests, for a total composite score between 0 and 12 (higher score indicates better performance).30
Physical Activity. Measured using actigraphy, namely a physical activity monitor adherent to the thigh (activ-PAL3TM, PAL Technologies Ltd., Glasgow, UK).31 Participants were instructed to wear the activPal for ≥ 1 week. Participants with a minimum of 5 days of wear were included in this analysis.
Data Analyses
Analyses were performed using SPSS software version 29.0.32 Continuous variables were summarized using mean (SD) or median and IQR using the weighted average method; categorical variables were summarized using frequencies and percentages. Baseline scores on outcome variables were compared by categorical hospitalization, posthospital syndrome, and postdischarge health care application factor variables using Mann-Whitney U tests. The differences between outcome variables from baseline to endpoint were then calculated to produce change scores. Relationships between the number of PAT sessions attended and baseline outcomes and outcome change scores were estimated using Spearman correlations. Relationships between categorical factors (hospitalization, posthospital syndrome, and postdischarge health care application) and outcome variable change scores (which were normally distributed) were examined using Mann-Whitney U tests. Relationships with continuous hospitalization (length of stay) and posthospital syndrome factors (medication changes) were estimated using Spearman correlations. Effect sizes (ES) were estimated with Cohen d; small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Missing data were handled using pairwise deletion.33 Therefore, sample sizes were reported for each analysis. For all statistical tests, P < .05 was considered significant.
Results
Twenty-four individuals completed the pilot intervention.15 Mean (SD) age was 73.6 (8.1) years (range, 64-93 years) and participants were predominantly White males (Table 1). Eight participants had a high school education only and 13 had more than a high school education. Diagnoses at admission included 9 patients with orthopedic/musculoskeletal conditions (6 were for joint replacement), 6 patients with vascular/pulmonary conditions, and 4 with gastrointestinal/renal/urological conditions. Of the 11 postsurgery participants, 7 were orthopedic, 4 were gastrointestinal, and 1 was peripheral vascular.

Baseline outcome scores did not differ significantly between groups, except individuals with moderate to severe pain interference reported a significantly lower IADL score (median [IQR] 4 [2-7]) than individuals with mild or moderate pain interference (median [IQR] 8 [7-8]; P = .02) (Table 2). The mean (SD) number of PAT sessions attended was 9.3 (3.7) (range, 3-19). There were no significant relationships between number of sessions attended and any baseline outcome variables or outcome change scores.

Hospitalization Factors
Participants who were postsurgery tended to have greater improvement than individuals who were nonpostsurgery in ADLs (median [IQR] 0 [0-1.5]; ES, 0.6; P = .10) and SPPB (median [IQR] 2 [1.5-9]; ES, 0.9; P = .07), but the improvements were not statistically significant (Table 3). Mean (SD) length of stay of the index hospitalization was 6.7 (6.1) days. Longer length of stay was significantly correlated with an increase in Nagi score (ρ, 0.45; 95% CI, 0.01-0.75). There were no other significant or trending relationships between length of stay and outcome variables.

Posthospital Syndrome Factors
The 16 participants with mild to moderate cognitive impairment had less improvement in ADLs (median [IQR] 0 [0-1]) than the 8 participants with no impairment (median [IQR] 0 [-0.75 to 0]; ES, -1.1; P = .04). Change in outcome variables from baseline to endpoint did not significantly differ between the 8 patients who reported a fall compared with the 13 who did not, nor were any trends observed. Change in outcome variables from baseline to endpoint also did not significantly differ between the 8 participants who reported no or mild pain interference compared with the 10 patients with moderate to severe pain interference, nor were any trends observed. Mean (SD) number of medication changes was 2.5 (1.6). Higher number of medication changes was significantly correlated with a decrease in Rosow-Breslau score (ρ, -0.47; 95% CI, -0.76 to -0.02). There were no other significant or trending relationships between number of medication changes and outcome variables.
Postdischarge Health Care Application Factors
The 16 participants who attended posthospital physical therapy trended towards less improvement in IADLs (median [IQR] 0 [-0.5 to 1.5]; ES, -0.7; P = .11) and SPPB (median [IQR] 2 [-3.0 to 4.5]; ES, -0.5; P = .15) than the 8 patients with no postdischarge physical therapy. Eleven participants were readmitted, while 13 had no readmissions in their medical records between baseline and endpoint. Participants with ≥ 1 readmission experienced a greater increase in Rosow-Breslau score (median [IQR] 0 [-0.5 to 1.0]) than those not readmitted (median [IQR] 0 [-1.25 to 0.25]; ES, 1.0; P = .03). Borderline greater improvement in number of steps was found in those not readmitted (median [IQR] 3365.6 [274.4-7710.9]) compared with those readmitted (median [IQR] 319.9 [-136.1 to 774.5]; ES, -1.3; P = .05). Patients who were readmitted also tended to have lower and not statistically significant improvements in SPPB (median [IQR] 1 [-4.0 to 5.3]) compared with those not readmitted (median [IQR] 2 [0.3-3.8]; ES, -0.5; P = .17) (Table 3).
Discussion
This study examined the association between hospitalization, posthospital syndrome, and postdischarge health care use in patients undergoing a VVC-based intervention following hospital discharge. Participants who had no or mild cognitive impairment, no readmissions, higher medication changes, and a shorter hospital length of stay tended to experience lower disability, including in mobility and ADLs. This suggests individuals who are less clinically complex may be more likely to benefit from this type of virtual rehabilitation program. These findings are consistent with clinical experiences; home-based programs to improve physical activity posthospital discharge can be challenging for those who were medically ill (and did not undergo a specific surgical procedure), cognitively impaired, and become acutely ill and trigger hospital readmission. 15 For example, the sample in this study had higher rates of falls, pain, and readmissions compared to previous research.2,3,34-39
The importance of posthospital syndrome in the context of recovery of function and health at home following hospitalization is well documented.16-18 The potential impact of posthospital syndrome on physical activity-focused interventions is less understood. In our analysis, participants with mild or moderate cognitive impairment tended to become more dependent in their ADLs, while those with no cognitive impairment tended to become more independent in their ADLs. This functional decline over time is perhaps expected in persons with cognitive impairment, but the significant difference with a large ES warrants further consideration on how to tailor interventions to better promote functional recovery in these individuals.40,41 While some cognitive decline may not be preventable, this finding supports the need to promote healthy cognitive aging, identify declines in cognition, and work to mitigate additional decline. Programs specifically designed to promote function and physical activity in older adults with cognitive impairment are needed, especially during care transitions.41-43
While participants reported that falls and pain interference did not have a significant impact on change in outcomes between baseline and endpoint, these areas need further investigation. Falls and pain have been associated with function and physical activity in older adults.42-46 Pain is common, yet underappreciated during older adult hospital-to-home transitions.11,12,45,46 There is a need for more comprehensive assessment of pain (including pain intensity) and qualitative research.
Hospitalization and postdischarge health care application factors may have a significant impact on home-telehealth physical activity intervention success. Individuals who were postsurgery tended to have greater improvements in ADLs and physical performance. Most postsurgery participants had joint replacement surgery. Postsurgery status may not be modifiable, but it is important to note expected differences in recovery between medical and surgical admissions and the need to tailor care based on admission diagnosis. Those with a longer length of hospital stay may be considered at higher risk of suboptimal outcomes postdischarge, which indicates an opportunity for targeting resources and support, in addition to efforts of reducing length of stay where possible.47
Readmissions were significantly related to a change in Rosow-Breslau mobility disability score. This may indicate the detrimental impact a readmission can have on increasing mobility and physical activity postdischarge, or the potential of this pilot program to impact readmissions by increasing mobility and physical activity, contrary to prior physical exercise interventions.5,7,9,48 With 5% to 79% of readmissions considered preventable, continued efforts and program dissemination and implementation to address preventable readmissions are warranted.49 Individuals with postdischarge physical therapy (prior to beginning the pilot program) tended to demonstrate less improvement in disability and physical performance. This relationship needs further investigation; the 2 groups did not appear to have significant differences at baseline, albeit with a small sample size. It is possible they experienced initial improvements with postdischarge physical therapy and plateaued or had little further reserve to improve upon entering the VVC program.
Strengths and Limitations
This pilot program provided evaluative data on the use of VVC to enhance function and physical activity in older adults posthospital discharge. It included individual (eg, fall, pain, cognitive impairment) and health service (eg, readmission, physical therapy) level factors as predictors of function and physical activity posthospitalization.5,7,9,15-19
The results of this pilot project stem from a small sample lacking diversity in terms of race, ethnicity, and sex. There was some variation in baseline and endpoints between participants, and when hospitalization, posthospital syndrome, and postdischarge health care application factors were collected. The majority of participants were recruited within a month postdischarge, and the program lasted about 6 months. Data collection was attempted at regular PAT contacts, but there was some variation in when visits occurred based on participant availability and preference. Some participants had missing data, which was handled using pairwise deletion.33 Larger studies are needed to confirm the findings of this study, particularly the trends that did not reach statistical significance. Home health services other than physical therapy (eg, nursing, occupational therapy) were not fully accounted for and should be considered in future research.
Conclusions
In patients undergoing a 6-month pilot VVC-based physical activity intervention posthospital discharge, improvements in mobility and disability were most likely in those who had no cognitive impairment and were not readmitted. Larger sample and qualitative investigations are necessary to optimize outcomes for patients who meet these clinical profiles.
Deconditioning among hospitalized older adults contributes to significant decline in posthospitalization functional ability, physical performance, and physical activity.1-10 Previous hospital-to-home interventions have targeted improving function and physical activity, including recent programs leveraging home telehealth as a feasible and potentially effective mode of delivering in-home exercise and rehabilitation.11-14 However, pilot interventions have shown mixed effectiveness.11,12,14 This study expands on a previously published intervention describing a pilot home telehealth program for veterans posthospital discharge that demonstrated significant 6-month improvement in physical activity as well as trends in physical function improvement, including among those with cognitive impairment.15 Factors that contribute to improved outcomes are the focus of the present study.
Key factors underlying the complexity of hospital-to-home transitions include hospitalization elements (ie, reason for admission and length of stay), associated posthospital syndromes (ie, postdischarge falls, medication changes, cognitive impairment, and pain), and postdischarge health care application (ie, physical therapy and hospital readmission).16-18 These factors may be associated with postdischarge functional ability, physical performance, and physical activity, but their direct influence on intervention outcomes is unclear (Figure 1).5,7,9,16-20 The objective of this study was to examine the influence of hospitalization, posthospital syndrome, and postdischarge health care application factors on outcomes of a US Department of Veterans Affairs (VA) Video Connect (VVC) intervention to enhance function and physical activity in older adults posthospital discharge.

health care application factors on physical activity, functional ability, and
physical performance intervention outcomes.
Methods
The previous analysis reported on patient characteristics, program feasibility, and preliminary outcomes.13,15 The current study reports on relationships between hospitalization, posthospital syndrome, and postdischarge health care application factors and change in key outcomes, namely postdischarge self-reported functional ability, physical performance, and physical activity from baseline to endpoint.
Participants provided written informed consent. The protocol and consent forms were approved by the VA Ann Arbor Healthcare System (VAAAHS) Research and Development Committee, and the project was registered on clinicaltrials.gov (NCT04045054).
