User login
Longer-term opioid use in workers’ comp cases highest in Louisiana
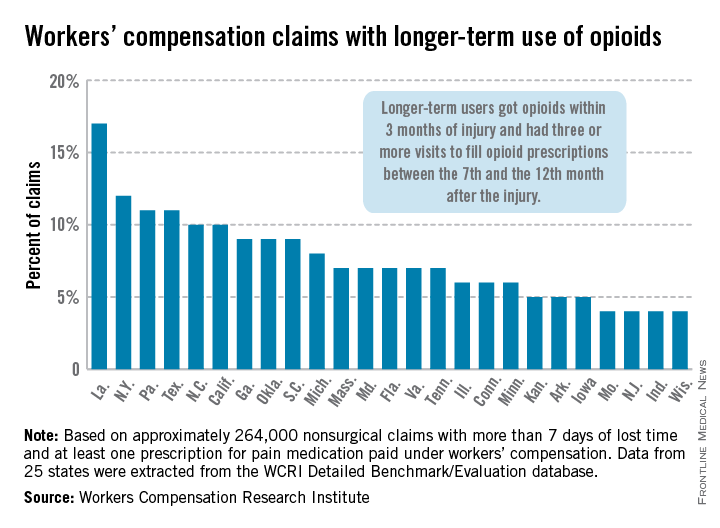
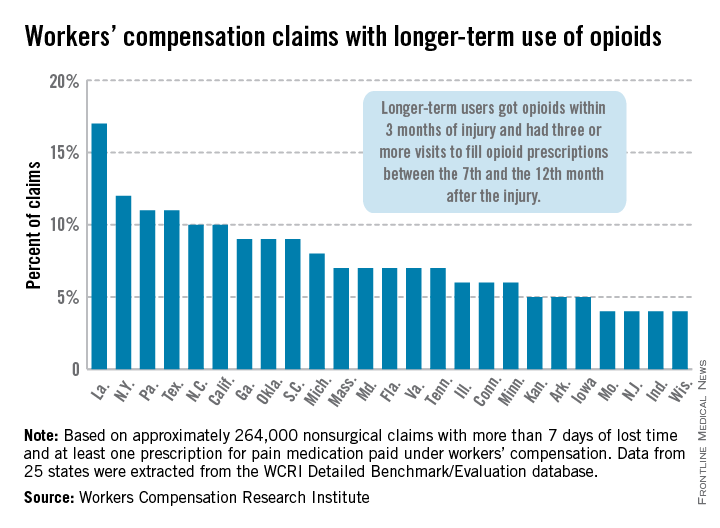
In Louisiana, opioid use lasted more than 6 months in 17% of nonsurgical workers’ compensation claims involving employees who received at least one prescription for pain medication, the Workers Compensation Research Institute reported.
In cases with more than 7 days of lost time, that was the highest rate seen among the 25 states in the study, with New York second at 12% and Pennsylvania and Texas tied for third at 11%. There were four states tied for the lowest rate, at 4%: Missouri, New Jersey, Indiana, and Wisconsin, according to the WCRI report.
Overall, use of narcotics for pain relief by injured workers in such cases ranged from 60% in New Jersey to 88% in Arkansas (median, 76%), while use of any pain medication ranged from 85% in Minnesota to 95% in Florida, Georgia, Tennessee, and Texas (median, 94%), the report showed.
The study involved claims with injuries that occurred from Oct. 1, 2009, through Sept. 30, 2010, with prescriptions filled through March 31, 2012. Longer-term users received a prescription for opioids within 3 months of their injury and had three or more visits to fill opioid prescriptions between the 7th and the 12th month after the injury.
The 25 states in the study "represent more than 70% of the workers’ compensation benefits paid in the United States," the WCRI noted.
The study was based on approximately 264,000 nonsurgical claims and more than 1.5 million prescriptions for pain medications. Data were extracted from the WCRI Detailed Benchmark/Evaluation database and consisted of detailed prescription transactions "collected from workers’ compensation payers and their medical bill review and pharmacy benefit management vendors," the report noted.
In Louisiana, opioid use lasted more than 6 months in 17% of nonsurgical workers’ compensation claims involving employees who received at least one prescription for pain medication, the Workers Compensation Research Institute reported.
In cases with more than 7 days of lost time, that was the highest rate seen among the 25 states in the study, with New York second at 12% and Pennsylvania and Texas tied for third at 11%. There were four states tied for the lowest rate, at 4%: Missouri, New Jersey, Indiana, and Wisconsin, according to the WCRI report.
Overall, use of narcotics for pain relief by injured workers in such cases ranged from 60% in New Jersey to 88% in Arkansas (median, 76%), while use of any pain medication ranged from 85% in Minnesota to 95% in Florida, Georgia, Tennessee, and Texas (median, 94%), the report showed.
The study involved claims with injuries that occurred from Oct. 1, 2009, through Sept. 30, 2010, with prescriptions filled through March 31, 2012. Longer-term users received a prescription for opioids within 3 months of their injury and had three or more visits to fill opioid prescriptions between the 7th and the 12th month after the injury.
The 25 states in the study "represent more than 70% of the workers’ compensation benefits paid in the United States," the WCRI noted.
The study was based on approximately 264,000 nonsurgical claims and more than 1.5 million prescriptions for pain medications. Data were extracted from the WCRI Detailed Benchmark/Evaluation database and consisted of detailed prescription transactions "collected from workers’ compensation payers and their medical bill review and pharmacy benefit management vendors," the report noted.

In Louisiana, opioid use lasted more than 6 months in 17% of nonsurgical workers’ compensation claims involving employees who received at least one prescription for pain medication, the Workers Compensation Research Institute reported.
In cases with more than 7 days of lost time, that was the highest rate seen among the 25 states in the study, with New York second at 12% and Pennsylvania and Texas tied for third at 11%. There were four states tied for the lowest rate, at 4%: Missouri, New Jersey, Indiana, and Wisconsin, according to the WCRI report.
Overall, use of narcotics for pain relief by injured workers in such cases ranged from 60% in New Jersey to 88% in Arkansas (median, 76%), while use of any pain medication ranged from 85% in Minnesota to 95% in Florida, Georgia, Tennessee, and Texas (median, 94%), the report showed.
The study involved claims with injuries that occurred from Oct. 1, 2009, through Sept. 30, 2010, with prescriptions filled through March 31, 2012. Longer-term users received a prescription for opioids within 3 months of their injury and had three or more visits to fill opioid prescriptions between the 7th and the 12th month after the injury.
The 25 states in the study "represent more than 70% of the workers’ compensation benefits paid in the United States," the WCRI noted.
The study was based on approximately 264,000 nonsurgical claims and more than 1.5 million prescriptions for pain medications. Data were extracted from the WCRI Detailed Benchmark/Evaluation database and consisted of detailed prescription transactions "collected from workers’ compensation payers and their medical bill review and pharmacy benefit management vendors," the report noted.

High-Altitude Illness
Patients participating in occupational and sports-related activities requiring ascent to high elevations are at risk of developing a range of high-altitude illnesses. Prompt recognition and treatment are paramount to improving outcomes and preventing life-threatening sequelae. High-elevation locations are the setting of many recreational activities for outdoor enthusiasts. As such, illnesses associated with high altitude may be encountered by those summiting peaks, traveling by air, or working in flight medicine or as part of an emergency rescue team. The altitude syndromes discussed in this review are acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). While these conditions do not represent all altitude-related illnesses, they are the primary pathological processes for which physicians should be familiar when working with high-altitude populations.
Physiological Response to Altitude
The Lake Louise Criteria
Acute Mountain Sickness
Acute mountain sickness comprises a constellation of symptoms caused by the atmospheric changes at elevations above approximately 2,500 m. It is the most common form of high-altitude illness, affecting 25% of travelers at moderate altitude and 50% to 85% above 4,000 m.3
Symptoms
The onset of symptoms (eg, headache, anorexia, nausea, vomiting, weakness) may occur at 2,000 m in the setting of rapid ascent—most commonly at 6 to 12 hours, but onset can range from 1 hour to 2 days after ascent. If symptoms begin after 3 days, other diagnoses should be considered. Symptoms of AMS are generally worse after the first night of sleep at elevation. On physical examination, vital signs are usually normal, though postural hypotension and tachycardia are possible. Oxygen saturation may be markedly decreased after rapid ascent, and chest auscultation may reveal rales in 20% of patients.4 Peripheral and facial edema may also be present. Funduscopic examination may show venous tortuosity and dilation, and retinal hemorrhage is common in ascents over 4,800 m.
Differential Diagnosis
The differential diagnosis for AMS is broad and includes hypothermia, dehydration, exhaustion, subarachnoid hemorrhage, intracranial mass, carbon monoxide poisoning, alcohol hangover, intoxication, central nervous system infection and migraine. Risk factors for developing AMS are a previous history of altitude illness, rapid ascent, and lack of previous acclimatization. Interestingly, physical fitness does not protect a person from developing AMS.5
Mechanism of AMS
The true mechanism of AMS is uncertain, but it is clear that a fall in barometric pressure results in hypobaric hypoxia. This is thought to lead to an increased blood volume in the brain and increased cerebral blood flow, possibly precipitating an enlarged brain. A mechanism related to vasogenic edema has been proposed due to patients’ clinical improvement with dexamethasone therapy.6 Acute mountain sickness does appear to be related to overall fluid balance, as an increase in reninangiotensin, aldosterone, and antidiuretic hormone has been observed in patients with the condition. Elevation of these hormones is contrary to the appropriate physiological response of diuresis.
Treatment
Treatment of AMS begins with descent from elevation as soon as possible. Descent should be at least 500 m from the aggravating elevation. Patients should remain at least 1 to 2 days at this lower elevation before attempting reascent. If descent is not feasible, any further ascent should be delayed until symptoms have resolved.
Dexamethasone. This glucocorticoid has been used clinically with good success, although the mechanism of action in unclear. The initial dose is 8 mg followed by 4 mg every 6 hours.3
Acetazolamide. A carbonic anhydrase inhibitor, acetazolamide acts to temper symptoms by causing an acidosis that increases ventilation and prevents periodic breathing and hypoxia during sleep. The standard dose is 250 mg twice daily.3
Oxygen. Supplemental oxygen provided at 1 to 2 L/min via nasal cannula for 12 to 24 hours may help to improve symptoms. A portable hyperbaric oxygen (HBO) bag (eg, a Gamow bag) can be used to create an effective altitude of approximately 1,500 to 2,000 m inside the bag. The patient is placed completely within the bag, the zipper is sealed shut, and the bag is inflated with a foot pump. Treatment in such a chamber can be provided in 1-hour increments and repeated as needed. However, if descent is possible, use of the HBO chamber should not prevent or delay descent.
Ibuprofen. Compared to placebo, studies have shown ibuprofen 600 mg three times a day reduces the severity of AMS.7
Prevention
Strategies to prevent AMS are similar to those used to treat the condition. These include gradual ascent and prophylactic drug therapy.
Gradual Ascent. Gradual ascent is the primary strategy to prevent AMS. At altitudes above 3,000 m, each subsequent night should not be spent at an elevation 300 m higher than the previous night.
Acetazolamide. Pretreatment with acetazolamide is indicated for patients with a history of altitude illness or who anticipate an abrupt ascent (eg, rescue workers). Acetazolamide has been shown in multiple studies to be effective in the prevention of AMS.8 Adverse side effects of acetazolamide include paresthesias and increased urinary frequency; the drug may also make carbonated beverages taste flat. The preventive dose is 125 mg twice daily, and should be started the day before ascent.
Dexamethasone. In addition to treating AMS, dexamethasone may be taken as a preventive in doses of 2 mg every 6 hours or 4 mg twice daily.3 However, unlike acetazolamide, which acts to facilitate acclimatization, dexamethasone only prevents symptoms. Thus, cessation of the drug can result in rebound AMS symptoms, and prolonged use can result in adrenal suppression.3 Therefore, it should not be used for more than 10 days.
Sumatriptan and Gabapentin. In recent studies, sumatriptan and gabapentin haven shown benefit in preventing AMS, 9,10 but further study is needed before either of these drugs can be recommended.
Ginkgo Biloba. While ginkgo biloba has been touted as an effective preventive treatment, studies have shown no benefit to its use.8
Ibuprofen. ibuprofen 600 mg three times daily can be initiated the day prior to ascent, and has been shown to decrease the incidence of AMS.7
High-Altitude Cerebral Edema
Mechanism of HACE
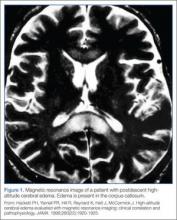
The exact mechanism of HACE is unclear. Magnetic resonance imaging of patients with the condition demonstrates cerebral edema primarily localized to the corpus callosum.11 These findings suggest an increased permeability in the blood-brain barrier, leading to vasogenic cerebral edema. Cases of death associated with HACE are the result of herniation. Fortunately, if the condition is recognized promptly and appropriate management is instituted, most patients will recover without permanent deficits.
Current recommendations for treating HACE are similar to treatment strategies for AMS.
Descent. A therapeutic priority, descent may prove challenging as the patient may be ataxic, have altered mental status, and have difficulty facilitating his or her own descent.
Oxygen. A portable HBO bag can be used to simulate descent until evacuation is possible. Supplemental oxygen should be applied immediately.