Intervention
The pilot program targeted older adults following recent hospital discharge from VAAAHS. Participants were eligible if they were aged ≥ 50 years, had been discharged following an inpatient stay in the past 1 to 2 weeks, evaluated by physical therapy during hospitalization with stated rehabilitation goals on discharge, and followed by a VAAAHS primary care physician. Participants were either recruited during hospital admission or shortly after discharge.13
An experienced physical activity trainer (PAT) supported the progression of participants’ rehabilitation goals via a home exercise program and coached the patient and caregiver to optimize functional ability, physical performance, and physical activity. The PAT was a nonlicensed research assistant with extensive experience in applying standard physical activity enhancement protocols (eg, increased walking) to older adults with comorbidities. Participation in the program lasted about 6 months. Initiation of the PAT program was delayed if the patient was already receiving postdischarge home-based or outpatient physical therapy. The PAT contacted the patient weekly via VVC for the first 6 weeks, then monthly for a total of 6 months. Each contact included information on optimal walking form, injury prevention, program progression, and ways to incorporate sit-to-stand transitions, nonsitting behavior, and walking into daily routines. The initial VVC contact lasted about 60 minutes and subsequent sessions lasted about 30 minutes.13
Demographic characteristics were self-reported by participants and included age, sex, race, years of education, and marital status. Clinical characteristics were obtained from each participant’s electronic health record (EHR), including copay status, index hospitalization length of stay, admission diagnosis, and postsurgery status (postsurgery vs nonpostsurgery). Intervention adherence was tracked as the number of PAT sessions attended.
Posthospital Syndrome Factors
Participant falls (categorized as those who reported a fall vs those who did not) and medication changes (number of changes reported, including new medication, discontinued medication, dose changes, medication changes, or changes in medication schedule) were reported by participants or caregivers during each VVC contact. Participants completed the Montreal Cognitive Assessment (MoCA) at baseline, and were dichotomized into 2 groups: no cognitive impairment (MoCA score ≥ 26) and mild to moderate cognitive impairment (MoCA score 10-25).13,21
Participants rated how much pain interfered with their normal daily activities since the previous VVC session on a 5-point Likert scale (1, not at all; to 5, extremely).22 Similar to prior research, participants were placed into 2 groups based on their mean pain interference score (individuals with scores from 1.0 to 2.0 in 1 group, and individuals with > 2.0 in another).23-25 Participants were separated into a no or mild pain interference group and a moderate to severe pain interference group. Hospital readmissions (VA and non-VA) and postdischarge physical therapy outcomes were obtained from the participant’s EHR, including primary care visits.
Outcomes
Outcomes were collected at baseline (posthospital discharge) and 6 months postenrollment.
Self-Reported Functional Ability. This measure is provided by participants or caregivers and measured by the Katz Index of Independence in Activities of Daily Living (ADL), Lawton and Brody Instrumental ADL Scale (IADL), Nagi Disability Model, and Rosow-Breslau Scale. The Katz ADL assesses the ability to complete 6 self-care activities and awards 1 point for independence and 0 if the individual is dependent (total score range, 0-6).26 The Lawton and Brody IADL measures an individual’s independence in 8 instrumental ADLs; it awards 1 point for independence and 0 if the individual is dependent (total score range, 0-8).27 The Nagi Disability Model evaluates an individual’s difficulty performing 5 tasks (total score range, 0-5) and tallies the number of items with a response other than “no difficulty at all” (higher total score indicates greater difficulty). 28 The Rosow-Breslau Scale is a 3-item measure of mobility disability; individual responses are 0 (no help) and 1 (requires help or unable); higher total score (range, 0-3) indicates greater disability.29
Physical Performance. Measured using the Short Physical Performance Battery (SPPB), which evaluates standing balance, sit to stand, and walking performance. Scores range from 0 to 4 on the balance, gait speed, and chair stand tests, for a total composite score between 0 and 12 (higher score indicates better performance).30
Physical Activity. Measured using actigraphy, namely a physical activity monitor adherent to the thigh (activ-PAL3TM, PAL Technologies Ltd., Glasgow, UK).31 Participants were instructed to wear the activPal for ≥ 1 week. Participants with a minimum of 5 days of wear were included in this analysis.
Data Analyses
Analyses were performed using SPSS software version 29.0.32 Continuous variables were summarized using mean (SD) or median and IQR using the weighted average method; categorical variables were summarized using frequencies and percentages. Baseline scores on outcome variables were compared by categorical hospitalization, posthospital syndrome, and postdischarge health care application factor variables using Mann-Whitney U tests. The differences between outcome variables from baseline to endpoint were then calculated to produce change scores. Relationships between the number of PAT sessions attended and baseline outcomes and outcome change scores were estimated using Spearman correlations. Relationships between categorical factors (hospitalization, posthospital syndrome, and postdischarge health care application) and outcome variable change scores (which were normally distributed) were examined using Mann-Whitney U tests. Relationships with continuous hospitalization (length of stay) and posthospital syndrome factors (medication changes) were estimated using Spearman correlations. Effect sizes (ES) were estimated with Cohen d; small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Missing data were handled using pairwise deletion.33 Therefore, sample sizes were reported for each analysis. For all statistical tests, P < .05 was considered significant.
Results
Twenty-four individuals completed the pilot intervention.15 Mean (SD) age was 73.6 (8.1) years (range, 64-93 years) and participants were predominantly White males (Table 1). Eight participants had a high school education only and 13 had more than a high school education. Diagnoses at admission included 9 patients with orthopedic/musculoskeletal conditions (6 were for joint replacement), 6 patients with vascular/pulmonary conditions, and 4 with gastrointestinal/renal/urological conditions. Of the 11 postsurgery participants, 7 were orthopedic, 4 were gastrointestinal, and 1 was peripheral vascular.

Baseline outcome scores did not differ significantly between groups, except individuals with moderate to severe pain interference reported a significantly lower IADL score (median [IQR] 4 [2-7]) than individuals with mild or moderate pain interference (median [IQR] 8 [7-8]; P = .02) (Table 2). The mean (SD) number of PAT sessions attended was 9.3 (3.7) (range, 3-19). There were no significant relationships between number of sessions attended and any baseline outcome variables or outcome change scores.

Hospitalization Factors
Participants who were postsurgery tended to have greater improvement than individuals who were nonpostsurgery in ADLs (median [IQR] 0 [0-1.5]; ES, 0.6; P = .10) and SPPB (median [IQR] 2 [1.5-9]; ES, 0.9; P = .07), but the improvements were not statistically significant (Table 3). Mean (SD) length of stay of the index hospitalization was 6.7 (6.1) days. Longer length of stay was significantly correlated with an increase in Nagi score (ρ, 0.45; 95% CI, 0.01-0.75). There were no other significant or trending relationships between length of stay and outcome variables.

Posthospital Syndrome Factors
The 16 participants with mild to moderate cognitive impairment had less improvement in ADLs (median [IQR] 0 [0-1]) than the 8 participants with no impairment (median [IQR] 0 [-0.75 to 0]; ES, -1.1; P = .04). Change in outcome variables from baseline to endpoint did not significantly differ between the 8 patients who reported a fall compared with the 13 who did not, nor were any trends observed. Change in outcome variables from baseline to endpoint also did not significantly differ between the 8 participants who reported no or mild pain interference compared with the 10 patients with moderate to severe pain interference, nor were any trends observed. Mean (SD) number of medication changes was 2.5 (1.6). Higher number of medication changes was significantly correlated with a decrease in Rosow-Breslau score (ρ, -0.47; 95% CI, -0.76 to -0.02). There were no other significant or trending relationships between number of medication changes and outcome variables.
Postdischarge Health Care Application Factors
The 16 participants who attended posthospital physical therapy trended towards less improvement in IADLs (median [IQR] 0 [-0.5 to 1.5]; ES, -0.7; P = .11) and SPPB (median [IQR] 2 [-3.0 to 4.5]; ES, -0.5; P = .15) than the 8 patients with no postdischarge physical therapy. Eleven participants were readmitted, while 13 had no readmissions in their medical records between baseline and endpoint. Participants with ≥ 1 readmission experienced a greater increase in Rosow-Breslau score (median [IQR] 0 [-0.5 to 1.0]) than those not readmitted (median [IQR] 0 [-1.25 to 0.25]; ES, 1.0; P = .03). Borderline greater improvement in number of steps was found in those not readmitted (median [IQR] 3365.6 [274.4-7710.9]) compared with those readmitted (median [IQR] 319.9 [-136.1 to 774.5]; ES, -1.3; P = .05). Patients who were readmitted also tended to have lower and not statistically significant improvements in SPPB (median [IQR] 1 [-4.0 to 5.3]) compared with those not readmitted (median [IQR] 2 [0.3-3.8]; ES, -0.5; P = .17) (Table 3).
Discussion
This study examined the association between hospitalization, posthospital syndrome, and postdischarge health care use in patients undergoing a VVC-based intervention following hospital discharge. Participants who had no or mild cognitive impairment, no readmissions, higher medication changes, and a shorter hospital length of stay tended to experience lower disability, including in mobility and ADLs. This suggests individuals who are less clinically complex may be more likely to benefit from this type of virtual rehabilitation program. These findings are consistent with clinical experiences; home-based programs to improve physical activity posthospital discharge can be challenging for those who were medically ill (and did not undergo a specific surgical procedure), cognitively impaired, and become acutely ill and trigger hospital readmission. 15 For example, the sample in this study had higher rates of falls, pain, and readmissions compared to previous research.2,3,34-39
The importance of posthospital syndrome in the context of recovery of function and health at home following hospitalization is well documented.16-18 The potential impact of posthospital syndrome on physical activity-focused interventions is less understood. In our analysis, participants with mild or moderate cognitive impairment tended to become more dependent in their ADLs, while those with no cognitive impairment tended to become more independent in their ADLs. This functional decline over time is perhaps expected in persons with cognitive impairment, but the significant difference with a large ES warrants further consideration on how to tailor interventions to better promote functional recovery in these individuals.40,41 While some cognitive decline may not be preventable, this finding supports the need to promote healthy cognitive aging, identify declines in cognition, and work to mitigate additional decline. Programs specifically designed to promote function and physical activity in older adults with cognitive impairment are needed, especially during care transitions.41-43
While participants reported that falls and pain interference did not have a significant impact on change in outcomes between baseline and endpoint, these areas need further investigation. Falls and pain have been associated with function and physical activity in older adults.42-46 Pain is common, yet underappreciated during older adult hospital-to-home transitions.11,12,45,46 There is a need for more comprehensive assessment of pain (including pain intensity) and qualitative research.
Hospitalization and postdischarge health care application factors may have a significant impact on home-telehealth physical activity intervention success. Individuals who were postsurgery tended to have greater improvements in ADLs and physical performance. Most postsurgery participants had joint replacement surgery. Postsurgery status may not be modifiable, but it is important to note expected differences in recovery between medical and surgical admissions and the need to tailor care based on admission diagnosis. Those with a longer length of hospital stay may be considered at higher risk of suboptimal outcomes postdischarge, which indicates an opportunity for targeting resources and support, in addition to efforts of reducing length of stay where possible.47
Readmissions were significantly related to a change in Rosow-Breslau mobility disability score. This may indicate the detrimental impact a readmission can have on increasing mobility and physical activity postdischarge, or the potential of this pilot program to impact readmissions by increasing mobility and physical activity, contrary to prior physical exercise interventions.5,7,9,48 With 5% to 79% of readmissions considered preventable, continued efforts and program dissemination and implementation to address preventable readmissions are warranted.49 Individuals with postdischarge physical therapy (prior to beginning the pilot program) tended to demonstrate less improvement in disability and physical performance. This relationship needs further investigation; the 2 groups did not appear to have significant differences at baseline, albeit with a small sample size. It is possible they experienced initial improvements with postdischarge physical therapy and plateaued or had little further reserve to improve upon entering the VVC program.
Strengths and Limitations
This pilot program provided evaluative data on the use of VVC to enhance function and physical activity in older adults posthospital discharge. It included individual (eg, fall, pain, cognitive impairment) and health service (eg, readmission, physical therapy) level factors as predictors of function and physical activity posthospitalization.5,7,9,15-19
The results of this pilot project stem from a small sample lacking diversity in terms of race, ethnicity, and sex. There was some variation in baseline and endpoints between participants, and when hospitalization, posthospital syndrome, and postdischarge health care application factors were collected. The majority of participants were recruited within a month postdischarge, and the program lasted about 6 months. Data collection was attempted at regular PAT contacts, but there was some variation in when visits occurred based on participant availability and preference. Some participants had missing data, which was handled using pairwise deletion.33 Larger studies are needed to confirm the findings of this study, particularly the trends that did not reach statistical significance. Home health services other than physical therapy (eg, nursing, occupational therapy) were not fully accounted for and should be considered in future research.
Conclusions
In patients undergoing a 6-month pilot VVC-based physical activity intervention posthospital discharge, improvements in mobility and disability were most likely in those who had no cognitive impairment and were not readmitted. Larger sample and qualitative investigations are necessary to optimize outcomes for patients who meet these clinical profiles.
- Liebzeit D, Bratzke L, Boltz M, Purvis S, King B. Getting back to normal: a grounded theory study of function in post-hospitalized older adults. Gerontologist. 2020;60:704-714. doi:10.1093/geront/gnz057
- Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83-91. doi:10.1016/s0167-4943(02)00061-4
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi:10.1371/journal.pone.0026951
- Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049-1054. doi:10.1093/gerona/58.11.m1049
- Huang HT, Chang CM, Liu LF, Lin HS, Chen CH. Trajectories and predictors of functional decline of hospitalised older patients. J Clin Nurs. 2013;22:1322-1331. doi:10.1111/jocn.12055
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171- 2179. doi:10.1111/j.1532-5415.2008.02023.x
- Helvik AS, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57:305-310. doi:10.1016/j.archger.2013.05.008
- Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol Biol Sci Med Sci. 2015;70:381-386. doi:10.1093/gerona/glu168
- Chen CC, Wang C, Huang GH. Functional trajectory 6 months posthospitalization: a cohort study of older hospitalized patients in Taiwan. Nurs Res. 2008;57:93-100. doi:10.1097/01.NNR.0000313485.18670.e2
- Kleinpell RM, Fletcher K, Jennings BM. Reducing functional decline in hospitalized elderly. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008. Accessed September 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK2629/
- Liebzeit D, Rutkowski R, Arbaje AI, Fields B, Werner NE. A scoping review of interventions for older adults transitioning from hospital to home. J Am Geriatr Soc. 2021;69:2950-2962. doi:10.1111/jgs.17323
- Hladkowicz E, Dumitrascu F, Auais M, et al. Evaluations of postoperative transitions in care for older adults: a scoping review. BMC Geriatr. 2022;22:329. doi:10.1186/s12877-022-02989-6
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi:10.1186/s12877-021-02454-w
- Dawson R, Oliveira JS, Kwok WS, et al. Exercise interventions delivered through telehealth to improve physical functioning for older adults with frailty, cognitive, or mobility disability: a systematic review and meta-analysis. Telemed J E Health. 2024;30:940-950. doi:10.1089/tmj.2023.0177
- Liebzeit D, Phillips KK, Hogikyan RV, Cigolle CT, Alexander NB. A pilot home-telehealth program to enhance functional ability, physical performance, and physical activity in older adult veterans post-hospital discharge. Res Gerontol Nurs. 2024;17:271-279. doi:10.3928/19404921-20241105-01
- Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100-102. doi:10.1056/NEJMp1212324
- Caraballo C, Dharmarajan K, Krumholz HM. Post hospital syndrome: is the stress of hospitalization causing harm? Rev Esp Cardiol (Engl Ed). 2019;72:896-898. doi:10.1016/j.rec.2019.04.010
- Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179:38- 45. doi:10.1001/jamainternmed.2018.5100
- Dutzi I, Schwenk M, Kirchner M, Jooss E, Bauer JM, Hauer K. Influence of cognitive impairment on rehabilitation received and its mediating effect on functional recovery. J Alzheimers Dis. 2021;84:745-756. doi:10.3233/JAD-210620
- Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence ofcognitiveimpairment. JAmMedDirAssoc. 2015;16:215-220. doi:10.1016/j.jamda.2014.09.009
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. doi:10.1111/j.1532-5415.2005.53221.x
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
- White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014;62:2350-2356. doi:10.1111/jgs.13135
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134. doi:10.1016/s0304-3959(00)00355-9
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368. doi:10.1016/j.pain.2004.04.017
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi:10.1001/jama.1963.03060120024016
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
- Alexander NB, Guire KE, Thelen DG, et al. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48:1408-1413. doi:10.1111/j.1532-5415.2000.tb02630.x
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556-559. doi:10.1093/geronj/21.4.556
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. doi:10.1093/geronj/49.2.m85
- Chan CS, Slaughter SE, Jones CA, Ickert C, Wagg AS. Measuring activity performance of older adults using the activPAL: a rapid review. Healthcare (Basel). 2017;5:94. doi:10.3390/healthcare5040094
- IBM SPSS software. IBM Corp; 2019. Accessed September 3, 2025. https://www.ibm.com/spss
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402-406. doi:10.4097/kjae.2013.64.5.402
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287-2295. doi:10.1056/NEJMsa1101942
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63:548-552. doi:10.1111/jgs.13317
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. doi:10.1056/NEJMsa0803563
- Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277-282. doi:10.1002/jhm.2152
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. J Am Geriatr Soc. 1994;42:269- 274. doi:10.1111/j.1532-5415.1994.tb01750.x
- Hoffman GJ, Liu H, Alexander NB, Tinetti M, Braun TM, Min LC. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276. doi:10.1001/jamanetworkopen.2019.4276
- Gill DP, Hubbard RA, Koepsell TD, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9:S63-S71. doi:10.1016/j.jalz.2012.10.010
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi:10.1159/000154929
- Patti A, Zangla D, Sahin FN, et al. Physical exercise and prevention of falls. Effects of a Pilates training method compared with a general physical activity program. Medicine (Baltimore). 2021;100:e25289. doi:10.1097/MD.0000000000025289
- Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. 2022;22:203. doi:10.1186/s12877-022-02879-x
- Ek S, Rizzuto D, Xu W, Calderón-Larrañaga A, Welmer AK. Predictors for functional decline after an injurious fall: a population-based cohort study. Aging Clin Exp Res. 2021;33:2183-2190. doi:10.1007/s40520-020-01747-1
- Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. 2022;16:736688. doi:10.3389/fnhum.2022.736688
- Ritchie CS, Patel K, Boscardin J, et al. Impact of persistent pain on function, cognition, and well-being of older adults. J Am Geriatr Soc. 2023;71:26-35. doi:10.1111/jgs.18125
- Han TS, Murray P, Robin J, et al. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Health Care. 2022;34:mzab160. doi:10.1093/intqhc/ mzab160
- Lærum-Onsager E, Molin M, Olsen CF, et al. Effect of nutritional and physical exercise intervention on hospital readmission for patients aged 65 or older: a systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2021;18:62. doi:10.1186/s12966-021-01123-w
- Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-E402. doi:10.1503/cmaj.101860
- Liebzeit D, Bratzke L, Boltz M, Purvis S, King B. Getting back to normal: a grounded theory study of function in post-hospitalized older adults. Gerontologist. 2020;60:704-714. doi:10.1093/geront/gnz057
- Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83-91. doi:10.1016/s0167-4943(02)00061-4
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi:10.1371/journal.pone.0026951
- Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049-1054. doi:10.1093/gerona/58.11.m1049
- Huang HT, Chang CM, Liu LF, Lin HS, Chen CH. Trajectories and predictors of functional decline of hospitalised older patients. J Clin Nurs. 2013;22:1322-1331. doi:10.1111/jocn.12055
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171- 2179. doi:10.1111/j.1532-5415.2008.02023.x
- Helvik AS, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57:305-310. doi:10.1016/j.archger.2013.05.008
- Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol Biol Sci Med Sci. 2015;70:381-386. doi:10.1093/gerona/glu168
- Chen CC, Wang C, Huang GH. Functional trajectory 6 months posthospitalization: a cohort study of older hospitalized patients in Taiwan. Nurs Res. 2008;57:93-100. doi:10.1097/01.NNR.0000313485.18670.e2
- Kleinpell RM, Fletcher K, Jennings BM. Reducing functional decline in hospitalized elderly. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008. Accessed September 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK2629/
- Liebzeit D, Rutkowski R, Arbaje AI, Fields B, Werner NE. A scoping review of interventions for older adults transitioning from hospital to home. J Am Geriatr Soc. 2021;69:2950-2962. doi:10.1111/jgs.17323
- Hladkowicz E, Dumitrascu F, Auais M, et al. Evaluations of postoperative transitions in care for older adults: a scoping review. BMC Geriatr. 2022;22:329. doi:10.1186/s12877-022-02989-6
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi:10.1186/s12877-021-02454-w
- Dawson R, Oliveira JS, Kwok WS, et al. Exercise interventions delivered through telehealth to improve physical functioning for older adults with frailty, cognitive, or mobility disability: a systematic review and meta-analysis. Telemed J E Health. 2024;30:940-950. doi:10.1089/tmj.2023.0177
- Liebzeit D, Phillips KK, Hogikyan RV, Cigolle CT, Alexander NB. A pilot home-telehealth program to enhance functional ability, physical performance, and physical activity in older adult veterans post-hospital discharge. Res Gerontol Nurs. 2024;17:271-279. doi:10.3928/19404921-20241105-01
- Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100-102. doi:10.1056/NEJMp1212324
- Caraballo C, Dharmarajan K, Krumholz HM. Post hospital syndrome: is the stress of hospitalization causing harm? Rev Esp Cardiol (Engl Ed). 2019;72:896-898. doi:10.1016/j.rec.2019.04.010
- Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179:38- 45. doi:10.1001/jamainternmed.2018.5100
- Dutzi I, Schwenk M, Kirchner M, Jooss E, Bauer JM, Hauer K. Influence of cognitive impairment on rehabilitation received and its mediating effect on functional recovery. J Alzheimers Dis. 2021;84:745-756. doi:10.3233/JAD-210620
- Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence ofcognitiveimpairment. JAmMedDirAssoc. 2015;16:215-220. doi:10.1016/j.jamda.2014.09.009
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. doi:10.1111/j.1532-5415.2005.53221.x
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
- White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014;62:2350-2356. doi:10.1111/jgs.13135
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134. doi:10.1016/s0304-3959(00)00355-9
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368. doi:10.1016/j.pain.2004.04.017
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi:10.1001/jama.1963.03060120024016
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
- Alexander NB, Guire KE, Thelen DG, et al. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48:1408-1413. doi:10.1111/j.1532-5415.2000.tb02630.x
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556-559. doi:10.1093/geronj/21.4.556
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. doi:10.1093/geronj/49.2.m85
- Chan CS, Slaughter SE, Jones CA, Ickert C, Wagg AS. Measuring activity performance of older adults using the activPAL: a rapid review. Healthcare (Basel). 2017;5:94. doi:10.3390/healthcare5040094
- IBM SPSS software. IBM Corp; 2019. Accessed September 3, 2025. https://www.ibm.com/spss
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402-406. doi:10.4097/kjae.2013.64.5.402
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287-2295. doi:10.1056/NEJMsa1101942
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63:548-552. doi:10.1111/jgs.13317
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. doi:10.1056/NEJMsa0803563
- Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277-282. doi:10.1002/jhm.2152
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. J Am Geriatr Soc. 1994;42:269- 274. doi:10.1111/j.1532-5415.1994.tb01750.x
- Hoffman GJ, Liu H, Alexander NB, Tinetti M, Braun TM, Min LC. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276. doi:10.1001/jamanetworkopen.2019.4276
- Gill DP, Hubbard RA, Koepsell TD, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9:S63-S71. doi:10.1016/j.jalz.2012.10.010
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi:10.1159/000154929
- Patti A, Zangla D, Sahin FN, et al. Physical exercise and prevention of falls. Effects of a Pilates training method compared with a general physical activity program. Medicine (Baltimore). 2021;100:e25289. doi:10.1097/MD.0000000000025289
- Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. 2022;22:203. doi:10.1186/s12877-022-02879-x
- Ek S, Rizzuto D, Xu W, Calderón-Larrañaga A, Welmer AK. Predictors for functional decline after an injurious fall: a population-based cohort study. Aging Clin Exp Res. 2021;33:2183-2190. doi:10.1007/s40520-020-01747-1
- Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. 2022;16:736688. doi:10.3389/fnhum.2022.736688
- Ritchie CS, Patel K, Boscardin J, et al. Impact of persistent pain on function, cognition, and well-being of older adults. J Am Geriatr Soc. 2023;71:26-35. doi:10.1111/jgs.18125
- Han TS, Murray P, Robin J, et al. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Health Care. 2022;34:mzab160. doi:10.1093/intqhc/ mzab160
- Lærum-Onsager E, Molin M, Olsen CF, et al. Effect of nutritional and physical exercise intervention on hospital readmission for patients aged 65 or older: a systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2021;18:62. doi:10.1186/s12966-021-01123-w
- Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-E402. doi:10.1503/cmaj.101860
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
GLP-1s Increase GERD Risk Over SGLT2 Inhibitors in T2D
in a cohort study of new users.