Dexamethasone. In treating HACE, dexamethasone may be administered at a loading dose of 8 mg, followed by 4 mg every 6 hours.3
Airway Management. If the patient has significantly altered mental status, appropriate airway management must be initiated.
High-Altitude Pulmonary Edema
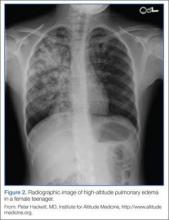
The most common cause of death from altitude illness is HAPE,12 a form of noncardiogenic pulmonary edema. This condition generally occurs at elevations above 3,000 m. Symptoms begin 2 to 5 days after ascent and progress in a typical pattern. A patient will initially experience a nonproductive cough and dyspnea at rest. The dyspnea worsens, and the cough becomes productive of pink, frothy sputum. Without medical intervention, lethargy, coma, and death may follow.
Symptoms of HAPE generally worsen following a night of sleep at elevation. Physical examination reveals crackles, tachycardia, tachypnea, and hypoxia. Diagnosis requires at least two of the following signs:
- Crackles or wheezing in at least one lung field
- Central cyanosis
- Tachypnea
- Tachycardia.
In addition to the above signs, at least two of the following symptoms must also be present:
- Dyspnea at rest
- Cough
- Weakness or decreased exercise performance
- Chest tightness
- Congestion.
Mechanism of HAPE
The mechanism of HAPE is better understood than that of AMS and HACE. In HAPE, high microvascular pressures in the lungs lead to elevated pulmonary vascular resistance and pulmonary artery pressure. Pulmonary edema ensues, but left ventricular function is preserved. Patients with a naturally low HVR, high pulmonary artery pressures at rest, preexisting pulmonary hypertension, or a previous history of HAPE are predisposed to developing the condition. Risk factors include heavy exertion, rapid ascent, cold, salt ingestion, and sleeping medications.
Treatment
Decent and warming of the patient as soon as possible, along with treatment outlined below, are essential.
Oxygen. Treatment of HAPE begins with supplemental oxygen to immediately lower pulmonary artery pressure. Oxygen should initially be administered at 4 to 6 L/min; if the patient improves clinically and can maintain oxygen saturations greater than 90%, oxygen may be decreased with a goal to maintain saturation above 90%.
Nifedipine. Following oxygen, descent, and warming, nifedipine can be used as an adjunctive therapy. The treatment dose for HAPE is 20 to 30 mg of the sustained release form every 12 hours.3
Salmeterol/Albuterol and Expiratory Positive Airway Pressure. The oral inhalers salmeterol or albuterol may be used for bronchodilation; however, there is little evidence to support their effectiveness in HAPE. Ventilation with expiratory positive airway pressure can be employed if available.
Prevention
For patients with a predisposition to HAPE, preventive measures should be considered prior to ascent. As with all forms of altitude illness, gradual ascent is the most effective prevention method available.
Phosphodiesterase Inhibitors. Phosphodiesterase inhibitors act via pulmonary vasodilation to prevent HAPE in some patients. Tadalafil at a dose of 10 mg twice daily or 20 mg once daily has been shown to reduce the incidence of HAPE.14 Alternatively, sildenafil 50 mg three times daily may be used.
Acetazolamide and β-Agonists. Although both acetazolamide and β-agonists such as albuterol have been theorized to aid in preventing HAPE, this has not been proven.15
Conclusion
Clinically, high-altitude illnesses range from subtle symptoms to severe, life threatening disease. Knowledge of these disease processes and clinical presentation prior to travel or work in a high-altitude setting is essential. Rapid recognition of symptoms and prompt, appropriate interventions, such as descent when necessary, can significantly improve the outcomes of these conditions.
Dr Haroutunian is an emergency physician, department of emergency medicine, Exempla St Joseph Hospital, Denver, Colorado. Dr Bono is professor and vice chairman, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
- Hackett PH, Oelz O. The Lake Louise consensus on the definition and qualification of altitude illness. In: Sutton JR, Coates G, Houston CS, eds. Hypoxia and Mountain Medicine. Burlington, VT: Queen City Printers; 1992:327-330.
- Roach RC, Bärtch P, Hackett PH, Oelz O, and the Lake Louise AMS Scoring Consensus Committee. The Lake Louise Acute Mountain Sickness Scoring System. In: Hypoxia and Molecular Medicine. Proceedings of the 8th International Hypoxia Symposium. Burlington, VT: Queen City Printers; 1993:272-274.
- Eide RP 3rd, Asplund CA. Altitude illness: update on prevention and treatment. Curr Sports Med Rep. 2012;11(3):124-130.
- Milzman DP, Damergis JA, Napoli AM. Rapid ascent changes in vitals at altitude. Ann Emerg Med. 2008;51(4):536.
- Bärtsch P, Swenson ER. Clinical practice: Acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294-2302.
- Hackett PH, Roach RC. Medical therapy of mountain illness. Ann Emerg Med. 1987;16(9):980-986.
- Lipman GS, Kanaan NC, Holck PS, Constance BB, Gertsch JH; PAINS Group. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012;59(6): 484-490.
- Seupaul RA, Welch JL, Malka ST, Emmett TW. Pharmacologic prophylaxis for acute mountain sickness: a systematic shortcut review. Ann Emerg Med. 2012; 59(4):307-317.
- Jafarian S, Gorouhi F, Salimi S, Lotfi J. Sumatriptan for prevention of acute mountain sickness: randomized clinical trial. Ann Neurol. 2007;62(3):273-277.
- Jafarian S, Abolfazli R, Gorouhi F, Rezaie S, Lotfi J. Gabapentin for prevention of hypobaric hypoxia-induced headache: randomized double-blind clinical trial. J Neurol Neurosurg Psychiatry. 2008;79(3): 321-323.
- Hackett PH, Yarnell PR, Hill R, Reynard K, Heit J, McCormick J. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA. 1998;280(22):1920-1925.
- Gallagher SA1, Hackett PH. High-altitude illness. Emerg Med Clin North Am. 2004;22(2):329-355.
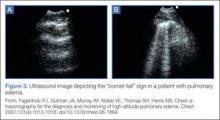
- Fagenholz PJ, Gutman JA, Murray AF, Noble VE, Thomas SH, Harris NS. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest. 2007;131(4): 1013-1018.
- Leshem E1, Caine Y, Rosenberg E, Maaravi Y, Hermesh H, Schwartz E. Tadalafil and acetazolamide versus acetazolamide for the prevention of severe high-altitude illness. J Travel Med. 2012;19(5): 308-310.
- Schoene RB. Illnesses at high altitude. Chest. 2008;134(2):402-416.
Patients participating in occupational and sports-related activities requiring ascent to high elevations are at risk of developing a range of high-altitude illnesses. Prompt recognition and treatment are paramount to improving outcomes and preventing life-threatening sequelae. High-elevation locations are the setting of many recreational activities for outdoor enthusiasts. As such, illnesses associated with high altitude may be encountered by those summiting peaks, traveling by air, or working in flight medicine or as part of an emergency rescue team. The altitude syndromes discussed in this review are acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). While these conditions do not represent all altitude-related illnesses, they are the primary pathological processes for which physicians should be familiar when working with high-altitude populations.
Physiological Response to Altitude
The Lake Louise Criteria
Acute Mountain Sickness
Acute mountain sickness comprises a constellation of symptoms caused by the atmospheric changes at elevations above approximately 2,500 m. It is the most common form of high-altitude illness, affecting 25% of travelers at moderate altitude and 50% to 85% above 4,000 m.3
Symptoms
The onset of symptoms (eg, headache, anorexia, nausea, vomiting, weakness) may occur at 2,000 m in the setting of rapid ascent—most commonly at 6 to 12 hours, but onset can range from 1 hour to 2 days after ascent. If symptoms begin after 3 days, other diagnoses should be considered. Symptoms of AMS are generally worse after the first night of sleep at elevation. On physical examination, vital signs are usually normal, though postural hypotension and tachycardia are possible. Oxygen saturation may be markedly decreased after rapid ascent, and chest auscultation may reveal rales in 20% of patients.4 Peripheral and facial edema may also be present. Funduscopic examination may show venous tortuosity and dilation, and retinal hemorrhage is common in ascents over 4,800 m.
Differential Diagnosis
The differential diagnosis for AMS is broad and includes hypothermia, dehydration, exhaustion, subarachnoid hemorrhage, intracranial mass, carbon monoxide poisoning, alcohol hangover, intoxication, central nervous system infection and migraine. Risk factors for developing AMS are a previous history of altitude illness, rapid ascent, and lack of previous acclimatization. Interestingly, physical fitness does not protect a person from developing AMS.5
Mechanism of AMS
The true mechanism of AMS is uncertain, but it is clear that a fall in barometric pressure results in hypobaric hypoxia. This is thought to lead to an increased blood volume in the brain and increased cerebral blood flow, possibly precipitating an enlarged brain. A mechanism related to vasogenic edema has been proposed due to patients’ clinical improvement with dexamethasone therapy.6 Acute mountain sickness does appear to be related to overall fluid balance, as an increase in reninangiotensin, aldosterone, and antidiuretic hormone has been observed in patients with the condition. Elevation of these hormones is contrary to the appropriate physiological response of diuresis.
Treatment
Treatment of AMS begins with descent from elevation as soon as possible. Descent should be at least 500 m from the aggravating elevation. Patients should remain at least 1 to 2 days at this lower elevation before attempting reascent. If descent is not feasible, any further ascent should be delayed until symptoms have resolved.
Dexamethasone. This glucocorticoid has been used clinically with good success, although the mechanism of action in unclear. The initial dose is 8 mg followed by 4 mg every 6 hours.3
Acetazolamide. A carbonic anhydrase inhibitor, acetazolamide acts to temper symptoms by causing an acidosis that increases ventilation and prevents periodic breathing and hypoxia during sleep. The standard dose is 250 mg twice daily.3
Oxygen. Supplemental oxygen provided at 1 to 2 L/min via nasal cannula for 12 to 24 hours may help to improve symptoms. A portable hyperbaric oxygen (HBO) bag (eg, a Gamow bag) can be used to create an effective altitude of approximately 1,500 to 2,000 m inside the bag. The patient is placed completely within the bag, the zipper is sealed shut, and the bag is inflated with a foot pump. Treatment in such a chamber can be provided in 1-hour increments and repeated as needed. However, if descent is possible, use of the HBO chamber should not prevent or delay descent.
Ibuprofen. Compared to placebo, studies have shown ibuprofen 600 mg three times a day reduces the severity of AMS.7
Prevention
Strategies to prevent AMS are similar to those used to treat the condition. These include gradual ascent and prophylactic drug therapy.
Gradual Ascent. Gradual ascent is the primary strategy to prevent AMS. At altitudes above 3,000 m, each subsequent night should not be spent at an elevation 300 m higher than the previous night.
Acetazolamide. Pretreatment with acetazolamide is indicated for patients with a history of altitude illness or who anticipate an abrupt ascent (eg, rescue workers). Acetazolamide has been shown in multiple studies to be effective in the prevention of AMS.8 Adverse side effects of acetazolamide include paresthesias and increased urinary frequency; the drug may also make carbonated beverages taste flat. The preventive dose is 125 mg twice daily, and should be started the day before ascent.
Dexamethasone. In addition to treating AMS, dexamethasone may be taken as a preventive in doses of 2 mg every 6 hours or 4 mg twice daily.3 However, unlike acetazolamide, which acts to facilitate acclimatization, dexamethasone only prevents symptoms. Thus, cessation of the drug can result in rebound AMS symptoms, and prolonged use can result in adrenal suppression.3 Therefore, it should not be used for more than 10 days.
Sumatriptan and Gabapentin. In recent studies, sumatriptan and gabapentin haven shown benefit in preventing AMS, 9,10 but further study is needed before either of these drugs can be recommended.
Ginkgo Biloba. While ginkgo biloba has been touted as an effective preventive treatment, studies have shown no benefit to its use.8
Ibuprofen. ibuprofen 600 mg three times daily can be initiated the day prior to ascent, and has been shown to decrease the incidence of AMS.7
High-Altitude Cerebral Edema
Mechanism of HACE
The exact mechanism of HACE is unclear. Magnetic resonance imaging of patients with the condition demonstrates cerebral edema primarily localized to the corpus callosum.11 These findings suggest an increased permeability in the blood-brain barrier, leading to vasogenic cerebral edema. Cases of death associated with HACE are the result of herniation. Fortunately, if the condition is recognized promptly and appropriate management is instituted, most patients will recover without permanent deficits.
Current recommendations for treating HACE are similar to treatment strategies for AMS.
Descent. A therapeutic priority, descent may prove challenging as the patient may be ataxic, have altered mental status, and have difficulty facilitating his or her own descent.
Oxygen. A portable HBO bag can be used to simulate descent until evacuation is possible. Supplemental oxygen should be applied immediately.
Dexamethasone. In treating HACE, dexamethasone may be administered at a loading dose of 8 mg, followed by 4 mg every 6 hours.3
Airway Management. If the patient has significantly altered mental status, appropriate airway management must be initiated.
High-Altitude Pulmonary Edema
The most common cause of death from altitude illness is HAPE,12 a form of noncardiogenic pulmonary edema. This condition generally occurs at elevations above 3,000 m. Symptoms begin 2 to 5 days after ascent and progress in a typical pattern. A patient will initially experience a nonproductive cough and dyspnea at rest. The dyspnea worsens, and the cough becomes productive of pink, frothy sputum. Without medical intervention, lethargy, coma, and death may follow.
Symptoms of HAPE generally worsen following a night of sleep at elevation. Physical examination reveals crackles, tachycardia, tachypnea, and hypoxia. Diagnosis requires at least two of the following signs:
- Crackles or wheezing in at least one lung field
- Central cyanosis
- Tachypnea
- Tachycardia.
In addition to the above signs, at least two of the following symptoms must also be present:
- Dyspnea at rest
- Cough
- Weakness or decreased exercise performance
- Chest tightness
- Congestion.
Mechanism of HAPE
The mechanism of HAPE is better understood than that of AMS and HACE. In HAPE, high microvascular pressures in the lungs lead to elevated pulmonary vascular resistance and pulmonary artery pressure. Pulmonary edema ensues, but left ventricular function is preserved. Patients with a naturally low HVR, high pulmonary artery pressures at rest, preexisting pulmonary hypertension, or a previous history of HAPE are predisposed to developing the condition. Risk factors include heavy exertion, rapid ascent, cold, salt ingestion, and sleeping medications.
Treatment
Decent and warming of the patient as soon as possible, along with treatment outlined below, are essential.
Oxygen. Treatment of HAPE begins with supplemental oxygen to immediately lower pulmonary artery pressure. Oxygen should initially be administered at 4 to 6 L/min; if the patient improves clinically and can maintain oxygen saturations greater than 90%, oxygen may be decreased with a goal to maintain saturation above 90%.
Nifedipine. Following oxygen, descent, and warming, nifedipine can be used as an adjunctive therapy. The treatment dose for HAPE is 20 to 30 mg of the sustained release form every 12 hours.3
Salmeterol/Albuterol and Expiratory Positive Airway Pressure. The oral inhalers salmeterol or albuterol may be used for bronchodilation; however, there is little evidence to support their effectiveness in HAPE. Ventilation with expiratory positive airway pressure can be employed if available.
Prevention
For patients with a predisposition to HAPE, preventive measures should be considered prior to ascent. As with all forms of altitude illness, gradual ascent is the most effective prevention method available.
Phosphodiesterase Inhibitors. Phosphodiesterase inhibitors act via pulmonary vasodilation to prevent HAPE in some patients. Tadalafil at a dose of 10 mg twice daily or 20 mg once daily has been shown to reduce the incidence of HAPE.14 Alternatively, sildenafil 50 mg three times daily may be used.
Acetazolamide and β-Agonists. Although both acetazolamide and β-agonists such as albuterol have been theorized to aid in preventing HAPE, this has not been proven.15
Conclusion
Clinically, high-altitude illnesses range from subtle symptoms to severe, life threatening disease. Knowledge of these disease processes and clinical presentation prior to travel or work in a high-altitude setting is essential. Rapid recognition of symptoms and prompt, appropriate interventions, such as descent when necessary, can significantly improve the outcomes of these conditions.
Dr Haroutunian is an emergency physician, department of emergency medicine, Exempla St Joseph Hospital, Denver, Colorado. Dr Bono is professor and vice chairman, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
Patients participating in occupational and sports-related activities requiring ascent to high elevations are at risk of developing a range of high-altitude illnesses. Prompt recognition and treatment are paramount to improving outcomes and preventing life-threatening sequelae. High-elevation locations are the setting of many recreational activities for outdoor enthusiasts. As such, illnesses associated with high altitude may be encountered by those summiting peaks, traveling by air, or working in flight medicine or as part of an emergency rescue team. The altitude syndromes discussed in this review are acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). While these conditions do not represent all altitude-related illnesses, they are the primary pathological processes for which physicians should be familiar when working with high-altitude populations.
Physiological Response to Altitude
The Lake Louise Criteria
Acute Mountain Sickness
Acute mountain sickness comprises a constellation of symptoms caused by the atmospheric changes at elevations above approximately 2,500 m. It is the most common form of high-altitude illness, affecting 25% of travelers at moderate altitude and 50% to 85% above 4,000 m.3
Symptoms
The onset of symptoms (eg, headache, anorexia, nausea, vomiting, weakness) may occur at 2,000 m in the setting of rapid ascent—most commonly at 6 to 12 hours, but onset can range from 1 hour to 2 days after ascent. If symptoms begin after 3 days, other diagnoses should be considered. Symptoms of AMS are generally worse after the first night of sleep at elevation. On physical examination, vital signs are usually normal, though postural hypotension and tachycardia are possible. Oxygen saturation may be markedly decreased after rapid ascent, and chest auscultation may reveal rales in 20% of patients.4 Peripheral and facial edema may also be present. Funduscopic examination may show venous tortuosity and dilation, and retinal hemorrhage is common in ascents over 4,800 m.
Differential Diagnosis
The differential diagnosis for AMS is broad and includes hypothermia, dehydration, exhaustion, subarachnoid hemorrhage, intracranial mass, carbon monoxide poisoning, alcohol hangover, intoxication, central nervous system infection and migraine. Risk factors for developing AMS are a previous history of altitude illness, rapid ascent, and lack of previous acclimatization. Interestingly, physical fitness does not protect a person from developing AMS.5
Mechanism of AMS
The true mechanism of AMS is uncertain, but it is clear that a fall in barometric pressure results in hypobaric hypoxia. This is thought to lead to an increased blood volume in the brain and increased cerebral blood flow, possibly precipitating an enlarged brain. A mechanism related to vasogenic edema has been proposed due to patients’ clinical improvement with dexamethasone therapy.6 Acute mountain sickness does appear to be related to overall fluid balance, as an increase in reninangiotensin, aldosterone, and antidiuretic hormone has been observed in patients with the condition. Elevation of these hormones is contrary to the appropriate physiological response of diuresis.
Treatment
Treatment of AMS begins with descent from elevation as soon as possible. Descent should be at least 500 m from the aggravating elevation. Patients should remain at least 1 to 2 days at this lower elevation before attempting reascent. If descent is not feasible, any further ascent should be delayed until symptoms have resolved.
Dexamethasone. This glucocorticoid has been used clinically with good success, although the mechanism of action in unclear. The initial dose is 8 mg followed by 4 mg every 6 hours.3
Acetazolamide. A carbonic anhydrase inhibitor, acetazolamide acts to temper symptoms by causing an acidosis that increases ventilation and prevents periodic breathing and hypoxia during sleep. The standard dose is 250 mg twice daily.3
Oxygen. Supplemental oxygen provided at 1 to 2 L/min via nasal cannula for 12 to 24 hours may help to improve symptoms. A portable hyperbaric oxygen (HBO) bag (eg, a Gamow bag) can be used to create an effective altitude of approximately 1,500 to 2,000 m inside the bag. The patient is placed completely within the bag, the zipper is sealed shut, and the bag is inflated with a foot pump. Treatment in such a chamber can be provided in 1-hour increments and repeated as needed. However, if descent is possible, use of the HBO chamber should not prevent or delay descent.
Ibuprofen. Compared to placebo, studies have shown ibuprofen 600 mg three times a day reduces the severity of AMS.7
Prevention
Strategies to prevent AMS are similar to those used to treat the condition. These include gradual ascent and prophylactic drug therapy.
Gradual Ascent. Gradual ascent is the primary strategy to prevent AMS. At altitudes above 3,000 m, each subsequent night should not be spent at an elevation 300 m higher than the previous night.
Acetazolamide. Pretreatment with acetazolamide is indicated for patients with a history of altitude illness or who anticipate an abrupt ascent (eg, rescue workers). Acetazolamide has been shown in multiple studies to be effective in the prevention of AMS.8 Adverse side effects of acetazolamide include paresthesias and increased urinary frequency; the drug may also make carbonated beverages taste flat. The preventive dose is 125 mg twice daily, and should be started the day before ascent.
Dexamethasone. In addition to treating AMS, dexamethasone may be taken as a preventive in doses of 2 mg every 6 hours or 4 mg twice daily.3 However, unlike acetazolamide, which acts to facilitate acclimatization, dexamethasone only prevents symptoms. Thus, cessation of the drug can result in rebound AMS symptoms, and prolonged use can result in adrenal suppression.3 Therefore, it should not be used for more than 10 days.
Sumatriptan and Gabapentin. In recent studies, sumatriptan and gabapentin haven shown benefit in preventing AMS, 9,10 but further study is needed before either of these drugs can be recommended.
Ginkgo Biloba. While ginkgo biloba has been touted as an effective preventive treatment, studies have shown no benefit to its use.8
Ibuprofen. ibuprofen 600 mg three times daily can be initiated the day prior to ascent, and has been shown to decrease the incidence of AMS.7
High-Altitude Cerebral Edema
Mechanism of HACE
The exact mechanism of HACE is unclear. Magnetic resonance imaging of patients with the condition demonstrates cerebral edema primarily localized to the corpus callosum.11 These findings suggest an increased permeability in the blood-brain barrier, leading to vasogenic cerebral edema. Cases of death associated with HACE are the result of herniation. Fortunately, if the condition is recognized promptly and appropriate management is instituted, most patients will recover without permanent deficits.
Current recommendations for treating HACE are similar to treatment strategies for AMS.
Descent. A therapeutic priority, descent may prove challenging as the patient may be ataxic, have altered mental status, and have difficulty facilitating his or her own descent.
Oxygen. A portable HBO bag can be used to simulate descent until evacuation is possible. Supplemental oxygen should be applied immediately.
Dexamethasone. In treating HACE, dexamethasone may be administered at a loading dose of 8 mg, followed by 4 mg every 6 hours.3
Airway Management. If the patient has significantly altered mental status, appropriate airway management must be initiated.
High-Altitude Pulmonary Edema
The most common cause of death from altitude illness is HAPE,12 a form of noncardiogenic pulmonary edema. This condition generally occurs at elevations above 3,000 m. Symptoms begin 2 to 5 days after ascent and progress in a typical pattern. A patient will initially experience a nonproductive cough and dyspnea at rest. The dyspnea worsens, and the cough becomes productive of pink, frothy sputum. Without medical intervention, lethargy, coma, and death may follow.
Symptoms of HAPE generally worsen following a night of sleep at elevation. Physical examination reveals crackles, tachycardia, tachypnea, and hypoxia. Diagnosis requires at least two of the following signs:
- Crackles or wheezing in at least one lung field
- Central cyanosis
- Tachypnea
- Tachycardia.
In addition to the above signs, at least two of the following symptoms must also be present:
- Dyspnea at rest
- Cough
- Weakness or decreased exercise performance
- Chest tightness
- Congestion.
Mechanism of HAPE
The mechanism of HAPE is better understood than that of AMS and HACE. In HAPE, high microvascular pressures in the lungs lead to elevated pulmonary vascular resistance and pulmonary artery pressure. Pulmonary edema ensues, but left ventricular function is preserved. Patients with a naturally low HVR, high pulmonary artery pressures at rest, preexisting pulmonary hypertension, or a previous history of HAPE are predisposed to developing the condition. Risk factors include heavy exertion, rapid ascent, cold, salt ingestion, and sleeping medications.
Treatment
Decent and warming of the patient as soon as possible, along with treatment outlined below, are essential.
Oxygen. Treatment of HAPE begins with supplemental oxygen to immediately lower pulmonary artery pressure. Oxygen should initially be administered at 4 to 6 L/min; if the patient improves clinically and can maintain oxygen saturations greater than 90%, oxygen may be decreased with a goal to maintain saturation above 90%.
Nifedipine. Following oxygen, descent, and warming, nifedipine can be used as an adjunctive therapy. The treatment dose for HAPE is 20 to 30 mg of the sustained release form every 12 hours.3
Salmeterol/Albuterol and Expiratory Positive Airway Pressure. The oral inhalers salmeterol or albuterol may be used for bronchodilation; however, there is little evidence to support their effectiveness in HAPE. Ventilation with expiratory positive airway pressure can be employed if available.
Prevention
For patients with a predisposition to HAPE, preventive measures should be considered prior to ascent. As with all forms of altitude illness, gradual ascent is the most effective prevention method available.
Phosphodiesterase Inhibitors. Phosphodiesterase inhibitors act via pulmonary vasodilation to prevent HAPE in some patients. Tadalafil at a dose of 10 mg twice daily or 20 mg once daily has been shown to reduce the incidence of HAPE.14 Alternatively, sildenafil 50 mg three times daily may be used.
Acetazolamide and β-Agonists. Although both acetazolamide and β-agonists such as albuterol have been theorized to aid in preventing HAPE, this has not been proven.15
Conclusion
Clinically, high-altitude illnesses range from subtle symptoms to severe, life threatening disease. Knowledge of these disease processes and clinical presentation prior to travel or work in a high-altitude setting is essential. Rapid recognition of symptoms and prompt, appropriate interventions, such as descent when necessary, can significantly improve the outcomes of these conditions.
Dr Haroutunian is an emergency physician, department of emergency medicine, Exempla St Joseph Hospital, Denver, Colorado. Dr Bono is professor and vice chairman, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
- Hackett PH, Oelz O. The Lake Louise consensus on the definition and qualification of altitude illness. In: Sutton JR, Coates G, Houston CS, eds. Hypoxia and Mountain Medicine. Burlington, VT: Queen City Printers; 1992:327-330.