Risks for GERD were higher overall for each GLP-1 RA type except lixisenatide, and risks for GERD complications were higher in ever-smokers, patients with obesity, and patients with gastric comorbidities.
“The findings were not entirely surprising,” principal author Laurent Azoulay, PhD, of McGill University and Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Quebec, Canada, told GI & Hepatology News. “There is a plausible biological mechanism through which GLP-1 RAs could increase the risk of GERD — namely, by delaying gastric emptying, which can lead to symptoms of reflux. Still, it’s always valuable to see whether the clinical data support what we suspect from a physiological standpoint.”
“As with any medication, it’s about balancing benefits and risks — and being proactive when side effects emerge,” he added.
The study was published online in Annals of Internal Medicine.
Duration of Use, Drug Action
Researchers designed an active comparator new-user cohort study emulating a target trial to estimate the effects of GLP-1 RAs compared with SGLT2 inhibitors on the risk for GERD and its complications among patients with T2D.
The study included 24,708 new users of GLP-1 RAs and 89,096 new users of SGLT2 inhibitors. Participants had a mean age of 56 years, and 55% were men. They initiated treatment with the drugs from January 2013 through December 2021, with follow-up through March 2022.
Three-year risk differences (RDs) and risk ratios (RRs) were estimated and weighted using propensity score fine stratification.
Overall, during follow-up, the incidence rate of GERD was 7.9 per 1000 person-years; 138 complications of GERD were observed, with over 90% of them being Barrett’s esophagus.
Over a median follow-up of 3 years, among GLP-1 RA users compared with SGLT2 inhibitor users, the RRs were 1.27 for GERD, with an RD of 0.7 per 100 patients, and 1.55 for complications, with an RD of 0.8 per 1000 patients.
Further analyses found that risks for GERD were higher overall for each GLP-1 RA type except lixisenatide, and risks for GERD complications were higher in ever-smokers, patients with obesity, and those with gastric comorbidities associated with gastric motility. The findings remained robust across sensitivity analyses addressing various types of biases.
The widening incidence curves with duration of use may indicate that mucosal injury and symptom severity correlate with reflux frequency and duration of esophageal acid exposure, the authors suggested.
GERD risk also was higher with long-acting GLP-1 RA use, suggesting that long-acting GLP-1 RAs (liraglutide, exenatide once weekly, dulaglutide, and semaglutide) may have more sustained delaying effects, they noted.
“These potential risks should be weighed against the established clinical benefits of this drug class, particularly in patients at high risk for gastroparesis and GERD,” the authors concluded.
“Given the mechanism through which these drugs may cause GERD, we can reasonably speculate that a similar effect might be observed in individuals without diabetes,” Azoulay added. “That said, a dedicated study would be needed to confirm that.”
Close Monitoring Advised
Caroline Collins, MD, assistant professor at Emory University School of Medicine in Atlanta, agreed with the findings and said the association between GLP-1s and GERD is consistent with what she has observed in her practice.
“I routinely counsel patients about the potential for GERD symptoms as well as other side effects before initiating GLP-1 therapy,” she told GI & Hepatology News. “Several patients on GLP-1s have reported new or worsening reflux symptoms after initiating therapy. Sometimes, we can lower the dose, and the GERD resolves. Other times initiating GERD treatment or discontinuing the medication is appropriate.”
“Patients with T2D are already at increased risk for delayed gastric emptying, which in itself is a contributor to GERD,” said Collins, who was not involved in the study. “Therefore, adding a GLP-1 RA, which further slows gastric motility, may compound this risk. I consider this when assessing which patients are the best candidates for these medications and often monitor more closely in patients with long-standing diabetes and other predisposing factors to GERD.”
Barrett’s esophagus and esophageal cancer generally occur over many years, she noted. “A median follow-up of 3 years may be insufficient to fully assess the long-term risks of serious complications.”
“Chronic cough, a common but often overlooked manifestation of GERD, was not included in the outcome definitions,” she added. Including chronic cough “may have captured a broader picture of reflux-related symptoms.”
The study was funded by a Foundation Scheme grant from the Canadian Institutes of Health Research. Azoulay holds a Distinguished Research Scholar award from the Fonds de recherche du Quebec – Sante and is the recipient of a William Dawson Scholar award from McGill University.
A version of this article appeared on Medscape.com.
in a cohort study of new users.
Risks for GERD were higher overall for each GLP-1 RA type except lixisenatide, and risks for GERD complications were higher in ever-smokers, patients with obesity, and patients with gastric comorbidities.
“The findings were not entirely surprising,” principal author Laurent Azoulay, PhD, of McGill University and Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Quebec, Canada, told GI & Hepatology News. “There is a plausible biological mechanism through which GLP-1 RAs could increase the risk of GERD — namely, by delaying gastric emptying, which can lead to symptoms of reflux. Still, it’s always valuable to see whether the clinical data support what we suspect from a physiological standpoint.”
“As with any medication, it’s about balancing benefits and risks — and being proactive when side effects emerge,” he added.
The study was published online in Annals of Internal Medicine.
Duration of Use, Drug Action
Researchers designed an active comparator new-user cohort study emulating a target trial to estimate the effects of GLP-1 RAs compared with SGLT2 inhibitors on the risk for GERD and its complications among patients with T2D.
The study included 24,708 new users of GLP-1 RAs and 89,096 new users of SGLT2 inhibitors. Participants had a mean age of 56 years, and 55% were men. They initiated treatment with the drugs from January 2013 through December 2021, with follow-up through March 2022.
Three-year risk differences (RDs) and risk ratios (RRs) were estimated and weighted using propensity score fine stratification.
Overall, during follow-up, the incidence rate of GERD was 7.9 per 1000 person-years; 138 complications of GERD were observed, with over 90% of them being Barrett’s esophagus.
Over a median follow-up of 3 years, among GLP-1 RA users compared with SGLT2 inhibitor users, the RRs were 1.27 for GERD, with an RD of 0.7 per 100 patients, and 1.55 for complications, with an RD of 0.8 per 1000 patients.
Further analyses found that risks for GERD were higher overall for each GLP-1 RA type except lixisenatide, and risks for GERD complications were higher in ever-smokers, patients with obesity, and those with gastric comorbidities associated with gastric motility. The findings remained robust across sensitivity analyses addressing various types of biases.
The widening incidence curves with duration of use may indicate that mucosal injury and symptom severity correlate with reflux frequency and duration of esophageal acid exposure, the authors suggested.
GERD risk also was higher with long-acting GLP-1 RA use, suggesting that long-acting GLP-1 RAs (liraglutide, exenatide once weekly, dulaglutide, and semaglutide) may have more sustained delaying effects, they noted.
“These potential risks should be weighed against the established clinical benefits of this drug class, particularly in patients at high risk for gastroparesis and GERD,” the authors concluded.
“Given the mechanism through which these drugs may cause GERD, we can reasonably speculate that a similar effect might be observed in individuals without diabetes,” Azoulay added. “That said, a dedicated study would be needed to confirm that.”
Close Monitoring Advised
Caroline Collins, MD, assistant professor at Emory University School of Medicine in Atlanta, agreed with the findings and said the association between GLP-1s and GERD is consistent with what she has observed in her practice.
“I routinely counsel patients about the potential for GERD symptoms as well as other side effects before initiating GLP-1 therapy,” she told GI & Hepatology News. “Several patients on GLP-1s have reported new or worsening reflux symptoms after initiating therapy. Sometimes, we can lower the dose, and the GERD resolves. Other times initiating GERD treatment or discontinuing the medication is appropriate.”
“Patients with T2D are already at increased risk for delayed gastric emptying, which in itself is a contributor to GERD,” said Collins, who was not involved in the study. “Therefore, adding a GLP-1 RA, which further slows gastric motility, may compound this risk. I consider this when assessing which patients are the best candidates for these medications and often monitor more closely in patients with long-standing diabetes and other predisposing factors to GERD.”
Barrett’s esophagus and esophageal cancer generally occur over many years, she noted. “A median follow-up of 3 years may be insufficient to fully assess the long-term risks of serious complications.”
“Chronic cough, a common but often overlooked manifestation of GERD, was not included in the outcome definitions,” she added. Including chronic cough “may have captured a broader picture of reflux-related symptoms.”
The study was funded by a Foundation Scheme grant from the Canadian Institutes of Health Research. Azoulay holds a Distinguished Research Scholar award from the Fonds de recherche du Quebec – Sante and is the recipient of a William Dawson Scholar award from McGill University.
A version of this article appeared on Medscape.com.
in a cohort study of new users.
Risks for GERD were higher overall for each GLP-1 RA type except lixisenatide, and risks for GERD complications were higher in ever-smokers, patients with obesity, and patients with gastric comorbidities.