- Roach RC, Bärtch P, Hackett PH, Oelz O, and the Lake Louise AMS Scoring Consensus Committee. The Lake Louise Acute Mountain Sickness Scoring System. In: Hypoxia and Molecular Medicine. Proceedings of the 8th International Hypoxia Symposium. Burlington, VT: Queen City Printers; 1993:272-274.
- Eide RP 3rd, Asplund CA. Altitude illness: update on prevention and treatment. Curr Sports Med Rep. 2012;11(3):124-130.
- Milzman DP, Damergis JA, Napoli AM. Rapid ascent changes in vitals at altitude. Ann Emerg Med. 2008;51(4):536.
- Bärtsch P, Swenson ER. Clinical practice: Acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294-2302.
- Hackett PH, Roach RC. Medical therapy of mountain illness. Ann Emerg Med. 1987;16(9):980-986.
- Lipman GS, Kanaan NC, Holck PS, Constance BB, Gertsch JH; PAINS Group. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012;59(6): 484-490.
- Seupaul RA, Welch JL, Malka ST, Emmett TW. Pharmacologic prophylaxis for acute mountain sickness: a systematic shortcut review. Ann Emerg Med. 2012; 59(4):307-317.
- Jafarian S, Gorouhi F, Salimi S, Lotfi J. Sumatriptan for prevention of acute mountain sickness: randomized clinical trial. Ann Neurol. 2007;62(3):273-277.
- Jafarian S, Abolfazli R, Gorouhi F, Rezaie S, Lotfi J. Gabapentin for prevention of hypobaric hypoxia-induced headache: randomized double-blind clinical trial. J Neurol Neurosurg Psychiatry. 2008;79(3): 321-323.
- Hackett PH, Yarnell PR, Hill R, Reynard K, Heit J, McCormick J. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA. 1998;280(22):1920-1925.
- Gallagher SA1, Hackett PH. High-altitude illness. Emerg Med Clin North Am. 2004;22(2):329-355.
- Fagenholz PJ, Gutman JA, Murray AF, Noble VE, Thomas SH, Harris NS. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest. 2007;131(4): 1013-1018.
- Leshem E1, Caine Y, Rosenberg E, Maaravi Y, Hermesh H, Schwartz E. Tadalafil and acetazolamide versus acetazolamide for the prevention of severe high-altitude illness. J Travel Med. 2012;19(5): 308-310.
- Schoene RB. Illnesses at high altitude. Chest. 2008;134(2):402-416.
- Hackett PH, Oelz O. The Lake Louise consensus on the definition and qualification of altitude illness. In: Sutton JR, Coates G, Houston CS, eds. Hypoxia and Mountain Medicine. Burlington, VT: Queen City Printers; 1992:327-330.
- Roach RC, Bärtch P, Hackett PH, Oelz O, and the Lake Louise AMS Scoring Consensus Committee. The Lake Louise Acute Mountain Sickness Scoring System. In: Hypoxia and Molecular Medicine. Proceedings of the 8th International Hypoxia Symposium. Burlington, VT: Queen City Printers; 1993:272-274.
- Eide RP 3rd, Asplund CA. Altitude illness: update on prevention and treatment. Curr Sports Med Rep. 2012;11(3):124-130.
- Milzman DP, Damergis JA, Napoli AM. Rapid ascent changes in vitals at altitude. Ann Emerg Med. 2008;51(4):536.
- Bärtsch P, Swenson ER. Clinical practice: Acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294-2302.
- Hackett PH, Roach RC. Medical therapy of mountain illness. Ann Emerg Med. 1987;16(9):980-986.
- Lipman GS, Kanaan NC, Holck PS, Constance BB, Gertsch JH; PAINS Group. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012;59(6): 484-490.
- Seupaul RA, Welch JL, Malka ST, Emmett TW. Pharmacologic prophylaxis for acute mountain sickness: a systematic shortcut review. Ann Emerg Med. 2012; 59(4):307-317.
- Jafarian S, Gorouhi F, Salimi S, Lotfi J. Sumatriptan for prevention of acute mountain sickness: randomized clinical trial. Ann Neurol. 2007;62(3):273-277.
- Jafarian S, Abolfazli R, Gorouhi F, Rezaie S, Lotfi J. Gabapentin for prevention of hypobaric hypoxia-induced headache: randomized double-blind clinical trial. J Neurol Neurosurg Psychiatry. 2008;79(3): 321-323.
- Hackett PH, Yarnell PR, Hill R, Reynard K, Heit J, McCormick J. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA. 1998;280(22):1920-1925.
- Gallagher SA1, Hackett PH. High-altitude illness. Emerg Med Clin North Am. 2004;22(2):329-355.
- Fagenholz PJ, Gutman JA, Murray AF, Noble VE, Thomas SH, Harris NS. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest. 2007;131(4): 1013-1018.
- Leshem E1, Caine Y, Rosenberg E, Maaravi Y, Hermesh H, Schwartz E. Tadalafil and acetazolamide versus acetazolamide for the prevention of severe high-altitude illness. J Travel Med. 2012;19(5): 308-310.
- Schoene RB. Illnesses at high altitude. Chest. 2008;134(2):402-416.
Emergency Imaging: What is the suspected diagnosis? Is additional imaging necessary, and if so, why?
A 25-year-old man with no significant past medical history presented with low-back pain that radiated down into his right thigh. The patient stated the pain began 1 week earlier when he was lifting weights and had increased in severity to the point where he was no longer able to walk or stand up straight. He had taken nonprescription nonsteroidal anti-inflammatory drugs but received no significant relief.
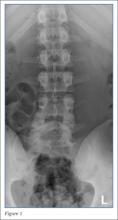
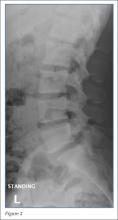
Radiographs of the lumbosacral spine were obtained; representative anteroposterior (AP) and lateral images are shown above (Figures 1 and 2).
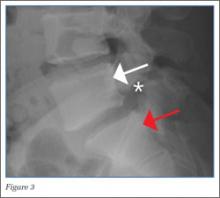
The lateral view of the lumbar spine demonstrates mild anterolisthesis of L5 on S1 with the posterior cortex of L5 (white arrow, Figure 3) anterior to the posterior cortex of S1 (red arrow, Figure 3). Normally, the posterior cortices of the adjacent vertebral bodies should align. Lucency is also noted in the region of the pars interarticularis (white asterisk, Figure 3). The combination of anterolisthesis and this lucency in a young patient suggests the diagnosis of spondylolysis (pars defect).
The pathophysiology of spondylolysis is still uncertain. Two theories have been proposed—underlying dysplastic pars interarticularis versus repetitive microtrauma resulting in stress factors are the two proposed underlying mechanism. If patients are genetically predisposed, underlying dysplasia probably contributes to the pathology, while microtrauma triggers the actual defect.2 Most patients respond well with conservative management.
When evaluating for spondylolysis, AP, lateral, 45-degree right and left oblique views, and collimated lateral views of the lumbosacral spine should be obtained. With this five-view study, up to 96.5% of pars defect can be identified.
In the general population, if spondylolysis is suspected and radiographs are negative, magnetic resonance imaging, computed tomography, and/or single-photon emission computed tomography bone scintigraphy can be used for further evaluation.4,5 In this case, the diagnosis was made based on radiographic imaging, and the patient was discharged with a scheduled follow-up with an orthopedic surgeon.
Dr Salama is a resident of radiology, resident of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Belfi is an assistant professor of radiology, Weill Cornell Medical College New York; and an assistant attending radiologist, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976). 2006;31(24):E907-E910. doi:10.1097/01.brs.0000245947.31473.0a.
- Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst. 2013;29(2):209-216. doi:10.1007/s00381-012-1942-2.
- Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984;153(3):627-629.
- Saraste H, Nilsson B, Broström LA, et al. Relationship between radiological and clinical variables in spondylolysis. Int Orthop. 1984;8(3):163-174. doi:10.1007/BF00269912.
- Lee JH, Ehara S, Tamakawa Y, Shimamura T. Spondylolysis of the upper lumbar spine: Radiological features. Clin Imaging. 1999;23(6):389-393. doi:10.1016/S0899-7071(99)00158-8.
A 25-year-old man with no significant past medical history presented with low-back pain that radiated down into his right thigh. The patient stated the pain began 1 week earlier when he was lifting weights and had increased in severity to the point where he was no longer able to walk or stand up straight. He had taken nonprescription nonsteroidal anti-inflammatory drugs but received no significant relief.
Radiographs of the lumbosacral spine were obtained; representative anteroposterior (AP) and lateral images are shown above (Figures 1 and 2).
The lateral view of the lumbar spine demonstrates mild anterolisthesis of L5 on S1 with the posterior cortex of L5 (white arrow, Figure 3) anterior to the posterior cortex of S1 (red arrow, Figure 3). Normally, the posterior cortices of the adjacent vertebral bodies should align. Lucency is also noted in the region of the pars interarticularis (white asterisk, Figure 3). The combination of anterolisthesis and this lucency in a young patient suggests the diagnosis of spondylolysis (pars defect).
The pathophysiology of spondylolysis is still uncertain. Two theories have been proposed—underlying dysplastic pars interarticularis versus repetitive microtrauma resulting in stress factors are the two proposed underlying mechanism. If patients are genetically predisposed, underlying dysplasia probably contributes to the pathology, while microtrauma triggers the actual defect.2 Most patients respond well with conservative management.
When evaluating for spondylolysis, AP, lateral, 45-degree right and left oblique views, and collimated lateral views of the lumbosacral spine should be obtained. With this five-view study, up to 96.5% of pars defect can be identified.
In the general population, if spondylolysis is suspected and radiographs are negative, magnetic resonance imaging, computed tomography, and/or single-photon emission computed tomography bone scintigraphy can be used for further evaluation.4,5 In this case, the diagnosis was made based on radiographic imaging, and the patient was discharged with a scheduled follow-up with an orthopedic surgeon.
Dr Salama is a resident of radiology, resident of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Belfi is an assistant professor of radiology, Weill Cornell Medical College New York; and an assistant attending radiologist, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
A 25-year-old man with no significant past medical history presented with low-back pain that radiated down into his right thigh. The patient stated the pain began 1 week earlier when he was lifting weights and had increased in severity to the point where he was no longer able to walk or stand up straight. He had taken nonprescription nonsteroidal anti-inflammatory drugs but received no significant relief.
Radiographs of the lumbosacral spine were obtained; representative anteroposterior (AP) and lateral images are shown above (Figures 1 and 2).
The lateral view of the lumbar spine demonstrates mild anterolisthesis of L5 on S1 with the posterior cortex of L5 (white arrow, Figure 3) anterior to the posterior cortex of S1 (red arrow, Figure 3). Normally, the posterior cortices of the adjacent vertebral bodies should align. Lucency is also noted in the region of the pars interarticularis (white asterisk, Figure 3). The combination of anterolisthesis and this lucency in a young patient suggests the diagnosis of spondylolysis (pars defect).
The pathophysiology of spondylolysis is still uncertain. Two theories have been proposed—underlying dysplastic pars interarticularis versus repetitive microtrauma resulting in stress factors are the two proposed underlying mechanism. If patients are genetically predisposed, underlying dysplasia probably contributes to the pathology, while microtrauma triggers the actual defect.2 Most patients respond well with conservative management.
When evaluating for spondylolysis, AP, lateral, 45-degree right and left oblique views, and collimated lateral views of the lumbosacral spine should be obtained. With this five-view study, up to 96.5% of pars defect can be identified.
In the general population, if spondylolysis is suspected and radiographs are negative, magnetic resonance imaging, computed tomography, and/or single-photon emission computed tomography bone scintigraphy can be used for further evaluation.4,5 In this case, the diagnosis was made based on radiographic imaging, and the patient was discharged with a scheduled follow-up with an orthopedic surgeon.
Dr Salama is a resident of radiology, resident of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Belfi is an assistant professor of radiology, Weill Cornell Medical College New York; and an assistant attending radiologist, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976). 2006;31(24):E907-E910. doi:10.1097/01.brs.0000245947.31473.0a.
- Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst. 2013;29(2):209-216. doi:10.1007/s00381-012-1942-2.
- Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984;153(3):627-629.
- Saraste H, Nilsson B, Broström LA, et al. Relationship between radiological and clinical variables in spondylolysis. Int Orthop. 1984;8(3):163-174. doi:10.1007/BF00269912.
- Lee JH, Ehara S, Tamakawa Y, Shimamura T. Spondylolysis of the upper lumbar spine: Radiological features. Clin Imaging. 1999;23(6):389-393. doi:10.1016/S0899-7071(99)00158-8.
- Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976). 2006;31(24):E907-E910. doi:10.1097/01.brs.0000245947.31473.0a.
- Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst. 2013;29(2):209-216. doi:10.1007/s00381-012-1942-2.
- Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984;153(3):627-629.
- Saraste H, Nilsson B, Broström LA, et al. Relationship between radiological and clinical variables in spondylolysis. Int Orthop. 1984;8(3):163-174. doi:10.1007/BF00269912.
- Lee JH, Ehara S, Tamakawa Y, Shimamura T. Spondylolysis of the upper lumbar spine: Radiological features. Clin Imaging. 1999;23(6):389-393. doi:10.1016/S0899-7071(99)00158-8.
Readability of Sports Medicine–Related Patient Education Materials From the American Academy of Orthopaedic Surgeons and the American Orthopaedic Society for Sports Medicine
Genetics and Other Factors May Help Senior Athletes Preserve Well-Functioning Hips Despite Reported Abnormalities
NEW ORLEANS—Genetics, cartilage type, and other factors may help senior athletes maintain well-functioning hips and stave off osteoarthritis even when radiographic results indicate abnormalities, according to research presented at the 2014 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
It is not known whether morphological abnormalities of the hip are compatible with life-long hip function and avoidance of osteoarthritis. Lucas Anderson, MD, from the University of Utah in Salt Lake City, and colleagues sought to investigate the prevalence of radiographic findings consistent with dysplasia and femoroacetabular impingement in senior athletes with well-functioning hips.
A total of 546 senior athletes (55% men, 45% women), average age 67 years (range 50 to 91) participated in this study. Two orthopedic surgeons independently evaluated 1,087 hips (excluding hip fractures) for radiographic signs of dysplasia and femoroacetabular impingement. Alpha angle was measured on frog-lateral and anteroposterior radiographs. Lateral center edge angle, acetabular index, and crossover sign were measured on anteroposterior films. Radiographic interpretations were averaged between 2 observers to assess prevalence of dysplasia, femoroacetabular impingement, and osteoarthritis. Cam femoroacetabular impingement was noted if the alpha angle was 50° or greater on either radiograph. Pincer femoroacetabular impingement was noted if lateral center edge angle was greater than 39°, acetabular index was less than 0°, and/or a positive crossover sign was detected. Dysplasia was noted if center edge angle was less than 20° and/or acetabular index was greater than 10°. A chi-squared analysis was used to assess for associations between osteoarthritis (Tönnis grade 2-3) and dysplasia and femoroacetabular impingement. Dysplasia and femoroacetabular impingement were then analyzed using a mixed-effect logistic regression model.
Nine percent of hips (99) had radiographic evidence for dysplasia; 3% (28) had a lateral center edge angle that was less than 20° and 8% (89) had an acetabular index that was greater than 10°. Just over 80% of hips had radiographic evidence of femoroacetabular impingement; 67% had isolated cam, 8% isolated pincer impingement, and 24% of hips had mixed femoroacetabular impingement. Osteoarthritis was present in 17% of hips; 93% of hips with osteoarthritis also had radiographic femoroacetabular impingement and 10% dysplasia. Hips with osteoarthritis were more likely to have radiographic evidence of femoroacetabular impingement (odds ratio = 3.7). However, 80% of the hips with findings of femoroacetabular impingement had no evidence of osteoarthritis despite the athletes’ age and lifelong activity levels. Femoroacetabular impingement was more prevalent in males than females (odds ratio = 10.7).
While the data suggest that senior athletes with femoroacetabular impingement are at a greater risk for having radiographic evidence of osteoarthritis, a substantial portion of the senior athletes in this study did not have osteoarthritis. While femoroacetabular impingement and dysplasia have historically been associated with development of early osteoarthritis, this study suggests that there may be other factors, such as genetics and cartilage type, which may play a joint-preserving role despite presence of pathomorphology in this series of high-functioning senior athletes.
NEW ORLEANS—Genetics, cartilage type, and other factors may help senior athletes maintain well-functioning hips and stave off osteoarthritis even when radiographic results indicate abnormalities, according to research presented at the 2014 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
It is not known whether morphological abnormalities of the hip are compatible with life-long hip function and avoidance of osteoarthritis. Lucas Anderson, MD, from the University of Utah in Salt Lake City, and colleagues sought to investigate the prevalence of radiographic findings consistent with dysplasia and femoroacetabular impingement in senior athletes with well-functioning hips.
A total of 546 senior athletes (55% men, 45% women), average age 67 years (range 50 to 91) participated in this study. Two orthopedic surgeons independently evaluated 1,087 hips (excluding hip fractures) for radiographic signs of dysplasia and femoroacetabular impingement. Alpha angle was measured on frog-lateral and anteroposterior radiographs. Lateral center edge angle, acetabular index, and crossover sign were measured on anteroposterior films. Radiographic interpretations were averaged between 2 observers to assess prevalence of dysplasia, femoroacetabular impingement, and osteoarthritis. Cam femoroacetabular impingement was noted if the alpha angle was 50° or greater on either radiograph. Pincer femoroacetabular impingement was noted if lateral center edge angle was greater than 39°, acetabular index was less than 0°, and/or a positive crossover sign was detected. Dysplasia was noted if center edge angle was less than 20° and/or acetabular index was greater than 10°. A chi-squared analysis was used to assess for associations between osteoarthritis (Tönnis grade 2-3) and dysplasia and femoroacetabular impingement. Dysplasia and femoroacetabular impingement were then analyzed using a mixed-effect logistic regression model.
Nine percent of hips (99) had radiographic evidence for dysplasia; 3% (28) had a lateral center edge angle that was less than 20° and 8% (89) had an acetabular index that was greater than 10°. Just over 80% of hips had radiographic evidence of femoroacetabular impingement; 67% had isolated cam, 8% isolated pincer impingement, and 24% of hips had mixed femoroacetabular impingement. Osteoarthritis was present in 17% of hips; 93% of hips with osteoarthritis also had radiographic femoroacetabular impingement and 10% dysplasia. Hips with osteoarthritis were more likely to have radiographic evidence of femoroacetabular impingement (odds ratio = 3.7). However, 80% of the hips with findings of femoroacetabular impingement had no evidence of osteoarthritis despite the athletes’ age and lifelong activity levels. Femoroacetabular impingement was more prevalent in males than females (odds ratio = 10.7).
While the data suggest that senior athletes with femoroacetabular impingement are at a greater risk for having radiographic evidence of osteoarthritis, a substantial portion of the senior athletes in this study did not have osteoarthritis. While femoroacetabular impingement and dysplasia have historically been associated with development of early osteoarthritis, this study suggests that there may be other factors, such as genetics and cartilage type, which may play a joint-preserving role despite presence of pathomorphology in this series of high-functioning senior athletes.
NEW ORLEANS—Genetics, cartilage type, and other factors may help senior athletes maintain well-functioning hips and stave off osteoarthritis even when radiographic results indicate abnormalities, according to research presented at the 2014 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
It is not known whether morphological abnormalities of the hip are compatible with life-long hip function and avoidance of osteoarthritis. Lucas Anderson, MD, from the University of Utah in Salt Lake City, and colleagues sought to investigate the prevalence of radiographic findings consistent with dysplasia and femoroacetabular impingement in senior athletes with well-functioning hips.
A total of 546 senior athletes (55% men, 45% women), average age 67 years (range 50 to 91) participated in this study. Two orthopedic surgeons independently evaluated 1,087 hips (excluding hip fractures) for radiographic signs of dysplasia and femoroacetabular impingement. Alpha angle was measured on frog-lateral and anteroposterior radiographs. Lateral center edge angle, acetabular index, and crossover sign were measured on anteroposterior films. Radiographic interpretations were averaged between 2 observers to assess prevalence of dysplasia, femoroacetabular impingement, and osteoarthritis. Cam femoroacetabular impingement was noted if the alpha angle was 50° or greater on either radiograph. Pincer femoroacetabular impingement was noted if lateral center edge angle was greater than 39°, acetabular index was less than 0°, and/or a positive crossover sign was detected. Dysplasia was noted if center edge angle was less than 20° and/or acetabular index was greater than 10°. A chi-squared analysis was used to assess for associations between osteoarthritis (Tönnis grade 2-3) and dysplasia and femoroacetabular impingement. Dysplasia and femoroacetabular impingement were then analyzed using a mixed-effect logistic regression model.
Nine percent of hips (99) had radiographic evidence for dysplasia; 3% (28) had a lateral center edge angle that was less than 20° and 8% (89) had an acetabular index that was greater than 10°. Just over 80% of hips had radiographic evidence of femoroacetabular impingement; 67% had isolated cam, 8% isolated pincer impingement, and 24% of hips had mixed femoroacetabular impingement. Osteoarthritis was present in 17% of hips; 93% of hips with osteoarthritis also had radiographic femoroacetabular impingement and 10% dysplasia. Hips with osteoarthritis were more likely to have radiographic evidence of femoroacetabular impingement (odds ratio = 3.7). However, 80% of the hips with findings of femoroacetabular impingement had no evidence of osteoarthritis despite the athletes’ age and lifelong activity levels. Femoroacetabular impingement was more prevalent in males than females (odds ratio = 10.7).
While the data suggest that senior athletes with femoroacetabular impingement are at a greater risk for having radiographic evidence of osteoarthritis, a substantial portion of the senior athletes in this study did not have osteoarthritis. While femoroacetabular impingement and dysplasia have historically been associated with development of early osteoarthritis, this study suggests that there may be other factors, such as genetics and cartilage type, which may play a joint-preserving role despite presence of pathomorphology in this series of high-functioning senior athletes.
Extreme Sports Provide Thrills But Also Increased Incidence of Head and Neck Injuries
NEW ORLEANS—Participation in extreme sports offers excitement not found in traditional team sports and also comes with significant risk, according to researchers reporting at the 2014 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
Since their conception during the mid-1970s, international participation in extreme sports has steadily grown. While many traditional sports have declined in participation, skateboarding has surged 49% to 14 million US participants and snowboarding now claims 7.2 million participants, up 51% from 1999. The recent death of extreme snowmobiler Caleb Moore at the 2013 Winter X games has demonstrated the serious risks associated with these sports.
In a presentation titled “Incidence of Head and Neck Injuries in Extreme Sports,” Vinay K. Sharma, MD, summarized the findings of a first-of-its-kind study. Dr. Sharma and colleagues reviewed 2000–2011 National Electronic Injury Surveillance System (NEISS) data for seven popular sports featured in the Winter and Summer X Games: surfing, mountain biking, motocross, skateboarding, snowboarding, snowmobiling, and snow skiing. Data from the NEISS database was collected for each individual sport per year and type of head and neck injury. Cumulative data for overall incidence and injuries over entire 11-year period was then calculated. National estimates were based off NEISS weighted calculations using US census data.
Of the over 4 million injuries reported for extreme sports participants between 2000–2011, 11.3% were head and neck injuries. Of all head and neck injuries reported in extreme sports, 83% were head injuries and 17% neck injuries. The 4 sports with the highest total incidence of reported head and neck injuries were skateboarding (129,600), snowboarding (97,527), skiing (83,313), and motocross (78,236). Severe head and neck injuries (cervical or skull fracture) had a reported total incidence of 2.5% of extreme sports head and neck injuries. Although the incidence of extreme sports HNI increased from year 2000 (34,065) to 2010 (40,042), this trend is not consistent from year to year.
According to Dr. Sharma and colleagues, a greater awareness of the dangers associated with extreme sports offers an opportunity for sports medicine and orthopedic physicians to advocate for safer equipment, improved on-site medical care, and further research regarding extreme sports injuries.
NEW ORLEANS—Participation in extreme sports offers excitement not found in traditional team sports and also comes with significant risk, according to researchers reporting at the 2014 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
Since their conception during the mid-1970s, international participation in extreme sports has steadily grown. While many traditional sports have declined in participation, skateboarding has surged 49% to 14 million US participants and snowboarding now claims 7.2 million participants, up 51% from 1999. The recent death of extreme snowmobiler Caleb Moore at the 2013 Winter X games has demonstrated the serious risks associated with these sports.
In a presentation titled “Incidence of Head and Neck Injuries in Extreme Sports,” Vinay K. Sharma, MD, summarized the findings of a first-of-its-kind study. Dr. Sharma and colleagues reviewed 2000–2011 National Electronic Injury Surveillance System (NEISS) data for seven popular sports featured in the Winter and Summer X Games: surfing, mountain biking, motocross, skateboarding, snowboarding, snowmobiling, and snow skiing. Data from the NEISS database was collected for each individual sport per year and type of head and neck injury. Cumulative data for overall incidence and injuries over entire 11-year period was then calculated. National estimates were based off NEISS weighted calculations using US census data.
Of the over 4 million injuries reported for extreme sports participants between 2000–2011, 11.3% were head and neck injuries. Of all head and neck injuries reported in extreme sports, 83% were head injuries and 17% neck injuries. The 4 sports with the highest total incidence of reported head and neck injuries were skateboarding (129,600), snowboarding (97,527), skiing (83,313), and motocross (78,236). Severe head and neck injuries (cervical or skull fracture) had a reported total incidence of 2.5% of extreme sports head and neck injuries. Although the incidence of extreme sports HNI increased from year 2000 (34,065) to 2010 (40,042), this trend is not consistent from year to year.
According to Dr. Sharma and colleagues, a greater awareness of the dangers associated with extreme sports offers an opportunity for sports medicine and orthopedic physicians to advocate for safer equipment, improved on-site medical care, and further research regarding extreme sports injuries.
NEW ORLEANS—Participation in extreme sports offers excitement not found in traditional team sports and also comes with significant risk, according to researchers reporting at the 2014 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
Since their conception during the mid-1970s, international participation in extreme sports has steadily grown. While many traditional sports have declined in participation, skateboarding has surged 49% to 14 million US participants and snowboarding now claims 7.2 million participants, up 51% from 1999. The recent death of extreme snowmobiler Caleb Moore at the 2013 Winter X games has demonstrated the serious risks associated with these sports.