“The findings were not entirely surprising,” principal author Laurent Azoulay, PhD, of McGill University and Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Quebec, Canada, told GI & Hepatology News. “There is a plausible biological mechanism through which GLP-1 RAs could increase the risk of GERD — namely, by delaying gastric emptying, which can lead to symptoms of reflux. Still, it’s always valuable to see whether the clinical data support what we suspect from a physiological standpoint.”
“As with any medication, it’s about balancing benefits and risks — and being proactive when side effects emerge,” he added.
The study was published online in Annals of Internal Medicine.
Duration of Use, Drug Action
Researchers designed an active comparator new-user cohort study emulating a target trial to estimate the effects of GLP-1 RAs compared with SGLT2 inhibitors on the risk for GERD and its complications among patients with T2D.
The study included 24,708 new users of GLP-1 RAs and 89,096 new users of SGLT2 inhibitors. Participants had a mean age of 56 years, and 55% were men. They initiated treatment with the drugs from January 2013 through December 2021, with follow-up through March 2022.
Three-year risk differences (RDs) and risk ratios (RRs) were estimated and weighted using propensity score fine stratification.
Overall, during follow-up, the incidence rate of GERD was 7.9 per 1000 person-years; 138 complications of GERD were observed, with over 90% of them being Barrett’s esophagus.
Over a median follow-up of 3 years, among GLP-1 RA users compared with SGLT2 inhibitor users, the RRs were 1.27 for GERD, with an RD of 0.7 per 100 patients, and 1.55 for complications, with an RD of 0.8 per 1000 patients.
Further analyses found that risks for GERD were higher overall for each GLP-1 RA type except lixisenatide, and risks for GERD complications were higher in ever-smokers, patients with obesity, and those with gastric comorbidities associated with gastric motility. The findings remained robust across sensitivity analyses addressing various types of biases.
The widening incidence curves with duration of use may indicate that mucosal injury and symptom severity correlate with reflux frequency and duration of esophageal acid exposure, the authors suggested.
GERD risk also was higher with long-acting GLP-1 RA use, suggesting that long-acting GLP-1 RAs (liraglutide, exenatide once weekly, dulaglutide, and semaglutide) may have more sustained delaying effects, they noted.
“These potential risks should be weighed against the established clinical benefits of this drug class, particularly in patients at high risk for gastroparesis and GERD,” the authors concluded.
“Given the mechanism through which these drugs may cause GERD, we can reasonably speculate that a similar effect might be observed in individuals without diabetes,” Azoulay added. “That said, a dedicated study would be needed to confirm that.”
Close Monitoring Advised
Caroline Collins, MD, assistant professor at Emory University School of Medicine in Atlanta, agreed with the findings and said the association between GLP-1s and GERD is consistent with what she has observed in her practice.
“I routinely counsel patients about the potential for GERD symptoms as well as other side effects before initiating GLP-1 therapy,” she told GI & Hepatology News. “Several patients on GLP-1s have reported new or worsening reflux symptoms after initiating therapy. Sometimes, we can lower the dose, and the GERD resolves. Other times initiating GERD treatment or discontinuing the medication is appropriate.”
“Patients with T2D are already at increased risk for delayed gastric emptying, which in itself is a contributor to GERD,” said Collins, who was not involved in the study. “Therefore, adding a GLP-1 RA, which further slows gastric motility, may compound this risk. I consider this when assessing which patients are the best candidates for these medications and often monitor more closely in patients with long-standing diabetes and other predisposing factors to GERD.”
Barrett’s esophagus and esophageal cancer generally occur over many years, she noted. “A median follow-up of 3 years may be insufficient to fully assess the long-term risks of serious complications.”
“Chronic cough, a common but often overlooked manifestation of GERD, was not included in the outcome definitions,” she added. Including chronic cough “may have captured a broader picture of reflux-related symptoms.”
The study was funded by a Foundation Scheme grant from the Canadian Institutes of Health Research. Azoulay holds a Distinguished Research Scholar award from the Fonds de recherche du Quebec – Sante and is the recipient of a William Dawson Scholar award from McGill University.
A version of this article appeared on Medscape.com.
Bariatric Surgery May Lower Long-Term CKD Risk
, according to a population-based study in Denmark.
Writing in BMC Nephrology, researchers reported patients with bariatric surgery had an increased 1-year risk for AKI and 10-year risk for nephrolithiasis, alongside a decreased 10-year risk for CKD (stages G3-5) and KFRT, compared with matched patients diagnosed with overweight/obesity who did not undergo surgery.
A Closer Look
Using national registry data, the team identified all adults who underwent Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) between January 1, 2006, and December 31, 2018. Each patient was age- and sex-matched (1:5) to patients with hospital-diagnosed overweight/obesity without bariatric surgery. Researchers also compared results against a population cohort matched solely by age and sex. Outcomes included cumulative risks for AKI, nephrolithiasis, CKD (G3-G5), and KFRT.
The cohort comprised 18,827 surgical patients (17,200 RYGB and 1627 SG) and 94,135 matched comparators. Median age was 41 years, 76% were women, and the median follow-up was 8.1 years. At baseline, the median estimated glomerular filtration rate (eGFR) was comparable (103 mL/min/1.73 m2) between both surgery and overweight/obesity control groups, as were A1c levels. There were fewer comorbidities in the population cohort matched only by age and sex than in the overweight/obesity comparison cohort.
Using multivariable Cox regression analyses, the researchers found the 1-year risk for AKI following bariatric surgery was 2.7%. At 10 years, risks were 3.5% for nephrolithiasis, 0.4% for CKD, and 0.2% for KFRT.
Adjusted hazard ratios (HRs) after bariatric surgery vs without bariatric surgery were higher for AKI (HR, 1.63) and nephrolithiasis (HR, 1.73) and lower for CKD (HR, 0.41) and KFRT (HR, 0.63). Results were consistent when compared against the population cohort.
By procedure, the 1-year AKI risk was 2.7% after RYGB and 2.4% after SG vs 2.5% in the overweight/obesity cohort and 1.1% in the population cohort. At 10 years, the risk for incident nephrolithiasis was 3.6% after RYGB and 1.2% after SG vs 2.4% and 1.3% in the overweight/obese and population cohorts, respectively. KFRT risk at 10 years was 0.2% after RYGB and 1.6% after SG vs 0.4% and 0.1% in the overweight/obesity and population cohorts, respectively.
“The increased short-term risk of AKI and nephrolithiasis was expected, given the physiological changes after bariatric surgery, but the long-term reduction in CKD and KFRT was both encouraging and clinically important,” said study investigator Christian Goul Sørensen, MD, Department of Clinical Epidemiology, Department of Clinical Medicine, Aarhus University and Aarhus University Hospital, Aarhus, Denmark. “It was also noteworthy that the results were consistent not only in the obesity-matched comparison cohort but also in the cohort matched solely on age and sex, which further strengthens the validity of our findings.”
RYGB and SG are known to help mitigate obesity-associated complications, such as hypertension, hyperlipidemia, and type 2 diabetes. Studies have suggested that there are improvements in eGFR after bariatric surgery. However, long-term evidence from routine clinical care has not been well studied. Furthermore, RYGB may lead to AKI due to a combination of preoperative, intraoperative, and postoperative factors.
“Obesity is a major driver of kidney disease, often in combination with comorbidities such as diabetes and hypertension,” Sorensen told GI & Hepatology News. “Patients and clinicians face complex decisions about surgery, and understanding both the short-term surgical risks and the long-term kidney benefits is crucial for informed counseling. As bariatric surgery becomes increasingly common worldwide, population-based evidence like this helps guide clinical practice and supports shared decision-making with patients.”
Consistent With Clinical Experience
Panduranga S. Rao, MD, professor of nephrology at the University of Michigan, Ann Arbor, Michigan, who was not involved in the study, called the results consistent with prior clinical experience. He highlighted the strong follow-up and detailed lab and comorbidity data, while noting that the decreasing use of RYGB may limit applicability going forward.
“However, one has to remain vigilant to the risk of nephrolithiasis in the patients who have undergone Roux-en-Y in the past,” he said.
The observation of decreasing risk for CKD with weight loss is particularly relevant, given the growing use of GLP-1s for weight loss, Rao added.
Srinivasan Beddhu, MD, professor of internal medicine and the scientific director of the Cardio-Renal & Metabolism Center at the University of Utah Health in Salt Lake City, said the large national cohort design and outcomes data offer further reassurance about the long-term kidney health effects of bariatric surgery.
“The message that the risks of AKI and nephrolithiasis are outweighed by the long-term kidney protective effects of bariatric surgery is important,” said Beddhu.
Alexander Chang, MD, associate professor and a practicing nephrologist at the Geisinger Health System in Danville, Pennsylvania, noted that the study provided more evidence about RYGB-associated kidney stone risk via fat malabsorption, which raises the levels of fatty acids that bind dietary calcium.
“Calcium normally precipitates with dietary oxalate, and thus, there can be an increase in urinary oxalate,” Chang explained. “There did not appear to be increased risk of nephrolithiasis with sleeve gastrectomy, consistent with other studies.
“This study emphasizes the importance of multidisciplinary care post-bariatric surgery to try to prevent complications such as kidney stones,” Chang added. “This can be tricky but requires trying different strategies to increase fluid intake and calcium citrate supplements with meals.”
The study was partly funded by a grant from the Novo Nordisk Foundation and the Independent Research Fund Denmark. One author reported receiving a speaking fee support from Novo Nordisk for conference attendance. The other authors declared no competing interests. Sørensen, Rao, Beddhu, and Chang had no financial disclosures.
A version of this article appeared on Medscape.com.
, according to a population-based study in Denmark.
Writing in BMC Nephrology, researchers reported patients with bariatric surgery had an increased 1-year risk for AKI and 10-year risk for nephrolithiasis, alongside a decreased 10-year risk for CKD (stages G3-5) and KFRT, compared with matched patients diagnosed with overweight/obesity who did not undergo surgery.
A Closer Look
Using national registry data, the team identified all adults who underwent Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) between January 1, 2006, and December 31, 2018. Each patient was age- and sex-matched (1:5) to patients with hospital-diagnosed overweight/obesity without bariatric surgery. Researchers also compared results against a population cohort matched solely by age and sex. Outcomes included cumulative risks for AKI, nephrolithiasis, CKD (G3-G5), and KFRT.
The cohort comprised 18,827 surgical patients (17,200 RYGB and 1627 SG) and 94,135 matched comparators. Median age was 41 years, 76% were women, and the median follow-up was 8.1 years. At baseline, the median estimated glomerular filtration rate (eGFR) was comparable (103 mL/min/1.73 m2) between both surgery and overweight/obesity control groups, as were A1c levels. There were fewer comorbidities in the population cohort matched only by age and sex than in the overweight/obesity comparison cohort.
Using multivariable Cox regression analyses, the researchers found the 1-year risk for AKI following bariatric surgery was 2.7%. At 10 years, risks were 3.5% for nephrolithiasis, 0.4% for CKD, and 0.2% for KFRT.
Adjusted hazard ratios (HRs) after bariatric surgery vs without bariatric surgery were higher for AKI (HR, 1.63) and nephrolithiasis (HR, 1.73) and lower for CKD (HR, 0.41) and KFRT (HR, 0.63). Results were consistent when compared against the population cohort.
By procedure, the 1-year AKI risk was 2.7% after RYGB and 2.4% after SG vs 2.5% in the overweight/obesity cohort and 1.1% in the population cohort. At 10 years, the risk for incident nephrolithiasis was 3.6% after RYGB and 1.2% after SG vs 2.4% and 1.3% in the overweight/obese and population cohorts, respectively. KFRT risk at 10 years was 0.2% after RYGB and 1.6% after SG vs 0.4% and 0.1% in the overweight/obesity and population cohorts, respectively.
“The increased short-term risk of AKI and nephrolithiasis was expected, given the physiological changes after bariatric surgery, but the long-term reduction in CKD and KFRT was both encouraging and clinically important,” said study investigator Christian Goul Sørensen, MD, Department of Clinical Epidemiology, Department of Clinical Medicine, Aarhus University and Aarhus University Hospital, Aarhus, Denmark. “It was also noteworthy that the results were consistent not only in the obesity-matched comparison cohort but also in the cohort matched solely on age and sex, which further strengthens the validity of our findings.”
RYGB and SG are known to help mitigate obesity-associated complications, such as hypertension, hyperlipidemia, and type 2 diabetes. Studies have suggested that there are improvements in eGFR after bariatric surgery. However, long-term evidence from routine clinical care has not been well studied. Furthermore, RYGB may lead to AKI due to a combination of preoperative, intraoperative, and postoperative factors.
“Obesity is a major driver of kidney disease, often in combination with comorbidities such as diabetes and hypertension,” Sorensen told GI & Hepatology News. “Patients and clinicians face complex decisions about surgery, and understanding both the short-term surgical risks and the long-term kidney benefits is crucial for informed counseling. As bariatric surgery becomes increasingly common worldwide, population-based evidence like this helps guide clinical practice and supports shared decision-making with patients.”
Consistent With Clinical Experience
Panduranga S. Rao, MD, professor of nephrology at the University of Michigan, Ann Arbor, Michigan, who was not involved in the study, called the results consistent with prior clinical experience. He highlighted the strong follow-up and detailed lab and comorbidity data, while noting that the decreasing use of RYGB may limit applicability going forward.
“However, one has to remain vigilant to the risk of nephrolithiasis in the patients who have undergone Roux-en-Y in the past,” he said.
The observation of decreasing risk for CKD with weight loss is particularly relevant, given the growing use of GLP-1s for weight loss, Rao added.
Srinivasan Beddhu, MD, professor of internal medicine and the scientific director of the Cardio-Renal & Metabolism Center at the University of Utah Health in Salt Lake City, said the large national cohort design and outcomes data offer further reassurance about the long-term kidney health effects of bariatric surgery.
“The message that the risks of AKI and nephrolithiasis are outweighed by the long-term kidney protective effects of bariatric surgery is important,” said Beddhu.
Alexander Chang, MD, associate professor and a practicing nephrologist at the Geisinger Health System in Danville, Pennsylvania, noted that the study provided more evidence about RYGB-associated kidney stone risk via fat malabsorption, which raises the levels of fatty acids that bind dietary calcium.
“Calcium normally precipitates with dietary oxalate, and thus, there can be an increase in urinary oxalate,” Chang explained. “There did not appear to be increased risk of nephrolithiasis with sleeve gastrectomy, consistent with other studies.
“This study emphasizes the importance of multidisciplinary care post-bariatric surgery to try to prevent complications such as kidney stones,” Chang added. “This can be tricky but requires trying different strategies to increase fluid intake and calcium citrate supplements with meals.”
The study was partly funded by a grant from the Novo Nordisk Foundation and the Independent Research Fund Denmark. One author reported receiving a speaking fee support from Novo Nordisk for conference attendance. The other authors declared no competing interests. Sørensen, Rao, Beddhu, and Chang had no financial disclosures.
A version of this article appeared on Medscape.com.
, according to a population-based study in Denmark.
Writing in BMC Nephrology, researchers reported patients with bariatric surgery had an increased 1-year risk for AKI and 10-year risk for nephrolithiasis, alongside a decreased 10-year risk for CKD (stages G3-5) and KFRT, compared with matched patients diagnosed with overweight/obesity who did not undergo surgery.
A Closer Look
Using national registry data, the team identified all adults who underwent Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) between January 1, 2006, and December 31, 2018. Each patient was age- and sex-matched (1:5) to patients with hospital-diagnosed overweight/obesity without bariatric surgery. Researchers also compared results against a population cohort matched solely by age and sex. Outcomes included cumulative risks for AKI, nephrolithiasis, CKD (G3-G5), and KFRT.
The cohort comprised 18,827 surgical patients (17,200 RYGB and 1627 SG) and 94,135 matched comparators. Median age was 41 years, 76% were women, and the median follow-up was 8.1 years. At baseline, the median estimated glomerular filtration rate (eGFR) was comparable (103 mL/min/1.73 m2) between both surgery and overweight/obesity control groups, as were A1c levels. There were fewer comorbidities in the population cohort matched only by age and sex than in the overweight/obesity comparison cohort.
Using multivariable Cox regression analyses, the researchers found the 1-year risk for AKI following bariatric surgery was 2.7%. At 10 years, risks were 3.5% for nephrolithiasis, 0.4% for CKD, and 0.2% for KFRT.
Adjusted hazard ratios (HRs) after bariatric surgery vs without bariatric surgery were higher for AKI (HR, 1.63) and nephrolithiasis (HR, 1.73) and lower for CKD (HR, 0.41) and KFRT (HR, 0.63). Results were consistent when compared against the population cohort.
By procedure, the 1-year AKI risk was 2.7% after RYGB and 2.4% after SG vs 2.5% in the overweight/obesity cohort and 1.1% in the population cohort. At 10 years, the risk for incident nephrolithiasis was 3.6% after RYGB and 1.2% after SG vs 2.4% and 1.3% in the overweight/obese and population cohorts, respectively. KFRT risk at 10 years was 0.2% after RYGB and 1.6% after SG vs 0.4% and 0.1% in the overweight/obesity and population cohorts, respectively.
“The increased short-term risk of AKI and nephrolithiasis was expected, given the physiological changes after bariatric surgery, but the long-term reduction in CKD and KFRT was both encouraging and clinically important,” said study investigator Christian Goul Sørensen, MD, Department of Clinical Epidemiology, Department of Clinical Medicine, Aarhus University and Aarhus University Hospital, Aarhus, Denmark. “It was also noteworthy that the results were consistent not only in the obesity-matched comparison cohort but also in the cohort matched solely on age and sex, which further strengthens the validity of our findings.”
RYGB and SG are known to help mitigate obesity-associated complications, such as hypertension, hyperlipidemia, and type 2 diabetes. Studies have suggested that there are improvements in eGFR after bariatric surgery. However, long-term evidence from routine clinical care has not been well studied. Furthermore, RYGB may lead to AKI due to a combination of preoperative, intraoperative, and postoperative factors.
“Obesity is a major driver of kidney disease, often in combination with comorbidities such as diabetes and hypertension,” Sorensen told GI & Hepatology News. “Patients and clinicians face complex decisions about surgery, and understanding both the short-term surgical risks and the long-term kidney benefits is crucial for informed counseling. As bariatric surgery becomes increasingly common worldwide, population-based evidence like this helps guide clinical practice and supports shared decision-making with patients.”
Consistent With Clinical Experience
Panduranga S. Rao, MD, professor of nephrology at the University of Michigan, Ann Arbor, Michigan, who was not involved in the study, called the results consistent with prior clinical experience. He highlighted the strong follow-up and detailed lab and comorbidity data, while noting that the decreasing use of RYGB may limit applicability going forward.
“However, one has to remain vigilant to the risk of nephrolithiasis in the patients who have undergone Roux-en-Y in the past,” he said.
The observation of decreasing risk for CKD with weight loss is particularly relevant, given the growing use of GLP-1s for weight loss, Rao added.
Srinivasan Beddhu, MD, professor of internal medicine and the scientific director of the Cardio-Renal & Metabolism Center at the University of Utah Health in Salt Lake City, said the large national cohort design and outcomes data offer further reassurance about the long-term kidney health effects of bariatric surgery.
“The message that the risks of AKI and nephrolithiasis are outweighed by the long-term kidney protective effects of bariatric surgery is important,” said Beddhu.
Alexander Chang, MD, associate professor and a practicing nephrologist at the Geisinger Health System in Danville, Pennsylvania, noted that the study provided more evidence about RYGB-associated kidney stone risk via fat malabsorption, which raises the levels of fatty acids that bind dietary calcium.
“Calcium normally precipitates with dietary oxalate, and thus, there can be an increase in urinary oxalate,” Chang explained. “There did not appear to be increased risk of nephrolithiasis with sleeve gastrectomy, consistent with other studies.
“This study emphasizes the importance of multidisciplinary care post-bariatric surgery to try to prevent complications such as kidney stones,” Chang added. “This can be tricky but requires trying different strategies to increase fluid intake and calcium citrate supplements with meals.”
The study was partly funded by a grant from the Novo Nordisk Foundation and the Independent Research Fund Denmark. One author reported receiving a speaking fee support from Novo Nordisk for conference attendance. The other authors declared no competing interests. Sørensen, Rao, Beddhu, and Chang had no financial disclosures.
A version of this article appeared on Medscape.com.
GLP-1 Use After Bariatric Surgery on the Rise
, a large retrospective cohort study showed.
GLP-1 initiation was also more common among women, those who underwent sleeve gastrectomy, and those with lower postoperative weight loss as measured by BMI.
“Some patients do not lose as much weight as expected, or they regain weight after a few years. In such cases, GLP-1 therapies are emerging as an important option for weight management,” said principal investigator Hemalkumar Mehta, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health in Baltimore.
“We also noted many personal stories circulating on social media in which patients shared their experiences using GLP-1 after bariatric surgery,” he told GI & Hepatology News.
But when the researchers reviewed the scientific literature, they found no published evidence on GLP-1 use in this setting and little or no data on outcomes with the newer drugs such as semaglutide and tirzepatide. “This gap motivated us to conduct the current study,” said Mehta. The study was published in JAMA Surgery.
The researchers analyzed data from a national multicenter database of electronic health records of approximately 113 million US adults to characterize the use of and factors associated with GLP-1 initiation after bariatric surgery.
Among 112,858 individuals undergoing bariatric surgery during the study period, the mean age was 45.2 years, and 78.9% were women.
By self-report race, 1.1% were Asian, 22.1% were Black or African American, 64.2% were White individuals, and 12.6% reported belonging to other races (American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or unknown).
A total of 15,749 individuals (14%) initiated GLP-1s post-surgery, with 3391 (21.5%) beginning within 2 years of surgery and the remainder initiating during postsurgical years 3-4 (32.3%), 5-6 (25.2%), or later (21%).
Notably, the proportion of GLP-1 use increased more in the more recent cohort, from 1.7% in the January 2015-December 2019 cohort to 12.6% from June 2020 to May 2025.
Differences Between Users and Nonusers
Those who initiated GLP-1s differed significantly from those who did not: GLP-1 users vs nonusers were younger (mean age, 44.9 years vs 45.2 years), and use was more common among women vs men (15.1% vs 9.7%), among Black or African American vs White patients (15.8% vs 13.5%), and among those who underwent sleeve gastrectomy vs Roux-en-Y gastric bypass (14.9% vs 12.1%).
Looked at another way, women (adjusted hazard ratio [aHR], 1.61), those undergoing sleeve gastrectomy (aHR, 1.42), and those with type 2 diabetes (aHR, 1.34) were more likely to initiate GLP-1s than their counterparts.