In a presentation titled “Incidence of Head and Neck Injuries in Extreme Sports,” Vinay K. Sharma, MD, summarized the findings of a first-of-its-kind study. Dr. Sharma and colleagues reviewed 2000–2011 National Electronic Injury Surveillance System (NEISS) data for seven popular sports featured in the Winter and Summer X Games: surfing, mountain biking, motocross, skateboarding, snowboarding, snowmobiling, and snow skiing. Data from the NEISS database was collected for each individual sport per year and type of head and neck injury. Cumulative data for overall incidence and injuries over entire 11-year period was then calculated. National estimates were based off NEISS weighted calculations using US census data.
Of the over 4 million injuries reported for extreme sports participants between 2000–2011, 11.3% were head and neck injuries. Of all head and neck injuries reported in extreme sports, 83% were head injuries and 17% neck injuries. The 4 sports with the highest total incidence of reported head and neck injuries were skateboarding (129,600), snowboarding (97,527), skiing (83,313), and motocross (78,236). Severe head and neck injuries (cervical or skull fracture) had a reported total incidence of 2.5% of extreme sports head and neck injuries. Although the incidence of extreme sports HNI increased from year 2000 (34,065) to 2010 (40,042), this trend is not consistent from year to year.
According to Dr. Sharma and colleagues, a greater awareness of the dangers associated with extreme sports offers an opportunity for sports medicine and orthopedic physicians to advocate for safer equipment, improved on-site medical care, and further research regarding extreme sports injuries.
Physicians are major source for frequent opioid misusers
Most people who misuse opioid pain relievers cite friends and relatives as their sources for the drugs, but more of the people who misuse these agents most often – those who take them from 200 to 365 days of the year – obtain their opioids from physicians’ prescriptions than from any other single source, according to a report published online March 3 in JAMA Internal Medicine.
"These results underscore the need for interventions targeting prescribing behaviors, in addition to those targeting medication sharing, selling, and diversion," the report’s authors warned.
It is a commonly cited statistic that most people who misuse opioid pain relievers obtain the drugs from family and friends for free, so many interventions to stop such misuse focus on patients. But few studies have examined whether the source of these drugs, and thus an appropriate target for interventions, might differ according to the frequency of misuse.
To study this issue, researchers analyzed data from the National Survey on Drug Use and Health, an annual survey that provides information on drug use among U.S. residents aged 12 years and older.
Survey data from 2008 through 2011 identified 11,018,735 respondents who said they misused an opioid pain reliever either by obtaining the drug without a prescription or by getting a prescription but taking the drug strictly because of the feeling or experience it provided. The source of the drug differed according to the frequency of use: As the days of use increased, the likelihood that the user obtained the drug from a friend or family member decreased, and the likelihood that he or she obtained the drug from a physician rose, said Christopher M. Jones, Pharm.D., and his associates at the National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta.
Among people who misused opioids only 1-29 days of the year, 54.4% said they got them from a friend or relative for free – the most popular source. In contrast, only 18% of this patient group said the opioids were prescribed by one or more physicians.
But the percentage of patients who obtained misused opioids through physician prescriptions steadily rose with increasing use.
Among those who misused the drugs 200-365 days a year, the top source was physician prescriptions, 27.3% of users, followed by opioids obtained for free from friends or relatives, 26.4%. A total of 23.2% of users said they bought their opioids from friends or relatives, while another 15% of frequent users bought their drugs from dealers or strangers.
"This pattern is similar to that of patients in opioid treatment programs, who cite dealers and physicians as frequent sources," Dr. Jones and his associates said in a Research Letter to the Editor (JAMA Intern. Med. 2014 March 3 [doi:10.1001/jamainternmed.2013.12809]).
"Many abusers of opioid pain relievers are going directly to doctors for their drugs," CDC Director Tom Frieden commented in a statement. "Health care providers need to screen for abuse risk and prescribe judiciously by checking past records in state prescription drug monitoring programs. It’s time we stop the source and treat the troubled."
"The essential steps health care providers can take to curb this serious health problem include more judicious prescribing, use of prescription-drug–monitoring programs, and screening patients for abuse before prescribing opioids," the study authors noted.
The federal government is encouraging the development of abuse-deterrent opioid formulations, the CDC noted, and requiring companies that make extended-release and long-acting opioids to offer prescribers educational programs about the understanding the risks of opioid therapy; choosing, managing, and monitoring patients; and counseling patients on safe use of opioids.
The Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
Most people who misuse opioid pain relievers cite friends and relatives as their sources for the drugs, but more of the people who misuse these agents most often – those who take them from 200 to 365 days of the year – obtain their opioids from physicians’ prescriptions than from any other single source, according to a report published online March 3 in JAMA Internal Medicine.
"These results underscore the need for interventions targeting prescribing behaviors, in addition to those targeting medication sharing, selling, and diversion," the report’s authors warned.
It is a commonly cited statistic that most people who misuse opioid pain relievers obtain the drugs from family and friends for free, so many interventions to stop such misuse focus on patients. But few studies have examined whether the source of these drugs, and thus an appropriate target for interventions, might differ according to the frequency of misuse.
To study this issue, researchers analyzed data from the National Survey on Drug Use and Health, an annual survey that provides information on drug use among U.S. residents aged 12 years and older.
Survey data from 2008 through 2011 identified 11,018,735 respondents who said they misused an opioid pain reliever either by obtaining the drug without a prescription or by getting a prescription but taking the drug strictly because of the feeling or experience it provided. The source of the drug differed according to the frequency of use: As the days of use increased, the likelihood that the user obtained the drug from a friend or family member decreased, and the likelihood that he or she obtained the drug from a physician rose, said Christopher M. Jones, Pharm.D., and his associates at the National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta.
Among people who misused opioids only 1-29 days of the year, 54.4% said they got them from a friend or relative for free – the most popular source. In contrast, only 18% of this patient group said the opioids were prescribed by one or more physicians.
But the percentage of patients who obtained misused opioids through physician prescriptions steadily rose with increasing use.
Among those who misused the drugs 200-365 days a year, the top source was physician prescriptions, 27.3% of users, followed by opioids obtained for free from friends or relatives, 26.4%. A total of 23.2% of users said they bought their opioids from friends or relatives, while another 15% of frequent users bought their drugs from dealers or strangers.
"This pattern is similar to that of patients in opioid treatment programs, who cite dealers and physicians as frequent sources," Dr. Jones and his associates said in a Research Letter to the Editor (JAMA Intern. Med. 2014 March 3 [doi:10.1001/jamainternmed.2013.12809]).
"Many abusers of opioid pain relievers are going directly to doctors for their drugs," CDC Director Tom Frieden commented in a statement. "Health care providers need to screen for abuse risk and prescribe judiciously by checking past records in state prescription drug monitoring programs. It’s time we stop the source and treat the troubled."
"The essential steps health care providers can take to curb this serious health problem include more judicious prescribing, use of prescription-drug–monitoring programs, and screening patients for abuse before prescribing opioids," the study authors noted.
The federal government is encouraging the development of abuse-deterrent opioid formulations, the CDC noted, and requiring companies that make extended-release and long-acting opioids to offer prescribers educational programs about the understanding the risks of opioid therapy; choosing, managing, and monitoring patients; and counseling patients on safe use of opioids.
The Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
Most people who misuse opioid pain relievers cite friends and relatives as their sources for the drugs, but more of the people who misuse these agents most often – those who take them from 200 to 365 days of the year – obtain their opioids from physicians’ prescriptions than from any other single source, according to a report published online March 3 in JAMA Internal Medicine.
"These results underscore the need for interventions targeting prescribing behaviors, in addition to those targeting medication sharing, selling, and diversion," the report’s authors warned.
It is a commonly cited statistic that most people who misuse opioid pain relievers obtain the drugs from family and friends for free, so many interventions to stop such misuse focus on patients. But few studies have examined whether the source of these drugs, and thus an appropriate target for interventions, might differ according to the frequency of misuse.
To study this issue, researchers analyzed data from the National Survey on Drug Use and Health, an annual survey that provides information on drug use among U.S. residents aged 12 years and older.
Survey data from 2008 through 2011 identified 11,018,735 respondents who said they misused an opioid pain reliever either by obtaining the drug without a prescription or by getting a prescription but taking the drug strictly because of the feeling or experience it provided. The source of the drug differed according to the frequency of use: As the days of use increased, the likelihood that the user obtained the drug from a friend or family member decreased, and the likelihood that he or she obtained the drug from a physician rose, said Christopher M. Jones, Pharm.D., and his associates at the National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta.
Among people who misused opioids only 1-29 days of the year, 54.4% said they got them from a friend or relative for free – the most popular source. In contrast, only 18% of this patient group said the opioids were prescribed by one or more physicians.
But the percentage of patients who obtained misused opioids through physician prescriptions steadily rose with increasing use.
Among those who misused the drugs 200-365 days a year, the top source was physician prescriptions, 27.3% of users, followed by opioids obtained for free from friends or relatives, 26.4%. A total of 23.2% of users said they bought their opioids from friends or relatives, while another 15% of frequent users bought their drugs from dealers or strangers.
"This pattern is similar to that of patients in opioid treatment programs, who cite dealers and physicians as frequent sources," Dr. Jones and his associates said in a Research Letter to the Editor (JAMA Intern. Med. 2014 March 3 [doi:10.1001/jamainternmed.2013.12809]).
"Many abusers of opioid pain relievers are going directly to doctors for their drugs," CDC Director Tom Frieden commented in a statement. "Health care providers need to screen for abuse risk and prescribe judiciously by checking past records in state prescription drug monitoring programs. It’s time we stop the source and treat the troubled."
"The essential steps health care providers can take to curb this serious health problem include more judicious prescribing, use of prescription-drug–monitoring programs, and screening patients for abuse before prescribing opioids," the study authors noted.
The federal government is encouraging the development of abuse-deterrent opioid formulations, the CDC noted, and requiring companies that make extended-release and long-acting opioids to offer prescribers educational programs about the understanding the risks of opioid therapy; choosing, managing, and monitoring patients; and counseling patients on safe use of opioids.
The Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
FROM JAMA INTERNAL MEDICINE
Major Finding: Opioid pain relievers were prescribed by a physician for 18% of people who used them only 1-29 days of the year, but that percentage steadily rose with increasing use, so that 27.3% of people who used the drugs 200-365 days/year obtained them via physician prescription, more than any other single source.
Data Source: An analysis of data on 11,018,735 survey respondents aged 12 years and older who reported misusing opioid pain relievers during a 4-year period.
Disclosures: The Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
Emergency Imaging: Wrist injury in a woman with multiple sclerosis
Case
A 54-year-old woman with history of active multiple sclerosis (MS) presented to the ED with left wrist pain after sustaining a mechanical fall 9 days earlier. On physical examination, the patient had minimal left wrist swelling with circumferential tenderness to palpation at both the distal radius and distal ulna, as well as snuff-box tenderness. Posterioranterior and lateral radiographs of the wrist were obtained (Figures 1 and 2).
|
|
|
What is the diagnosis?
Radiographs of the left wrist demonstrated cortically based sclerosis of the ulna, triquetrum, and hamate; no fracture or dislocation was seen. This pattern of cortical bone formation, described as the “dripping candle wax” sign, is characteristic for melorheostosis, a rare nonhereditary mixed sclerosing bone dysplasia.
|
|
|
Sclerosing bone dysplasias include a wide range of both hereditary and nonhereditary skeletal abnormalities that result from a disturbance in the bone ossification pathway. These dysplasias can be categorized as disruptions in endochondral bone formation, disruptions in intramembranous bone formation, or mixed. First described by Leri and Joanny in 1922, there have been approximately 400 reported cases of melorheostosis since its initial identification.1,2
In melorheostosis, the distribution of lesions follows sclerotomes, skeletal regions supplied by a single spinal sensory nerve. The condition manifests as cortical and medullary hyperostosis involving one side of the bone (eg, medial or lateral) with a clear distinction between affected bone and the adjacent normal bone. As in this case, the radiographic appearance of melorheostosis is almost always sufficient for diagnosis.
Melorheostosis has no gender predilection and is usually diagnosed in late adolescence or early adulthood.5 While the etiology is unknown, genetic analyses have found a common loss-of-function mutation on chromosome 12q (LEMD3) associated with several sclerosing bone dysplasias, including melorheostosis, suggesting a common etiology.3 In addition, since melorheostosis typically has a sclerotomal distribution, some theories postulate that it represents an acquired defect of the spinal sensory nerves.1
Patients generally present with pain, stiffness, and occasionally joint swelling in the involved regions. Although any bone can be involved, the extremities are most often affected, with the disease frequently polyostotic, but rarely bilateral.4,5 In hyperostosis involving a joint, muscular atrophy, tendon and ligament shortening, and muscle contractures may be seen, which can limit range of motion.6 Some cases of leg-length discrepancy have also been described. Skeletal lesions may progress, but there is no reported risk of pathological fracture or malignant degeneration.7
Treatment is dependent on the patient’s age, location of involved bone, and specific symptoms. The major goals of treatment are pain relief and preserving or restoring full range of motion. Therapy is generally focused on conservative techniques such as analgesics, braces, and physical therapy. Occasionally, surgical treatment is necessary, including soft-tissue release, fasciotomy, tendon lengthening, and arthroplasty.8
In the ED setting, it is important to recognize melorheostosis as the source of pain as this condition may be confused with osseous neoplasm. Based on this patient’s underlying partially treated MS flare and the potential for recurring fall, admission was recommended for intravenous corticosteroids. The patient, however, was only amenable to a wrist splint and refused further treatment.