The overall median presurgical BMI was 42. On analyzing obesity classification based on BMI, the researchers found that the chances of GLP-1 use were 1.73 times higher among class 1 obesity patients (BMI, 30.0-34.9), 2.19 times higher among class 2 obesity patients (BMI, 35.0-39.9), and 2.69 times higher among patients with class 3 obesity (BMI ≥ 40) than among overweight patients (BMI, 25.0-29.9).
The median post-surgery BMI for GLP-1 users at drug initiation was 36.7. Each one-unit increase in postsurgical BMI was associated with an 8% increase in the likelihood of GLP-1 initiation (aHR, 1.08).
“Importantly, our study did not specifically evaluate the effectiveness of GLP-1 therapy on weight loss after surgery,” Mehta noted. That issue and others, such as optimal timing for initiating GLP-1s, are currently under investigation.
In a related editorial, Kate Lauer, MD, of the University of Wisconsin-Madison and colleagues noted that the study had several limitations. It relied on data prior to the USFDA approvals of semaglutide and tirzepatide, the two most prescribed GLP-1s currently, potentially limiting its applicability to current practice.
Furthermore, the prescribing data did not capture dose, titration schedules, or adherence, which are “critical for understanding treatment efficacy,” they wrote. “Nonetheless, the findings highlight two important trends: (1) GLP-1s are being increasingly used as an adjunct after bariatric surgery, and (2) there is substantial variability in the timing of their initiation.”
‘Logical’ to Use GLP-1s Post Surgery
Commenting on the study findings for GI & Hepatology News, Louis Aronne, MD, director of the Comprehensive Weight Control Center at Weill Cornell Medicine in New York City, who was not involved in the study, said, “I think it is perfectly logical to use GLP-1s in patients who have had bariatric surgery.”
In this study, weight loss in those who took GLP-1s was about 12% (from a median BMI of 42 pre-surgery to 36.7 when a GLP-1 was initiated), which is significantly less than average, Aronne noted. “The patients still had Class 2 obesity.”
“Obesity is the same as other metabolic diseases,” he added. “We have to use common sense and good medical judgment when treating patients. If surgery isn’t completely effective and weight loss is inadequate, I would recommend medications.”
Of note, his team has found that lower doses of GLP-1s are required in those who have had surgery than in those who have not. “My opinion is that patients who have undergone bariatric surgery seem to be more sensitive to the medications than the average patient, but this hasn’t been carefully studied.”
To prepare patients for the possible use of GLP1s post-surgery, he suggested telling those with very high BMI that “they may need medication in addition to the procedure in order to get the best result.”
Mehta added, “Ultimately, the decision to start GLP-1s after surgery is shared between patients and clinicians. Given the amount of media coverage on GLP-1 therapies, it is not surprising that more patients are initiating these discussions with their doctors.”
Mehta is supported by the US National Institute on Aging and reported receiving grants from the institute for this study; no other funding was reported. Lauer reported receiving grants from the US National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a large retrospective cohort study showed.
GLP-1 initiation was also more common among women, those who underwent sleeve gastrectomy, and those with lower postoperative weight loss as measured by BMI.
“Some patients do not lose as much weight as expected, or they regain weight after a few years. In such cases, GLP-1 therapies are emerging as an important option for weight management,” said principal investigator Hemalkumar Mehta, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health in Baltimore.
“We also noted many personal stories circulating on social media in which patients shared their experiences using GLP-1 after bariatric surgery,” he told GI & Hepatology News.
But when the researchers reviewed the scientific literature, they found no published evidence on GLP-1 use in this setting and little or no data on outcomes with the newer drugs such as semaglutide and tirzepatide. “This gap motivated us to conduct the current study,” said Mehta. The study was published in JAMA Surgery.
The researchers analyzed data from a national multicenter database of electronic health records of approximately 113 million US adults to characterize the use of and factors associated with GLP-1 initiation after bariatric surgery.
Among 112,858 individuals undergoing bariatric surgery during the study period, the mean age was 45.2 years, and 78.9% were women.
By self-report race, 1.1% were Asian, 22.1% were Black or African American, 64.2% were White individuals, and 12.6% reported belonging to other races (American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or unknown).
A total of 15,749 individuals (14%) initiated GLP-1s post-surgery, with 3391 (21.5%) beginning within 2 years of surgery and the remainder initiating during postsurgical years 3-4 (32.3%), 5-6 (25.2%), or later (21%).
Notably, the proportion of GLP-1 use increased more in the more recent cohort, from 1.7% in the January 2015-December 2019 cohort to 12.6% from June 2020 to May 2025.
Differences Between Users and Nonusers
Those who initiated GLP-1s differed significantly from those who did not: GLP-1 users vs nonusers were younger (mean age, 44.9 years vs 45.2 years), and use was more common among women vs men (15.1% vs 9.7%), among Black or African American vs White patients (15.8% vs 13.5%), and among those who underwent sleeve gastrectomy vs Roux-en-Y gastric bypass (14.9% vs 12.1%).
Looked at another way, women (adjusted hazard ratio [aHR], 1.61), those undergoing sleeve gastrectomy (aHR, 1.42), and those with type 2 diabetes (aHR, 1.34) were more likely to initiate GLP-1s than their counterparts.
The overall median presurgical BMI was 42. On analyzing obesity classification based on BMI, the researchers found that the chances of GLP-1 use were 1.73 times higher among class 1 obesity patients (BMI, 30.0-34.9), 2.19 times higher among class 2 obesity patients (BMI, 35.0-39.9), and 2.69 times higher among patients with class 3 obesity (BMI ≥ 40) than among overweight patients (BMI, 25.0-29.9).
The median post-surgery BMI for GLP-1 users at drug initiation was 36.7. Each one-unit increase in postsurgical BMI was associated with an 8% increase in the likelihood of GLP-1 initiation (aHR, 1.08).
“Importantly, our study did not specifically evaluate the effectiveness of GLP-1 therapy on weight loss after surgery,” Mehta noted. That issue and others, such as optimal timing for initiating GLP-1s, are currently under investigation.
In a related editorial, Kate Lauer, MD, of the University of Wisconsin-Madison and colleagues noted that the study had several limitations. It relied on data prior to the USFDA approvals of semaglutide and tirzepatide, the two most prescribed GLP-1s currently, potentially limiting its applicability to current practice.
Furthermore, the prescribing data did not capture dose, titration schedules, or adherence, which are “critical for understanding treatment efficacy,” they wrote. “Nonetheless, the findings highlight two important trends: (1) GLP-1s are being increasingly used as an adjunct after bariatric surgery, and (2) there is substantial variability in the timing of their initiation.”
‘Logical’ to Use GLP-1s Post Surgery
Commenting on the study findings for GI & Hepatology News, Louis Aronne, MD, director of the Comprehensive Weight Control Center at Weill Cornell Medicine in New York City, who was not involved in the study, said, “I think it is perfectly logical to use GLP-1s in patients who have had bariatric surgery.”
In this study, weight loss in those who took GLP-1s was about 12% (from a median BMI of 42 pre-surgery to 36.7 when a GLP-1 was initiated), which is significantly less than average, Aronne noted. “The patients still had Class 2 obesity.”
“Obesity is the same as other metabolic diseases,” he added. “We have to use common sense and good medical judgment when treating patients. If surgery isn’t completely effective and weight loss is inadequate, I would recommend medications.”
Of note, his team has found that lower doses of GLP-1s are required in those who have had surgery than in those who have not. “My opinion is that patients who have undergone bariatric surgery seem to be more sensitive to the medications than the average patient, but this hasn’t been carefully studied.”
To prepare patients for the possible use of GLP1s post-surgery, he suggested telling those with very high BMI that “they may need medication in addition to the procedure in order to get the best result.”
Mehta added, “Ultimately, the decision to start GLP-1s after surgery is shared between patients and clinicians. Given the amount of media coverage on GLP-1 therapies, it is not surprising that more patients are initiating these discussions with their doctors.”
Mehta is supported by the US National Institute on Aging and reported receiving grants from the institute for this study; no other funding was reported. Lauer reported receiving grants from the US National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a large retrospective cohort study showed.
GLP-1 initiation was also more common among women, those who underwent sleeve gastrectomy, and those with lower postoperative weight loss as measured by BMI.
“Some patients do not lose as much weight as expected, or they regain weight after a few years. In such cases, GLP-1 therapies are emerging as an important option for weight management,” said principal investigator Hemalkumar Mehta, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health in Baltimore.
“We also noted many personal stories circulating on social media in which patients shared their experiences using GLP-1 after bariatric surgery,” he told GI & Hepatology News.
But when the researchers reviewed the scientific literature, they found no published evidence on GLP-1 use in this setting and little or no data on outcomes with the newer drugs such as semaglutide and tirzepatide. “This gap motivated us to conduct the current study,” said Mehta. The study was published in JAMA Surgery.
The researchers analyzed data from a national multicenter database of electronic health records of approximately 113 million US adults to characterize the use of and factors associated with GLP-1 initiation after bariatric surgery.
Among 112,858 individuals undergoing bariatric surgery during the study period, the mean age was 45.2 years, and 78.9% were women.
By self-report race, 1.1% were Asian, 22.1% were Black or African American, 64.2% were White individuals, and 12.6% reported belonging to other races (American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or unknown).
A total of 15,749 individuals (14%) initiated GLP-1s post-surgery, with 3391 (21.5%) beginning within 2 years of surgery and the remainder initiating during postsurgical years 3-4 (32.3%), 5-6 (25.2%), or later (21%).
Notably, the proportion of GLP-1 use increased more in the more recent cohort, from 1.7% in the January 2015-December 2019 cohort to 12.6% from June 2020 to May 2025.
Differences Between Users and Nonusers
Those who initiated GLP-1s differed significantly from those who did not: GLP-1 users vs nonusers were younger (mean age, 44.9 years vs 45.2 years), and use was more common among women vs men (15.1% vs 9.7%), among Black or African American vs White patients (15.8% vs 13.5%), and among those who underwent sleeve gastrectomy vs Roux-en-Y gastric bypass (14.9% vs 12.1%).
Looked at another way, women (adjusted hazard ratio [aHR], 1.61), those undergoing sleeve gastrectomy (aHR, 1.42), and those with type 2 diabetes (aHR, 1.34) were more likely to initiate GLP-1s than their counterparts.
The overall median presurgical BMI was 42. On analyzing obesity classification based on BMI, the researchers found that the chances of GLP-1 use were 1.73 times higher among class 1 obesity patients (BMI, 30.0-34.9), 2.19 times higher among class 2 obesity patients (BMI, 35.0-39.9), and 2.69 times higher among patients with class 3 obesity (BMI ≥ 40) than among overweight patients (BMI, 25.0-29.9).
The median post-surgery BMI for GLP-1 users at drug initiation was 36.7. Each one-unit increase in postsurgical BMI was associated with an 8% increase in the likelihood of GLP-1 initiation (aHR, 1.08).
“Importantly, our study did not specifically evaluate the effectiveness of GLP-1 therapy on weight loss after surgery,” Mehta noted. That issue and others, such as optimal timing for initiating GLP-1s, are currently under investigation.
In a related editorial, Kate Lauer, MD, of the University of Wisconsin-Madison and colleagues noted that the study had several limitations. It relied on data prior to the USFDA approvals of semaglutide and tirzepatide, the two most prescribed GLP-1s currently, potentially limiting its applicability to current practice.
Furthermore, the prescribing data did not capture dose, titration schedules, or adherence, which are “critical for understanding treatment efficacy,” they wrote. “Nonetheless, the findings highlight two important trends: (1) GLP-1s are being increasingly used as an adjunct after bariatric surgery, and (2) there is substantial variability in the timing of their initiation.”
‘Logical’ to Use GLP-1s Post Surgery
Commenting on the study findings for GI & Hepatology News, Louis Aronne, MD, director of the Comprehensive Weight Control Center at Weill Cornell Medicine in New York City, who was not involved in the study, said, “I think it is perfectly logical to use GLP-1s in patients who have had bariatric surgery.”
In this study, weight loss in those who took GLP-1s was about 12% (from a median BMI of 42 pre-surgery to 36.7 when a GLP-1 was initiated), which is significantly less than average, Aronne noted. “The patients still had Class 2 obesity.”
“Obesity is the same as other metabolic diseases,” he added. “We have to use common sense and good medical judgment when treating patients. If surgery isn’t completely effective and weight loss is inadequate, I would recommend medications.”
Of note, his team has found that lower doses of GLP-1s are required in those who have had surgery than in those who have not. “My opinion is that patients who have undergone bariatric surgery seem to be more sensitive to the medications than the average patient, but this hasn’t been carefully studied.”
To prepare patients for the possible use of GLP1s post-surgery, he suggested telling those with very high BMI that “they may need medication in addition to the procedure in order to get the best result.”
Mehta added, “Ultimately, the decision to start GLP-1s after surgery is shared between patients and clinicians. Given the amount of media coverage on GLP-1 therapies, it is not surprising that more patients are initiating these discussions with their doctors.”
Mehta is supported by the US National Institute on Aging and reported receiving grants from the institute for this study; no other funding was reported. Lauer reported receiving grants from the US National Institutes of Health.
A version of this article first appeared on Medscape.com.
Wegovy Approved for MASH With Fibrosis, No Cirrhosis
The once-weekly 2.4 mg semaglutide subcutaneous injection is given in conjunction with a reduced calorie diet and increased physical activity.
Among people living with overweight or obesity globally, 1 in 3 also have MASH.
The accelerated approval was based on part-one results from the ongoing two-part, phase-3 ESSENCE trial, in which Wegovy demonstrated a significant improvement in liver fibrosis with no worsening of steatohepatitis, as well as resolution of steatohepatitis with no worsening of liver fibrosis, compared with placebo at week 72. Those results were published online in April in The New England Journal of Medicine.
For the trial, 800 participants were randomly assigned to either Wegovy (534 participants) or placebo (266 participants) in addition to lifestyle changes. The mean age was 56 years and the mean BMI was 34. Most patients were white individuals (67.5%) and women (57.1%), and 55.9% of the patients had type 2 diabetes; 250 patients (31.3%) had stage II fibrosis and 550 (68.8%) had stage III fibrosis. Participants were on stable doses of lipid-lowering, glucose-management, and weight-loss medications.
At week 72, the first primary endpoint showed 63% of the 534 people treated with Wegovy achieved resolution of steatohepatitis and no worsening of liver fibrosis compared with 34% of 266 individuals treated with placebo — a statistically significant difference.
The second primary endpoint showed 37% of people treated with Wegovy achieved improvement in liver fibrosis and no worsening of steatohepatitis compared with 22% of those treated with placebo, also a significant difference.
A confirmatory secondary endpoint at week 72 showed 33% of patients treated with Wegovy achieved both resolution of steatohepatitis and improvement in liver fibrosis compared with 16% of those treated with placebo — a statistically significant difference in response rate of 17%.
In addition, 83.5% of the patients in the semaglutide group maintained the target dose of 2.4 mg until week 72.
Wegovy is also indicated, along with diet and physical activity, to reduce the risk for major cardiovascular events in adults with known heart disease and with either obesity or overweight. It is also indicated for adults and children aged 12 years or older with obesity, and some adults with overweight who also have weight-related medical problems, to help them lose excess body weight and keep the weight off.
What’s Next for Wegovy?
In February 2025, Novo Nordisk filed for regulatory approval in the EU, followed by regulatory submission in Japan in May 2025. Also in May, the FDA accepted a filing application for oral semaglutide 25 mg.
Furthermore, “There’s an expected readout of part 2 of ESSENCE in 2029, which aims to demonstrate treatment with Wegovy lowers the risk of liver-related clinical events, compared to placebo, in patients with MASH and F2 or F3 fibrosis at week 240,” a Novo Nordisk spokesperson told GI & Hepatology News.
Although the company has the technology to produce semaglutide as a pill or tablet, she said, “the US launch of oral semaglutide for obesity will be contingent on portfolio prioritization and manufacturing capacity.” The company has not yet submitted the 50 mg oral semaglutide to regulatory authorities.
“The oral form requires more active pharmaceutical ingredient (API),” she noted. “Given that we have a fixed amount of API, the injectable form enables us to treat more patients. We are currently expanding our oral and injectable production capacities globally with the aim of serving as many patients as possible. It requires time to build, install, validate, and ramp-up these production processes.”
A version of this article appeared on Medscape.com.
The once-weekly 2.4 mg semaglutide subcutaneous injection is given in conjunction with a reduced calorie diet and increased physical activity.
Among people living with overweight or obesity globally, 1 in 3 also have MASH.
The accelerated approval was based on part-one results from the ongoing two-part, phase-3 ESSENCE trial, in which Wegovy demonstrated a significant improvement in liver fibrosis with no worsening of steatohepatitis, as well as resolution of steatohepatitis with no worsening of liver fibrosis, compared with placebo at week 72. Those results were published online in April in The New England Journal of Medicine.
For the trial, 800 participants were randomly assigned to either Wegovy (534 participants) or placebo (266 participants) in addition to lifestyle changes. The mean age was 56 years and the mean BMI was 34. Most patients were white individuals (67.5%) and women (57.1%), and 55.9% of the patients had type 2 diabetes; 250 patients (31.3%) had stage II fibrosis and 550 (68.8%) had stage III fibrosis. Participants were on stable doses of lipid-lowering, glucose-management, and weight-loss medications.
At week 72, the first primary endpoint showed 63% of the 534 people treated with Wegovy achieved resolution of steatohepatitis and no worsening of liver fibrosis compared with 34% of 266 individuals treated with placebo — a statistically significant difference.
The second primary endpoint showed 37% of people treated with Wegovy achieved improvement in liver fibrosis and no worsening of steatohepatitis compared with 22% of those treated with placebo, also a significant difference.
A confirmatory secondary endpoint at week 72 showed 33% of patients treated with Wegovy achieved both resolution of steatohepatitis and improvement in liver fibrosis compared with 16% of those treated with placebo — a statistically significant difference in response rate of 17%.
In addition, 83.5% of the patients in the semaglutide group maintained the target dose of 2.4 mg until week 72.
Wegovy is also indicated, along with diet and physical activity, to reduce the risk for major cardiovascular events in adults with known heart disease and with either obesity or overweight. It is also indicated for adults and children aged 12 years or older with obesity, and some adults with overweight who also have weight-related medical problems, to help them lose excess body weight and keep the weight off.
What’s Next for Wegovy?
In February 2025, Novo Nordisk filed for regulatory approval in the EU, followed by regulatory submission in Japan in May 2025. Also in May, the FDA accepted a filing application for oral semaglutide 25 mg.
Furthermore, “There’s an expected readout of part 2 of ESSENCE in 2029, which aims to demonstrate treatment with Wegovy lowers the risk of liver-related clinical events, compared to placebo, in patients with MASH and F2 or F3 fibrosis at week 240,” a Novo Nordisk spokesperson told GI & Hepatology News.
Although the company has the technology to produce semaglutide as a pill or tablet, she said, “the US launch of oral semaglutide for obesity will be contingent on portfolio prioritization and manufacturing capacity.” The company has not yet submitted the 50 mg oral semaglutide to regulatory authorities.
“The oral form requires more active pharmaceutical ingredient (API),” she noted. “Given that we have a fixed amount of API, the injectable form enables us to treat more patients. We are currently expanding our oral and injectable production capacities globally with the aim of serving as many patients as possible. It requires time to build, install, validate, and ramp-up these production processes.”
A version of this article appeared on Medscape.com.
The once-weekly 2.4 mg semaglutide subcutaneous injection is given in conjunction with a reduced calorie diet and increased physical activity.
Among people living with overweight or obesity globally, 1 in 3 also have MASH.
The accelerated approval was based on part-one results from the ongoing two-part, phase-3 ESSENCE trial, in which Wegovy demonstrated a significant improvement in liver fibrosis with no worsening of steatohepatitis, as well as resolution of steatohepatitis with no worsening of liver fibrosis, compared with placebo at week 72. Those results were published online in April in The New England Journal of Medicine.
For the trial, 800 participants were randomly assigned to either Wegovy (534 participants) or placebo (266 participants) in addition to lifestyle changes. The mean age was 56 years and the mean BMI was 34. Most patients were white individuals (67.5%) and women (57.1%), and 55.9% of the patients had type 2 diabetes; 250 patients (31.3%) had stage II fibrosis and 550 (68.8%) had stage III fibrosis. Participants were on stable doses of lipid-lowering, glucose-management, and weight-loss medications.
At week 72, the first primary endpoint showed 63% of the 534 people treated with Wegovy achieved resolution of steatohepatitis and no worsening of liver fibrosis compared with 34% of 266 individuals treated with placebo — a statistically significant difference.
The second primary endpoint showed 37% of people treated with Wegovy achieved improvement in liver fibrosis and no worsening of steatohepatitis compared with 22% of those treated with placebo, also a significant difference.
A confirmatory secondary endpoint at week 72 showed 33% of patients treated with Wegovy achieved both resolution of steatohepatitis and improvement in liver fibrosis compared with 16% of those treated with placebo — a statistically significant difference in response rate of 17%.
In addition, 83.5% of the patients in the semaglutide group maintained the target dose of 2.4 mg until week 72.
Wegovy is also indicated, along with diet and physical activity, to reduce the risk for major cardiovascular events in adults with known heart disease and with either obesity or overweight. It is also indicated for adults and children aged 12 years or older with obesity, and some adults with overweight who also have weight-related medical problems, to help them lose excess body weight and keep the weight off.
What’s Next for Wegovy?
In February 2025, Novo Nordisk filed for regulatory approval in the EU, followed by regulatory submission in Japan in May 2025. Also in May, the FDA accepted a filing application for oral semaglutide 25 mg.
Furthermore, “There’s an expected readout of part 2 of ESSENCE in 2029, which aims to demonstrate treatment with Wegovy lowers the risk of liver-related clinical events, compared to placebo, in patients with MASH and F2 or F3 fibrosis at week 240,” a Novo Nordisk spokesperson told GI & Hepatology News.
Although the company has the technology to produce semaglutide as a pill or tablet, she said, “the US launch of oral semaglutide for obesity will be contingent on portfolio prioritization and manufacturing capacity.” The company has not yet submitted the 50 mg oral semaglutide to regulatory authorities.
“The oral form requires more active pharmaceutical ingredient (API),” she noted. “Given that we have a fixed amount of API, the injectable form enables us to treat more patients. We are currently expanding our oral and injectable production capacities globally with the aim of serving as many patients as possible. It requires time to build, install, validate, and ramp-up these production processes.”
A version of this article appeared on Medscape.com.