Dr Escalon is second-year postgraduate resident, the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Dr Loftus is an assistant professor of radiology, New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Ihde LL, Forrester DM, Gottsegen CJ, Masih S, et al. Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis. Radiographics. 2011;31(7):1865-1882.
- Suresh S, Muthukumar T, Saifuddin A. Classical and unusual imaging appearances of melorheostosis. Clin Radiol. 2010;65(8):593-600.
- Bansal A. The dripping candle wax sign. Radiology. 2008;246(2):638-640.
- Birtane M, Eryavuz M, Unalan H, Tüzün F. Melorheostosis: report of a new case with linear seleroderma. Clin Rheumatol. 1998;17(6):543-545.
- Siegel A, Williams H. Linear scleroderma and melorheostosis. Br J Radiol. 1992;65(771):266-268.
- Tekin L, Akarsu S, Durmuş O, Kiralp MZ. Melorheostosis in the hand and forearm. Am J Phys Med Rehabil. 2012;91(1):96.
- Soffa DJ, Sire DJ, Dodson JH. Melorheostosis with Linear Sclerodermatous Skin Changes. Radiology. 1975;114(3):577,578.
- Jain VK, Arya RK, Bharadwaj M, Kumar S. Melorheostosis: clinicopathological features, diagnosis, and management. Orthopedics. 2009;32(7):512
Case
A 54-year-old woman with history of active multiple sclerosis (MS) presented to the ED with left wrist pain after sustaining a mechanical fall 9 days earlier. On physical examination, the patient had minimal left wrist swelling with circumferential tenderness to palpation at both the distal radius and distal ulna, as well as snuff-box tenderness. Posterioranterior and lateral radiographs of the wrist were obtained (Figures 1 and 2).
|
|
|
What is the diagnosis?
Radiographs of the left wrist demonstrated cortically based sclerosis of the ulna, triquetrum, and hamate; no fracture or dislocation was seen. This pattern of cortical bone formation, described as the “dripping candle wax” sign, is characteristic for melorheostosis, a rare nonhereditary mixed sclerosing bone dysplasia.
|
|
|
Sclerosing bone dysplasias include a wide range of both hereditary and nonhereditary skeletal abnormalities that result from a disturbance in the bone ossification pathway. These dysplasias can be categorized as disruptions in endochondral bone formation, disruptions in intramembranous bone formation, or mixed. First described by Leri and Joanny in 1922, there have been approximately 400 reported cases of melorheostosis since its initial identification.1,2
In melorheostosis, the distribution of lesions follows sclerotomes, skeletal regions supplied by a single spinal sensory nerve. The condition manifests as cortical and medullary hyperostosis involving one side of the bone (eg, medial or lateral) with a clear distinction between affected bone and the adjacent normal bone. As in this case, the radiographic appearance of melorheostosis is almost always sufficient for diagnosis.
Melorheostosis has no gender predilection and is usually diagnosed in late adolescence or early adulthood.5 While the etiology is unknown, genetic analyses have found a common loss-of-function mutation on chromosome 12q (LEMD3) associated with several sclerosing bone dysplasias, including melorheostosis, suggesting a common etiology.3 In addition, since melorheostosis typically has a sclerotomal distribution, some theories postulate that it represents an acquired defect of the spinal sensory nerves.1
Patients generally present with pain, stiffness, and occasionally joint swelling in the involved regions. Although any bone can be involved, the extremities are most often affected, with the disease frequently polyostotic, but rarely bilateral.4,5 In hyperostosis involving a joint, muscular atrophy, tendon and ligament shortening, and muscle contractures may be seen, which can limit range of motion.6 Some cases of leg-length discrepancy have also been described. Skeletal lesions may progress, but there is no reported risk of pathological fracture or malignant degeneration.7
Treatment is dependent on the patient’s age, location of involved bone, and specific symptoms. The major goals of treatment are pain relief and preserving or restoring full range of motion. Therapy is generally focused on conservative techniques such as analgesics, braces, and physical therapy. Occasionally, surgical treatment is necessary, including soft-tissue release, fasciotomy, tendon lengthening, and arthroplasty.8
In the ED setting, it is important to recognize melorheostosis as the source of pain as this condition may be confused with osseous neoplasm. Based on this patient’s underlying partially treated MS flare and the potential for recurring fall, admission was recommended for intravenous corticosteroids. The patient, however, was only amenable to a wrist splint and refused further treatment.
Dr Escalon is second-year postgraduate resident, the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Dr Loftus is an assistant professor of radiology, New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Case
A 54-year-old woman with history of active multiple sclerosis (MS) presented to the ED with left wrist pain after sustaining a mechanical fall 9 days earlier. On physical examination, the patient had minimal left wrist swelling with circumferential tenderness to palpation at both the distal radius and distal ulna, as well as snuff-box tenderness. Posterioranterior and lateral radiographs of the wrist were obtained (Figures 1 and 2).
|
|
|
What is the diagnosis?
Radiographs of the left wrist demonstrated cortically based sclerosis of the ulna, triquetrum, and hamate; no fracture or dislocation was seen. This pattern of cortical bone formation, described as the “dripping candle wax” sign, is characteristic for melorheostosis, a rare nonhereditary mixed sclerosing bone dysplasia.
|
|
|
Sclerosing bone dysplasias include a wide range of both hereditary and nonhereditary skeletal abnormalities that result from a disturbance in the bone ossification pathway. These dysplasias can be categorized as disruptions in endochondral bone formation, disruptions in intramembranous bone formation, or mixed. First described by Leri and Joanny in 1922, there have been approximately 400 reported cases of melorheostosis since its initial identification.1,2
In melorheostosis, the distribution of lesions follows sclerotomes, skeletal regions supplied by a single spinal sensory nerve. The condition manifests as cortical and medullary hyperostosis involving one side of the bone (eg, medial or lateral) with a clear distinction between affected bone and the adjacent normal bone. As in this case, the radiographic appearance of melorheostosis is almost always sufficient for diagnosis.
Melorheostosis has no gender predilection and is usually diagnosed in late adolescence or early adulthood.5 While the etiology is unknown, genetic analyses have found a common loss-of-function mutation on chromosome 12q (LEMD3) associated with several sclerosing bone dysplasias, including melorheostosis, suggesting a common etiology.3 In addition, since melorheostosis typically has a sclerotomal distribution, some theories postulate that it represents an acquired defect of the spinal sensory nerves.1
Patients generally present with pain, stiffness, and occasionally joint swelling in the involved regions. Although any bone can be involved, the extremities are most often affected, with the disease frequently polyostotic, but rarely bilateral.4,5 In hyperostosis involving a joint, muscular atrophy, tendon and ligament shortening, and muscle contractures may be seen, which can limit range of motion.6 Some cases of leg-length discrepancy have also been described. Skeletal lesions may progress, but there is no reported risk of pathological fracture or malignant degeneration.7
Treatment is dependent on the patient’s age, location of involved bone, and specific symptoms. The major goals of treatment are pain relief and preserving or restoring full range of motion. Therapy is generally focused on conservative techniques such as analgesics, braces, and physical therapy. Occasionally, surgical treatment is necessary, including soft-tissue release, fasciotomy, tendon lengthening, and arthroplasty.8
In the ED setting, it is important to recognize melorheostosis as the source of pain as this condition may be confused with osseous neoplasm. Based on this patient’s underlying partially treated MS flare and the potential for recurring fall, admission was recommended for intravenous corticosteroids. The patient, however, was only amenable to a wrist splint and refused further treatment.
Dr Escalon is second-year postgraduate resident, the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Dr Loftus is an assistant professor of radiology, New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Ihde LL, Forrester DM, Gottsegen CJ, Masih S, et al. Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis. Radiographics. 2011;31(7):1865-1882.
- Suresh S, Muthukumar T, Saifuddin A. Classical and unusual imaging appearances of melorheostosis. Clin Radiol. 2010;65(8):593-600.
- Bansal A. The dripping candle wax sign. Radiology. 2008;246(2):638-640.
- Birtane M, Eryavuz M, Unalan H, Tüzün F. Melorheostosis: report of a new case with linear seleroderma. Clin Rheumatol. 1998;17(6):543-545.
- Siegel A, Williams H. Linear scleroderma and melorheostosis. Br J Radiol. 1992;65(771):266-268.
- Tekin L, Akarsu S, Durmuş O, Kiralp MZ. Melorheostosis in the hand and forearm. Am J Phys Med Rehabil. 2012;91(1):96.
- Soffa DJ, Sire DJ, Dodson JH. Melorheostosis with Linear Sclerodermatous Skin Changes. Radiology. 1975;114(3):577,578.
- Jain VK, Arya RK, Bharadwaj M, Kumar S. Melorheostosis: clinicopathological features, diagnosis, and management. Orthopedics. 2009;32(7):512
- Ihde LL, Forrester DM, Gottsegen CJ, Masih S, et al. Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis. Radiographics. 2011;31(7):1865-1882.
- Suresh S, Muthukumar T, Saifuddin A. Classical and unusual imaging appearances of melorheostosis. Clin Radiol. 2010;65(8):593-600.
- Bansal A. The dripping candle wax sign. Radiology. 2008;246(2):638-640.
- Birtane M, Eryavuz M, Unalan H, Tüzün F. Melorheostosis: report of a new case with linear seleroderma. Clin Rheumatol. 1998;17(6):543-545.
- Siegel A, Williams H. Linear scleroderma and melorheostosis. Br J Radiol. 1992;65(771):266-268.
- Tekin L, Akarsu S, Durmuş O, Kiralp MZ. Melorheostosis in the hand and forearm. Am J Phys Med Rehabil. 2012;91(1):96.
- Soffa DJ, Sire DJ, Dodson JH. Melorheostosis with Linear Sclerodermatous Skin Changes. Radiology. 1975;114(3):577,578.
- Jain VK, Arya RK, Bharadwaj M, Kumar S. Melorheostosis: clinicopathological features, diagnosis, and management. Orthopedics. 2009;32(7):512
Challenges in Sports Medicine and Orthopedics
Dr Patterson, editor of “Challenges in Sports Medicine and Orthopedics,” is a sports medicine physician at Florida Sports Injury in Clermont, Florida. Dr Patterson is board certified in family medicine and spinal cord injury medicine, and is a member of the faculty of sports and exercise medicine of the Royal College of Surgeons in Ireland.
|
|
A 5-year-old boy presented to the ED after sustaining an injury to his left leg during play with a friend. He was unable to bear weight on the left foot and had a visible deformity to his lower extremity. The left foot was neurovascularly intact. Radiographs were completed (Figures 1 and 2).
What is your interpretation of the following radiographs?
Answer
|
|
The radiographs revealed a 20˚ anteriorly (apex posterior) angulated fracture through the metaphysis of the distal tibia and fibula. Angulated distal tibia fractures in adults are usually fixed with surgery; however, in children, displaced or angulated fractures to long bones such as the tibia stimulate a significant amount of growth. In treating pediatric patients, there is a greater amount of acceptable angulation the closer a fracture is to the end of bone.1
The patient in this case was placed in a long leg cast, and the fractures were reduced with three-point fixation technique. He remained in the cast for 5 weeks; thereafter, a below-the-knee orthopedic walking boot was placed for 3 weeks. The radiographs in Figures 3 and 4, taken 8 weeks after initiation of treatment, show a healed distal tibia and fibula fracture with an acceptable 7˚ of anterior angulation.
Dr Patterson, editor of “Challenges in Sports Medicine and Orthopedics,” is a sports medicine physician at Florida Sports Injury in Clermont, Florida. Dr Patterson is board certified in family medicine and spinal cord injury medicine, and is a member of the faculty of sports and exercise medicine of the Royal College of Surgeons in Ireland.
|
|
A 5-year-old boy presented to the ED after sustaining an injury to his left leg during play with a friend. He was unable to bear weight on the left foot and had a visible deformity to his lower extremity. The left foot was neurovascularly intact. Radiographs were completed (Figures 1 and 2).
What is your interpretation of the following radiographs?
Answer
|
|
The radiographs revealed a 20˚ anteriorly (apex posterior) angulated fracture through the metaphysis of the distal tibia and fibula. Angulated distal tibia fractures in adults are usually fixed with surgery; however, in children, displaced or angulated fractures to long bones such as the tibia stimulate a significant amount of growth. In treating pediatric patients, there is a greater amount of acceptable angulation the closer a fracture is to the end of bone.1
The patient in this case was placed in a long leg cast, and the fractures were reduced with three-point fixation technique. He remained in the cast for 5 weeks; thereafter, a below-the-knee orthopedic walking boot was placed for 3 weeks. The radiographs in Figures 3 and 4, taken 8 weeks after initiation of treatment, show a healed distal tibia and fibula fracture with an acceptable 7˚ of anterior angulation.
Dr Patterson, editor of “Challenges in Sports Medicine and Orthopedics,” is a sports medicine physician at Florida Sports Injury in Clermont, Florida. Dr Patterson is board certified in family medicine and spinal cord injury medicine, and is a member of the faculty of sports and exercise medicine of the Royal College of Surgeons in Ireland.
|
|
A 5-year-old boy presented to the ED after sustaining an injury to his left leg during play with a friend. He was unable to bear weight on the left foot and had a visible deformity to his lower extremity. The left foot was neurovascularly intact. Radiographs were completed (Figures 1 and 2).
What is your interpretation of the following radiographs?
Answer
|
|
The radiographs revealed a 20˚ anteriorly (apex posterior) angulated fracture through the metaphysis of the distal tibia and fibula. Angulated distal tibia fractures in adults are usually fixed with surgery; however, in children, displaced or angulated fractures to long bones such as the tibia stimulate a significant amount of growth. In treating pediatric patients, there is a greater amount of acceptable angulation the closer a fracture is to the end of bone.1
The patient in this case was placed in a long leg cast, and the fractures were reduced with three-point fixation technique. He remained in the cast for 5 weeks; thereafter, a below-the-knee orthopedic walking boot was placed for 3 weeks. The radiographs in Figures 3 and 4, taken 8 weeks after initiation of treatment, show a healed distal tibia and fibula fracture with an acceptable 7˚ of anterior angulation.
Military’s top doc: Medical lessons from Iraq saved lives in Boston bombings
Dr. Jonathan Woodson fielded e-mails in his Pentagon office last April from nervous colleagues in Boston.
His fellow vascular surgeons there wanted to know if more attacks were coming in the Marathon bombings. "When you work at the Pentagon, everyone thinks you have total situational awareness," said Dr. Woodson, assistant secretary of defense for health affairs.
His former colleagues, however, also wanted tips on how to care for bombing victims, and knew that military surgeons like Dr. Woodson had answers. Before President Obama tapped him in 2010 to become the military’s top doctor and oversee the Pentagon’s $50 billion-plus medical budget, he’d scrubbed in on battlefield cases in Kosovo, Iraq, and Afghanistan, in addition to performing his vascular surgery and administrative duties at Boston University and Boston Medical Center.
"We’ve been dealing with [improvised explosive device] injuries for over a decade," said Dr. Woodson, a brigadier general in the U.S. Army Reserve.
"The good news is, I had trained some of the folks who worked" on the bombing victims, and "they knew what my techniques were." Still, there were discussions about particular fixes; hypertrophic bone growths, infections, and other wound complications; and ways to help young people recover from devastating injuries. Many of the lessons came from Dr. Woodson’s military service.
The back-and-forth about the Boston attacks highlights how important it is for military and civilian physicians to cross-pollinate. In the past, "there’s always been a cadre of usually academic surgeons and physicians who maintain contact with the military. I think [it] is very important to formalize these connections. One of my strategic imperatives is to make sure we invest in partnerships [with] academic medical centers and key professional organizations" so that war innovations make it into civilian practice, and, in turn, are remembered for future conflicts.
"There’s the potential for this expertise to be lost," said Dr. Woodson, who was finishing a paper with two colleagues on the history of military medical innovations (Semin. Vasc. Surg. 2010;23:235-42) when he was nominated to serve as the secretary of defense’s principal adviser on health issues.
There’ve been many innovations in recent years, including the recognition that extensive fasciotomies can save badly damaged limbs.
At the start of Operation Iraqi Freedom in 2003, Dr. Woodson was in northern Kuwait running a makeshift U.S. military hospital out of a commandeered Kuwaiti facility. "As the troops went over the berm, we were taking the first wave of casualties," performing surgery in full chemical-attack gear. "Luckily," the incoming Scud missiles weren’t very accurate. It was a humbling experience," he said.
A young soldier was helicoptered in with a crushed leg after a hit to his armored vehicle. "We did an interposition graft to repair the popliteal artery," and, because of severe swelling, extensive fasciotomies to prevent compartment syndrome; the soldier still had his leg when he was airlifted out. Although not standard practice at the time, that case and others like it quickly demonstrated "the benefit of doing four-compartment fasciotomies early on to preserve limbs. Fasciotomies are now [used routinely] to address these injuries," Dr. Woodson said.
Tourniquets – which "prior to this conflict had a very bad reputation" – have proved their worth in recent battles as well, a lesson that helped save lives in Boston. Likewise, the military has learned the value of temporary vascular shunts, narrow tubing – most often Argyle carotid artery shunts – to bypass tears or reconnect severed vessels when there’s no time for a definitive repair. "We [also] came to understand very early on the need for aggressive blood replacement. Previously, protocols called for lactated Ringer’s or saline infusions, but we realized they just lead to massive soft tissue edema, increased lung water," and other problems, he said.
Currently, Dr. Woodson and his military colleagues are helping coach the rehabilitation of the Boston bombing victims. Rehab has seen "major advances in the military that are now finding their way into civilian practice," including improved prosthetics; better understanding of rehabilitation psychology; and recognition of subtle traumatic brain, eye, ear, and other injuries that can derail recovery efforts, he said.
"Because soldiers were athletes before [their injury], they often have aggressive goals in mind. Some want to return to swimming, surfing, or skiing. We try to meet those goals ... to get them fully reincorporated into the lives they want to lead"; adaptive sports programs help, among other things. It’s a very appropriate strategy for the Boston bombing survivors, since many were athletes, he said.
Dr. Jonathan Woodson fielded e-mails in his Pentagon office last April from nervous colleagues in Boston.
His fellow vascular surgeons there wanted to know if more attacks were coming in the Marathon bombings. "When you work at the Pentagon, everyone thinks you have total situational awareness," said Dr. Woodson, assistant secretary of defense for health affairs.
His former colleagues, however, also wanted tips on how to care for bombing victims, and knew that military surgeons like Dr. Woodson had answers. Before President Obama tapped him in 2010 to become the military’s top doctor and oversee the Pentagon’s $50 billion-plus medical budget, he’d scrubbed in on battlefield cases in Kosovo, Iraq, and Afghanistan, in addition to performing his vascular surgery and administrative duties at Boston University and Boston Medical Center.
"We’ve been dealing with [improvised explosive device] injuries for over a decade," said Dr. Woodson, a brigadier general in the U.S. Army Reserve.
"The good news is, I had trained some of the folks who worked" on the bombing victims, and "they knew what my techniques were." Still, there were discussions about particular fixes; hypertrophic bone growths, infections, and other wound complications; and ways to help young people recover from devastating injuries. Many of the lessons came from Dr. Woodson’s military service.
The back-and-forth about the Boston attacks highlights how important it is for military and civilian physicians to cross-pollinate. In the past, "there’s always been a cadre of usually academic surgeons and physicians who maintain contact with the military. I think [it] is very important to formalize these connections. One of my strategic imperatives is to make sure we invest in partnerships [with] academic medical centers and key professional organizations" so that war innovations make it into civilian practice, and, in turn, are remembered for future conflicts.
"There’s the potential for this expertise to be lost," said Dr. Woodson, who was finishing a paper with two colleagues on the history of military medical innovations (Semin. Vasc. Surg. 2010;23:235-42) when he was nominated to serve as the secretary of defense’s principal adviser on health issues.
There’ve been many innovations in recent years, including the recognition that extensive fasciotomies can save badly damaged limbs.
At the start of Operation Iraqi Freedom in 2003, Dr. Woodson was in northern Kuwait running a makeshift U.S. military hospital out of a commandeered Kuwaiti facility. "As the troops went over the berm, we were taking the first wave of casualties," performing surgery in full chemical-attack gear. "Luckily," the incoming Scud missiles weren’t very accurate. It was a humbling experience," he said.
A young soldier was helicoptered in with a crushed leg after a hit to his armored vehicle. "We did an interposition graft to repair the popliteal artery," and, because of severe swelling, extensive fasciotomies to prevent compartment syndrome; the soldier still had his leg when he was airlifted out. Although not standard practice at the time, that case and others like it quickly demonstrated "the benefit of doing four-compartment fasciotomies early on to preserve limbs. Fasciotomies are now [used routinely] to address these injuries," Dr. Woodson said.
Tourniquets – which "prior to this conflict had a very bad reputation" – have proved their worth in recent battles as well, a lesson that helped save lives in Boston. Likewise, the military has learned the value of temporary vascular shunts, narrow tubing – most often Argyle carotid artery shunts – to bypass tears or reconnect severed vessels when there’s no time for a definitive repair. "We [also] came to understand very early on the need for aggressive blood replacement. Previously, protocols called for lactated Ringer’s or saline infusions, but we realized they just lead to massive soft tissue edema, increased lung water," and other problems, he said.
Currently, Dr. Woodson and his military colleagues are helping coach the rehabilitation of the Boston bombing victims. Rehab has seen "major advances in the military that are now finding their way into civilian practice," including improved prosthetics; better understanding of rehabilitation psychology; and recognition of subtle traumatic brain, eye, ear, and other injuries that can derail recovery efforts, he said.
"Because soldiers were athletes before [their injury], they often have aggressive goals in mind. Some want to return to swimming, surfing, or skiing. We try to meet those goals ... to get them fully reincorporated into the lives they want to lead"; adaptive sports programs help, among other things. It’s a very appropriate strategy for the Boston bombing survivors, since many were athletes, he said.
Dr. Jonathan Woodson fielded e-mails in his Pentagon office last April from nervous colleagues in Boston.
His fellow vascular surgeons there wanted to know if more attacks were coming in the Marathon bombings. "When you work at the Pentagon, everyone thinks you have total situational awareness," said Dr. Woodson, assistant secretary of defense for health affairs.
His former colleagues, however, also wanted tips on how to care for bombing victims, and knew that military surgeons like Dr. Woodson had answers. Before President Obama tapped him in 2010 to become the military’s top doctor and oversee the Pentagon’s $50 billion-plus medical budget, he’d scrubbed in on battlefield cases in Kosovo, Iraq, and Afghanistan, in addition to performing his vascular surgery and administrative duties at Boston University and Boston Medical Center.
"We’ve been dealing with [improvised explosive device] injuries for over a decade," said Dr. Woodson, a brigadier general in the U.S. Army Reserve.
"The good news is, I had trained some of the folks who worked" on the bombing victims, and "they knew what my techniques were." Still, there were discussions about particular fixes; hypertrophic bone growths, infections, and other wound complications; and ways to help young people recover from devastating injuries. Many of the lessons came from Dr. Woodson’s military service.
The back-and-forth about the Boston attacks highlights how important it is for military and civilian physicians to cross-pollinate. In the past, "there’s always been a cadre of usually academic surgeons and physicians who maintain contact with the military. I think [it] is very important to formalize these connections. One of my strategic imperatives is to make sure we invest in partnerships [with] academic medical centers and key professional organizations" so that war innovations make it into civilian practice, and, in turn, are remembered for future conflicts.
"There’s the potential for this expertise to be lost," said Dr. Woodson, who was finishing a paper with two colleagues on the history of military medical innovations (Semin. Vasc. Surg. 2010;23:235-42) when he was nominated to serve as the secretary of defense’s principal adviser on health issues.
There’ve been many innovations in recent years, including the recognition that extensive fasciotomies can save badly damaged limbs.
At the start of Operation Iraqi Freedom in 2003, Dr. Woodson was in northern Kuwait running a makeshift U.S. military hospital out of a commandeered Kuwaiti facility. "As the troops went over the berm, we were taking the first wave of casualties," performing surgery in full chemical-attack gear. "Luckily," the incoming Scud missiles weren’t very accurate. It was a humbling experience," he said.
A young soldier was helicoptered in with a crushed leg after a hit to his armored vehicle. "We did an interposition graft to repair the popliteal artery," and, because of severe swelling, extensive fasciotomies to prevent compartment syndrome; the soldier still had his leg when he was airlifted out. Although not standard practice at the time, that case and others like it quickly demonstrated "the benefit of doing four-compartment fasciotomies early on to preserve limbs. Fasciotomies are now [used routinely] to address these injuries," Dr. Woodson said.
Tourniquets – which "prior to this conflict had a very bad reputation" – have proved their worth in recent battles as well, a lesson that helped save lives in Boston. Likewise, the military has learned the value of temporary vascular shunts, narrow tubing – most often Argyle carotid artery shunts – to bypass tears or reconnect severed vessels when there’s no time for a definitive repair. "We [also] came to understand very early on the need for aggressive blood replacement. Previously, protocols called for lactated Ringer’s or saline infusions, but we realized they just lead to massive soft tissue edema, increased lung water," and other problems, he said.
Currently, Dr. Woodson and his military colleagues are helping coach the rehabilitation of the Boston bombing victims. Rehab has seen "major advances in the military that are now finding their way into civilian practice," including improved prosthetics; better understanding of rehabilitation psychology; and recognition of subtle traumatic brain, eye, ear, and other injuries that can derail recovery efforts, he said.
"Because soldiers were athletes before [their injury], they often have aggressive goals in mind. Some want to return to swimming, surfing, or skiing. We try to meet those goals ... to get them fully reincorporated into the lives they want to lead"; adaptive sports programs help, among other things. It’s a very appropriate strategy for the Boston bombing survivors, since many were athletes, he said.